A Modified Wet Transfer Method for Eliminating Interfacial Impurities in Graphene
Abstract
:1. Introduction
2. Experiments
2.1. Chemical Vapor Deposition of Graphene
2.2. Substrate Treatment
2.3. Raman Spectroscopy
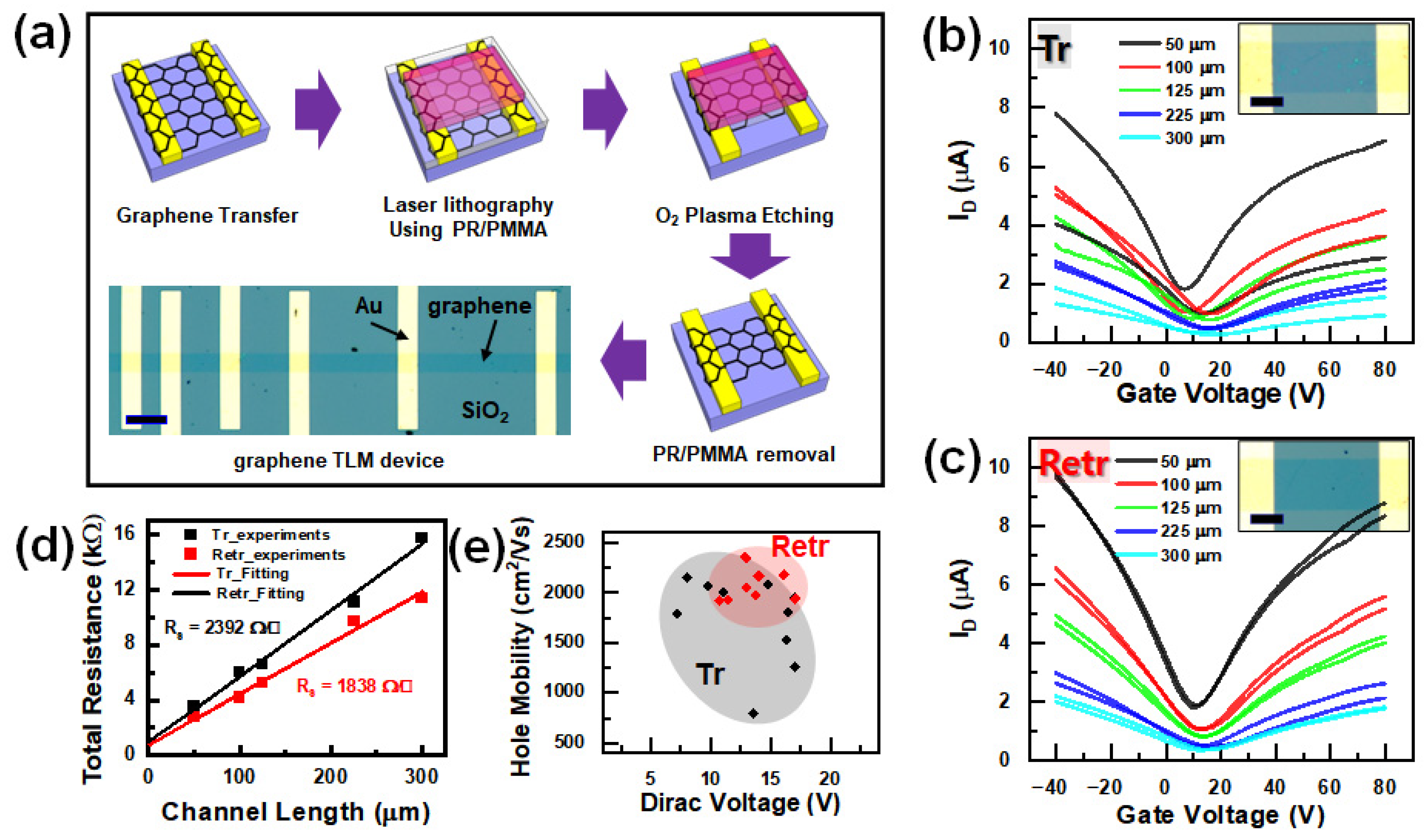
2.4. Device Fabrication
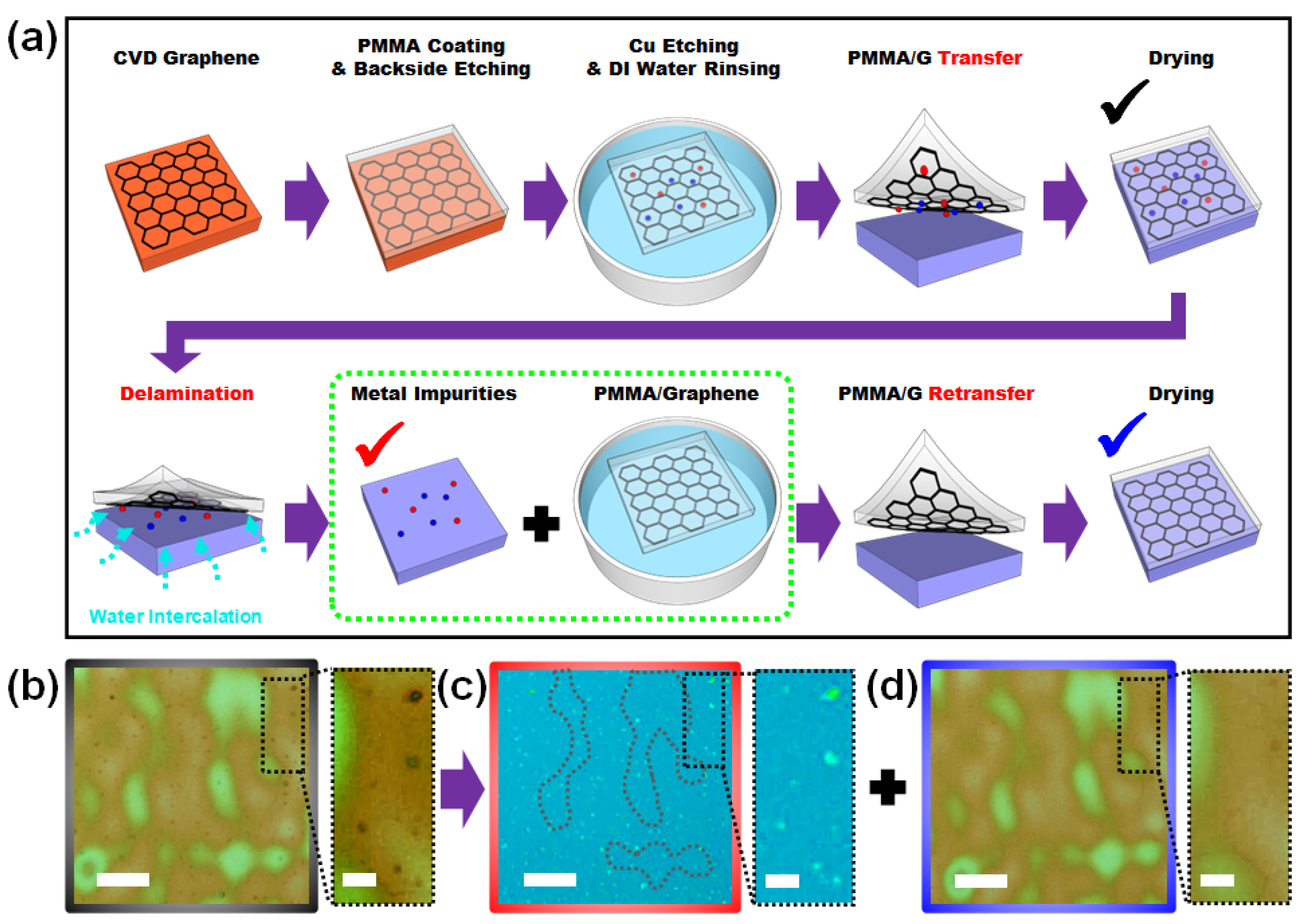
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Biswal, M.; Park, H.J.; Jin, S.; Qu, D.; Hong, S.; Zhu, Z.; Qiu, L.; Luo, D.; Liu, X.; et al. Highly oriented monolayer graphene grown on a Cu/Ni (111) alloy foil. ACS Nano 2018, 12, 6117–6127. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.T.; Zhao, W.W.; Chen, W.Y.; Jiang, J.; Nan, H.Y.; Guo, X.T.; Liang, Z.; Chen, Y.M.; Chen, Y.F.; Ni, Z.H. The influence of chemical solvents on the properties of CVD graphene. J. Raman Spectrosc. 2015, 46, 21–24. [Google Scholar] [CrossRef]
- Wang, M.; Yang, E.H.; Vajtai, R.; Kono, J.; Ajayan, P.M. Effects of etchants in the transfer of chemical vapor deposited graphene. J. Appl. Phys. 2018, 123, 195103. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lu, C.C.; Yeh, C.H.; Jin, C.; Suenaga, K.; Chiu, P.W. Graphene annealing: How clean can it be? Nano Lett. 2012, 12, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Pirkle, A.; Chan, J.; Venugopal, A.; Hinojos, D.; Magnuson, C.W.; McDonnell, S.; Colombo, L.; Vogel, E.M.; Ruoff, R.S.; Wallace, R.M. The effect of chemical residues on the physical and electrical properties of chemical vapor deposited graphene transferred to SiO2. Appl. Phys. Lett. 2011, 99, 122108. [Google Scholar] [CrossRef]
- Lupina, G.; Kitzmann, J.; Costina, I.; Lukosius, M.; Wenger, C.; Wolff, A.; Vaziri, S.; Ostling, M.; Pasternak, I.; Krajewska, A.; et al. Residual metallic contamination of transferred chemical vapor deposited graphene. ACS Nano 2015, 9, 4776–4785. [Google Scholar] [CrossRef]
- Pumera, M.; Ambrosi, A.; Chng, E.L. Impurities in graphenes and carbon nanotubes and their influence on the redox properties. Chem. Sci. 2012, 3, 3347–3355. [Google Scholar] [CrossRef]
