Seed Nano-Priming with Calcium Oxide Maintains the Redox State by Boosting the Antioxidant Defense System in Water-Stressed Carom (Trachyspermum ammi L.) Plants to Confer Drought Tolerance
Abstract
1. Introduction
2. Materials and Methods
2.1. Priming Experiment and Experimental Layout
2.2. Determination of Malondialdehyde Contents
2.3. Hydrogen Peroxide Contents Analysis
2.4. Determination of Electrolyte Leakage
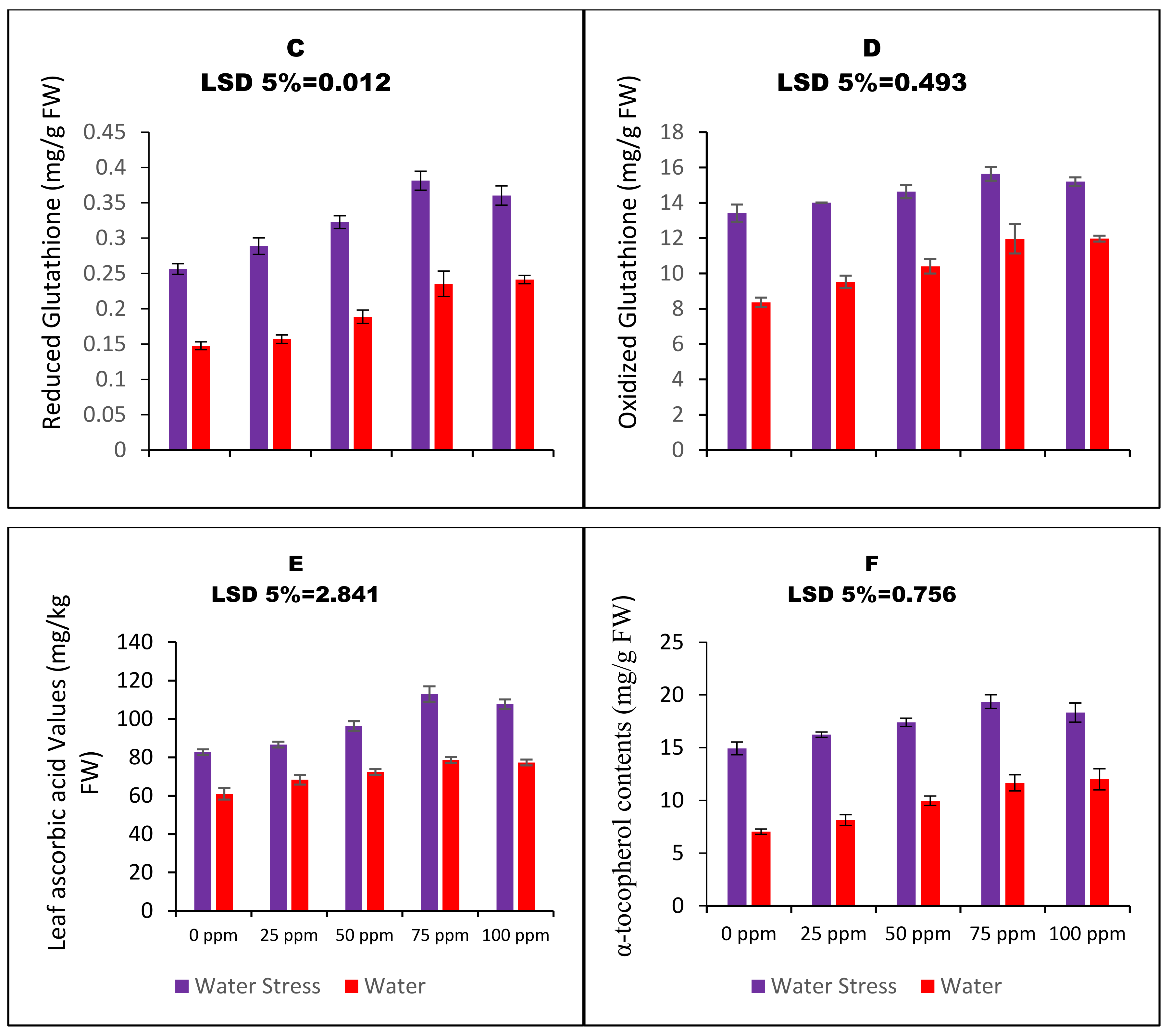
2.5. Determination of Oxidized and Reduced States of Glutathione
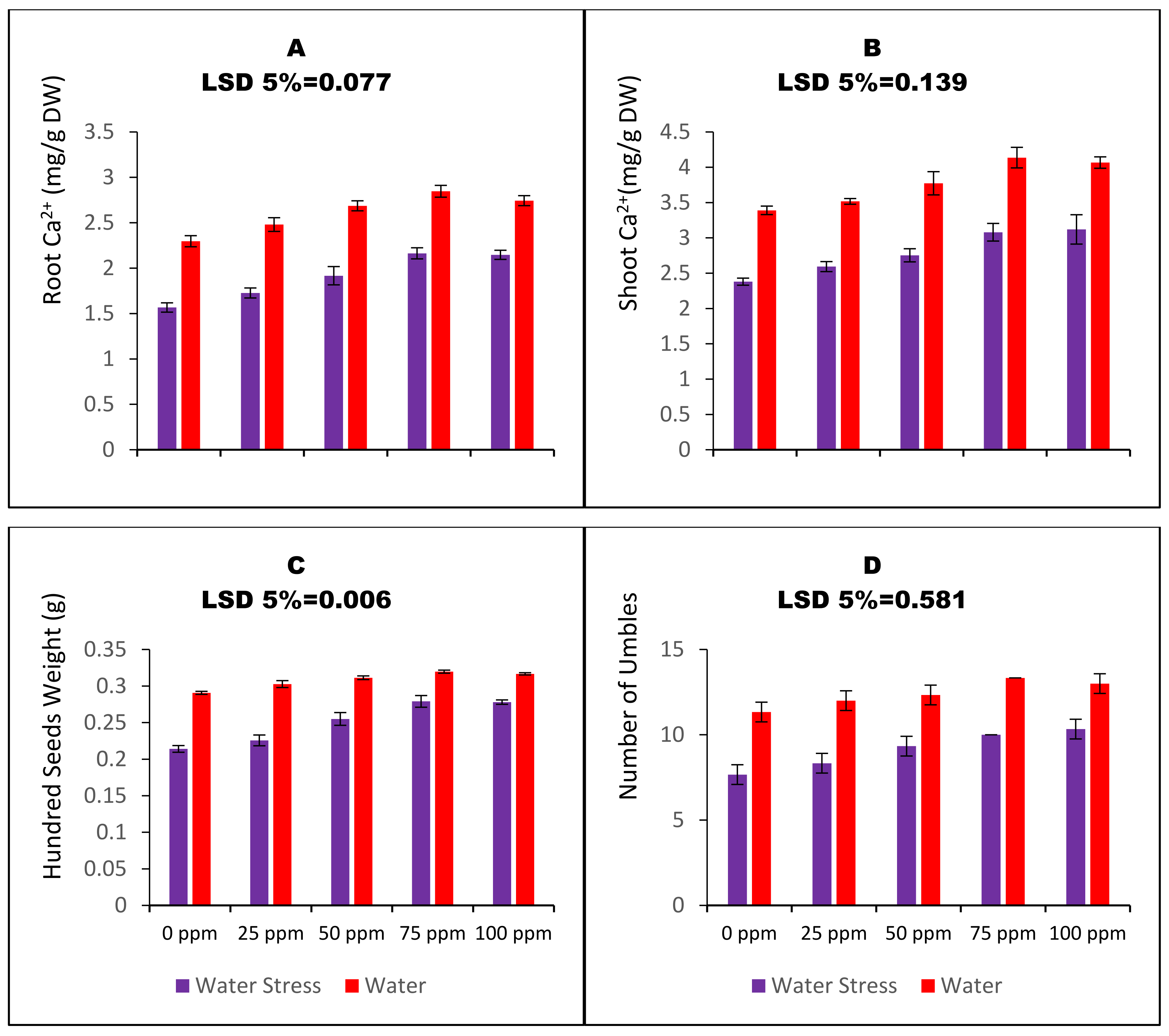
2.6. Shoot and Root Calcium Contents
2.7. Determination of Tocopherols
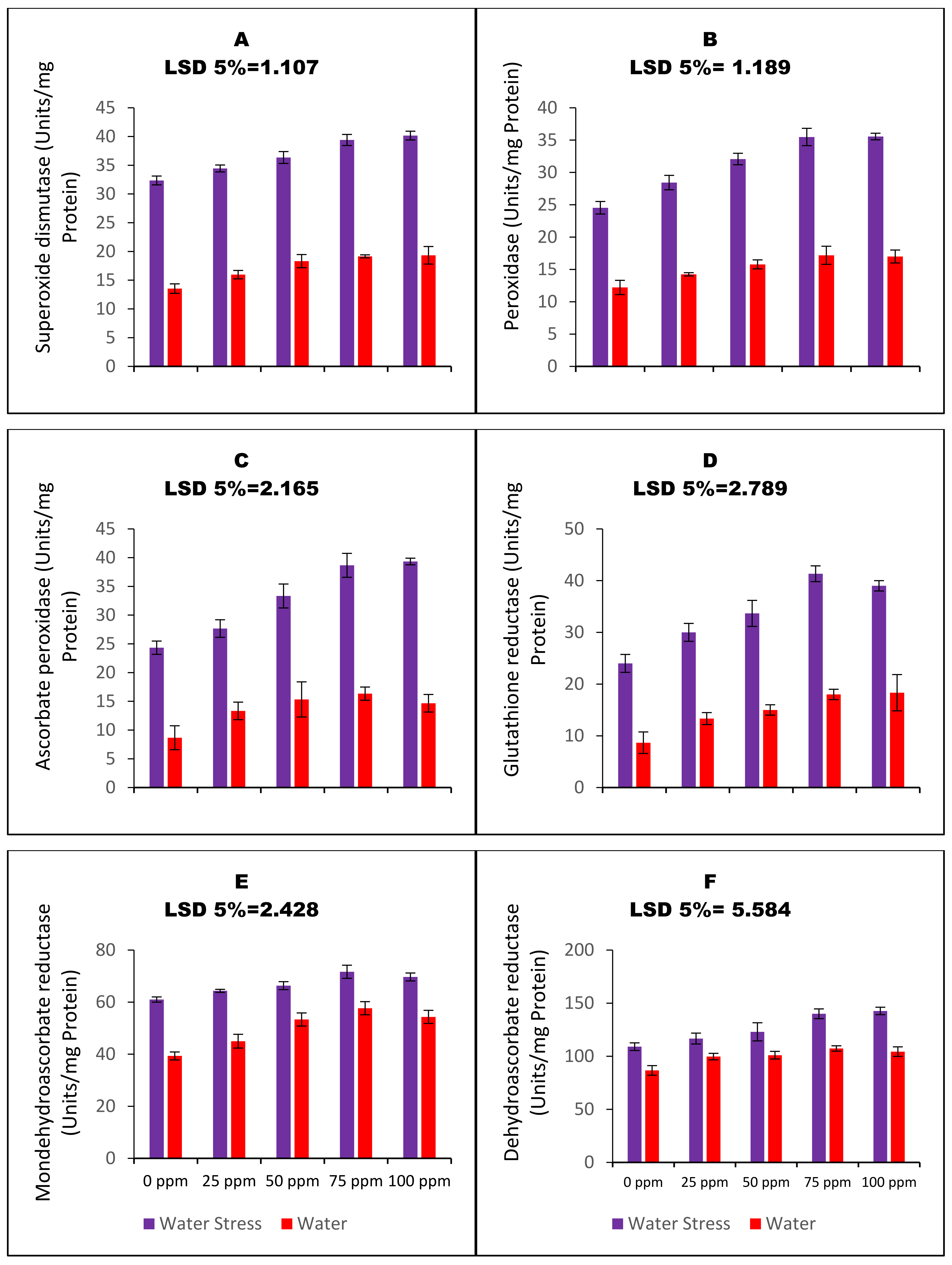
2.8. Determination of Superoxide Dismutase (SOD) and Peroxidase (POD) Activity
2.9. Determination of Glutathione S-Transferase Activity and Ascorbate Glutathione Pathway Enzymes
2.10. Determination of Ascorbic Acid (AsA) Contents
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazhar, M.W.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Bhatti, K.H.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Ajaib, M.; Hussain, I.; Hussain, T.; Parveen, A.; Thind, S.; Sardar, T.; Akram, R.; et al. Synergistic application of calcium oxide nanoparticles and farmyard manure induces cadmium tolerance in mung bean (Vigna radiata L.) by influencing physiological and biochemical parameters. PLoS ONE 2023, 18, e0282531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R.; Shahid, A.; Shokralla, S.; Al-Ghobari, H.; Alataway, A.; Dewidar, A.Z.; El-Sabrout, A.M.; et al. Seed Priming with Iron Oxide Nanoparticles Raises Biomass Production and Agronomic Profile of Water-Stressed Flax Plants. Agronomy 2022, 12, 982. [Google Scholar] [CrossRef]
- Naz, R.; Gul, F.; Zahoor, S.; Nosheen, A.; Yasmin, H.; Keyani, R.; Shahid, M.; Hassan, M.N.; Siddiqui, M.H.; Batool, S.; et al. Interactive effects of hydrogen sulphide and silicon enhance drought and heat tolerance by modulating hormones, antioxidant defence enzymes and redox status in barley (Hordeum vulgare L.). Plant Biol. 2022, 24, 684–696. [Google Scholar] [CrossRef]
- Raja, V.; Wani, U.M.; Wani, Z.A.; Jan, N.; Kottakota, C.; Reddy, M.K.; Kaul, T.; John, R. Pyramiding ascorbate–glutathione pathway in Lycopersicum esculentum confers tolerance to drought and salinity stress. Plant Cell Rep. 2022, 41, 619–637. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline metabolism as regulatory hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar] [CrossRef]
