Cytotoxicity of Carbon Nanotubes, Graphene, Fullerenes, and Dots
Abstract
:1. Introduction
2. Cytotoxicity Issues
- Materials characterization, and theoretical considerations,
- Cell viability,
- Drug release.
3. Material Characterization
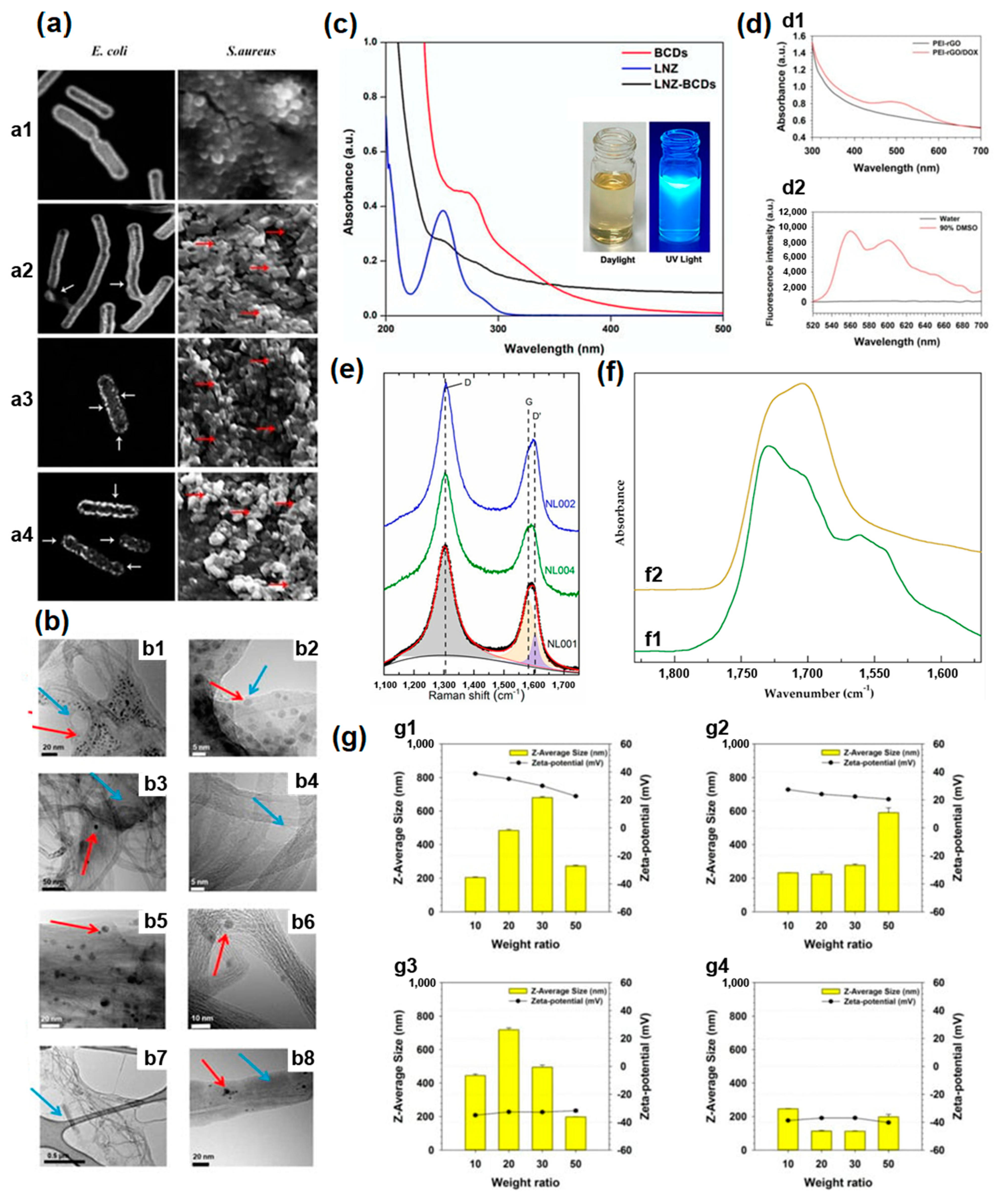
3.1. Microscopy
3.2. Spectroscopy
3.2.1. Optical Absorption Spectroscopy
3.2.2. Raman Spectroscopy
3.2.3. Fourier Transformed Infrared Spectroscopy
3.3. Other Methods
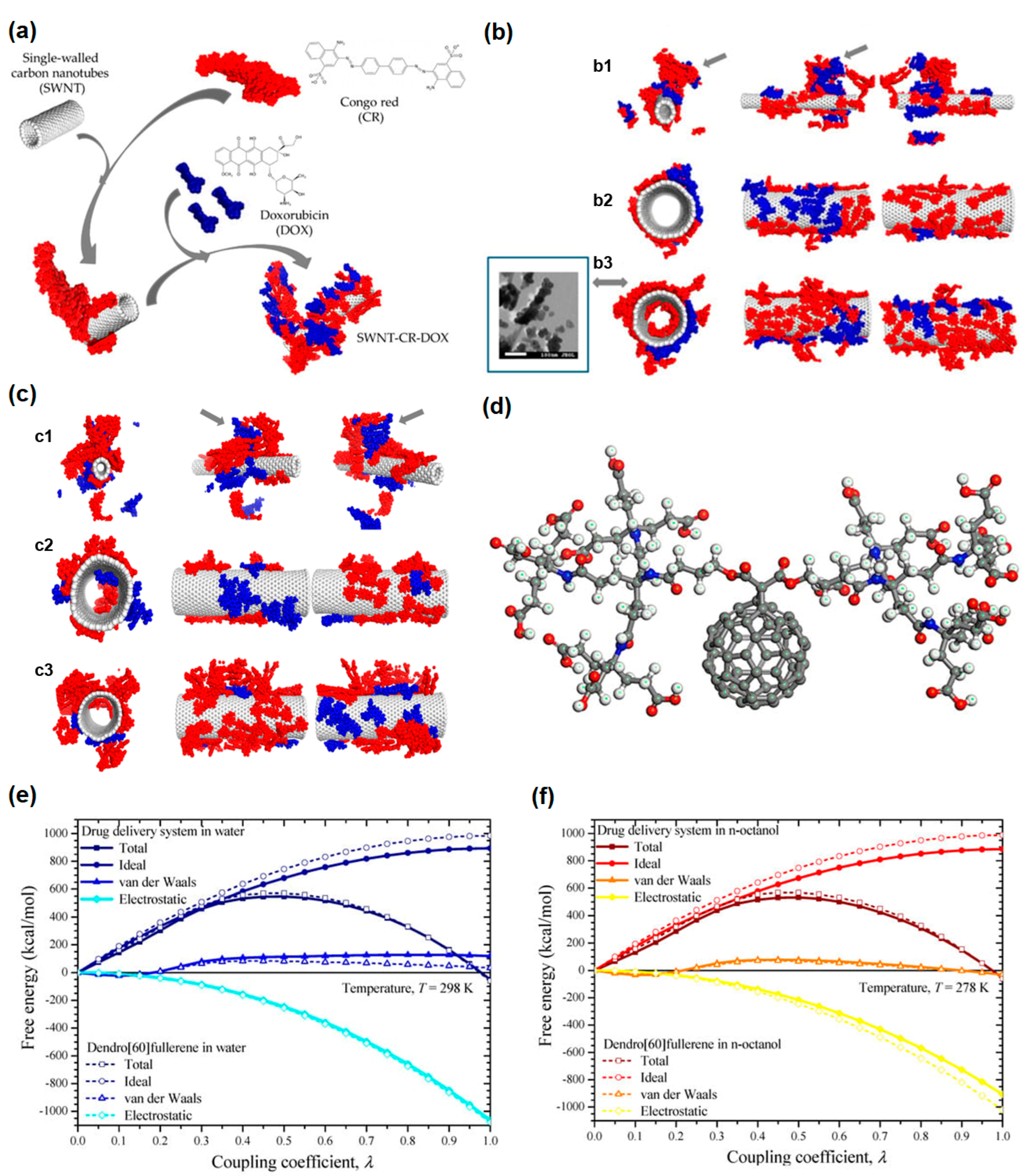
4. Theoretical Methods
5. Biological Effect of Nanomaterials upon Bacteria Cells In Vitro and In Vivo
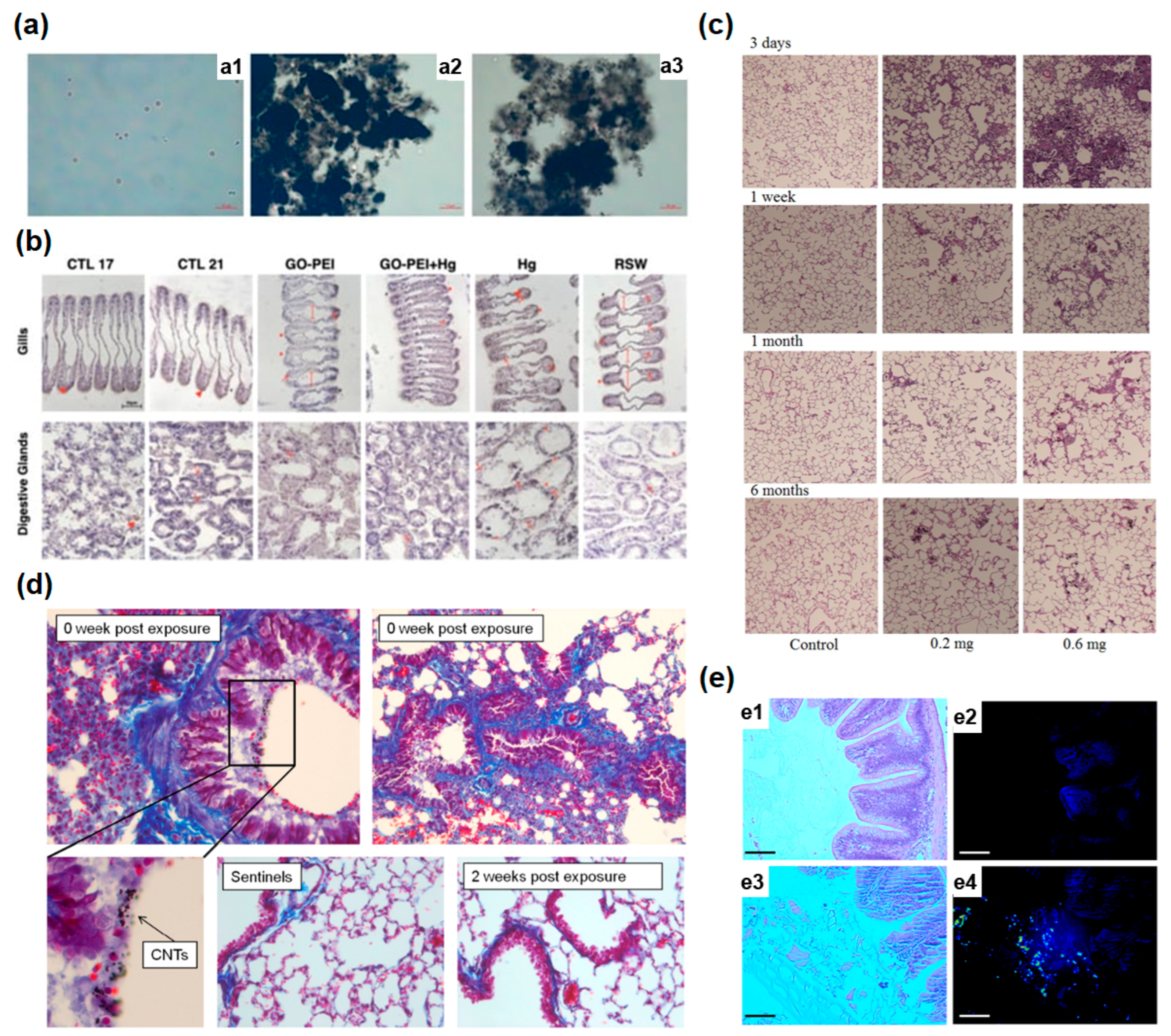