- Kim, J.H.; Haidari, M.M.; Choi, J.S.; Kim, H.; Yu, Y.J.; Park, J. Facile dry surface cleaning of graphene by UV treatment. J. Korean Phys. Soc. 2018, 72, 1045–1051. [Google Scholar] [CrossRef]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on gas impermeability of graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Flak, D.; Braun, A.; Mun, B.S.; Döbeli, M.; Graule, T.; Rekas, M. Electronic structure and surface properties of non-stoichiometric Fe2O3-δ (α and γ) and its application in gas sensing. Procedia Eng. 2012, 47, 257–260. [Google Scholar] [CrossRef]
- Cao, X.; Chen, Y.; Jiao, S.; Fang, Z.; Xu, M.; Liu, X.; Li, L.; Pang, G.; Feng, S. Magnetic photocatalysts with ap–n junction: Fe3O4 nanoparticle and FeWO4 nanowire heterostructures. Nanoscale 2014, 6, 12366–12370. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Ichimura, M. Electrochemical deposition of Cu-doped p-type iron oxide thin films. Semicond. Sci. Technol. 2018, 33, 105006. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Xu, X.; Dubuisson, E.; Bao, Q.; Lu, J.; Loh, K.P. Electrochemical delamination of CVD-grown graphene film: Toward the recyclable use of copper catalyst. ACS Nano 2011, 5, 9927–9933. [Google Scholar] [CrossRef]
- Banszerus, L.; Schmitz, M.; Engels, S.; Goldsche, M.; Watanabe, K.; Taniguchi, T.; Beschoten, B.; Stampfer, C. Ballistic transport exceeding 28 μm in CVD grown graphene. Nano Lett. 2016, 16, 1387–1391. [Google Scholar] [CrossRef]
- Liang, X.; Sperling, B.A.; Calizo, I.; Cheng, G.; Hacker, C.A.; Zhang, Q.; Obeng, Y.; Yan, K.; Peng, H.; Li, Q.; et al. Toward clean and crackless transfer of graphene. ACS Nano 2011, 5, 9144–9153. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Chen, G.; Fan, C.; Liu, X. Recent progress in the transfer of graphene films and nanostructures. Small Methods 2021, 5, 2100771. [Google Scholar] [CrossRef]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-step fabrication of graphene oxide enhanced magnetic composite gel for highly efficient dye adsorption and catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Shangguan, Q.; Zhao, Y.; Song, Z.; Wang, J.; Yang, H.; Chen, J.; Liu, C.; Cheng, S.; Yang, W.; Yi, Z. High sensitivity active adjustable graphene absorber for refractive index sensing applications. Diam. Relat. Mater. 2022, 128, 109273. [Google Scholar] [CrossRef]