- Lee, B.-R.; La, V.H.; Park, S.-H.; Mamun, A.; Bae, D.-W.; Kim, T.-H. H2O2-Responsive Hormonal Status Involves Oxidative Burst Signaling and Proline Metabolism in Rapeseed Leaves. Antioxidants 2022, 11, 566. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Akram, R.; Shahid, A. Foliar application of iron glutamate improves yield and growth of tomatoes compared to iron sulphate and L-glutamate. Int. J. Veg. Sci. 2022, 28, 511–520. [Google Scholar] [CrossRef]
- Kaur, H.; Hussain, S.J.; Kaur, G.; Poor, P.; Alamri, S.; Siddiqui, M.H.; Khan, M.I.R. Salicylic Acid Improves Nitrogen Fixation, Growth, Yield and Antioxidant Defence Mechanisms in Chickpea Genotypes Under Salt Stress. J. Plant Growth Regul. 2022, 41, 2034–2047. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R. Efficacy of zinc oxide nanoparticles on bitter gourd growth, yield, and some phytochemicals through seed priming. Int. J. Veg. Sci. 2022, 29, 145–155. [Google Scholar] [CrossRef]
- Thakur, M.; Tiwari, S.; Kataria, S.; Anand, A. Recent advances in seed priming strategies for enhancing planting value of vegetable seeds. Sci. Hortic. 2022, 305, 111355. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Tania, S.S.; Imran, S.; Rauf, F.; Kibria, M.G.; Ye, W.; Hasanuzzaman, M.; Murata, Y. Seed Priming with Nanoparticles: An Emerging Technique for Improving Plant Growth, Development, and Abiotic Stress Tolerance. J. Soil Sci. Plant Nutr. 2022, 22, 4047–4062. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R. Seed priming with Calcium oxide nanoparticles improves germination, biomass, antioxidant defence and yield traits of canola plants under drought stress. South Afr. J. Bot. 2022, 151, 889–899. [Google Scholar] [CrossRef]
- Nabgan, W.; Nabgan, B.; Ikram, M.; Jadhav, A.H.; Ali, M.W.; Ul-Hamid, A.; Nam, H.; Lakshminarayana, P.; Kumar, A.; Bahari, M.B.; et al. Synthesis and catalytic properties of calcium oxide obtained from organic ash over a titanium nanocatalyst for biodiesel production from dairy scum. Chemosphere 2022, 290, 133296. [Google Scholar] [CrossRef]
- Nazir, M.M.; Li, Q.; Noman, M.; Ulhassan, Z.; Ali, S.; Ahmed, T.; Zeng, F.; Zhang, G. Calcium Oxide Nanoparticles Have the Role of Alleviating Arsenic Toxicity of Barley. Front. Plant Sci. 2022, 13, 843795. [Google Scholar] [CrossRef] [PubMed]
