Recent Advances in ZnO Nanomaterial-Mediated Biological Applications and Action Mechanisms
Abstract
:1. Introduction
2. The Structure and Structure-Dependent Properties of ZnO Nanoparticles
3. The Common Synthesis Methods of ZnO Nanoparticles
4. Bio-Application and Action Mechanisms of ZnO Nanomaterials
4.1. ZnO Nanomaterials for Anti-Bacterial and Anti-Fungal Applications
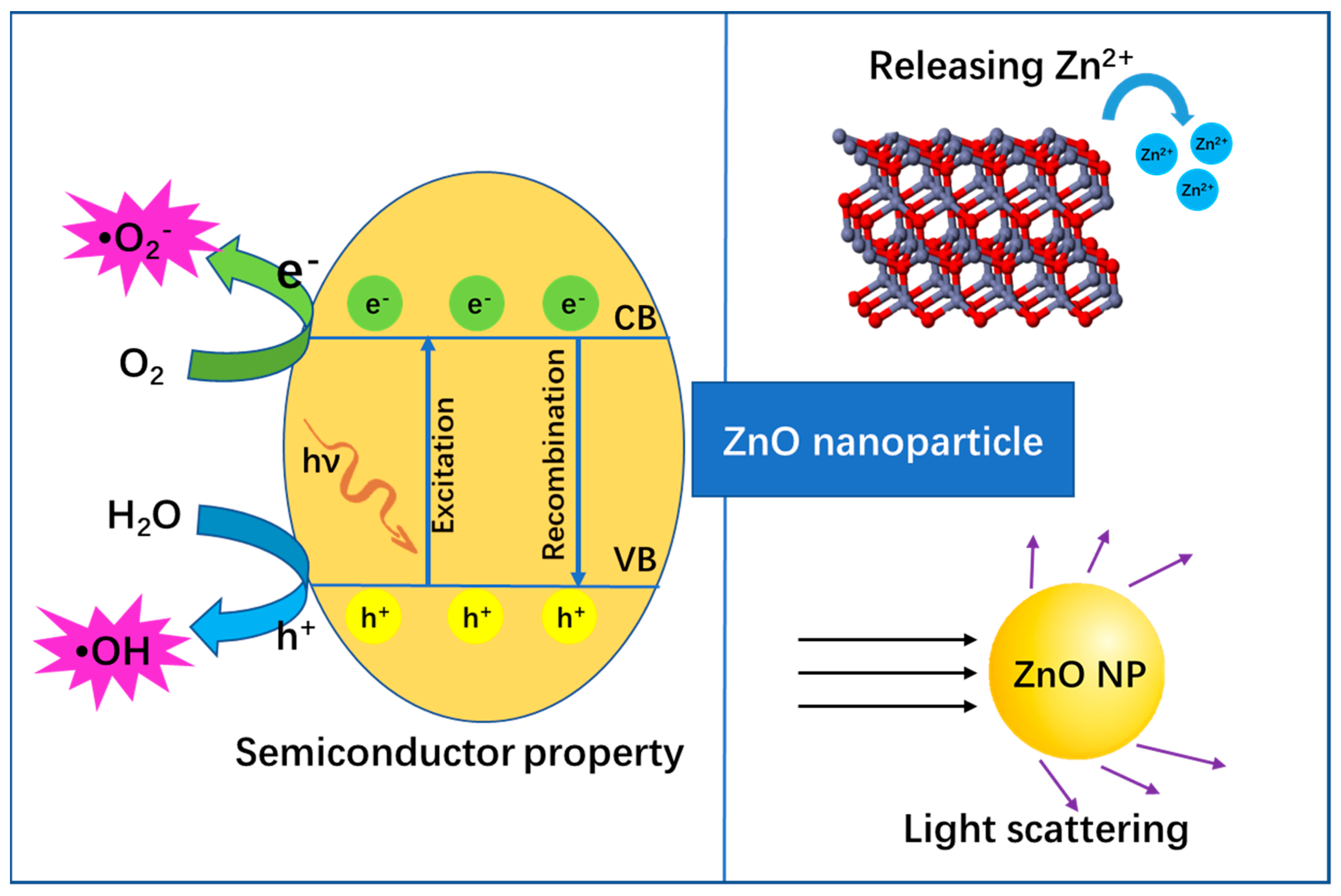
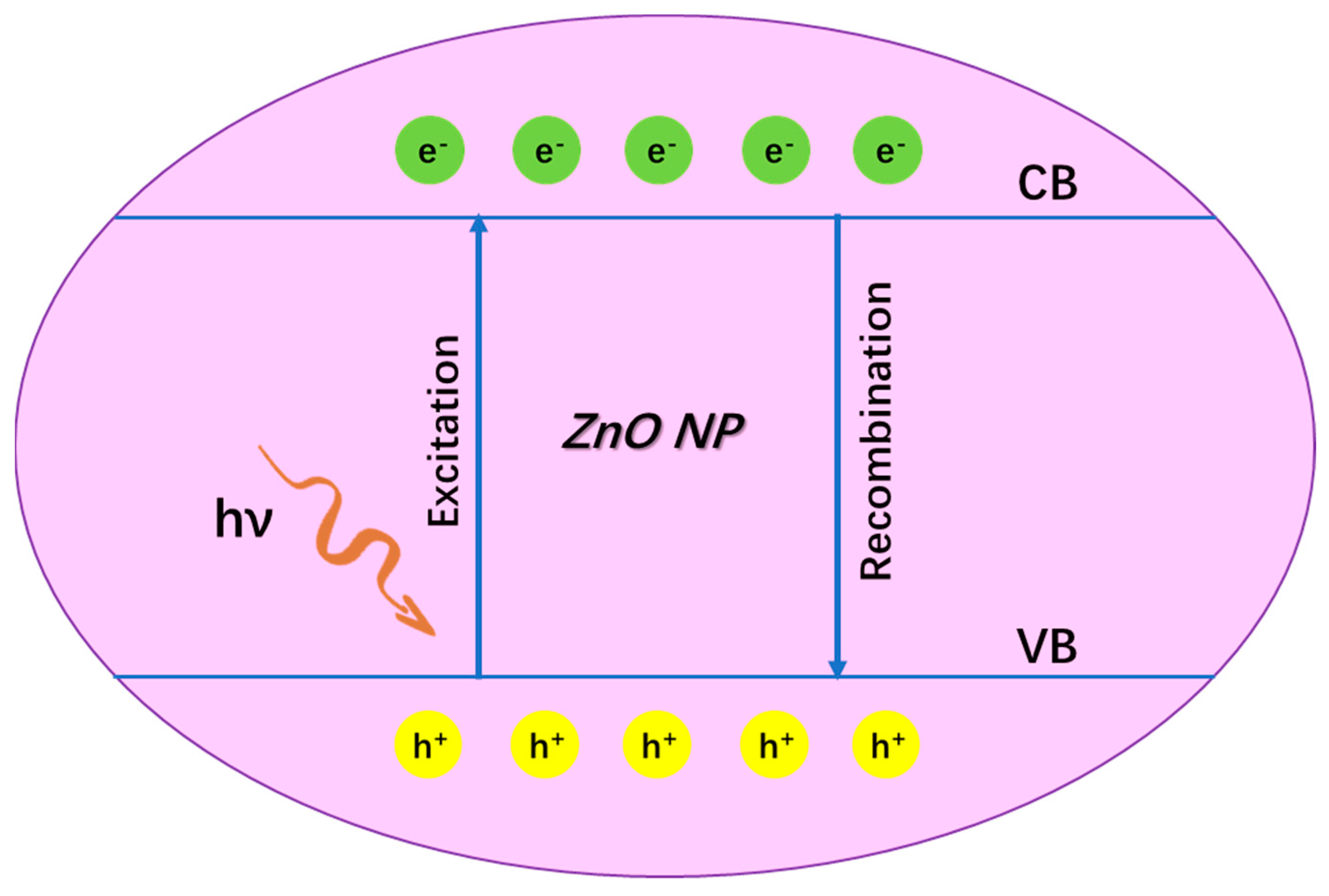
4.1.1. Anti-Bacterial Mechanisms
4.1.2. Anti-Bacterial and Anti-Fungal Applications
4.2. ZnO Nanomaterials for Antitumor Applications
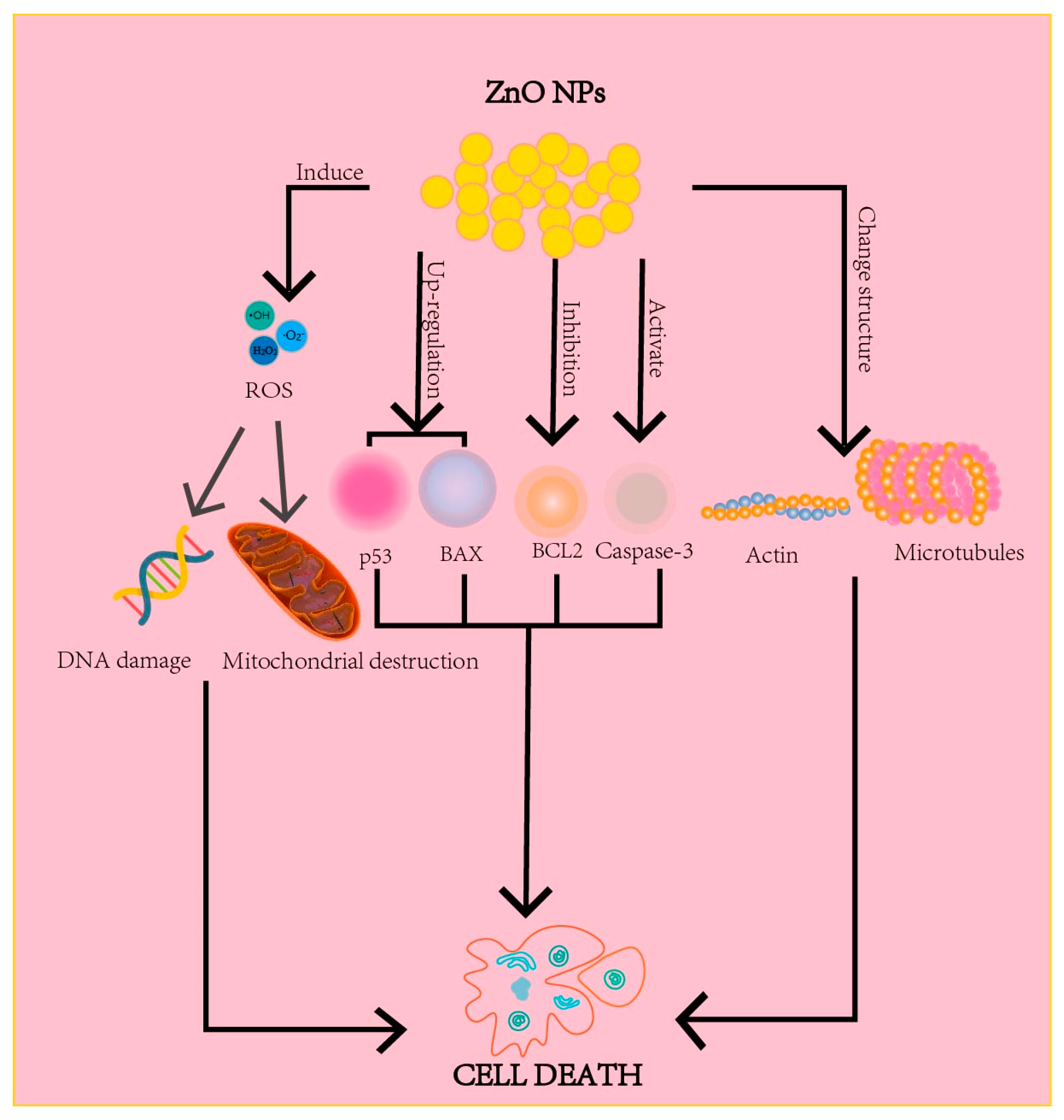
4.2.1. Anti-Cancer Mechanisms