5.1. Biological Effect of Nanomaterials upon Bacteria
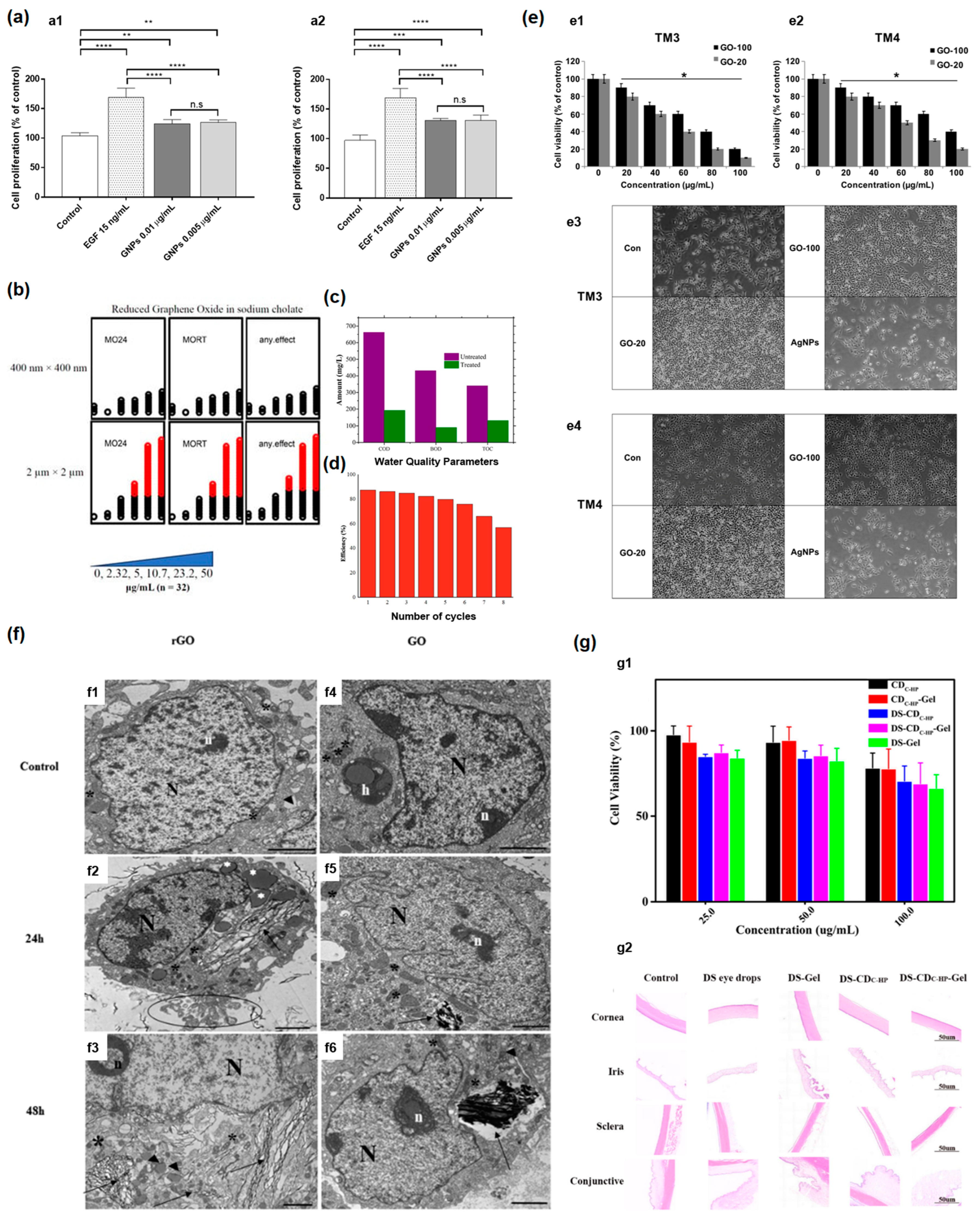
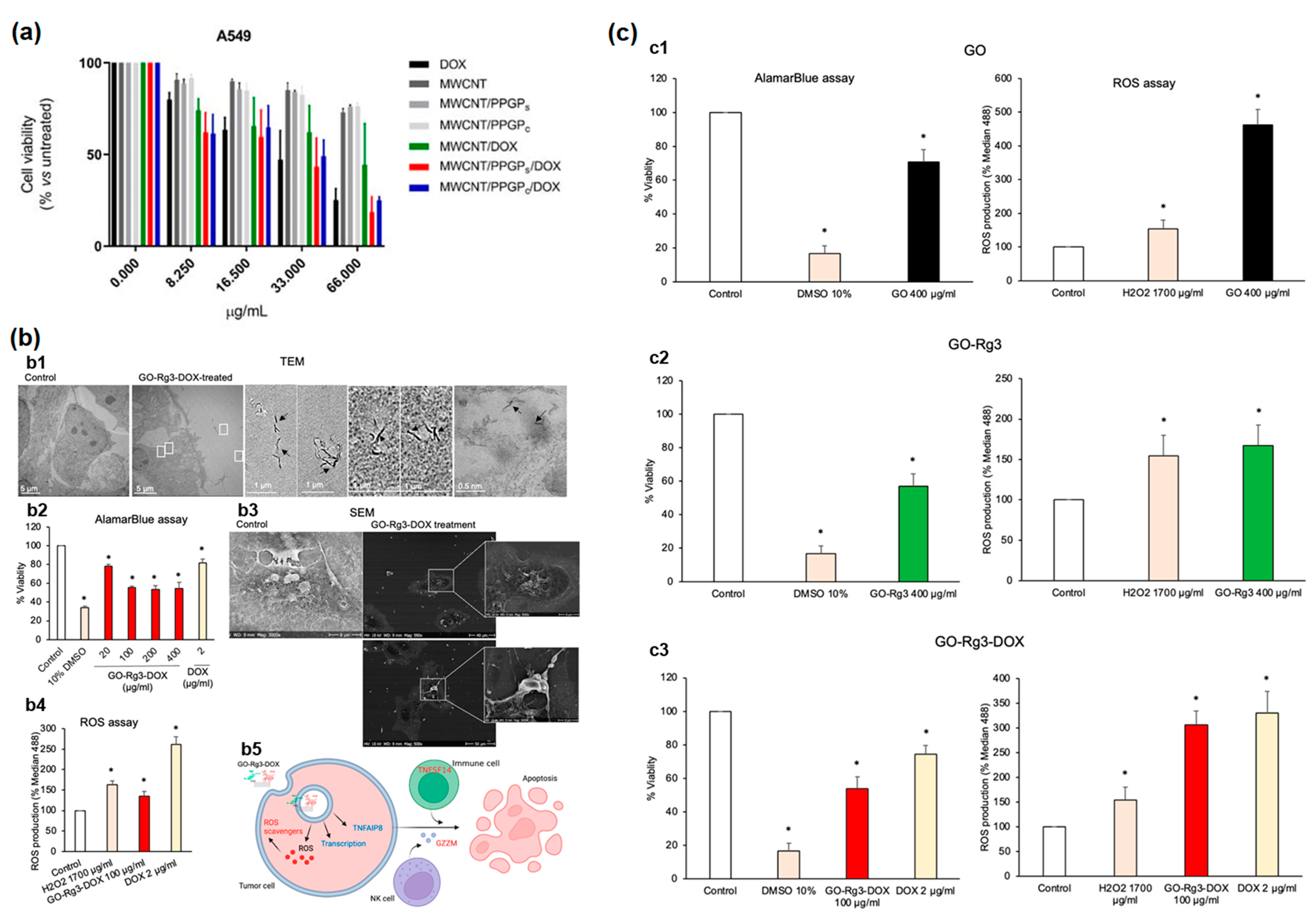
5.2. Biological Effect of Nanomaterials upon Cell (In Vitro)
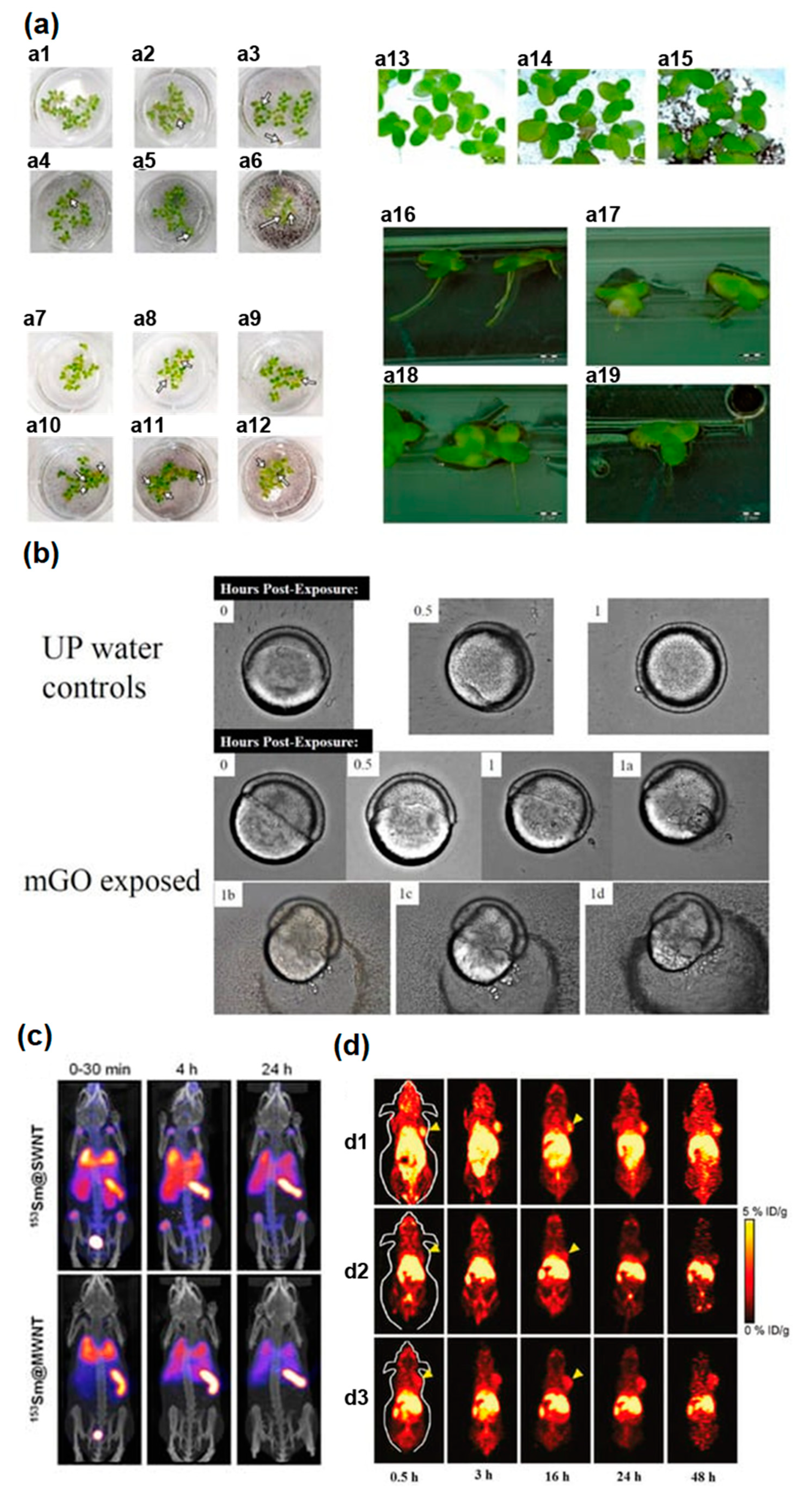
5.3. Biological Effect of Nano Materials upon In Vivo
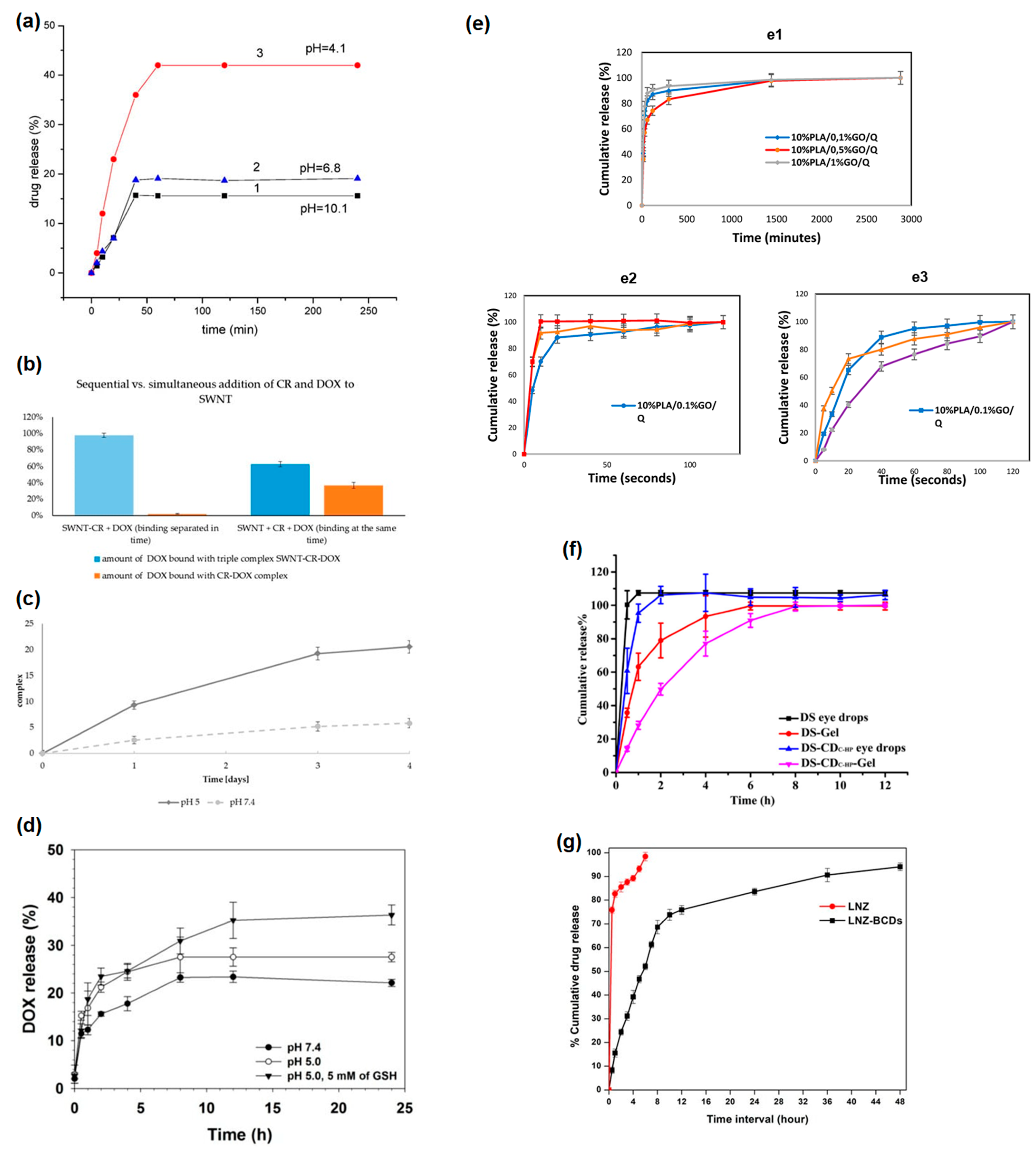
6. Different Biological Effects on Drug and Nanomaterial + Drug
6.1. Drug Toxicity and Nanomaterial + Drug Toxicity
6.2. Synergy or Antagonistic Effect
6.3. Advantages and Disadvantages of Using Nanomaterials
- BCDs have a small size, suitable optical and photoluminescence properties, and good photostability, and that is why they are promising nanocarriers of LNZ,
- LNZ–BCDs nanocomposites show biphase release, which is important for improving tissue healing,
- LNZ–BCDs nanocomposites were shown to have good biocompatibility and low cytotoxicity for human cells,
- LNZ–BCDs nanocomposites have good antibacterial properties,
- LNZ–BCDs nanocomposites have increased cell proliferation, which improves tissue regeneration and healing effect,
- LNZ–BCDs nanocomposites can be considered as a replacement for toxic nanoparticles in biomedical applications and for drug delivery to mend humans [67].
7. Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| BCD | bovine serum albumin carbon dots |
| BOD | biological oxygen demand |
| CD | carbon dot |
| CNT | carbon nanotube |
| COD | chemical oxygen demand |
| CR | Congo Red |
| CT | computer tomograhy |
| DOX | doxorubicin |
| DS | diclofenac sodium |
| FT-IR | Fourier transformed infrared |
| GO | graphene oxid |
| HE | Hematoxylin–eosin |
| LNZ | linezolid |
| MWCNT | multi-walled carbon nanotubes |
| NOTA | 1,4,7-triazacyclo nonane-1,4,7 triacetic acid |
| PEG | polyethylene glycol |
| PEI | poly(ethylene imine) |
| PELI | protein expression level index |
| PET | positron emission tomography |
| Q | Quercitine |
| rGO | reduced graphene oxide |
| ROS | reactive oxygen species |
| SEM | scanning electron microscopy |
| SPECT | single-photon emission computed tomography |
| SRLS | self-assembled ribbon-like structures |
| SWCNT | single-walled carbon nanotube |
| TEM | transmission electron microscopy |
| TOC | total organic carbon |
References
- Horie, M.; Stowe, M.; Kambara, T.; Lee, B.W.; Endoh, S.; Maru, J.; Oyabu, T.; Myojo, T.; Ogami, A.; Uchida, K.; et al. Pulmonary Inflammation of Well-Dispersed Multi-Wall Carbon Nanotubes Following Intratracheal Instillation: Toxicity by Fiber of 1–5 µm in Length. Materials 2012, 5, 2833–2849. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.T.; Adamcakova-Dodd, A.; Altmaier, R.; Thorne, P.S. Assessment of the Aerosol Generation and Toxicity of Carbon Nanotubes. Nanomaterials 2014, 4, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Matsuda, Y.; Haniu, H. The Role of Autophagy as a Mechanism of Toxicity Induced by Multi-Walled Carbon Nanotubes in Human Lung Cells. Int. J. Mol. Sci. 2015, 16, 40–48. [Google Scholar] [CrossRef]
- Revel, M.; Fournier, M.; Robidoux, P.Y. Single-Walled Carbon Nanotubes Toxicity to the Freshwater Amphipod Hyalella Azteca: Influence of Sediment and Exposure Duration. J. Xenobiot. 2015, 5, 5086. [Google Scholar] [CrossRef]
- Bisesi, J.H., Jr.; Ngo, T.; Ponnavolu, S.; Liu, K.; Lavelle, C.M.; Afrooz, A.R.M.N.; Saleh, N.B.; Ferguson, P.L.; Denslow, N.D.; Sabo-Attwood, T. Examination of Single-Walled Carbon Nanotubes Uptake and Toxicity from Dietary Exposure: Tracking Movement and Impacts in the Gastrointestinal System. Nanomaterials 2015, 5, 1066–1086. [Google Scholar] [CrossRef]
- Wang, Y.; Mortimer, M.; Chang, C.H.; Holden, P.A. Alginic Acid-Aided Dispersion of Carbon Nanotubes, Graphene, and Boron Nitride Nanomaterials for Microbial Toxicity Testing. Nanomaterials 2018, 8, 76. [Google Scholar] [CrossRef]
- Pikula, K.; Chaika, V.; Zakharenko, A.; Markina, Z.; Vedyagin, A.; Kuznetsov, V.; Gusev, A.; Park, S.; Golokhvast, K. Comparison of the Level and Mechanisms of Toxicity of Carbon Nanotubes, Carbon Nanofibers, and Silicon Nanotubes in Bioassay with Four Marine Microalgae. Nanomaterials 2020, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Orsi, M.; Al Hatem, C.; Leinardi, R.; Huaux, F. Carbon Nanotubes under Scrutiny: Their Toxicity and Utility in Mesothelioma Research. Appl. Sci. 2020, 10, 4513. [Google Scholar] [CrossRef]
- Wei, H.; Peng, Z.; Yang, C.; Tian, Y.; Sun, L.; Wang, G.; Liu, M. Three-Dimensional Au/Ag Nanoparticle/Crossed Carbon Nanotube SERS Substrate for the Detection of Mixed Toxic Molecules. Nanomaterials 2021, 11, 2026. [Google Scholar] [CrossRef]
- Lu, J.-H.; Hou, W.-C.; Tsai, M.-H.; Chang, Y.-T.; Chao, H.-R. The Impact of Background-Level Carboxylated Single-Walled Carbon Nanotubes (SWCNTs−COOH) on Induced Toxicity in Caenorhabditis elegans and Human Cells. Int. J. Environ. Res. Public Health 2022, 19, 1218. [Google Scholar] [CrossRef]
- Pinto, F.; Lourenço, A.F.; Pedrosa, J.F.S.; Gonçalves, L.; Ventura, C.; Vital, N.; Bettencourt, A.; Fernandes, S.N.; da Rosa, R.R.; Godinho, M.H.; et al. Analysis of the In Vitro Toxicity of Nanocelluloses in Human Lung Cells as Compared to Multi-Walled Carbon Nanotubes. Nanomaterials 2022, 12, 1432. [Google Scholar] [CrossRef]
- Gupta, S.S.; Singh, K.P.; Gupta, S.; Dusinska, M.; Rahman, Q. Do Carbon Nanotubes and Asbestos Fibers Exhibit Common Toxicity Mechanisms? Nanomaterials 2022, 12, 1708. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, M.; Florek, E.; Mrówczyński, R. Assessment of Pristine Carbon Nanotubes Toxicity in Rodent Models. Int. J. Mol. Sci. 2022, 23, 15343. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, N.; Mohebbi, M. Anti-Infective and Toxicity Properties of Carbon Based Materials: Graphene and Functionalized Carbon Nanotubes. Microorganisms 2022, 10, 2439. [Google Scholar] [CrossRef] [PubMed]
- Willemse, C.M.; Tlhomelang, K.; Jahed, N.; Baker, P.G.; Iwuoha, E.I. Metallo-Graphene Nanocomposite Electrocatalytic Platform for the Determination of Toxic Metal Ions. Sensors 2011, 11, 3970–3987. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, S.; Strojny-Cieślak, B.; Wierzbicki, M.; Kutwin, M.; Sawosz, E.; Kamaszewski, M.; Matuszewski, A.; Sosnowska, M.; Szczepaniak, J.; Daniluk, K.; et al. Comparison of the Toxicity of Pristine Graphene and Graphene Oxide, Using Four Biological Models. Materials 2021, 14, 4250. [Google Scholar] [CrossRef]
- Coppola, F.; Soares, A.M.V.M.; Figueira, E.; Pereira, E.; Marques, P.A.A.P.; Polese, G.; Freitas, R. The Influence of Temperature Increase on the Toxicity of Mercury Remediated Seawater Using the Nanomaterial Graphene Oxide on the Mussel Mytilus galloprovincialis. Nanomaterials 2021, 11, 1978. [Google Scholar] [CrossRef]
- Zakharova, O.V.; Mastalygina, E.E.; Golokhvast, K.S.; Gusev, A.A. Graphene Nanoribbons: Prospects of Application in Biomedicine and Toxicity. Nanomaterials 2021, 11, 2425. [Google Scholar] [CrossRef]
- Jiang, T.; Amadei, C.A.; Lin, Y.; Gou, N.; Rahman, S.M.; Lan, J.; Vecitis, C.D.; Gu, A.Z. Dependence of Graphene Oxide (GO) Toxicity on Oxidation Level, Elemental Composition, and Size. Int. J. Mol. Sci. 2021, 22, 10578. [Google Scholar] [CrossRef]
- Achawi, S.; Feneon, B.; Pourchez, J.; Forest, V. Structure–Activity Relationship of Graphene-Based Materials: Impact of the Surface Chemistry, Surface Specific Area and Lateral Size on Their In Vitro Toxicity. Nanomaterials 2021, 11, 2963. [Google Scholar] [CrossRef]
- Salesa, B.; Tuñón-Molina, A.; Cano-Vicent, A.; Assis, M.; Andrés, J.; Serrano-Aroca, Á. Graphene Nanoplatelets: In Vivo and In Vitro Toxicity, Cell Proliferative Activity, and Cell Gene Expression. Appl. Sci. 2022, 12, 720. [Google Scholar] [CrossRef]
- Lopez, R.M.; White, J.R.; Truong, L.; Tanguay, R.L. Size- and Oxidation-Dependent Toxicity of Graphene Oxide Nanomaterials in Embryonic Zebrafish. Nanomaterials 2022, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Ghulam, A.N.; dos Santos, O.A.L.; Hazeem, L.; Pizzorno Backx, B.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, L.; Cheng, S.; Zhu, L.; Liu, L.; Wen, P.; Zhou, L.; Xue, W.; Lu, S.; Zhang, W.; et al. An Overview of Light-Mediated Impact of Graphene Oxide on Algae: Photo-Transform, Toxicity and Mechanism. Water 2022, 14, 2997. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Mahmoud, A.H.M.; Mekhemer, G.A.H.; Shawky, A.M.; Soliman, M.E.S.; Moussa, N.A.M. Adsorption Behavior of Toxic Carbon Dichalcogenides (CX2; X = O, S, or Se) on β12 Borophene and Pristine Graphene Sheets: A DFT Study. Nanomaterials 2022, 12, 3411. [Google Scholar] [CrossRef]
- Yousaf, M.; Akram, M.; Bhatti, I.A.; Ahmad, M.; Usman, M.; Khan, M.U.; Sarwar, A.; Sultan, M.; Sohoo, I. On-Site Application of Solar-Activated Membrane (Cr–Mn-Doped TiO2@Graphene Oxide) for the Rapid Degradation of Toxic Textile Effluents. Membranes 2022, 12, 1178. [Google Scholar] [CrossRef]
- Rahimi, S.; van Leeuwen, D.; Roshanzamir, F.; Pandit, S.; Shi, L.; Sasanian, N.; Nielsen, J.; Esbjörner, E.K.; Mijakovic, I. Ginsenoside Rg3 Reduces the Toxicity of Graphene Oxide Used for pH-Responsive Delivery of Doxorubicin to Liver and Breast Cancer Cells. Pharmaceutics 2023, 15, 391. [Google Scholar] [CrossRef]
- Mollaamin, F.; Monajjemi, M. Doping of Graphene Nanostructure with Iron, Nickel and Zinc as Selective Detector for the Toxic Gas Removal: A Density Functional Theory Study. C 2023, 9, 20. [Google Scholar] [CrossRef]