- Mirniyam, G.; Rahimmalek, M.; Arzani, A.; Matkowski, A.; Gharibi, S.; Szumny, A. Changes in Essential Oil Composition, Polyphenolic Compounds and Antioxidant Capacity of Ajowan (Trachyspermum ammi L.) Populations in Response to Water Deficit. Foods 2022, 11, 3084. [Google Scholar] [CrossRef]
- Modareskia, M.; Fattahi, M.; Mirjalili, M.H. Thymol screening, phenolic contents, antioxidant and antibacterial activities of Iranian populations of Trachyspermum ammi (L.) Sprague (Apiaceae). Sci. Rep. 2022, 12, 15645. [Google Scholar] [CrossRef]
- Naquvi, K.J.; Ansari, S.H.; Salma, A.; Ahamad, J.; Najib, S. A Review on Phytochemical Investigations and Biological Activities of Trachyspermum ammi (L.) Sprague. Res. J. Pharm. Technol. 2022, 15, 2364–2370. [Google Scholar] [CrossRef]
- Kim, N.H.; Jacob, P.; Dangl, J.L. Con-Ca2+-tenating plant immune responses via calcium-permeable cation channels. New Phytol. 2022, 234, 813–818. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R. Seed priming with zinc oxide nanoparticles improves growth, osmolyte accumulation, antioxidant defence and yield quality of water-stressed mung bean plants. Arid. Land Res. Manag. 2022, 37, 222–246. [Google Scholar] [CrossRef]
- Ali, Q.; Shahid, S.; Ali, S.; El-Esawi, M.A.; Hussain, A.I.; Perveen, R.; Iqbal, N.; Rizwan, M.; Nasser Alyemeni, M.; El-Serehy, H.A.; et al. Fertigation of Ajwain (Trachyspermum ammi L.) with Fe-Glutamate confers better plant performance and drought tolerance in comparison with FeSO4. Sustainability 2020, 12, 7119. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Campos, P.S.; Quartin, V.N.; Ramalho, J.; Nunes, M.A. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Wolf, B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Baker, J.L., Jr. The effectiveness of alpha-tocopherol (vitamin E) in reducing the incidence of spherical contracture around breast implants. Plast. Reconstr. Surg. 1981, 68, 696–698. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Hasanuzzaman, M.; Fujita, M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 2013, 22, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foster, J.G.; Hess, J.L. Responses of Superoxide Dismutase and Glutathione Reductase Activities in Cotton Leaf Tissue Exposed to an Atmosphere Enriched in Oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C.; Asada, K. Thylakoid-Bound Ascorbate Peroxidase in Spinach Chloroplasts and Photoreduction of Its Primary Oxidation Product Monodehydroascorbate Radicals in Thylakoids. Plant Cell Physiol. 