4.2.2. Anti-Cancer Applications
Liver Cancer Treatment
Breast Cancer Treatment
Lung Cancer Treatment
4.3. ZnO Nanomaterials for Anti-Inflammation
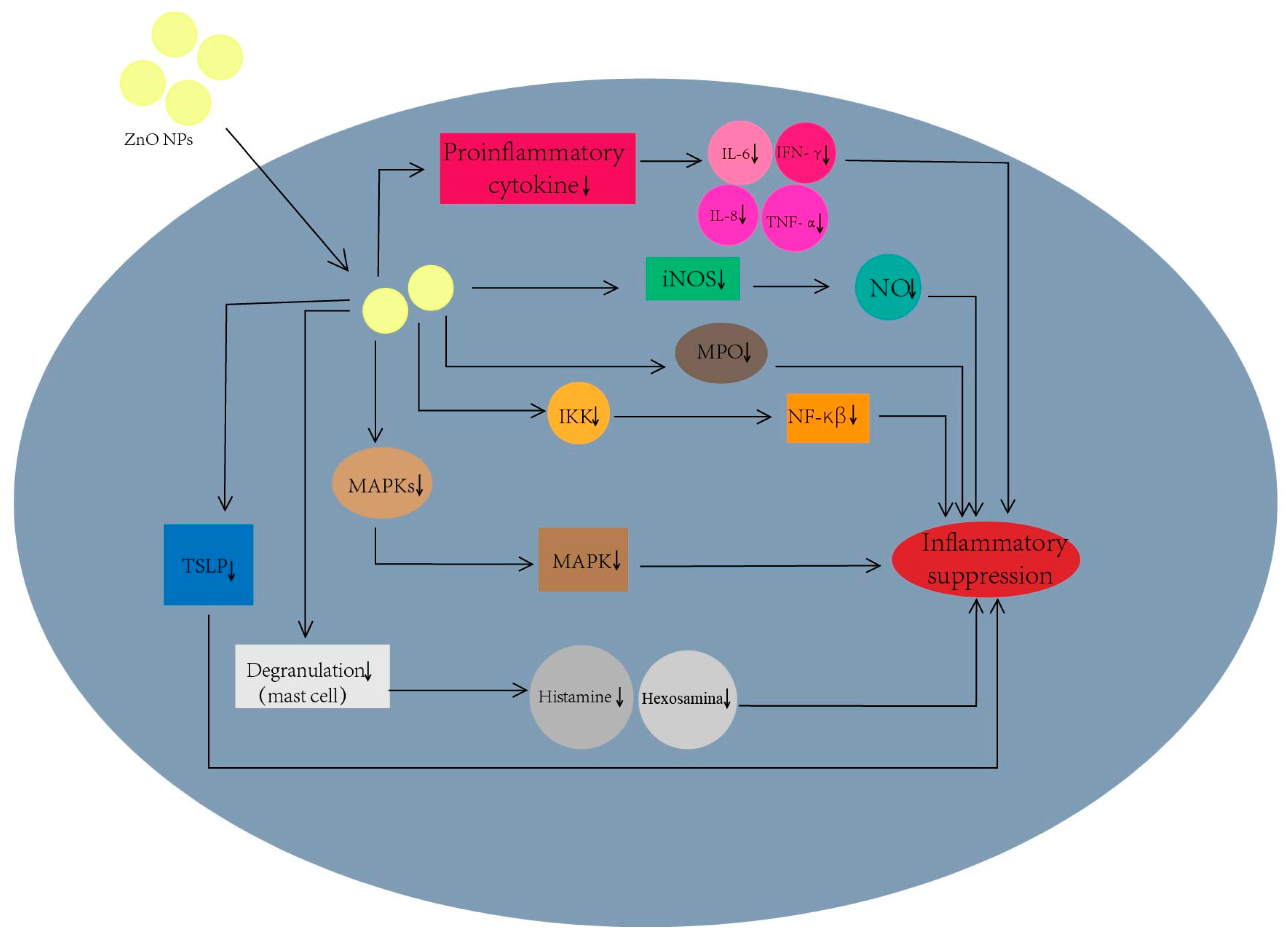
4.3.1. Anti-Inflammatory Mechanisms
4.3.2. Anti-Inflammatory Applications
4.4. ZnO Nanomaterials for Skin Care
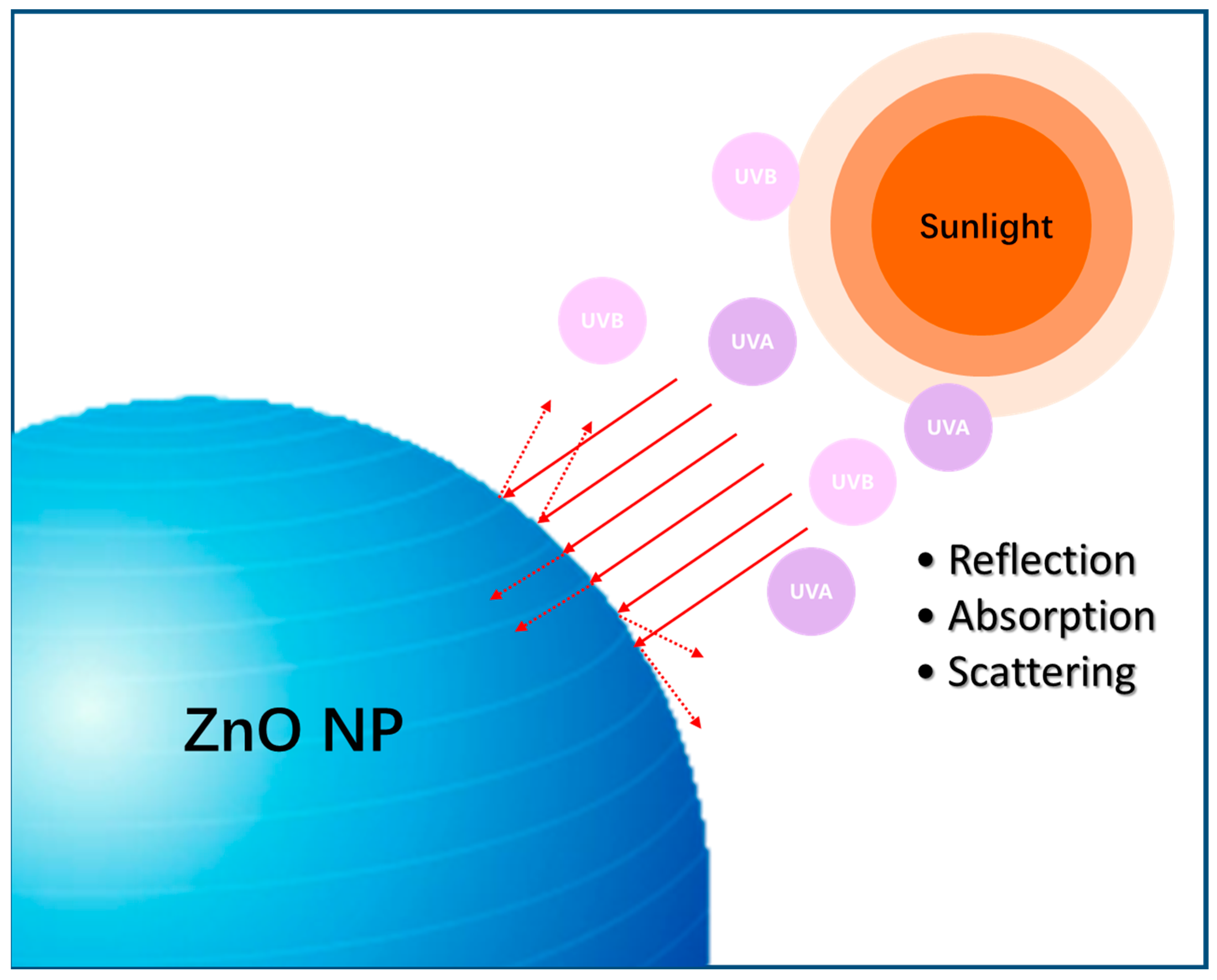
4.4.1. Sunscreen Mechanism
4.4.2. Skin Care Applications
4.5. ZnO Nanomaterials for Bioimaging
4.5.1. Imaging Mechanisms
4.5.2. Imaging Applications
4.6. ZnO Nanomaterials for Food Packaging
4.6.1. Material Performance Evaluation
4.6.2. Food Packaging Application
5. Biosafety of ZnO Nanomaterials
6. Conclusions and Outlook
- (i).