- Ye, Z.; Wu, P.; Wang, H.; Jiang, S.; Huang, M.; Lei, D.; Wu, F. Multimode tunable terahertz absorber based on a quarter graphene disk structure. Results Phys. 2023, 48, 106420. [Google Scholar] [CrossRef]
- Goniszewski, S.; Adabi, M.; Shaforost, O.; Hanham, S.M.; Hao, L.; Klein, N.J. Correlation of p-doping in CVD Graphene with Substrate Surface Charges. Sci. Rep. 2016, 6, 22858. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Granick, S. Hydrophobicity at a Janus interface. Science 2002, 295, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sacher, E.; Meunier, M. The effects of hydrogen bonds on the adhesion of inorganic oxide particles on hydrophilic silicon surfaces. J. Appl. Phys. 1999, 86, 1744–1748. [Google Scholar] [CrossRef]
- Si, C.; Liu, Z.; Duan, W.; Liu, F. First-principles calculations on the effect of doping and biaxial tensile strain on electron-phonon coupling in graphene. Phys. Rev. Lett. 2013, 111, 196802. [Google Scholar] [CrossRef]
- Ji, E.; Kim, M.J.; Lee, J.Y.; Sung, D.; Kim, N.; Park, J.W.; Hong, S.; Lee, G.H. Substrate effect on doping and degradation of graphene. Carbon 2021, 184, 651–658. [Google Scholar] [CrossRef]
- Haidari, M.M.; Kim, H.; Kim, J.H.; Lee, S.; Yu, Y.J.; Kim, J.T.; Choi, C.G.; Choi, J.S. Graphene laminated Cu nanoparticle arrays by spontaneous formation through dewetting. J. Ind. Eng. Chem. 2018, 64, 367–372. [Google Scholar] [CrossRef]
- Ferrante, C.; Virga, A.; Benfatto, L.; Martinati, M.; De Fazio, D.; Sassi, U.; Fasolato, C.; Ott, A.K.; Postorino, P.; Yoon, D.; et al. Raman spectroscopy of graphene under ultrafast laser excitation. Nat. Commun. 2018, 9, 308. [Google Scholar] [CrossRef]
- Hatel, R.; El Majdoub, S.; Bakour, A.; Khenfouch, M.; Baitoul, M. Graphene oxide/Fe3O4 nanorods composite: Structural and Raman investigation. J. Phys. Conf. Ser. 2018, 1081, 012006. [Google Scholar] [CrossRef]