1992, 33, 541–553. [Google Scholar]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Qiu, Y.; Xi, J.; Du, L.; Suttle, J.C.; Poovaiah, B.W. Coupling calcium/calmodulin-mediated signaling and herbivore-induced plant response through calmodulin-binding transcription factor AtSR1/CAMTA3. Plant Mol. Biol. 2012, 79, 89–99. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003, 8, 505–512. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Qin, F.; Osakabe, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18822–18827. [Google Scholar] [CrossRef]
- Marcec, M.J.; Tanaka, K. Crosstalk between Calcium and ROS Signaling during Flg22-Triggered Immune Response in Arabidopsis Leaves. Plants 2022, 11, 14. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Ghosh, S.; Bheri, M.; Bisht, D.; Pandey, G.K. Calcium signaling and transport machinery: Potential for development of stress tolerance in plants. Curr. Plant Biol. 2022, 29, 100235. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Devika, O.S.; Singh, S.; Sarkar, D.; Barnwal, P.; Suman, J.; Rakshit, A. Seed Priming: A Potential Supplement in Integrated Resource Management Under Fragile Intensive Ecosystems. Front. Sustain. Food Syst. 2021, 5, 654001. [Google Scholar] [CrossRef]
- Sarkar, D.; Rakshit, A. Safeguarding the fragile rice–wheat ecosystem of the Indo-Gangetic Plains through bio-priming and bioaugmentation interventions. FEMS Microbiol. Ecol. 2020, 96, fiaa221. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Ni, F.; Rizwan, M.; Fahad, S.; Hu, L. Morpho-physiological and biochemical responses of tolerant and sensitive rapeseed cultivars to drought stress during early seedling growth stage. Acta Physiol. Plant. 2019, 41, 25. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Réthoré, E.; Pluchon, S.; Ali, N.; Billiot, B.; Yvin, J.C. Calcium application enhances drought stress tolerance in sugar beet and promotes plant biomass and beetroot sucrose concentration. Int. J. Mol. Sci. 2019, 20, 3777. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, T.; Masuda, T.; Lv, C.; Sun, L.; Qu, G.; Zhao, G. Chitinase III in pomegranate seeds (Punica granatum Linn.): A high-capacity calcium-binding protein in amyloplasts. Plant J. 2011, 68, 765–776. [Google Scholar] [CrossRef]