- Biosafety evaluation and improvement need further and deeper research. Although toxicity studies of ZnO nanoparticles have been extensively published, the reports about long-term effects are still not enough. It is not difficult to see that the acute toxicity of ZnO nanoparticles is low according to many reports. Thus, the chronic toxicity and long-term effects are more valuable to be concerned with. Meanwhile, the genetic toxicity evaluation of ZnO nanoparticles also needs to be further strengthened, which is closely related to the health of future generations. In addition, for the improvement of material biosafety, we can make material modifications such as alterations of the surface reactive property to improve its biocompatibility and minimize the adverse effects [173].
- (ii).
- Strengthening toxicity mechanisms study and establishing standardization. To date, the understanding of the toxicity mechanisms of ZnO nanoparticles is not deep enough, and the research results are diverse, even contradictory, in different models. The absence of key nanotoxicity information and the lack of consensus among researchers on the experimental evaluation index is unbeneficial to regulation control and enforcement in use. Therefore, the experimental protocols of toxicity mechanisms are demanded, and guidance and standardization for nanotoxicity evaluation that includes cytotoxicity, in vivo toxicity, genetic toxicity and so on should be established.
- (iii).
- The optimization of the synthesis. On the one hand, for existing mature synthesis methods, one can optimize the experimental parameters such as pH, temperature and ingredient proportion to rationally regulate the physicochemical properties of ZnO nanoparticles so as to obtain the particle with desired size, shape and specific surface area, etc. On the other hand, the yield demand of ZnO nanoparticles has increased dramatically due to their large-scale commercial use. Thus, simple, low-cost and eco-friendly new synthesis methods such as popular green synthesis are currently an urgent task.
- (iv).
- Proper surface functionalization of ZnO nanoparticles. The surface of a nanoparticle is closely related with its dispersibility, biocompatibility and biotoxicity in a physiological environment. Meanwhile, the modification of the surface with various functional biomolecules, such as aptamers and antibodies, is helpful to improve the selectivity and targeting ability to pathogenic tissue and so on. Thus, the proper surface functionalization of ZnO nanoparticles is significant to enhance its therapeutic efficacy for diseases such as tumors.
- (v).
- Deepening the research on the action mechanism. It is not difficult to see that the action mechanisms are more than one path of each ZnO nanomaterial-mediated application. For example, the ZnO-mediated antibacterial mechanisms cover not only ROS generation but also Zn2+ release, disturbance of the cell membrane and so on. So, which path is dominant? Or is there an inner link between these paths? Questions such as this need to be further explained.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO Nanostructures in Active Antibacterial Food Packaging: Preparation Methods, Antimicrobial Mechanisms, Safety Issues, Future Prospects, and Challenges. Food Rev. Int. 2020, 38, 537–565. [Google Scholar] [CrossRef]
- Zhong, L.; Yun, K. Graphene oxide-modified ZnO particles: Synthesis, characterization, and antibacterial properties. Int. J. Nanomed. 2015, 10, 79–92. [Google Scholar] [CrossRef]
- Lallo da Silva, B.; Caetano, B.L.; Chiari-Andreo, B.G.; Pietro, R.; Chiavacci, L.A. Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf. B 2019, 177, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef]
- Gad, S.S.; Fayez, A.M.; Abdelaziz, M.; Abou El-Ezz, D. Amelioration of autoimmunity and inflammation by zinc oxide nanoparticles in experimental rheumatoid arthritis. N-S Arch. Pharmacol. 2021, 394, 1975–1981. [Google Scholar] [CrossRef]
- Feltis, B.N.; Elbaz, A.; Wright, P.F.; Mackay, G.A.; Turney, T.W.; Lopata, A.L. Characterizing the inhibitory action of zinc oxide nanoparticles on allergic-type mast cell activation. Mol. Immunol. 2015, 66, 139–146. [Google Scholar] [CrossRef]
- de Lucas-Gil, E.; Del Campo, A.; Pascual, L.; Monte-Serrano, M.; Menendez, J.; Fernandez, J.F.; Rubio-Marcos, F. The fight against multidrug-resistant organisms: The role of ZnO crystalline defects. Mater. Sci. Eng. C 2019, 99, 575–581. [Google Scholar] [CrossRef]
- Vimala, K.; Shanthi, K.; Sundarraj, S.; Kannan, S. Synergistic effect of chemo-photothermal for breast cancer therapy using folic acid (FA) modified zinc oxide nanosheet. J. Colloid Interface Sci. 2017, 488, 92–108. [Google Scholar] [CrossRef]
- Yin, X.; Fan, X.; Zhou, Z.; Li, Q. Encapsulation of berberine decorated ZnO nano-colloids into injectable hydrogel using for diabetic wound healing. Front. Chem. 2022, 10, 964662. [Google Scholar] [CrossRef]
- Sarkar, S.; Debnath, S.K.; Srivastava, R.; Kulkarni, A.R. Continuous flow scale-up of biofunctionalized defective ZnO quantum dots: A safer inorganic ingredient for skin UV protection. Acta Biomater. 2022, 147, 377–390. [Google Scholar] [CrossRef]
- Du, T.; Zhao, C.; ur Rehman, F.; Lai, L.; Li, X.; Sun, Y.; Luo, S.; Jiang, H.; Selke, M.; Wang, X. Rapid and multimodal in vivo bioimaging of cancer cells through in situ biosynthesis of Zn&Fe nanoclusters. Nano Res. 2017, 10, 2626–2632. [Google Scholar] [CrossRef]
- Janani, N.; Zare, E.N.; Salimi, F.; Makvandi, P. Antibacterial tragacanth gum-based nanocomposite films carrying ascorbic acid antioxidant for bioactive food packaging. Carbohydr. Polym. 2020, 247, 116678. [Google Scholar] [CrossRef] [PubMed]
- Secu, C.E.; Sima, M. Photoluminescence and thermoluminescence of ZnO nano-needle arrays and films. Opt. Mater. 2009, 31, 876–880. [Google Scholar] [CrossRef]
- Sharma, P.; Jang, N.Y.; Lee, J.W.; Park, B.C.; Kim, Y.K.; Cho, N.H. Application of ZnO-Based Nanocomposites for Vaccines and Cancer Immunotherapy. Pharmaceutics 2019, 11, 493. [Google Scholar] [CrossRef]
- Xie, J.; Gong, L.; Zhu, S.; Yong, Y.; Gu, Z.; Zhao, Y. Emerging Strategies of Nanomaterial-Mediated Tumor Radiosensitization. Adv. Mater. 2019, 31, e1802244. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Lu, C. Synthesis and characterization of nano-sized ZnO powders by direct precipitation method. Chem. Eng. J. 2008, 144, 509–513. [Google Scholar] [CrossRef]
- Dhanya, R.; Suganthi, K.S.; Rajan, K.S. Studies on Scale-up of Synthesis of ZnO Nanoparticles. Asian J. Chem. 2014, 26, 4273–4276. [Google Scholar] [CrossRef]
- Maroufi, S.; Nekouei, R.K.; Assefi, M.; Sahajwalla, V. Waste-cleaning waste: Synthesis of ZnO porous nano-sheets from batteries for dye degradation. Environ. Sci. Pollut. Res. 2018, 25, 28594–28600. [Google Scholar] [CrossRef]
- Raj, C.J.; Joshi, R.K.; Varma, K.B.R. Synthesis from zinc oxalate, growth mechanism and optical properties of ZnO nano/micro structures. Cryst. Res. Technol. 2011, 46, 1181–1188. [Google Scholar] [CrossRef]