- Ming, Z.; Feng, S.; Yilihamu, A.; Ma, Q.; Yang, S.; Yang, S.-T. Toxicity of Pristine and Chemically Functionalized Fullerenes to White Rot Fungus Phanerochaete chrysosporium. Nanomaterials 2018, 8, 120. [Google Scholar] [CrossRef]
- Emelyantsev, S.; Prazdnova, E.; Chistyakov, V.; Alperovich, I. Biological Effects of C60 Fullerene Revealed with Bacterial Biosensor—Toxic or Rather Antioxidant? Biosensors 2019, 9, 81. [Google Scholar] [CrossRef]
- Liao, S.; Liu, G.; Tan, B.; Qi, M.; Li, J.; Li, X.; Zhu, C.; Huang, J.; Yin, Y.; Tang, Y. Fullerene C60 Protects Against Intestinal Injury from Deoxynivalenol Toxicity by Improving Antioxidant Capacity. Life 2021, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Đurašević, S.; Pejić, S.; Grigorov, I.; Nikolić, G.; Mitić-Ćulafić, D.; Dragićević, M.; Đorđević, J.; Todorović Vukotić, N.; Đorđević, N.; Todorović, A.; et al. Effects of C60 Fullerene on Thioacetamide-Induced Rat Liver Toxicity and Gut Microbiome Changes. Antioxidants 2021, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Jing, Z.; Xia, F.; Zhang, J.Z.; Li, Y. Toxic Effect of Fullerene and Its Derivatives upon the Transmembrane β2-Adrenergic Receptors. Molecules 2022, 27, 4562. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-Y.; Chen, Y.-J.; Kang, C.-H.; Lin, H.-Y.; Huang, C.-C.; Hsu, P.-H.; Lin, H.-J. Toxic or Not Toxic, That Is the Carbon Quantum Dot’s Question: A Comprehensive Evaluation with Zebrafish Embryo, Eleutheroembryo, and Adult Models. Polymers 2021, 13, 1598. [Google Scholar] [CrossRef]
- Havrdová, M.; Urbančič, I.; Bartoň Tománková, K.; Malina, L.; Štrancar, J.; Bourlinos, A.B. Self-Targeting of Carbon Dots into the Cell Nucleus: Diverse Mechanisms of Toxicity in NIH/3T3 and L929 Cells. Int. J. Mol. Sci. 2021, 22, 5608. [Google Scholar] [CrossRef]
- Shabbir, H.; Wojtaszek, K.; Rutkowski, B.; Csapó, E.; Bednarski, M.; Adamiec, A.; Głuch-Lutwin, M.; Mordyl, B.; Druciarek, J.; Kotańska, M.; et al. Milk-Derived Carbon Quantum Dots: Study of Biological and Chemical Properties Provides Evidence of Toxicity. Molecules 2022, 27, 8728. [Google Scholar] [CrossRef] [PubMed]
- Qiang, S.; Zhang, L.; Li, Z.; Liang, J.; Li, P.; Song, J.; Guo, K.; Wang, Z.; Fan, Q. New Insights into the Cellular Toxicity of Carbon Quantum Dots to Escherichia coli. Antioxidants 2022, 11, 2475. [Google Scholar] [CrossRef]
- Arul, V.; Radhakrishnan, K.; Sampathkumar, N.; Vinoth Kumar, J.; Abirami, N.; Inbaraj, B.S. Detoxification of Toxic Organic Dye by Heteroatom-Doped Fluorescent Carbon Dots Prepared by Green Hydrothermal Method Using Garcinia mangostana Extract. Agronomy 2023, 13, 205. [Google Scholar] [CrossRef]
- Tan, J.M.; Saifullah, B.; Kura, A.U.; Fakurazi, S.; Hussein, M.Z. Incorporation of Levodopa into Biopolymer Coatings Based on Carboxylated Carbon Nanotubes for pH-Dependent Sustained Release Drug Delivery. Nanomaterials 2018, 8, 389. [Google Scholar] [CrossRef]
- Jagusiak, A.; Chłopaś, K.; Zemanek, G.; Jemioła-Rzemińska, M.; Piekarska, B.; Stopa, B.; Pańczyk, T. Self-Assembled Supramolecular Ribbon-Like Structures Complexed to Single Walled Carbon Nanotubes as Possible Anticancer Drug Delivery Systems. Int. J. Mol. Sci. 2019, 20, 2064. [Google Scholar] [CrossRef]
- Pennetta, C.; Floresta, G.; Graziano, A.C.E.; Cardile, V.; Rubino, L.; Galimberti, M.; Rescifina, A.; Barbera, V. Functionalization of Single and Multi-Walled Carbon Nanotubes with Polypropylene Glycol Decorated Pyrrole for the Development of Doxorubicin Nano-Conveyors for Cancer Drug Delivery. Nanomaterials 2020, 10, 1073. [Google Scholar] [CrossRef]
- Jagusiak, A.; Chlopas, K.; Zemanek, G.; Wolski, P.; Panczyk, T. Controlled Release of Doxorubicin from the Drug Delivery Formulation Composed of Single-Walled Carbon Nanotubes and Congo Red: A Molecular Dynamics Study and Dynamic Light Scattering Analysis. Pharmaceutics 2020, 12, 622. [Google Scholar] [CrossRef]
- Tangoulis, V.; Lalioti, N.; Parthenios, J.; Langford, N.; Valsami-Jones, E.; Kakoulidou, C.; Psomas, G.; Bekiari, V. Facile Method to Prepare pH-Sensitive PEI-Functionalized Carbon Nanotubes as Rationally Designed Vehicles for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Delivery. C 2020, 6, 62. [Google Scholar] [CrossRef]
- Torres-Ávalos, J.A.; Cajero-Zul, L.R.; Vázquez-Lepe, M.; López-Dellamary, F.A.; Martínez-Richa, A.; Barrera-Rivera, K.A.; López-Serrano, F.; Nuño-Donlucas, S.M. Synthesis of Poly(methacrylic acid-co-butyl acrylate) Grafted onto Functionalized Carbon Nanotube Nanocomposites for Drug Delivery. Polymers 2021, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Kralova, K. Advances in Drug Delivery Nanosystems Using Graphene-Based Materials and Carbon Nanotubes. Materials 2021, 14, 1059. [Google Scholar] [CrossRef]
- Kofoed Andersen, C.; Khatri, S.; Hansen, J.; Slott, S.; Pavan Parvathaneni, R.; Mendes, A.C.; Chronakis, I.S.; Hung, S.-C.; Rajasekaran, N.; Ma, Z.; et al. Carbon Nanotubes—Potent Carriers for Targeted Drug Delivery in Rheumatoid Arthritis. Pharmaceutics 2021, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Rahamathulla, M.; Bhosale, R.R.; Osmani, R.A.M.; Mahima, K.C.; Johnson, A.P.; Hani, U.; Ghazwani, M.; Begum, M.Y.; Alshehri, S.; Ghoneim, M.M.; et al. Carbon Nanotubes: Current Perspectives on Diverse Applications in Targeted Drug Delivery and Therapies. Materials 2021, 14, 6707. [Google Scholar] [CrossRef]
- Anisimov, R.A.; Gorin, D.A.; Abalymov, A.A. 3D Cell Spheroids as a Tool for Evaluating the Effectiveness of Carbon Nanotubes as a Drug Delivery and Photothermal Therapy Agents. C 2022, 8, 56. [Google Scholar] [CrossRef]
- Thakur, C.K.; Karthikeyan, C.; Abou-Dahech, M.S.; Altabakha, M.M.A.M.; Al Shahwan, M.J.S.; Ashby, C.R., Jr.; Tiwari, A.K.; Babu, R.J.; Moorthy, N.S.H.N. Microwave-Assisted Functionalization of Multi-Walled Carbon Nanotubes for Biosensor and Drug Delivery Applications. Pharmaceutics 2023, 15, 335. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.; Park, J.; Kim, T.-I. Effect of pH-Responsive Charge-Conversional Polymer Coating to Cationic Reduced Graphene Oxide Nanostructures for Tumor Microenvironment-Targeted Drug Delivery Systems. Nanomaterials 2019, 9, 1289. [Google Scholar] [CrossRef]
- Sharma, H.; Mondal, S. Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine. Int. J. Mol. Sci. 2020, 21, 6280. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, A.-M.; Karaçelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydoğan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.-M.; et al. Electrically Triggered Drug Delivery from Novel Electrospun Poly(Lactic Acid)/Graphene Oxide/Quercetin Fibrous Scaffolds for Wound Dressing Applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Colombi Ciacchi, L.; Wei, G. Recent Advances in the Synthesis of Graphene-Based Nanomaterials for Controlled Drug Delivery. Appl. Sci. 2017, 7, 1175. [Google Scholar] [CrossRef]
- Shi, G.; Liu, T.; Kopecki, Z.; Cowin, A.; Lee, I.; Pai, J.-H.; Lowe, S.E.; Zhong, Y.L. A Multifunctional Wearable Device with a Graphene/Silver Nanowire Nanocomposite for Highly Sensitive Strain Sensing and Drug Delivery. C 2019, 5, 17. [Google Scholar] [CrossRef]
- Lu, Y.-J.; Lan, Y.-H.; Chuang, C.-C.; Lu, W.-T.; Chan, L.-Y.; Hsu, P.-W.; Chen, J.-P. Injectable Thermo-Sensitive Chitosan Hydrogel Containing CPT-11-Loaded EGFR-Targeted Graphene Oxide and SLP2 shRNA for Localized Drug/Gene Delivery in Glioblastoma Therapy. Int. J. Mol. Sci. 2020, 21, 7111. [Google Scholar] [CrossRef] [PubMed]
- Jihad, M.A.; Noori, F.T.M.; Jabir, M.S.; Albukhaty, S.; AlMalki, F.A.; Alyamani, A.A. Polyethylene Glycol Functionalized Graphene Oxide Nanoparticles Loaded with Nigella sativa Extract: A Smart Antibacterial Therapeutic Drug Delivery System. Molecules 2021, 26, 3067. [Google Scholar] [CrossRef] [PubMed]
- Babić Radić, M.M.; Filipović, V.V.; Vukomanović, M.; Nikodinović Runić, J.; Tomić, S.L. Degradable 2-Hydroxyethyl Methacrylate/Gelatin/Alginate Hydrogels Infused by Nanocolloidal Graphene Oxide as Promising Drug Delivery and Scaffolding Biomaterials. Gels 2022, 8, 22. [Google Scholar] [CrossRef]