- Zhao, S.; Surwade, S.P.; Li, Z.; Liu, H. Photochemical oxidation of CVD-grown single layer graphene. Nanotechnology 2012, 23, 355703. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Pumera, M. The CVD graphene transfer procedure introduces metallic impurities which alter the graphene electrochemical properties. Nanoscale 2014, 6, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Ahn, G.; Shim, J.; Lee, Y.S.; Ryu, S. Optical separation of mechanical strain from charge doping in graphene. Nat. Commun. 2012, 3, 1024. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.B.; Mackenzie, D.M.; Caridad, J.M.; Bøggild, P.; Booth, T.J. Transfer induced compressive strain in graphene: Evidence from Raman spectroscopic mapping. Microelectron. Eng. 2014, 121, 113–117. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.S.; Byun, I.S.; Lee, D.H.; Lee, M.J.; Park, B.H.; Lee, C.; Yoon, D.; Cheong, H.; Lee, K.H.; et al. Friction anisotropy-driven domain imaging on exfoliated monolayer graphene. Science 2011, 333, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Bendiab, N.; Renard, J.; Schwarz, C.; Reserbat-Plantey, A.; Djevahirdjian, L.; Bouchiat, V.; Coraux, J.; Marty, L. Unravelling external perturbation effects on the optical phonon response of graphene. J. Raman Spectrosc. 2018, 49, 130–145. [Google Scholar] [CrossRef]
- Zabel, J.; Nair, R.R.; Ott, A.; Georgiou, T.; Geim, A.K.; Novoselov, K.S.; Casiraghi, C. Raman spectroscopy of graphene and bilayer under biaxial strain: Bubbles and balloons. Nano Lett. 2012, 12, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, R.; Lin, P.C.; Faraji, F.; Hassani, N.; Bana, H.; Zarkua, Z.; Nair, M.N.; Tsai, H.C.; Auge, M.; Junge, F.; et al. Breakdown of universal scaling for nanometer-sized bubbles in graphene. Nano Lett. 2021, 21, 8103–8110. [Google Scholar] [CrossRef]
- Wang, M.; Huang, M.; Luo, D.; Li, Y.; Choe, M.; Seong, W.K.; Kim, M.; Jin, S.; Wang, M.; Chatterjee, S.; et al. Single-crystal, large-area, fold-free monolayer graphene. Nature 2021, 596, 519–524. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Giubileo, F.; Romeo, F.; Sabatino, P.; Carapella, G.; Iemmo, L.; Schroeder, T.; Lupina, G. Graphene field effect transistors with niobium contacts and asymmetric transfer characteristics. Nanotechnology 2015, 26, 475202. [Google Scholar] [CrossRef] [PubMed]