- Wang, H.; Li, C.; Zhao, H.; Liu, J. Preparation of nano-sized flower-like ZnO bunches by a direct precipitation method. Adv. Powder Technol. 2013, 24, 599–604. [Google Scholar] [CrossRef]
- Yildiz, Ö. A Novel Synthesis Process of ZnO Nano Powders in Granule Form. Trans. Ind. Ceram. Soc. 2015, 74, 41–48. [Google Scholar] [CrossRef]
- Ulyankina, A.; Molodtsova, T.; Gorshenkov, M.; Leontyev, I.; Zhigunov, D.; Konstantinova, E.; Lastovina, T.; Tolasz, J.; Henych, J.; Licciardello, N.; et al. Photocatalytic degradation of ciprofloxacin in water at nano-ZnO prepared by pulse alternating current electrochemical synthesis. J. Water Process. Eng. 2021, 40, 101809. [Google Scholar] [CrossRef]
- Bai, X.; Yi, L.; Liu, D.; Nie, E.; Sun, C.; Feng, H.; Jin, Y.; Jiao, Z.; Sun, X. Electrodeposition from ZnO nano-rods to nano-sheets with only zinc nitrate electrolyte and its photoluminescence. Appl. Surf. Sci. 2011, 257, 10317–10321. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, C.; Zhang, H.; Lu, G. Effects of bath temperature on the morphology of ZnO nano-rods and its optical properties. Mater. Lett. 2015, 148, 1–4. [Google Scholar] [CrossRef]
- An, D.; Tong, X.; Liu, J.; Wang, Q.; Zhou, Q.; Dong, J.; Li, Y. Template-free hydrothermal synthesis of ZnO micro/nano-materials and their application in acetone sensing properties. Superlattices Microstruct. 2015, 77, 1–11. [Google Scholar] [CrossRef]
- Ben Saad, L.; Soltane, L.; Sediri, F. Nano-ZnO Sand Flowers and Rods: Hydrothermal Synthesis and Optical Properties. Russ. J. Phys. Chem. A 2019, 93, 2269–2274. [Google Scholar] [CrossRef]
- Du, Y.; Yang, Y.; Wang, X.; Li, X.; Zhou, Q. Hydrothermal synthesis and optical properties of CTAB modified nano-ZnO. Integr. Ferroelectr. 2019, 200, 161–167. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Wang, J.; Zhang, B.; Liu, L.; Liu, H.; Yang, J. Supercritical hydrothermal synthesis of nano-zinc oxide: Process and mechanism. Ceram. Int. 2022, 48, 22629–22646. [Google Scholar] [CrossRef]
- Wen, M.; Yang, B.; Yan, H.; Fu, Z.; Cai, C.; Liu, K.; Chen, Y.; Xu, J.; Fu, S.; Zhang, S. Morphology-controlled synthesis of flowerlike ZnO nano/microstructures and their photocatalytic property. J. Nanosci. Nanotechnol. 2009, 9, 2038–2044. [Google Scholar] [CrossRef]
- Xiang-Qi, L.I.; Qing-Fei, F.A.N.; Guang-Li, L.I.; Yao-Han, H.; Zhao, G.A.O.; Xi-Mei, F.A.N.; Chao-Liang, Z.; Zuo-Wan, Z. Syntheses of ZnO Nano-Arrays and Spike-Shaped CuO/ZnO Heterostructure. Acta Phys.-Chim. Sin. 2015, 31, 783–792. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Zheng, J.; Li, L.; Zhu, Z. Shuttle-like ZnO nano/microrods: Facile synthesis, optical characterization and high formaldehyde sensing properties. Appl. Surf. Sci. 2011, 258, 711–718. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, F.; Zhang, S.; Wang, W.; Wang, X.; Yang, Y. Study on hydrothermal synthesis and photoelectric properties of nano-ZnO. Ferroelectrics 2019, 549, 212–219. [Google Scholar] [CrossRef]
- Kumar, D.; Singh Mehata, M. Synthesis of diamagnetic ZnO nano-crystallites via sol-gel method and their photocatalytic activity. Indian J. Eng. Mater. Sci. 2022, 29, 437–444. [Google Scholar] [CrossRef]
- Fu, D.; Han, G.; Meng, C. Size-controlled synthesis and photocatalytic degradation properties of nano-sized ZnO nanorods. Mater. Lett. 2012, 72, 53–56. [Google Scholar] [CrossRef]
- John, R.; Shanmugaraj, S.; Rajaram, R.; Endo, T. Synthesis and characterization of nano ZnO and CdO. J. Ceram. Soc. JPN 2010, 118, 329–332. [Google Scholar] [CrossRef]
- Noroozi, A.H.; Sadeghi, M.; Tehrani, A.A.; Sarabadani, P.; Rajabaifar, S.; Mirzaee, M. Synthesis and Characterization of Zinc Oxide Nano-particles by Solid State Chemical Reaction Method. J. Clust. Sci. 2013, 24, 757–770. [Google Scholar] [CrossRef]
- Ali, M.; Friedenberger, N.; Spasova, M.; Winterer, M. A Novel Approach for Chemical Vapor Synthesis of ZnO Nanocrystals: Optimization of Yield, Crystallinity. Chem. Vap. Depos. 2009, 15, 192–198. [Google Scholar] [CrossRef]
- Riahi-Noori, N.; Sarraf-Mamoory, R.; Alizadeh, P.; Mehdikhanic, A. Synthesis of ZnO nano powder by a gel combustion method. J. Ceram. Process. Res. 2008, 9, 246–249. [Google Scholar]
- Teng, H.; Xu, S.; Meng, W. Controllable Synthesis of Different Dimensions Nano-ZnO by Microemulsion and Photocatalytic Activity. J. Inorg. Mater. 2010, 25, 1034–1040. [Google Scholar] [CrossRef]
- Luković Golić, D.; Ćirković, J.; Šćepanović, M.; Srećković, T.; Longo, E.; Varela, J.A.; Daneu, N.; Stamenković, V.; Branković, G.; Branković, Z. The modification of structural and optical properties of nano- and submicron ZnO powders by variation of solvothermal syntheses conditions. J. Nanopart. Res. 2014, 16, 2670. [Google Scholar] [CrossRef]
- Moulahi, A.; Sediri, F. Controlled synthesis of nano-ZnO via hydro/solvothermal process and study of their optical properties. Optik 2016, 127, 7586–7593. [Google Scholar] [CrossRef]
- Song, Y.L.; Zhang, T.J.; Jie Du, H.; Ji, P.F.; Li, Y.; Zhou, F.Q. Synthesis, structures and temperature-dependent photoluminescence from ZnO nano/micro-rods on Zn foil. Mater. Lett. 2016, 176, 139–142. [Google Scholar] [CrossRef]
- Agouram, S.; Bushiri, M.J.; Montenegro, D.N.; Reig, C.; Martinez-Tomas, M.C.; Munoz-Sanjose, V. Synthesis and characterization of ZnO nano and micro structures grown by low temperature spray pyrolysis and vapor transport. J. Nanosci. Nanotechnol. 2012, 12, 6792–6799. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhai, F.; Liu, Y.; Cao, M.; Wang, F.; Zhang, X. Synthesis and photoluminescence study on ZnO nano-particles. Chin. Phys. 2007, 16, 2769–2772. [Google Scholar]
- Seo, S.H.; Kang, H.C. Self-assembled ZnO hexagonal nano-disks grown by radio-frequency magnetron sputtering. Mater. Lett. 2013, 94, 34–37. [Google Scholar] [CrossRef]
- Sun, J.H.; Kang, H.C. Synthesis and characterization of self-assembled ZnO nano-dots grown on SiNx/Si(001) substrates by radio frequency magnetron sputtering. Thin Solid Films 2010, 518, 6522–6525. [Google Scholar] [CrossRef]
- Khanna, P.K.; Kate, K.; Dhanabalan, K.; Banerjee, S.; Reji, N.; Shinde, S.D.; Jain, G.H. Sono-chemical synthesis of ZnO nano-particles and their application in hydrogen sulphide gas sensing. J. Nanosci. Nanotechnol. 2012, 12, 2791–2796. [Google Scholar] [CrossRef]
- Shi, L.-E.; Fang, X.-J.; Zhang, Z.-L.; Zhou, T.; Jiang, D.; Wu, H.-H.; Tang, Z.-X. Preparation of nano-ZnO using sonication method and its antibacterial characteristics. Int. J. Food Sci. Technol. 2012, 47, 1866–1871. [Google Scholar] [CrossRef]
- Song, H.; Zhu, K.; Liu, Y.; Zhai, X. Microwave-assisted synthesis of ZnO and its photocatalytic activity in degradation of CTAB. Russ. J. Phys. Chem. A 2017, 91, 59–62. [Google Scholar] [CrossRef]
- Lv, H.; Sang, D.; Li, H.; Du, X.; Li, D.; Zou, G. Thermal Evaporation Synthesis and Properties of ZnO Nano/Microstructures Using Carbon Group Elements as the Reducing Agents. Nanoscale Res. Lett. 2010, 5, 620–624. [Google Scholar] [CrossRef]