- Oliveira, A.M.L.; Machado, M.; Silva, G.A.; Bitoque, D.B.; Tavares Ferreira, J.; Pinto, L.A.; Ferreira, Q. Graphene Oxide Thin Films with Drug Delivery Function. Nanomaterials 2022, 12, 1149. [Google Scholar] [CrossRef]
- Croitoru, A.-M.; Moroșan, A.; Tihăuan, B.; Oprea, O.; Motelică, L.; Trușcă, R.; Nicoară, A.I.; Popescu, R.-C.; Savu, D.; Mihăiescu, D.E.; et al. Novel Graphene Oxide/Quercetin and Graphene Oxide/Juglone Nanostructured Platforms as Effective Drug Delivery Systems with Biomedical Applications. Nanomaterials 2022, 12, 1943. [Google Scholar] [CrossRef]
- Giusto, E.; Žárská, L.; Beirne, D.F.; Rossi, A.; Bassi, G.; Ruffini, A.; Montesi, M.; Montagner, D.; Ranc, V.; Panseri, S. Graphene Oxide Nanoplatforms to Enhance Cisplatin-Based Drug Delivery in Anticancer Therapy. Nanomaterials 2022, 12, 2372. [Google Scholar] [CrossRef]
- Gilpin, V.; Surandhiran, D.; Scott, C.; Devine, A.; Cundell, J.H.; Gill, C.I.R.; Pourshahidi, L.K.; Davis, J. Lasered Graphene Microheaters Modified with Phase-Change Composites: New Approach to Smart Patch Drug Delivery. Micromachines 2022, 13, 1132. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Arjmand, F.; Pettinari, C.; Tabassum, S. Ru(II)(η6-p-cymene) Conjugates Loaded onto Graphene Oxide: An Effective pH-Responsive Anticancer Drug Delivery System. Molecules 2022, 27, 7592. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, G.I. Fullerene Derivatives for Drug Delivery against COVID-19: A Molecular Dynamics Investigation of Dendro[60]fullerene as Nanocarrier of Molnupiravir. Nanomaterials 2022, 12, 2711. [Google Scholar] [CrossRef]
- Wang, L.; Pan, H.; Gu, D.; Sun, H.; Chen, K.; Tan, G.; Pan, W. A Novel Carbon Dots/Thermo-Sensitive In Situ Gel for a Composite Ocular Drug Delivery System: Characterization, Ex-Vivo Imaging, and In Vivo Evaluation. Int. J. Mol. Sci. 2021, 22, 9934. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int. J. Mol. Sci. 2021, 22, 11783. [Google Scholar] [CrossRef]
- Chai, Y.; Feng, Y.; Zhang, K.; Li, J. Preparation of Fluorescent Carbon Dots Composites and Their Potential Applications in Biomedicine and Drug Delivery—A Review. Pharmaceutics 2022, 14, 2482. [Google Scholar] [CrossRef]
- Ghataty, D.S.; Amer, R.I.; Amer, M.A.; Abdel Rahman, M.F.; Shamma, R.N. Green Synthesis of Highly Fluorescent Carbon Dots from Bovine Serum Albumin for Linezolid Drug Delivery as Potential Wound Healing Biomaterial: Bio-Synergistic Approach, Antibacterial Activity, and In Vitro and Ex Vivo Evaluation. Pharmaceutics 2023, 15, 234. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kang, J.S.; Park, K.C. Carbon Nanotube Electron Emitter for X-ray Imaging. Materials 2012, 5, 2353–2359. [Google Scholar] [CrossRef]
- Khazi-Syed, A.; Hasan, M.T.; Campbell, E.; Gonzalez-Rodriguez, R.; Naumov, A.V. Single-Walled Carbon Nanotube-Assisted Antibiotic Delivery and Imaging in S. epidermidis Strains Addressing Antibiotic Resistance. Nanomaterials 2019, 9, 1685. [Google Scholar] [CrossRef]
- Li, H.; Yu, Y.; Peng, J.; Wu, Y.; Zhang, Y. Resolution Improvement of Light Field Imaging via a Nematic Liquid Crystal Microlens with Added Multi-Walled Carbon Nanotubes. Sensors 2020, 20, 5557. [Google Scholar] [CrossRef]
- Rivera, E.J.; Sethi, R.; Qu, F.; Krishnamurthy, R.; Muthupillai, R.; Alford, M.; Swanson, M.A.; Eaton, S.S.; Eaton, G.R.; Wilson, L.J. Nitroxide Radicals@US-Tubes: New Spin Labels for Biomedical Applications. Adv. Funct. Mater. 2012, 22, 3691–3698. [Google Scholar] [CrossRef]
- Rivera, E.J.; Tran, L.A.; Hernández-Rivera, M.; Yoon, D.; Mikos, A.G.; Rusakova, I.A.; Cheong, B.Y.; da Graça Cabreira-Hansen, M.; Willerson, J.T.; Perin, E.C.; et al. Bismuth@US-tubes as a potential contrast agent for X-ray imaging applications. J. Mater. Chem. B 2013, 1, 4792. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, J.; Hartman, K.; Kissell, K.; Mackeyev, Y.; Pheasant, S.; Young, S.; Van der Heide, P.; Mikos, A.; Wilson, L. Single-Molecule I2@US-Tube Nanocapsules: A New X-ray Contrast-Agent Design. Adv. Mater. 2007, 19, 573–576. [Google Scholar] [CrossRef]
- Munari, S.D.; Sandoval, S.; Pach, E.; Ballesteros, B.; Tobias, G.; Anthony, D.C.; Davis, B.G. In vivo behaviour of glyco-NaI@SWCNT ‘nanobottles’. Inorg. Chim. Acta 2019, 495, 118933. [Google Scholar] [CrossRef]
- D’Accolti, L.; Gajewska, A.; Kierkowicz, M.; Martincic, M.; Nacci, A.; Sandoval, S.; Ballesteros, B.; Tobias, G.; Ros, T.D.; Fusco, C. Epoxidation of Carbon Nanocapsules: Decoration of Single-Walled Carbon Nanotubes Filled with Metal Halides. Nanomaterials 2018, 8, 137. [Google Scholar] [CrossRef]
- Wang, J.T.W.; Klippstein, R.; Martincic, M.; Pach, E.; Feldman, R.; Šefl, M.; Michel, Y.; Asker, D.; Sosabowski, J.K.; Kalbac, M.; et al. Neutron Activated 153Sm Sealed in Carbon Nanocapsules for in Vivo Imaging and Tumor Radiotherapy. ACS Nano 2020, 14, 129–141. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Hong, H.; Cai, W.; Liu, Z. Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat. Protoc. 2013, 8, 2392–2403. [Google Scholar] [CrossRef]
- de Garibay, A.P.R.; Spinato, C.; Klippstein, R.; Bourgognon, M.; Martincic, M.; Pach, E.; Ballesteros, B.; Ménard-Moyon, C.; Al-Jamal, K.T.; Tobias, G.; et al. Evaluation of the immunological profile of antibody-functionalized metal-filled single-walled carbon nanocapsules for targeted radiotherapy. Sci. Rep. 2017, 7, 42605. [Google Scholar] [CrossRef]
- Spinato, C.; de Garibay, A.P.R.; Kierkowicz, M.; Pach, E.; Martincic, M.; Klippstein, R.; Bourgognon, M.; Wang, J.T.W.; Ménard-Moyon, C.; Al-Jamal, K.T.; et al. Design of antibody-functionalized carbon nanotubes filled with radioactivable metals towards a targeted anticancer therapy. Nanoscale 2016, 8, 12626–12638. [Google Scholar] [CrossRef]
- Serpell, C.J.; Rutte, R.N.; Geraki, K.; Pach, E.; Martincic, M.; Kierkowicz, M.; Munari, S.D.; Wals, K.; Raj, R.; Ballesteros, B.; et al. Carbon nanotubes allow capture of krypton, barium and lead for multichannel biological X-ray fluorescence imaging. Nat. Commun. 2016, 7, 13118. [Google Scholar] [CrossRef]
- Foti, A.; Venkatesan, S.; Lebental, B.; Zucchi, G.; Ossikovski, R. Comparing Commercial Metal-Coated AFM Tips and Home-Made Bulk Gold Tips for Tip-Enhanced Raman Spectroscopy of Polymer Functionalized Multiwalled Carbon Nanotubes. Nanomaterials 2022, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.V. A Library of Doped-Graphene Images via Transmission Electron Microscopy. C 2018, 4, 34. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Kim, J.; Lee, Z. Contrast Transfer Function-Based Exit-Wave Reconstruction and Denoising of Atomic-Resolution Transmission Electron Microscopy Images of Graphene and Cu Single Atom Substitutions by Deep Learning Framework. Nanomaterials 2020, 10, 1977. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, A.B.G.; Kusmartsev, F.V.; Kusmartseva, A.; Alkallas, F.H.; AlFaify, S.; Shkir, M. Raman Spectroscopy Imaging of Exceptional Electronic Properties in Epitaxial Graphene Grown on SiC. Nanomaterials 2020, 10, 2234. [Google Scholar] [CrossRef]
- Chen, M.; Shao, Q.; He, W.; Wei, D.; Hu, C.; Shi, J.; Liu, K.; Wang, H.; Xie, C.; Zhang, X. Electrically Controlled Liquid Crystal Microlens Array Based on Single-Crystal Graphene Coupling Alignment for Plenoptic Imaging. Micromachines 2020, 11, 1039. [Google Scholar] [CrossRef]
- Khambampati, A.K.; Rahman, S.A.; Sharma, S.K.; Kim, W.Y.; Kim, K.Y. Imaging Conductivity Changes in Monolayer Graphene Using Electrical Impedance Tomography. Micromachines 2020, 11, 1074. [Google Scholar] [CrossRef]
- Lee, B.H.; McKinney, R.L.; Hasan, M.T.; Naumov, A.V. Graphene Quantum Dots as Intracellular Imaging-Based Temperature Sensors. Materials 2021, 14, 616. [Google Scholar] [CrossRef]
- Silkin, V.M.; Kogan, E.; Gumbs, G. Screening in Graphene: Response to External Static Electric Field and an Image-Potential Problem. Nanomaterials 2021, 11, 1561. [Google Scholar] [CrossRef]