- Berciaud, S.; Ryu, S.; Brus, L.E.; Heinz, T.F. Probing the intrinsic properties of exfoliated graphene: Raman spectroscopy of free-standing monolayers. Nano Lett. 2009, 9, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Nguyen, Y.; Chen, T.W.; Yen, Z.L.; Hofmann, M.; Hsieh, Y.P. Neutral scatterers dominate carrier transport in CVD graphene with ionic impurities. Carbon 2020, 165, 163–168. [Google Scholar] [CrossRef]
- Schwierz, F. Graphene transistors. Nat. Nanotechnol. 2010, 5, 487–496. [Google Scholar] [CrossRef] [PubMed]


| Transfer Method | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Conventional wet transfer | Easy and scalable | Presence of metallic impurities | [8] |
| Electrochemical delamination | Free of metallic impurities; metal catalyst reusable | Additional instruments required; damage caused by bubbles | [8,15] |
| Mechanical delamination | Free of metallic impurities; metal catalyst reusable | Time-consuming; hard to scale up; may cause mechanical damage | [16] |
| RCA clean | Free of metallic impurities | Use of hazardous solution; time-consuming; generation of metal waste | [17] |
| Modified wet transfer (This work) | Free of metallic impurities; easy and scalable | Generation of metal waste |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, D.J.; Haidari, M.M.; Kim, J.H.; Ko, J.-Y.; Yi, Y.; Choi, J.S. A Modified Wet Transfer Method for Eliminating Interfacial Impurities in Graphene. Nanomaterials 2023, 13, 1494. https://doi.org/10.3390/nano13091494
Jang DJ, Haidari MM, Kim JH, Ko J-Y, Yi Y, Choi JS. A Modified Wet Transfer Method for Eliminating Interfacial Impurities in Graphene. Nanomaterials. 2023; 13(9):1494. https://doi.org/10.3390/nano13091494
Chicago/Turabian StyleJang, Dong Jin, Mohd Musaib Haidari, Jin Hong Kim, Jin-Yong Ko, Yoonsik Yi, and Jin Sik Choi. 2023. "A Modified Wet Transfer Method for Eliminating Interfacial Impurities in Graphene" Nanomaterials 13, no. 9: 1494. https://doi.org/10.3390/nano13091494