- Park, N.-K.; Han, G.B.; Lee, J.D.; Ryu, S.O.; Lee, T.J.; Chang, W.C.; Chang, C.H. The growth of ZnO nano-wire by a thermal evaporation method with very small amount of oxygen. Curr. Appl. Phys. 2006, 6, e176–e181. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Xu, B.; Jiang, F.; Li, J. Shape-controlled synthesis of ZnO nano- and micro-structures. J. Cryst. Growth 2005, 280, 509–515. [Google Scholar] [CrossRef]
- Chen, C.; Yu, B.; Liu, P.; Liu, J.; Wang, L. Investigation of nano-sized ZnO particles fabricated by various synthesis routes. J. Ceram. Process. Res. 2011, 12, 420–425. [Google Scholar]
- Gu, Z.; Paranthaman, M.P.; Xu, J.; Pan, Z.W. Aligned ZnO nanorod arrays grown directly on zinc foils and zinc spheres by a low-temperature oxidization method. ACS Nano 2009, 3, 273–278. [Google Scholar] [CrossRef]
- Prasad, A.R.; Williams, L.; Garvasis, J.; Shamsheera, K.O.; Basheer, S.M.; Kuruvilla, M.; Joseph, A. Applications of phytogenic ZnO nanoparticles: A review on recent advancements. J. Mol. Liq. 2021, 331, 115805. [Google Scholar] [CrossRef]
- Essawy, A.A.; Alsohaimi, I.H.; Alhumaimess, M.S.; Hassan, H.M.A.; Kamel, M.M. Green synthesis of spongy Nano-ZnO productive of hydroxyl radicals for unconventional solar-driven photocatalytic remediation of antibiotic enriched wastewater. J. Environ. Manag. 2020, 271, 110961. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Kiew, S.F.; Boakye-Ansah, S.; Lau, S.Y.; Barhoum, A.; Danquah, M.K.; Rodrigues, J. Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale 2022, 14, 2534–2571. [Google Scholar] [CrossRef]
- Svetlichnyi, V.; Shabalina, A.; Lapin, I.; Goncharova, D.; Nemoykina, A. ZnO nanoparticles obtained by pulsed laser ablation and their composite with cotton fabric: Preparation and study of antibacterial activity. Appl. Surf. Sci. 2016, 372, 20–29. [Google Scholar] [CrossRef]
- Sharma, S.C. ZnO nano-flowers from Carica papaya milk: Degradation of Alizarin Red-S dye and antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus. Optik 2016, 127, 6498–6512. [Google Scholar] [CrossRef]
- Happy, A.; Soumya, M.; Venkat Kumar, S.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem.-Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Iqbal, G.; Faisal, S.; Khan, S.; Shams, D.F.; Nadhman, A. Photo-inactivation and efflux pump inhibition of methicillin resistant Staphylococcus aureus using thiolated cobalt doped ZnO nanoparticles. J. Photochem. Photobiol. B 2019, 192, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Jha, E.; Panda, P.K.; Das, J.K.; Thirumurugan, A.; Suar, M.; Parashar, S. Molecular aspects of core-shell intrinsic defect induced enhanced antibacterial activity of ZnO nanocrystals. Nanomedicine 2018, 13, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Subramani, K.; Petchi Muthu Raju, S.A.K.; Rangaraj, S.; Venkatachalam, R. Psidium guajava leaf extract-mediated synthesis of ZnO nanoparticles under different processing parameters for hydrophobic and antibacterial finishing over cotton fabrics. Prog. Org. Coat. 2018, 124, 80–91. [Google Scholar] [CrossRef]
- Babitha, N.; Priya, L.S.; Christy, S.R.; Manikandan, A.; Dinesh, A.; Durka, M.; Arunadevi, S. Enhanced Antibacterial Activity and Photo-Catalytic Properties of ZnO Nanoparticles: Pedalium Murex Plant Extract-Assisted Synthesis. J. Nanosci. Nanotechnol. 2019, 19, 2888–2894. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Salimikia, I. Phytosynthesis of zinc oxide nanoparticles and its antibacterial, antiquorum sensing, antimotility, and antioxidant capacities against multidrug resistant bacteria. J. Ind. Eng. Chem. 2019, 72, 457–473. [Google Scholar] [CrossRef]
- Datta, A.; Patra, C.; Bharadwaj, H.; Kaur, S.; Dimri, N.; Khajuria, R. Green Synthesis of Zinc Oxide Nanoparticles Using Parthenium hysterophorus Leaf Extract and Evaluation of their Antibacterial Properties. J. Biotechnol. Biomater. 2017, 7, 1000271. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Jafar Ahamed, A.; Karthikeyan, C.; Vijaya Kumar, P. Enhancement of antibacterial and anticancer properties of pure and REM doped ZnO nanoparticles synthesized using Gymnema sylvestre leaves extract. SN Appl. Sci. 2019, 1, 355. [Google Scholar] [CrossRef]
- Mahendiran, D.; Subash, G.; Arumai Selvan, D.; Rehana, D.; Senthil Kumar, R.; Kalilur Rahiman, A. Biosynthesis of Zinc Oxide Nanoparticles Using Plant Extracts of Aloe vera and Hibiscus sabdariffa: Phytochemical, Antibacterial, Antioxidant and Anti-proliferative Studies. BioNanoScience 2017, 7, 530–545. [Google Scholar] [CrossRef]
- Nguyen, V.; Vu, V.; Nguyen, T.; Nguyen, T.; Tran, V.; Nguyen-Tri, P. Antibacterial Activity of TiO2- and ZnO-Decorated with Silver Nanoparticles. J. Compos. Sci. 2019, 3, 61. [Google Scholar] [CrossRef]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 2019, 124, 1132–1136. [Google Scholar] [CrossRef]
- Afzal, G.; Jamal, A.; Kiran, S.; Mustafa, G.; Ahmad, F.; Saeed, S.; Ahmad, H.I.; Dawood, S. Aerva javanica Mediated Synthesis, Characterization and Antimicrobial Evaluation of Zinc Oxide Nanoparticles. J. Anim. Plant Sci. 2021, 32, 547–553. [Google Scholar] [CrossRef]
- Ahmed, J.; Ali, M.; Sheikh, H.M.; Al-Kattan, M.O.; Farhana; Haroon, U.; Safaeishakib, M.; Akbar, M.; Kamal, A.; Zubair, M.S.; et al. Biocontrol of Fruit Rot of Litchi chinensis Using Zinc Oxide Nanoparticles Synthesized in Azadirachta indica. Micromachines 2022, 13, 1461. [Google Scholar] [CrossRef] [PubMed]
- Gondal, M.A.; Alzahrani, A.J.; Randhawa, M.A.; Siddiqui, M.N. Morphology and antifungal effect of nano-ZnO and nano-Pd-doped nano-ZnO against Aspergillus and Candida. J. Environ. Sci. Health A 2012, 47, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, N.; Abdi, Y.; Haghighi, F. Light-induced antifungal activity of TiO2 nanoparticles/ZnO nanowires. Appl. Surf. Sci. 2011, 257, 10096–10100. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Sivasankarapillai, V.S.; Natarajan, V.K.; Eldesoky, G.E.; Wabaidur, S.M.; Eswaran, M.; Dhanusuraman, R. Biocidal activity of ZnO NPs against pathogens and antioxidant activity—A greener approach by Citrus hystrix leaf extract as bio-reductant. Biochem. Eng. J. 2023, 192, 108818. [Google Scholar] [CrossRef]
- Liu, H.; Qiao, Z.; Jang, Y.O.; Kim, M.G.; Zou, Q.; Lee, H.J.; Koo, B.; Kim, S.H.; Yun, K.; Kim, H.S.; et al. Diatomaceous earth/zinc oxide micro-composite assisted antibiotics in fungal therapy. Nano Converg. 2021, 8, 32. [Google Scholar] [CrossRef]
- Munnawar, I.; Iqbal, S.S.; Anwar, M.N.; Batool, M.; Tariq, S.; Faitma, N.; Khan, A.L.; Khan, A.U.; Nazar, U.; Jamil, T.; et al. Synergistic effect of Chitosan-Zinc Oxide Hybrid Nanoparticles on antibiofouling and water disinfection of mixed matrix polyethersulfone nanocomposite membranes. Carbohydr. Polym. 2017, 175, 661–670. [Google Scholar] [CrossRef]