- Ahmed, R.; Uddin, K.; Quddus, A.; Samad, M.Y.A.; Hossain, M.A.M.; Haque, A.N.A. Impact of Foliar Application of Zinc and Zinc Oxide Nanoparticles on Growth, Yield, Nutrient Uptake and Quality of Tomato. Horticulturae 2023, 9, 162. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Selwey, W.A.; Ibrahim, A.A.; Shady, M.; Alsadon, A.A. Foliar Applications of ZnO and SiO2 Nanoparticles Mitigate Water Deficit and Enhance Potato Yield and Quality Traits. Agronomy 2023, 13, 466. [Google Scholar] [CrossRef]
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized Biodiesel Production from Waste Cooking Oil (WCO) using Calcium Oxide (CaO) Nano-catalyst. Sci. Rep. 2019, 9, 18982. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free. Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, R. Modulation of Ethylene and Ascorbic Acid on Reactive Oxygen Species Scavenging in Plant Salt Response. Front. Plant Sci. 2019, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, H.I.; Sofy, M.R. Role of Ascorbic acid, Glutathione and Proline Applied as Singly or in Sequence Combination in Improving Chickpea Plant through Physiological Change and Antioxidant Defense under Different Levels of Irrigation Intervals. Molecules 2020, 25, 1702. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Mallhi, Z.I.; Khan, N.; Rizwan, M.; Ali, S.; Ahmad, A.; Hussain, A.; Alsahli, A.A.; Alyemeni, M.N. Combined Citric Acid and Glutathione Augments Lead (Pb) Stress Tolerance and Phytoremediation of Castorbean through Antioxidant Machinery and Pb Uptake. Sustainability 2021, 13, 4073. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Polash, M.A.S.; Chowdhury, M.B.; Afrin, S.; Burritt, D.J.; Murata, Y.; Hossain, M.A.; Hossain, M.A. Exogenous Glutathione-Mediated Drought Stress Tolerance in Rice (Oryza sativa L.) is Associated with Lower Oxidative Damage and Favorable Ionic Homeostasis. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 955–971. [Google Scholar] [CrossRef]
- Zhou, Y.; Diao, M.; Chen, X.; Cui, J.; Pang, S.; Li, Y.; Hou, C.; Liu, H.-Y. Application of exogenous glutathione confers salinity stress tolerance in tomato seedlings by modulating ions homeostasis and polyamine metabolism. Sci. Hortic. 2019, 250, 45–58. [Google Scholar] [CrossRef]
- Chen, J.; Duan, R.-X.; Hu, W.-J.; Zhang, N.-N.; Lin, X.-Y.; Zhang, J.-H.; Zheng, H.-L. Unravelling calcium-alleviated aluminium toxicity in Arabidopsis thaliana: Insights into regulatory mechanisms using proteomics. J. Proteom. 2019, 199, 15–30. [Google Scholar] [CrossRef]