- Li, W.; Jiang, N.; Wu, B.; Liu, Y.; Zhang, L.; He, J. Chlorine Modulation Fluorescent Performance of Seaweed-Derived Graphene Quantum Dots for Long-Wavelength Excitation Cell-Imaging Application. Molecules 2021, 26, 4994. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Lin, Y.-S.; Wu, P.-C.; Chang, C.-Y.; Wang, J.-Y.; Chen, P.-C.; Hsieh, M.-H.; Kao, H.-F.; Lin, S.-H.; Chang, C.-C. Two-Photon–Near Infrared-II Antimicrobial Graphene-Nanoagent for Ultraviolet–Near Infrared Imaging and Photoinactivation. Int. J. Mol. Sci. 2022, 23, 3230. [Google Scholar] [CrossRef]
- Gollavelli, G.; Ghule, A.V.; Ling, Y.-C. Multimodal Imaging and Phototherapy of Cancer and Bacterial Infection by Graphene and Related Nanocomposites. Molecules 2022, 27, 5588. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Lee, Y.-K.; Shaik, M.R.; Alrashood, S.T.; Ekhzaimy, A.A. Nanocomposites of Nitrogen-Doped Graphene Oxide and Manganese Oxide for Photodynamic Therapy and Magnetic Resonance Imaging. Int. J. Mol. Sci. 2022, 23, 15087. [Google Scholar] [CrossRef] [PubMed]
- Jamlos, M.A.; Jamlos, M.F.; Mustafa, W.A.; Othman, N.A.; Rohani, M.N.K.H.; Saidi, S.A.; Sarip, M.S.M.; Mohd Nawi, M.A.H. Reduced Graphene Oxide UWB Array Sensor: High Performance for Brain Tumor Imaging and Detection. Nanomaterials 2023, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Yang, K.; Zhang, Y.; Engle, J.W.; Feng, L.; Yang, Y.; Tapas, R.; Goel, N.S.; Bean, J.; Theuer, C.P.; et al. In vivo targeting and imaging of tumour vasculature with radiolabeled, antibody-conjugated nanographene. ACS Nano 2012, 6, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, R.; Kondo, K.; Miura, R.; Yamana, K.; Isozaki, H.; Shimada, R.; Kawamura, S.; Hirano, H.; Nishimura, T.; Tarutani, N.; et al. Theranostic Agent Combining Fullerene Nanocrystals and Gold Nanoparticles for Photoacoustic Imaging and Photothermal Therapy. Int. J. Mol. Sci. 2022, 23, 4686. [Google Scholar] [CrossRef]
- Mancini, F.; Menichetti, A.; Degli Esposti, L.; Montesi, M.; Panseri, S.; Bassi, G.; Montalti, M.; Lazzarini, L.; Adamiano, A.; Iafisco, M. Fluorescent Carbon Dots from Food Industry By-Products for Cell Imaging. J. Funct. Biomater. 2023, 14, 90. [Google Scholar] [CrossRef]
- Green, A.A.; Hersam, M.C. Properties and Application of Double-Walled Carbon Nanotubes Sorted by Outer-Wall Electronic Type. ASC Nano 2011, 5, 4927–4934. [Google Scholar] [CrossRef]
- Brozena, A.H.; Moskowitz, J.; Shao, B.; Deng, S.; Liao, H.; Gaskell, K.J.; Wang, Y. Outer Wall Selectively Oxidized, Water-Soluble Double-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2010, 132, 3932. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Fedoseeva, Y.; Okotrub, A.V.; Flahaut, E.; Asanov, I.P.; Koroteev, V.O.; Yaya, A.; Ewels, C.; Chuvilin, A.; Felten, A.; et al. Stability of Fluorinated Double-Walled Carbon Nanotubes Produced by Different Fluorination Techniques. Chem. Mater. 2010, 22, 4197–4203. [Google Scholar] [CrossRef]
- Piao, Y.; Chen, C.F.; Green, A.A.; Kwon, H.; Hersam, M.C.; Lee, C.S.; Schatz, G.C.; Wang, Y. Optical and Electrical Properties of Inner Tubes in Outer Wall-Selectively Functionalized Double-Wall Carbon Nanotubes. J. Phys. Chem. Lett. 2011, 2, 1577–1582. [Google Scholar] [CrossRef]
- Ellis, B.D.; Dyker, C.A.; Decken, A.; Macdonald, C.L.B. The synthesis, characterisation and electronic structure of N-heterocyclic carbene adducts of PI cations. Chem. Commun. 2005, 1965–1967. [Google Scholar] [CrossRef]
- Collins, P.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622. [Google Scholar] [CrossRef] [PubMed]
- Ayala, P.; Arenal, R.; Loiseau, A.; Rubio, A.; Pichler, T. The physical and chemical properties of heteronanotubes. Rev. Mod. Phys. 2010, 82, 1843. [Google Scholar] [CrossRef]
- Yi, J.-Y.; Bernholc, J. Atomic structure and doping of microtubules. Phys. Rev. B 1993, 47, 1708. [Google Scholar] [CrossRef] [PubMed]
- Ayala, P.; Grüneis, A.; Gemming, T.; Grimm, D.; Kramberger, C.; Rümmeli, M.H.; Freire, F.L., Jr.; Kuzmany, H.; Pfeiffer, R.; Barreiro, A.; et al. Tailoring N-Doped Single and Double Wall Carbon Nanotubes from a Nondiluted Carbon/Nitrogen Feedstock. J. Phys. Chem. C 2007, 111, 2879. [Google Scholar] [CrossRef]
- Elias, A.L.; Ayala, P.; Zamudio, A.; Grobosch, M.; Cruz-Silva, E.; Romo-Herrera, J.M.; Campos-Delgado, J.; Terrones, H.; Pichler, T.; Terrones, M. Spectroscopic characterization of N-doped single-walled carbon nanotube strands: An X-ray photoelectron spectroscopy and Raman study. J. Nanosci. Nanotechnol. 2010, 10, 3959. [Google Scholar] [CrossRef]
- Glerup, M.; Steinmetz, J.; Samaille, D.; Stéphan, O.; Enouz, S.; Loiseau, A.; Roth, S.; Bernier, P. Synthesis of N-doped SWNT using the arc-discharge procedure. Chem. Phys. Lett. 2004, 387, 193. [Google Scholar] [CrossRef]
- Keskar, G.; Rao, R.; Luo, J.; Hudson, J.; Chen, J.; Rao, A.M. Growth, nitrogen doping and characterization of isolated single-wall carbon nanotubes using liquid precursor. Chem. Phys. Lett. 2005, 412, 269. [Google Scholar] [CrossRef]
- Krstić, V.; Rikken, G.L.J.A.; Bernier, P.; Roth, S.; Glerup, M. Nitrogen doping of metallic single-walled carbon nanotubes:n-type conduction and dipole scattering. Europhys. Lett. 2007, 77, 37001. [Google Scholar] [CrossRef]
- Lin, H.; Lagoute, J.; Chacon, C.; Arenal, R.; Stéphan, O.; Repain, V.; Girard, Y.; Enouz, S.; Bresson, L.; Rousset, S.; et al. Combined STM/STS, TEM/EELS investigation of CNx-SWNTs. Phys. Status Solidi B 2008, 245, 1986. [Google Scholar] [CrossRef]
- Lin, H.; Arenal, R.; Enouz-Vedrenne, S.; Stephan, O.; Loiseau, A. Nitrogen Configuration in Individual CNx-SWNTs Synthesized by Laser Vaporization Technique. J. Phys. Chem. C 2009, 113, 9509. [Google Scholar] [CrossRef]
- Maciel, I.O.; Anderson, N.; Pimenta, M.A.; Hartschuh, A.; Qian, H.; Terrones, M.; Terrones, H.; Campos-Delgado, J.; Rao, A.M.; Novotny, L.; et al. Electron and phonon renormalization near charged defects in carbon nanotubes. Nat. Mater. 2008, 7, 878. [Google Scholar] [CrossRef] [PubMed]
- Susi, T.; Zhu, Z.; Ruiz-Soria, G.; Arenal, R.; Ayala, P.; Nasibulin, A.G.; Lin, H.; Jiang, H.; Stephan, O.; Pichler, T.; et al. Nitrogen-doped SWCNT synthesis using ammonia and carbon monoxide. Phys. Status Solidi B 2010, 247, 2726. [Google Scholar] [CrossRef]
- Villalpando-Paez, F.; Zamudio, A.; Elias, A.L.; Son, H.; Barros, E.B.; Chou, S.G.; Kim, Y.A.; Muramatsu, H.; Hayashi, T.; Kong, J.; et al. Synthesis and characterization of long strands of nitrogen-doped single-walled carbon nanotubes. Chem. Phys. Lett. 2006, 424, 345. [Google Scholar] [CrossRef]
- Wiltshire, J.G.; Li, L.-J.; Herz, L.M.; Nicholas, R.J.; Glerup, M.; Sauvajol, J.-L.; Khlobystov, A.N. Chirality-dependent boron-mediated growth of nitrogen-doped single-walled carbon nanotubes. Phys. Rev. B 2005, 72, 205431. [Google Scholar] [CrossRef]
- Ayala, P.; Plank, W.; Grüneis, A.; Kauppinen, E.I.; Rümmeli, M.H.; Kuzmany, H.; Pichler, T. A one step approach to B-doped single-walled carbon nanotubes. J. Mater. Chem. 2008, 18, 5676. [Google Scholar] [CrossRef]
- Ayala, P.; Reppert, J.; Grobosch, M.; Knupfer, M.; Pichler, T.; Rao, A.M. Evidence for substitutional boron in doped single-walled carbon nanotubes. Appl. Phys. Lett. 2010, 96, 183110. [Google Scholar] [CrossRef]
- Borowiak-Palen, E.; Pichler, T.; Fuentes, G.G.; Graff, A.; Kalenczuk, R.J.; Knupfer, M.; Fink, J. Efficient production of B-substituted single-wall carbon nanotubes. Chem. Phys. Lett. 2003, 378, 516. [Google Scholar] [CrossRef]
- Borowiak-Palen, E.; Pichler, T.; Graff, A.; Kalenczuk, R.J.; Knupfer, M.; Fink, J. Synthesis and electronic properties of B-doped single wall carbon nanotubes. Carbon 2004, 42, 1123. [Google Scholar] [CrossRef]
- Daothong, S.; Parjanne, J.; Kauppinen, E.; Valkeapää, M.; Pichler, T.; Singjai, P.; Ayala, P. Study of the role of Fe based catalysts on the growth of B-doped SWCNTs synthesized by CVD. Phys. Status Solidi B 2009, 246, 2518. [Google Scholar] [CrossRef]
- Fuentes, G.G.; Borowiak-Palen, E.; Knupfer, M.; Pichler, T.; Fink, J.; Wirtz, L.; Rubio, A. Formation and electronic properties of BC3 single-wall nanotubes upon boron substitution of carbon nanotubes. Phys. Rev. B 2004, 69, 245403. [Google Scholar] [CrossRef]