- Roy, A.; Joshi, M.; Butola, B.S. Preparation and antimicrobial assessment of zinc-montmorillonite intercalates based HDPE nanocomposites: A cost-effective and safe bioactive plastic. J. Clean. Prod. 2019, 212, 1518–1525. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ghose, R. Synthesis of zinc oxide nanoparticles by homogeneous precipitation method and its application in antifungal activity against Candida albicans. Ceram. Int. 2015, 41, 967–975. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Krishnamoorthy, N.; Eldesoky, G.E.; Wabaidur, S.M.; Islam, M.A.; Dhanusuraman, R.; Ponnusamy, V.K. One-pot green synthesis of ZnO nanoparticles using Scoparia Dulcis plant extract for antimicrobial and antioxidant activities. Appl. Nanosci. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tryfon, P.; Kamou, N.N.; Ntalli, N.; Mourdikoudis, S.; Karamanoli, K.; Karfaridis, D.; Menkissoglu-Spiroudi, U.; Dendrinou-Samara, C. Coated Cu-doped ZnO and Cu nanoparticles as control agents against plant pathogenic fungi and nematodes. NanoImpact 2022, 28, 100430. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent Advances in Zinc Oxide Nanoparticles (ZnO NPs) for Cancer Diagnosis, Target Drug Delivery, and Treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Phull, A.-R.; Zia, M. Elemental zinc to zinc nanoparticles: Is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnol. Rev. 2018, 7, 413–441. [Google Scholar] [CrossRef]
- Anitha, R.; Ramesh, K.V.; Ravishankar, T.N.; Sudheer Kumar, K.H.; Ramakrishnappa, T. Cytotoxicity, antibacterial and antifungal activities of ZnO nanoparticles prepared by the Artocarpus gomezianus fruit mediated facile green combustion method. J. Sci. Adv. Mater. Devices 2018, 3, 440–451. [Google Scholar] [CrossRef]
- Fu, W.; Zhou, W.; Chu, P.K.; Yu, X.F. Inherent Chemotherapeutic Anti-Cancer Effects of Low-Dimensional Nanomaterials. Chemistry 2019, 25, 10995–11006. [Google Scholar] [CrossRef]
- Puisney, C.; Baeza-Squiban, A.; Boland, S. Mechanisms of uptake and translocation of nanomaterials in the lung. Adv. Exp. Med. Biol. 2018, 1048, 21–36. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Yang, R.; Wu, R.; Mei, J.; Hu, F.-R.; Lei, C.-J. Zinc oxide nanoparticles promotes liver cancer cell apoptosis through inducing autophagy and promoting p53. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1557–1563. [Google Scholar]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Khan, M.M.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857. [Google Scholar] [CrossRef]
- Rahimi Kalateh Shah Mohammad, G.; Seyedi, S.M.R.; Karimi, E.; Homayouni-Tabrizi, M. The cytotoxic properties of zinc oxide nanoparticles on the rat liver and spleen, and its anticancer impacts on human liver cancer cell lines. J. Biochem. Mol. Toxicol. 2019, 33, e22324. [Google Scholar] [CrossRef] [PubMed]
- Alabyadh, T.; Albadri, R.; Es-Haghi, A.; Yazdi, M.E.T.; Ajalli, N.; Rahdar, A.; Thakur, V.K. ZnO/CeO2 Nanocomposites: Metal-Organic Framework-Mediated Synthesis, Characterization, and Estimation of Cellular Toxicity toward Liver Cancer Cells. J. Funct. Biomater. 2022, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Jasim Makkawi, A.J.; Aysa, N.H.; Gassim, F.-A.G. Anticancer Activity of Zinc Oxide and Zinc Oxide/Cadmium Sulfide Nanocomposites. Asian J. Pharm. Clin. Res. 2019, 12, 535–539. [Google Scholar] [CrossRef]
- Abarna, B.; Preethi, T.; Rajarajeswari, G.R. Single-pot solid-state synthesis of ZnO/chitosan composite for photocatalytic and antitumour applications. J. Mater. Sci. Mater. Electron. 2019, 30, 21355–21368. [Google Scholar] [CrossRef]
- Sanad, F.; Nabih, S.; Goda, M.A. A Lot of Promise for ZnO-5FU Nanoparticles Cytotoxicity against Breast Cancer Cell Lines. J. Nanomed. Nanotechnol. 2018, 9, 1000486. [Google Scholar] [CrossRef]
- Bisht, G.; Rayamajhi, S.; Kc, B.; Paudel, S.N.; Karna, D.; Shrestha, B.G. Synthesis, Characterization, and Study of In Vitro Cytotoxicity of ZnO-Fe3O4 Magnetic Composite Nanoparticles in Human Breast Cancer Cell Line (MDA-MB-231) and Mouse Fibroblast (NIH 3T3). Nanoscale Res. Lett. 2016, 11, 537. [Google Scholar] [CrossRef]
- Martinez-Carmona, M.; Gun’ko, Y.; Vallet-Regi, M. ZnO Nanostructures for Drug Delivery and Theranostic Applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef]
- Hu, C.; Du, W. Zinc oxide nanoparticles (ZnO NPs combined with cisplatin and gemcitabine inhibits tumor activity of NSCLC cells. Aging 2020, 12, 25767–25777. [Google Scholar] [CrossRef]
- Rupa, E.J.; Arunkumar, L.; Han, Y.; Kang, J.P.; Ahn, J.C.; Jung, S.K.; Kim, M.; Kim, J.Y.; Yang, D.C.; Lee, G.J. Dendropanax morbifera Extract-Mediated ZnO Nanoparticles Loaded with Indole-3-Carbinol for Enhancement of Anticancer Efficacy in the A549 Human Lung Carcinoma Cell Line. Materials 2020, 13, 3197. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, J.S.; Zhang, P.; Chen, J.; Kong, J.L.; Sun, L.H.; Xiong, H.M.; Mohwald, H. Self-Assembled ZnO Nanoparticle Capsules for Carrying and Delivering Isotretinoin to Cancer Cells. ACS Appl. Mater. Interfaces 2017, 9, 18474–18481. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO Quantum Dots-Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442–22450. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Shanmugam, V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorg. Chem. 2020, 94, 103423. [Google Scholar] [CrossRef] [PubMed]
- Mobarez, E.; Azoz, H.; Alkalamawy, N.; Nada, A.-F. Evaluation the Effectiveness of Green Zinc Oxide Nanoparticles on the Anti-Inflammatory Effect of Dexamethasone and Its Side Effects in Rats. SVU-Int. J. Vet. Sci. 2018, 1, 25–54. [Google Scholar] [CrossRef]
- Xia, T.; Lai, W.; Han, M.; Han, M.; Ma, X.; Zhang, L. Dietary ZnO nanoparticles alters intestinal microbiota and inflammation response in weaned piglets. Oncotarget 2017, 8, 64878–64891. [Google Scholar] [CrossRef]
- Abd El-Baset, S.A.; Mazen, N.F.; Abdul-Maksoud, R.S.; Kattaia, A.A.A. The therapeutic prospect of zinc oxide nanoparticles in experimentally induced diabetic nephropathy. Tissue Barriers 2022, 11, 2069966. [Google Scholar] [CrossRef]
- Surendra, B.S.; Mallikarjunaswamy, C.; Pramila, S.; Rekha, N.D. Bio-mediated synthesis of ZnO nanoparticles using Lantana camara flower extract: Its characterizations, photocatalytic, electrochemical and anti-inflammatory applications. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100442. [Google Scholar] [CrossRef]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V.K. Synthesis and optimization of zinc oxide nanoparticles using Kalanchoe pinnata towards the evaluation of its anti-inflammatory activity. J. Drug Deliv. Sci. Technol. 2019, 54, 101291. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Cha, S.J.; Yang, I.J.; Sreekanth, T.V.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Thatoi, P.; Kerry, R.G.; Gouda, S.; Das, G.; Pramanik, K.; Thatoi, H.; Patra, J.K. Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species, Heritiera fomes and Sonneratia apetala and investigation of their biomedical applications. J. Photochem. Photobiol. B 2016, 163, 311–318. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A. Green Synthesis and Characterization of ZnO Nanoparticles Using Pelargonium odoratissimum (L.) Aqueous Leaf Extract and Their Antioxidant, Antibacterial and Anti-inflammatory Activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Singh, D.; Yadav, P.; Verma, A. Ameliorative effect of biofabricated ZnO nanoparticles of Trianthema portulacastrum Linn. on dermal wounds via removal of oxidative stress and inflammation. RSC Adv. 2018, 8, 21621–21635. [Google Scholar] [CrossRef] [PubMed]