- Salleh, M.S.; Nordin, M.S.; Puteh, A.B. Germination performance and biochemical changes under drought stress of primed rice seeds. Seed Sci. Technol. 2020, 48, 333–343. [Google Scholar] [CrossRef]
- Kiran, K.R.; Deepika, V.B.; Swathy, P.S.; Prasad, K.; Kabekkodu, S.P.; Murali, T.S.; Satyamoorthy, K.; Muthusamy, A. ROS-dependent DNA damage and repair during germination of NaCl primed seeds. J. Photochem. Photobiol. B Biol. 2020, 213, 112050. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Cai, C.; Fan, S.; Luo, H. Physiological Response Characteristics of Moso Bamboo under Drought Stress Based on Calcium Signal. Forests 2021, 12, 1699. [Google Scholar] [CrossRef]
- Shan, C.; Wang, T.; Zhou, Y.; Wang, W. Hydrogen sulfide is involved in the regulation of ascorbate and glutathione metabolism by jasmonic acid in Arabidopsis thaliana. Biol. Plant. 2018, 62, 188–193. [Google Scholar] [CrossRef]
- Ahmad, P.; Sarwat, M.; Bhat, N.A.; Wani, M.R.; Kazi, A.G.; Tran, L.-S.P. Alleviation of Cadmium Toxicity in Brassica juncea L. (Czern. & Coss.) by Calcium Application Involves Various Physiological and Biochemical Strategies. PLoS ONE 2015, 10, e0114571. [Google Scholar]
- Szarka, A.; Tomasskovics, B.; Bánhegyi, G. The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int. J. Mol. Sci. 2012, 13, 4458–4483. [Google Scholar] [CrossRef] [PubMed]
- Kadam, U.S.; Schulz, B.; Irudayaraj, J.M. Multiplex single-cell quantification of rare RNA transcripts from protoplasts in a model plant system. Plant J. 2017, 90, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
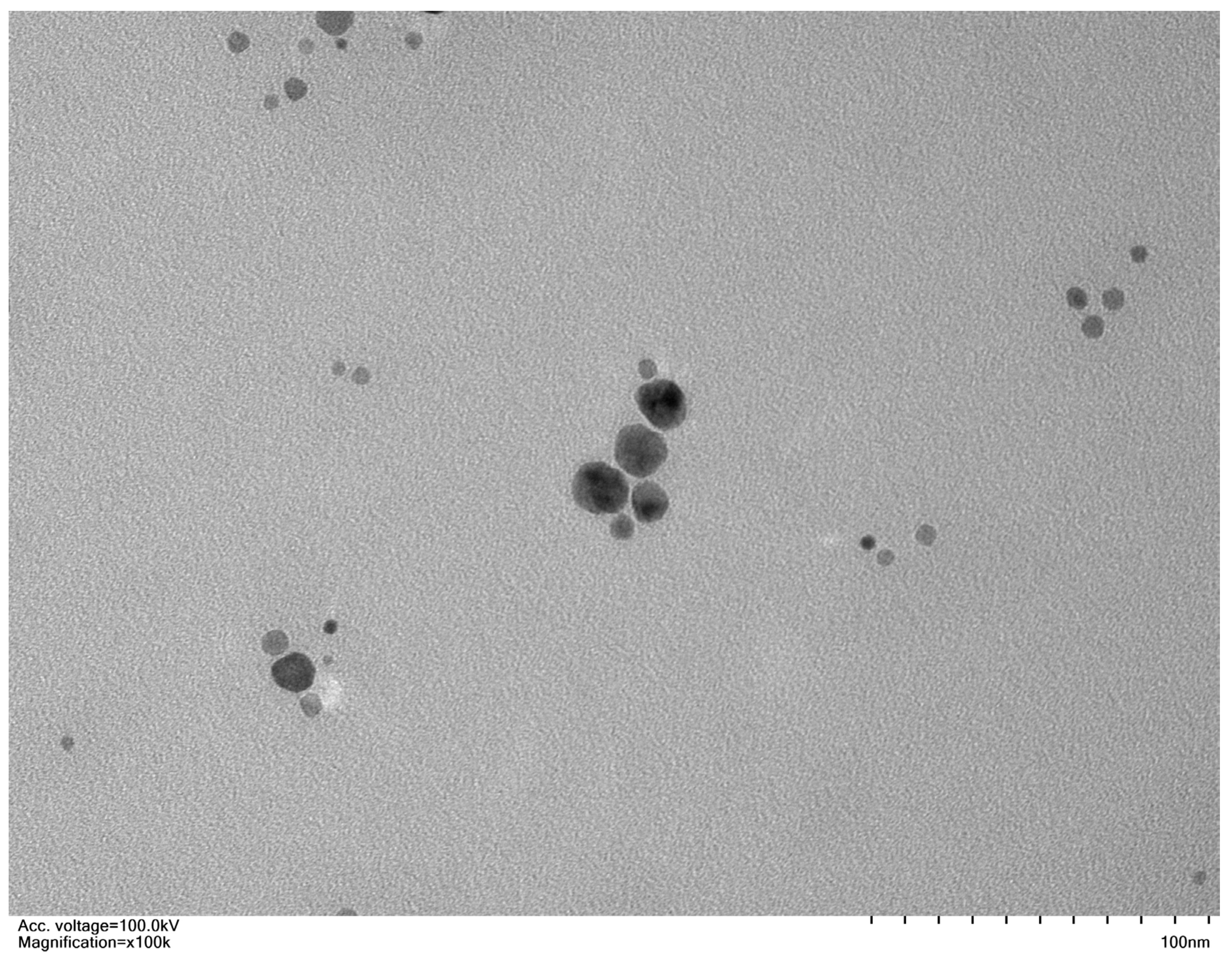
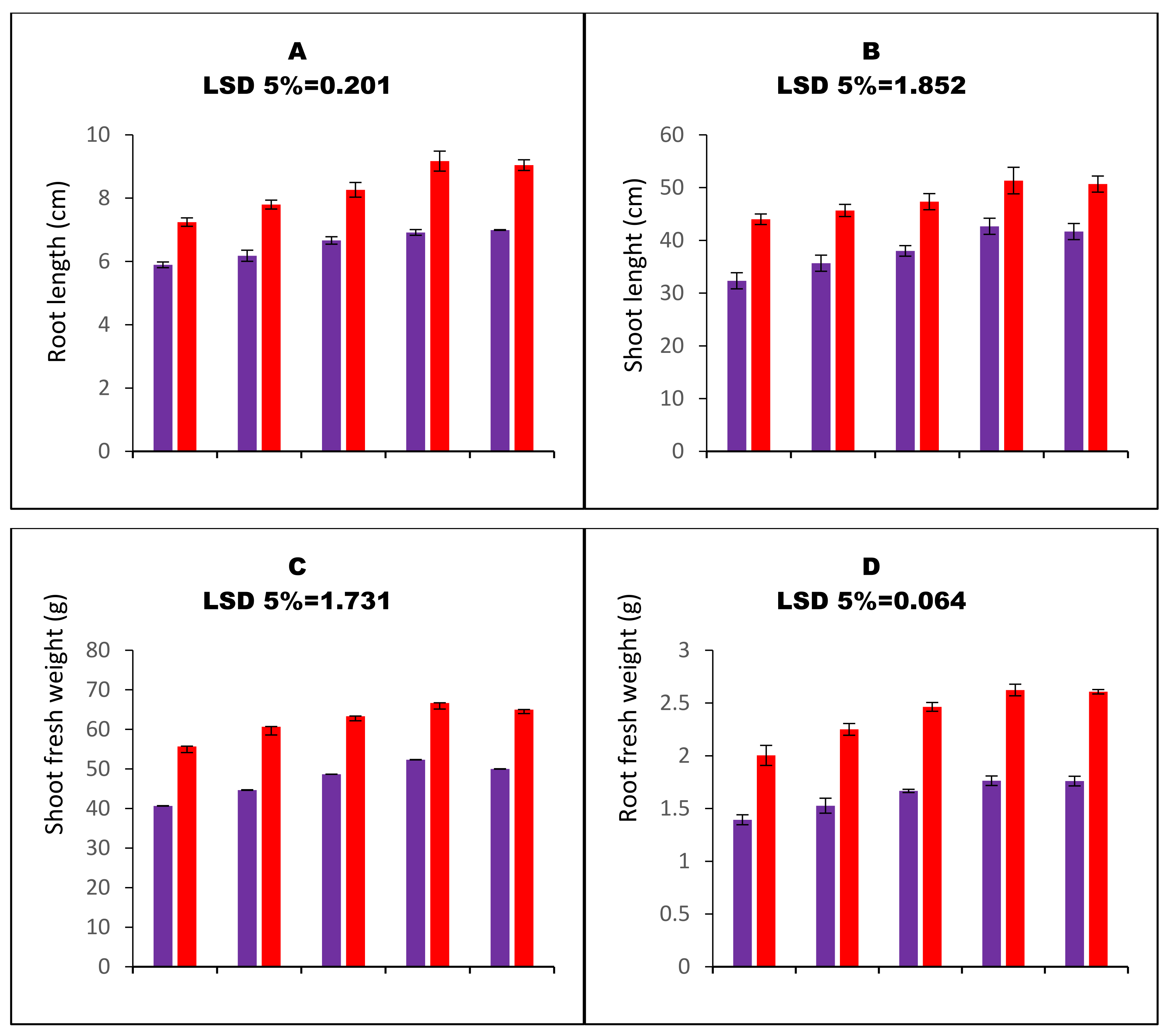
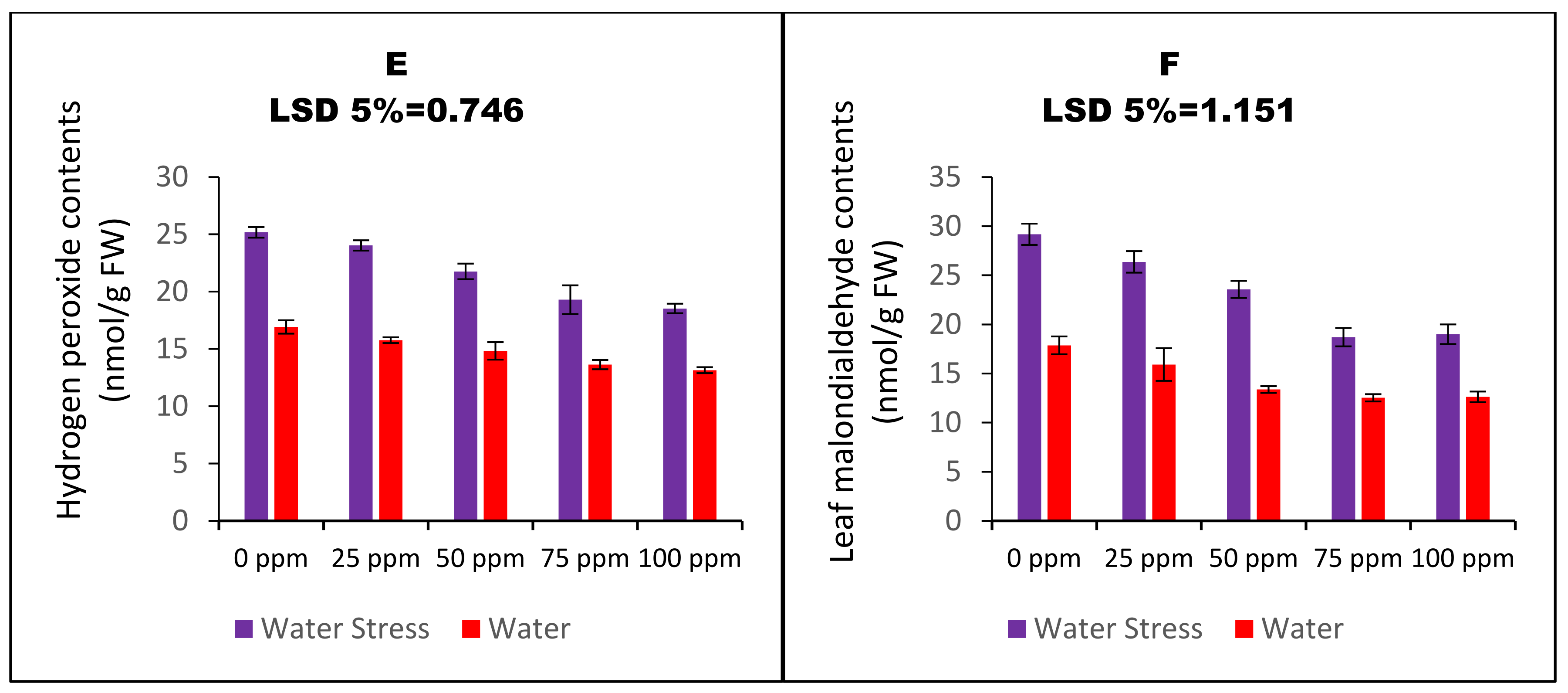
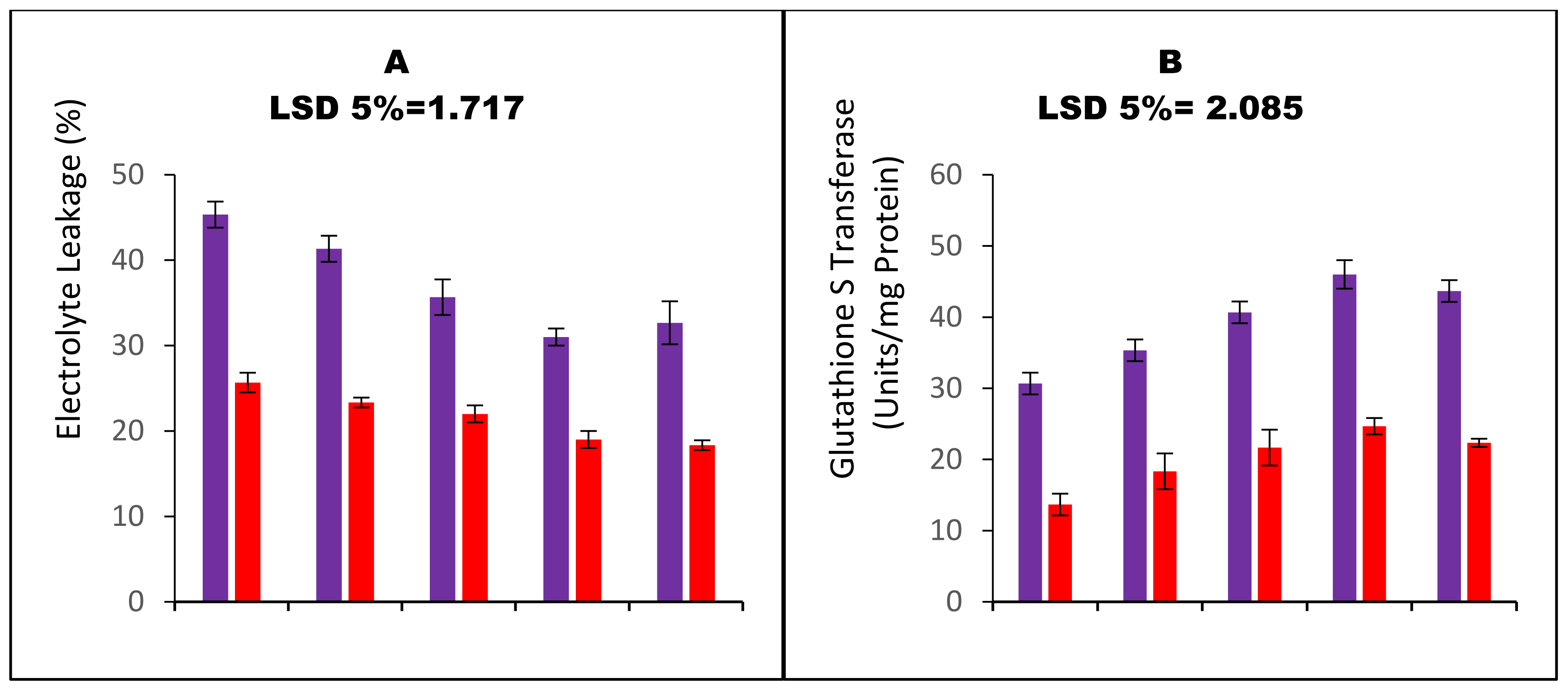
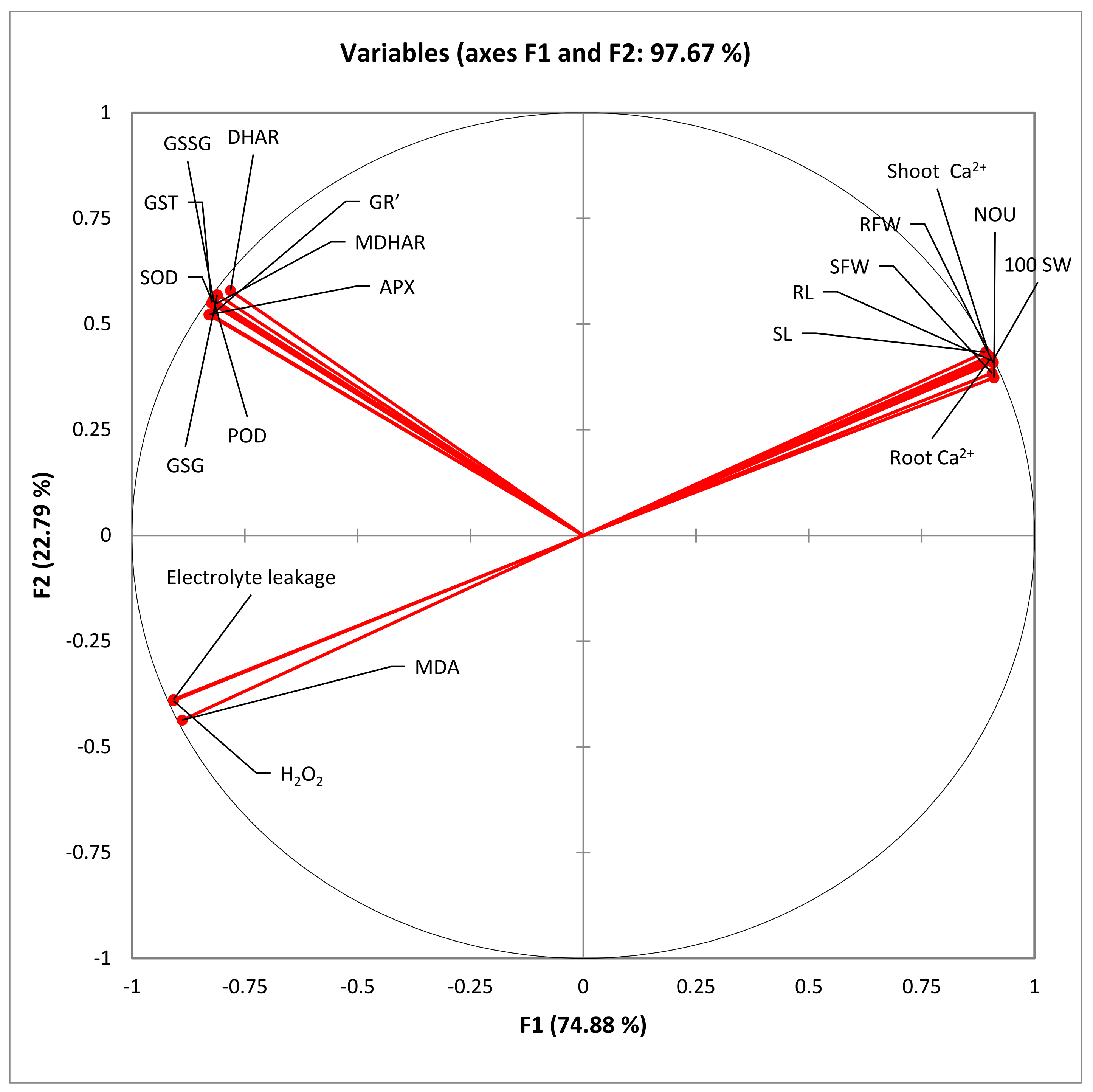
| Variables | a H2O2 Values | MDA Contents | E Leakage | SOD Activity | POD Activity | GST Activity | APX Activity | GR’ Activity | MDHAR Activity | DHAR activity | GSG Activity | GSSG Activity | Root Ca2+ | Shoot Ca2+ | 100 SW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O2 | 1 | ||||||||||||||
| MDA | 0.977 * | 1 | |||||||||||||
| E Leakage | 0.977 * | 0.978 * | 1 | ||||||||||||
| SOD | 0.521 * | 0.494 * | 0.536 * | 1 | |||||||||||
| POD | 0.515 * | 0.488 * | 0.532 * | 0.988 * | 1 | ||||||||||
| GST | 0.546 * | 0.492 * | 0.538 * | 0.976 * | 0.972 * | 1 | |||||||||