- Gai, P.L.; Stephan, O.; McGuire, K.; Rao, A.M.; Dresselhaus, M.S.; Dresselhaus, G.; Colliex, C. Structural systematics in boron-doped single wall carbon nanotube. J. Mater. Chem. 2004, 14, 669. [Google Scholar] [CrossRef]
- McGuire, K.; Gothard, N.; Gai, P.L.; Dresselhaus, M.S.; Sumanasekera, G.; Rao, A.M. Synthesis and Raman characterization of boron-doped single-walled carbon nanotubes. Carbon 2005, 43, 219. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, X.; Liu, R.; Shen, S.; Wu, F.; Xie, A. Tuning the Dielectric and Microwaves Absorption Properties of N-Doped Carbon Nanotubes by Boron Insertion. Nanomaterials 2021, 11, 1164. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution Properties of Single-Walled Carbon Nanotubes. Science 1998, 282, 95. [Google Scholar] [CrossRef]
- Rao, A.M.; Eklund, P.C.; Bandow, S.; Thess, A.; Smalley, R.E. Evidence for charge transfer in doped carbon nanotube bundles from Raman scattering. Nature 1997, 388, 257. [Google Scholar] [CrossRef]
- Lee, R.S.; Kim, H.J.; Fischer, J.E.; Thess, A.; Smalley, R.E. Conductivity enhancement in single-walled carbon nanotube bundles doped with K and Br. Nature 1997, 388, 255. [Google Scholar] [CrossRef]
- Bandow, S.; Rao, A.; Sumanasekera, G.; Eklund, P.; Kokai, F.; Takahashi, K.; Yudasaka, M.; Iijima, S. Evidence for anomalously small charge transfer in doped single-wall carbon nanohorn aggregates with Li, K and Br. Appl. Phys. A 2000, 71, 561. [Google Scholar] [CrossRef]
- Kukovecz, A.; Pichler, T.; Pfeiffer, R.; Kramberger, C.; Kuzmany, H. Diameter selective doping of single wall carbon nanotubes. Phys. Chem. Chem. Phys. 2003, 5, 582. [Google Scholar] [CrossRef]
- Pichler, T.; Kukovecz, A.; Kuzmany, H.; Kataura, H. Charge transfer in doped single-walled carbon nanotubes. Synthet. Met. 2003, 136, 717. [Google Scholar] [CrossRef]
- De Blauwe, K.; Kramberger, C.; Plank, W.; Kataura, H.; Pichler, T. Raman response of FeCl3 intercalated single-wall carbon nanotubes at high doping. Phys. Status Solidi B 2009, 246, 2732. [Google Scholar] [CrossRef]
- Grigorian, L.; Williams, K.A.; Fang, S.; Sumanasekera, G.U.; Loper, A.L.; Dickey, E.C.; Pennycook, S.J.; Eklund, P.C. Reversible Intercalation of Charged Iodine Chains into Carbon Nanotube Ropes. Phys. Rev. Lett. 1998, 80, 5560. [Google Scholar] [CrossRef]
- Sumanasekera, G.U.; Allen, J.L.; Fang, S.L.; Loper, A.L.; Rao, A.M.; Eklund, P.C. Electrochemical Oxidation of Single Wall Carbon Nanotube Bundles in Sulfuric Acid. J. Phys. Chem. B 1999, 103, 4292. [Google Scholar] [CrossRef]
- Kukovecz, A.; Pichler, T.; Pfeiffera, R.; Kuzmany, H. Diameter selective charge transfer in p- and n-doped single wall carbon nanotubes synthesized by the HiPCO method. Chem. Commun. 2002, 1730. [Google Scholar] [CrossRef]
- Bendiab, N.; Anglaret, E.; Bantignies, J.-L.; Zahab, A.; Sauvajol, J.L.; Petit, P.; Mathis, C.; Lefrant, S. Stoichiometry dependence of the Raman spectrum of alkali-doped single-wall carbon nanotubes. Phys. Rev. B 2001, 64, 245424. [Google Scholar] [CrossRef]
- Iwasa, Y.; Fudo, H.; Yatsu, Y.; Mitani, T.; Kataura, H.; Achiba, Y. Phase stability of doped carbon nanotubes. Synthet. Met. 2001, 121, 1203. [Google Scholar] [CrossRef]
- Rao, A.; Bandow, S.; Richter, E.; Eklund, P. Raman spectroscopy of pristine and doped single wall carbon nanotubes. Thin Solid Films 1998, 331, 141. [Google Scholar] [CrossRef]
- Kazaoui, S.; Minami, N.; Jacquemin, R.; Kataura, H.; Achiba, Y. Amphoteric doping of single-wall carbon-nanotube thin films as probed by optical absorption spectroscopy. Phys. Rev. B 1999, 60, 13339. [Google Scholar] [CrossRef]
- Jouguelet, E.; Mathis, C.; Petit, P. Controlling the electronic properties of single-wall carbon nanotubes by chemical doping. Chem. Phys. Lett. 2000, 318, 561. [Google Scholar] [CrossRef]
- Petit, P.; Mathis, C.; Journet, C.; Bernier, P. Tuning and monitoring the electronic structure of carbon nanotubes. Chem. Phys. Lett. 1999, 305, 370. [Google Scholar] [CrossRef]
- Yuan, J.; Ji, G.; Chen, X.; Wei, D.; Zhao, F.; Wu, Q. Phase transition, thermodynamics properties and IR spectrum of α- and γ-RDX: First principles and MD studies. Chem. Phys. Lett. 2016, 644, 250–254. [Google Scholar] [CrossRef]
- Jacquemin, R.; Kazaoui, S.; Yu, D.; Hassanien, A.; Minami, N.; Kataura, H.; Achiba, Y. Doping mechanism in single-wall carbon nanotubes studied by optical absorption. Synthet. Met. 2000, 115, 283. [Google Scholar] [CrossRef]
- Minami, N.; Kazaoui, S.; Jacquemin, R.; Yamawaki, H.; Aoki, K.; Kataura, H.; Achiba, Y. Optical properties of semiconducting and metallic single wall carbon nanotubes: Effects of doping and high pressure. Synthet. Met. 2001, 116, 405–409. [Google Scholar] [CrossRef]
- Bower, C.; Suzuki, S.; Tanigaki, K.; Zhou, O. Synthesis and structure of pristine and alkali-metal-intercalated single-walled carbon nanotubes. Appl. Phys. A 1998, 67, 47. [Google Scholar] [CrossRef]
- Liu, X.; Pichler, T.; Knupfer, M.; Fink, J. Electronic and optical properties of alkali-metal-intercalated single-wall carbon nanotubes. Phys. Rev. B 2003, 67, 125403. [Google Scholar] [CrossRef]
- Yanagi, K.; Iakoubovskii, K.; Matsui, H.; Okamoto, H.; Miyata, Y.; Maniwa, Y.; Kazaoui, S.; Minami, N.; Kataura, H. Photosensitive Function of Encapsulated Dye in Carbon Nanotubes. Am. Chem. Soc. 2007, 129, 4992–4997. [Google Scholar] [CrossRef]
- Pichler, T.; Sing, M.; Knupfer, M.; Golden, M.; Fink, J. Potassium intercalated bundles of single-wall carbon nanotubes: Electronic structure and optical properties. Solid State Commun. 1999, 109, 721. [Google Scholar] [CrossRef]
- Suzuki, S.; Bower, C.; Zhou, O. In-situ TEM and EELS studies of alkali–metal intercalation with single-walled carbon nanotubes. Chem. Phys. Lett. 1998, 285, 230. [Google Scholar] [CrossRef]
- Liu, X.; Pichler, T.; Knupfer, M.; Fink, J.; Kataura, H. Electronic properties of FeCl3-intercalated single-wall carbon nanotubes. Phys. Rev. B 2004, 70, 205405. [Google Scholar] [CrossRef]
- Ruzicka, B.; Degiorgi, L.; Gaal, R.; Thien-Nga, L.; Bacsa, R.; Salvetat, J.-P.; Forró, L. Optical and dc conductivity study of potassium-doped single-walled carbon nanotube films. Phys. Rev. B 2000, 61, R2468. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C. Phemenology of Filling, Investigation of Growth Kinetics and Electronic Properties for Applications of Filled Single-Walled Carbon Nanotubes. Nanomaterials 2023, 13, 314. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Brzhezinskaya, M.M.; Vinogradov, A.S.; Suzdalev, I.P.; Maksimov, Y.V.; Imshennik, V.K.; Novichikhin, S.; Krestinin, A.V.; Yashina, L.; Lukashin, A.V.; et al. The forming and properties of one-dimensional FeHal2 (Hal=Cl, Br, I) nanocrystals in channels of single-walled carbon nanotubes. Russ. Nanotechnol. 2009, 4, 77–87. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C. Applications of Filled Single-Walled Carbon Nanotubes: Progress, Challenges, and Perspectives. Nanomaterials 2021, 11, 2863. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Kim, J.-H. Differential Cytotoxicity of Different Sizes of Graphene Oxide Nanoparticles in Leydig (TM3) and Sertoli (TM4) Cells. Nanomaterials 2019, 9, 139. [Google Scholar] [CrossRef]
- Cebadero-Domínguez, O.; Ferrández-Gómez, B.; Sánchez-Ballester, S.; Moreno, J.; Jos, A.; Cameán, A.M. In vitro toxicity evaluation of graphene oxide and reduced graphene oxide on Caco-2 cells. Toxicology Reports 2022, 9, 1130–1138. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharlamova, M.V.; Kramberger, C. Cytotoxicity of Carbon Nanotubes, Graphene, Fullerenes, and Dots. Nanomaterials 2023, 13, 1458. https://doi.org/10.3390/nano13091458
Kharlamova MV, Kramberger C. Cytotoxicity of Carbon Nanotubes, Graphene, Fullerenes, and Dots. Nanomaterials. 2023; 13(9):1458. https://doi.org/10.3390/nano13091458
Chicago/Turabian StyleKharlamova, Marianna V., and Christian Kramberger. 2023. "Cytotoxicity of Carbon Nanotubes, Graphene, Fullerenes, and Dots" Nanomaterials 13, no. 9: 1458. https://doi.org/10.3390/nano13091458
APA StyleKharlamova, M. V., & Kramberger, C. (2023). Cytotoxicity of Carbon Nanotubes, Graphene, Fullerenes, and Dots. Nanomaterials, 13(9), 1458. https://doi.org/10.3390/nano13091458