- Ashwini, J.; Aswathy, T.R.; Rahul, A.B.; Thara, G.M.; Nair, A.S. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Acacia caesia Bark Extract and Its Photocatalytic and Antimicrobial Activities. Catalysts 2021, 11, 1507. [Google Scholar] [CrossRef]
- Yao, S.; Feng, X.; Lu, J.; Zheng, Y.; Wang, X.; Volinsky, A.A.; Wang, L.N. Antibacterial activity and inflammation inhibition of ZnO nanoparticles embedded TiO(2) nanotubes. Nanotechnology 2018, 29, 244003. [Google Scholar] [CrossRef] [PubMed]
- Séby, F. Metal and metal oxide nanoparticles in cosmetics and skin care products. Compr. Anal. Chem. 2021, 93, 381–427. [Google Scholar]
- Osmond-McLeod, M.J.; Oytam, Y.; Kirby, J.K.; Gomez-Fernandez, L.; Baxter, B.; McCall, M.J. Dermal absorption and short-term biological impact in hairless mice from sunscreens containing zinc oxide nano- or larger particles. Nanotoxicology 2014, 8 (Suppl. S1), 72–84. [Google Scholar] [CrossRef]
- Miljković, S.; Tomić, M.; Hut, I.; Pelemis, S. Nanomaterials for Skin Care. In Commercialization of Nanotechnologies—A Case Study Approach; Springer: Cham, Switzerland, 2018; pp. 205–226. [Google Scholar]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Cosma, P. Nanomaterials in sun-care products. In Nanocosmetics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 349–373. [Google Scholar]
- Al-Hilli, S.M.; Willander, M. Optical Properties of Zinc Oxide Nano-particles Embedded in Dielectric Medium for UV region: Numerical Simulation. J. Nanopart. Res. 2006, 8, 79–97. [Google Scholar] [CrossRef]
- Egambaram, O.P.; Kesavan Pillai, S.; Ray, S.S. Materials Science Challenges in Skin UV Protection: A Review. Photochem. Photobiol. 2020, 96, 779–797. [Google Scholar] [CrossRef]
- Filipe, P.; Silva, J.N.; Silva, R.; Cirne de Castro, J.L.; Marques Gomes, M.; Alves, L.C.; Santus, R.; Pinheiro, T. Stratum corneum is an effective barrier to TiO2 and ZnO nanoparticle percutaneous absorption. Skin Pharmacol. Physiol. 2009, 22, 266–275. [Google Scholar] [CrossRef]
- Kim, S.-H.; Heo, Y.; Choi, S.-J.; Kim, Y.-J.; Kim, M.-S.; Kim, H.; Jo, E.; Song, C.-W.; Lee, K. Safety evaluation of zinc oxide nanoparticles in terms of acute dermal toxicity, dermal irritation and corrosion, and skin sensitization. Mol. Cell Toxicol. 2016, 12, 93–99. [Google Scholar] [CrossRef]
- Holmes, A.M.; Kempson, I.; Turnbull, T.; Paterson, D.; Roberts, M.S. Penetration of Zinc into Human Skin after Topical Application of Nano Zinc Oxide Used in Commercial Sunscreen Formulations. ACS Appl. Bio Mater. 2020, 3, 3640–3647. [Google Scholar] [CrossRef] [PubMed]
- Ezealisiji, K.M.; Siwe-Noundou, X.; Maduelosi, B.; Nwachukwu, N.; Krause, R.W.M. Green synthesis of zinc oxide nanoparticles using Solanum torvum (L.) leaf extract and evaluation of the toxicological profile of the ZnO nanoparticles–hydrogel composite in Wistar albino rats. Int. Nano Lett. 2019, 9, 99–107. [Google Scholar] [CrossRef]
- Leite-Silva, V.R.; Sanchez, W.Y.; Studier, H.; Liu, D.C.; Mohammed, Y.H.; Holmes, A.M.; Ryan, E.M.; Haridass, I.N.; Chandrasekaran, N.C.; Becker, W.; et al. Human skin penetration and local effects of topical nano zinc oxide after occlusion and barrier impairment. Eur. J. Pharm. Biopharm. 2016, 104, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Lin, Y.-H.; Hou, W.-C.; Li, M.-H.; Chang, J.-W. Exposure to ZnO/TiO2 Nanoparticles Affects Health Outcomes in Cosmetics Salesclerks. Int. J. Environ. Res. Public Health 2020, 17, 6088. [Google Scholar] [CrossRef]
- Holmes, A.M.; Song, Z.; Moghimi, H.R.; Roberts, M.S. Relative Penetration of Zinc Oxide and Zinc Ions into Human Skin after Application of Different Zinc Oxide Formulations. ACS Nano 2016, 10, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Sogne, V.; Meier, F.; Klein, T.; Contado, C. Investigation of zinc oxide particles in cosmetic products by means of centrifugal and asymmetrical flow field-flow fractionation. J. Chromatogr. A 2017, 1515, 196–208. [Google Scholar] [CrossRef]
- Girigoswami, K.; Viswanathan, M.; Murugesan, R.; Girigoswami, A. Studies on polymer-coated zinc oxide nanoparticles: UV-blocking efficacy and in vivo toxicity. Mater. Sci. Eng. C-Mater. 2015, 56, 501–510. [Google Scholar] [CrossRef]
- Hameed, A.; Fatima, G.R.; Malik, K. Scope of Nanotechnology in Cosmetics: Dermatology and Skin Care Products. J. Med. Chem. Sci. 2019, 2, 9–16. [Google Scholar] [CrossRef]
- Niska, K.; Zielinska, E.; Radomski, M.W.; Inkielewicz-Stepniak, I. Metal nanoparticles in dermatology and cosmetology: Interactions with human skin cells. Chem.-Biol. Interact. 2018, 295, 38–51. [Google Scholar] [CrossRef]
- Gupta, S.; Bansal, R.; Gupta, S.; Jindal, N.; Jindal, A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013, 4, 267–272. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Senthilkumar, O.; Yamauchi, K.; Sato, M.; Morito, S.; Ohba, T.; Nakamura, M.; Fujita, Y. Preparation of ZnO nanoparticles for bio-imaging applications. Phys. Status Solidi B 2009, 246, 885–888. [Google Scholar] [CrossRef]
- Neogi, A. Zno Nanoparticles in Hydrogel Polymer Network for Bio-Imaging. Glob. J. Nanomed. 2017, 1, 555572. [Google Scholar] [CrossRef]
- Yan, N.; Tang, B.Z.; Wang, W.X. In Vivo Bioimaging of Silver Nanoparticle Dissolution in the Gut Environment of Zooplankton. ACS Nano 2018, 12, 12212–12223. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, S.; Jothimani, B.; Sridhar, T.M.; Santhosh, A.; Venkatachalapathy, B. Synthesis of Hexagonal ZnO-PQ7 Nano Disks Conjugated with Folic Acid to Image MCF–7 Cancer Cells. J. Fluoresc. 2017, 27, 21–29. [Google Scholar] [CrossRef]
- Yao, J.; Li, P.; Li, L.; Yang, M. Biochemistry and biomedicine of quantum dots: From biodetection to bioimaging, drug discovery, diagnostics, and therapy. Acta Biomater. 2018, 74, 36–55. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Zheng, W.; Li, Y. Silica-Coated Ga(III)-Doped ZnO: Yb(3+), Tm(3+) Upconversion Nanoparticles for High-Resolution in vivo Bioimaging using Near-Infrared to Near-Infrared Upconversion Emission. Inorg. Chem. 2019, 58, 8230–8236. [Google Scholar] [CrossRef]
- Matsuyama, K.; Ihsan, N.; Irie, K.; Mishima, K.; Okuyama, T.; Muto, H. Bioimaging application of highly luminescent silica-coated ZnO-nanoparticle quantum dots with biotin. J. Colloid Interface Sci. 2013, 399, 19–25. [Google Scholar] [CrossRef]
- Manaia, E.B.; Kiatkoski Kaminski, R.C.; Caetano, B.L.; Briois, V.; Chiavacci, L.A.; Bourgaux, C. Surface modified Mg-doped ZnO QDs for biological imaging. Eur. J. Nanomed. 2015, 7, 109–120. [Google Scholar] [CrossRef]
- Zang, Z.; Tang, X. Enhanced fluorescence imaging performance of hydrophobic colloidal ZnO nanoparticles by a facile method. J. Alloys Compd. 2015, 619, 98–101. [Google Scholar] [CrossRef]
- Gulia; Rita Kakkar, S. ZnO Quantum Dots for Biomedical Applications. Adv. Mater. Lett. 2013, 4, 876–887. [Google Scholar] [CrossRef]