| APX | 0.550 * | 0.518 * | 0.564 * | 0.977 * | 0.980 * | 0.969 * | 1 | ||||||||
| GR | 0.553 * | 0.490 * | 0.527 * | 0.944 * | 0.952 * | 0.973 * | 0.933 * | 1 | |||||||
| MDHAR | 0.538 * | 0.476 * | 0.534 * | 0.975 * | 0.965 * | 0.984 * | 0.965 * | 0.963 * | 1 | ||||||
| DHAR | 0.479 * | 0.458 * | 0.488 * | 0.969 * | 0.965 * | 0.956 * | 0.950 * | 0.924 * | 0.935 * | 1 | |||||
| GSG | 0.511 * | 0.472 * | 0.511 * | 0.974 * | 0.985 * | 0.973 * | 0.958 * | 0.970 * | 0.965 * | 0.961 * | 1 | ||||
| GSSG | 0.527 * | 0.481 * | 0.520 * | 0.970 * | 0.980 * | 0.976 * | 0.953 * | 0.980 * | 0.968 * | 0.952 * | 0.995 * | 1 | |||
| Root Ca2+ | −0.975 * | −0.974 * | −0.978 * | −0.511 * | −0.507 * | −0.508 * | −0.521 * | −0.531 * | −0.514 * | −0.460 * | −0.496 * | −0.511 * | 1 | ||
| Shoot Ca2+ | −0.985 * | −0.984 * | −0.972 * | −0.499 * | −0.496 * | −0.510 * | −0.520 * | −0.535 * | −0.509 * | −0.453 * | −0.491 * | −0.507 * | 0.989 * | 1 | |
| 100 SW | −0.978 * | −0.990 * | −0.980 * | −0.518 * | −0.521 * | −0.516 * | −0.538 * | −0.526 * | −0.508 * | −0.472 * | −0.507 * | −0.519 * | 0.988 * | 0.990 * | 1 |
| NOU | −0.971 * | −0.968 * | −0.956 * | −0.540 * | −0.538 * | −0.540 * | −0.552 * | −0.560 * | −0.543 * | −0.499 * | −0.529 * | −0.537 * | 0.972 * | 0.980 * | 0.975 * |
| Variation Source | a df | 100 SW | NOU | RL | SL | RFW | Ascorbic Acid | α-Tocopherols |
|---|---|---|---|---|---|---|---|---|
| Water Stress (WS) | 1 | 0.025 b,*** (0.000) | 80.033 *** (0.000) | 23.603 *** (0.000) | 710.533 *** (0.000) | 4.416 *** (0.000) | 4966.345 *** (0.000) | 421.12 *** (0.000) |
| CaONPs Seed Priming (SP) | 4 | 0.002 *** (0.000) | 5.383 *** (0.000) | 2.490 *** (0.000) | 82.383 *** (0.000) | 0.267 *** (0.000) | 602.8 *** (0.000) | 22.418 *** (0.000) |
| WS X SP | 4 | 0.001 *** (0.000) | 0.283 ns (0.335) | 0.202 *** (0.000) | 2.116 ns (0.451) | 0.015 ** (0.003) | 63.553 *** (0.000) | 0.722 ns (0.162) |
| Error | 20 | 0.00 | 0.233 | 0.028 | 2.366 | 0.002 | 5.566 | 0.394 |
| Variation Source | df | Electrolyte Leakage | H2O2 | MDA | SOD | POD | GST | APX |
| Water Stress (WS) | 1 | 1809.633 *** (0.000) | 357.213 *** (0.000) | 594.075 *** (0.000) | 2788.633 *** (0.000) | 1904.192 *** (0.000) | 2745.63 *** (0.000) | 2707.5 *** (0.000) |
| CaONPs Seed Priming (SP) | 4 | 121.05 *** (0.000) | 29.275 *** (0.000) | 70.365 *** (0.000) | 48.783 *** (0.000) | 69.852 *** (0.000) | 164.616 *** (0.000) | 129.58 *** (0.000) |
| WS X SP | 4 | 15.216 *** (0.000) | 2.824 *** (0.000) | 8.878 *** (0.000) | 2.164 ns (0.070) | 10.733 *** (0.000) | 7.05 ns (0.089) | 28.916 *** ns (0.000) |
| Error | 20 | 2.033 | 0.384 | 0.914 | 0.846 | 0.974 | 3 | 0.001 |
| Variation Source | df | GR | MDHAR | DHAR | GSG | GSSG | Root Calcium | Shoot Calcium |
| Water Stress (WS) | 1 | 2688.533 *** (0.000) | 2083.333 *** (0.000) | 5253.633 *** (0.000) | 5096.33 *** (0.000) | 127.965 *** (0.000) | 3.745 *** (0.000) | 7.370 *** (0.000) |
| CaONPs Seed Priming (SP) | 4 | 177.2 *** (0.000) | 203.883 *** (0.000) | 717.616 *** (0.000) | 492.283 *** (0.000) | 9.015 *** (0.000) | 0.342 ***(0.000) | 0.619 ***(0.000) |
| WS X SP | 4 | 15.2 ns (0.051) | 20.416 ** (0.005) | 115.716 ** (0.004) | 67.783 *** (0.000) | 0.741 * (0.010) | 0.007 ns (0.181) | 0.004 ns (0.8512) |
| Error | 20 | 5.36 | 4.066 | 2.528 | 6.133 | 0.168 | 0.004 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Atiq Hussain, S.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Seed Nano-Priming with Calcium Oxide Maintains the Redox State by Boosting the Antioxidant Defense System in Water-Stressed Carom (Trachyspermum ammi L.) Plants to Confer Drought Tolerance. Nanomaterials 2023, 13, 1453. https://doi.org/10.3390/nano13091453
Mazhar MW, Ishtiaq M, Maqbool M, Atiq Hussain S, Casini R, Abd-ElGawad AM, Elansary HO. Seed Nano-Priming with Calcium Oxide Maintains the Redox State by Boosting the Antioxidant Defense System in Water-Stressed Carom (Trachyspermum ammi L.) Plants to Confer Drought Tolerance. Nanomaterials. 2023; 13(9):1453. https://doi.org/10.3390/nano13091453
Chicago/Turabian StyleMazhar, Muhammad Waqas, Muhammad Ishtiaq, Mehwish Maqbool, Syed Atiq Hussain, Ryan Casini, Ahmed M. Abd-ElGawad, and Hosam O. Elansary. 2023. "Seed Nano-Priming with Calcium Oxide Maintains the Redox State by Boosting the Antioxidant Defense System in Water-Stressed Carom (Trachyspermum ammi L.) Plants to Confer Drought Tolerance" Nanomaterials 13, no. 9: 1453. https://doi.org/10.3390/nano13091453
APA StyleMazhar, M. W., Ishtiaq, M., Maqbool, M., Atiq Hussain, S., Casini, R., Abd-ElGawad, A. M., & Elansary, H. O. (2023). Seed Nano-Priming with Calcium Oxide Maintains the Redox State by Boosting the Antioxidant Defense System in Water-Stressed Carom (Trachyspermum ammi L.) Plants to Confer Drought Tolerance. Nanomaterials, 13(9), 1453. https://doi.org/10.3390/nano13091453