- Xiong, H.M.; Xu, Y.; Ren, Q.G.; Xia, Y.Y. Stable aqueous ZnO@polymer core-shell nanoparticles with tunable photoluminescence and their application in cell imaging. J. Am. Chem. Soc. 2008, 130, 7522–7523. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, N.; Qurishi, Y.; Atreya, H.S.; Srivastava, C. ZnO coated CoFe2O4 nanoparticles for multimodal bio-imaging. RSC Adv. 2016, 6, 18843–18851. [Google Scholar] [CrossRef]
- Lei, G.; Yang, S.; Cao, R.; Zhou, P.; Peng, H.; Peng, R.; Zhang, X.; Yang, Y.; Li, Y.; Wang, M.; et al. In Situ Preparation of Amphibious ZnO Quantum Dots with Blue Fluorescence Based on Hyperbranched Polymers and their Application in Bio-Imaging. Polymers 2020, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, A.P.S.; Venkataprasanna, K.S.; Pannerselvam, B.; Asokan, V.; Jeniffer, R.S.; Venkatasubbu, G.D. Multifunctional ZnO/SiO(2) Core/Shell Nanoparticles for Bioimaging and Drug Delivery Application. J. Fluoresc. 2020, 30, 1075–1083. [Google Scholar] [CrossRef]
- Kaur, P.; Choudhury, D. Functionality of receptor targeted zinc-insulin quantum clusters in skin tissue augmentation and bioimaging. J. Drug Target. 2021, 29, 541–550. [Google Scholar] [CrossRef]
- Giannousi, K.; Karageorgou, M.E.; Oikonomou, I.M.; Komninou, P.; Dendrinou-Samara, C. ZnO NPs immobilized by Alizarin as in vitro predictive and imaging biomarkers for protein amyloidosis. J. Inorg. Biochem. 2022, 236, 111971. [Google Scholar] [CrossRef]
- Wanas, W.; Abd El-Kaream, S.A.; Ebrahim, S.; Soliman, M.; Karim, M. Cancer bioimaging using dual mode luminescence of graphene/FA-ZnO nanocomposite based on novel green technique. Sci. Rep. 2023, 13, 27. [Google Scholar] [CrossRef]
- Wu, Y.L.; Lim, C.S.; Fu, S.; Tok, A.I.Y.; Lau, H.M.; Boey, F.Y.C.; Zeng, X.T. Surface modifications of ZnO quantum dots for bio-imaging. Nanotechnology 2007, 18, 215604. [Google Scholar] [CrossRef]
- Youn, S.M.; Choi, S.J. Food Additive Zinc Oxide Nanoparticles: Dissolution, Interaction, Fate, Cytotoxicity, and Oral Toxicity. Int. J. Mol. Sci. 2022, 23, 6074. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan based ZnO nanoparticles loaded gallic-acid films for active food packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Zinc Oxide Nanoparticles for Food Packaging Applications. In Antimicrobial Food Packaging; Academic Press: San Diego, CA, USA, 2016; pp. 425–431. [Google Scholar]
- Priyadarshi, R.; Negi, Y.S. Effect of Varying Filler Concentration on Zinc Oxide Nanoparticle Embedded Chitosan Films as Potential Food Packaging Material. J. Polym. Environ. 2016, 25, 1087–1098. [Google Scholar] [CrossRef]
- Zahiri Oghani, F.; Tahvildari, K.; Nozari, M. Novel Antibacterial Food Packaging Based on Chitosan Loaded ZnO Nano Particles Prepared by Green Synthesis from Nettle Leaf Extract. J. Inorg. Organomet. Polym. Mater. 2020, 31, 43–54. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Wu, H.; Tong, C.; Pang, J.; Wu, C. Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocoll. 2020, 107, 105942. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S.H.; Roufegarinejad, L.; Hamishehkar, H. Application of Reinforced ZnO Nanoparticle-Incorporated Gelatin Bionanocomposite Film with Chitosan Nanofiber for Packaging of Chicken Fillet and Cheese as Food Models. Food Bioprocess Technol. 2019, 12, 1205–1219. [Google Scholar] [CrossRef]
- Esmailzadeh, H.; Sangpour, P.; Shahraz, F.; Hejazi, J.; Khaksar, R. Effect of nanocomposite packaging containing ZnO on growth of Bacillus subtilis and Enterobacter aerogenes. Mater. Sci. Eng. C 2016, 58, 1058–1063. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Hu, Z.; Li, G.; Hu, L.; Chen, X.; Hu, Y. Chitosan-based films with antioxidant of bamboo leaves and ZnO nanoparticles for application in active food packaging. Int. J. Biol. Macromol. 2021, 189, 363–369. [Google Scholar] [CrossRef]
- Sarojini, K.S.; Indumathi, M.P.; Rajarajeswari, G.R. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar] [CrossRef]
- Yu, F.; Fei, X.; He, Y.; Li, H. Poly(lactic acid)-based composite film reinforced with acetylated cellulose nanocrystals and ZnO nanoparticles for active food packaging. Int. J. Biol. Macromol. 2021, 186, 770–779. [Google Scholar] [CrossRef]
- Jayakumar, A.; Heera, K.V.; Sumi, T.S.; Joseph, M.; Mathew, S.; Praveen, G.; Nair, I.C.; Radhakrishnan, E.K. Starch-PVA composite films with zinc-oxide nanoparticles and phytochemicals as intelligent pH sensing wraps for food packaging application. Int. J. Biol. Macromol. 2019, 136, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhou, X.; Dai, Q.; Qin, Z. Antibacterial, Antioxidation, UV-Blocking, and Biodegradable Soy Protein Isolate Food Packaging Film with Mangosteen Peel Extract and ZnO Nanoparticles. Nanomaterials 2021, 11, 3337. [Google Scholar] [CrossRef] [PubMed]
- Dhapte, V.; Gaikwad, N.; More, P.V.; Banerjee, S.; Dhapte, V.V.; Kadam, S.; Khanna, P.K. Transparent ZnO/polycarbonate nanocomposite for food packaging application. Nanocomposites 2015, 1, 106–112. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, M.; Mukherjee, A.; Chattopadhyay, D.; Acharya, K. Biosynthesis and safety evaluation of ZnO nanoparticles. Bioprocess Biosyst. Eng. 2014, 37, 165–171. [Google Scholar] [CrossRef]
- Yu, J.; Choi, S.J. Particle Size and Biological Fate of ZnO Do Not Cause Acute Toxicity, but Affect Toxicokinetics and Gene Expression Profiles in the Rat Livers after Oral Administration. Int. J. Mol. Sci. 2021, 22, 1698. [Google Scholar] [CrossRef]
- Jung, E.B.; Yu, J.; Choi, S.J. Interaction between ZnO Nanoparticles and Albumin and Its Effect on Cytotoxicity, Cellular Uptake, Intestinal Transport, Toxicokinetics, and Acute Oral Toxicity. Nanomaterials 2021, 11, 2922. [Google Scholar] [CrossRef]
- Kong, T.; Zhang, S.H.; Zhang, J.L.; Hao, X.Q.; Yang, F.; Zhang, C.; Yang, Z.J.; Zhang, M.Y.; Wang, J. Acute and Cumulative Effects of Unmodified 50-nm Nano-ZnO on Mice. Biol. Trace Elem. Res. 2018, 185, 124–134. [Google Scholar] [CrossRef]
- Kong, T.; Zhang, S.H.; Zhang, C.; Zhang, J.L.; Yang, F.; Wang, G.Y.; Yang, Z.J.; Bai, D.Y.; Zhang, M.Y.; Wang, J.; et al. Long-Term Effects of Unmodified 50 nm ZnO in Mice. Biol. Trace Elem. Res. 2019, 189, 478–489. [Google Scholar] [CrossRef]
- Kong, T.; Zhang, S.H.; Zhang, C.; Zhang, J.L.; Yang, F.; Wang, G.Y.; Yang, Z.J.; Bai, D.Y.; Shi, Y.Y.; Liu, T.Q.; et al. The Effects of 50 nm Unmodified Nano-ZnO on Lipid Metabolism and Semen Quality in Male Mice. Biol. Trace Elem. Res. 2020, 194, 432–442. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Dhiman, N.; Tiwari, R.; Arjaria, N.; Prakash, J.; Jagdale, P.; Ayanur, A.; Singh, D.; Patnaik, S.; Kumar, M. Sub-acute oral exposure of zinc oxide nanoparticles causes alteration in iron homeostasis through acute phase response: A protective effect by surface modification. J. Trace Elem. Med. Biol. 2019, 52, 270–287. [Google Scholar] [CrossRef]


| Nanomaterials | Synthetic Method | Particle Size | Type of Microbe | Anti-Microbial Outcomes | Ref. |
|---|---|---|---|---|---|
| GPTMS-ZnO nanoparticle | Sol-gel | Varying sizes from 5.3 to 38.2 nm | Staphylococcus aureus | Antibacterial activity increased with decreased particle size | [3] |
| ZnO nanocrystalline | HEBM | Varying sizes from 20 to 250 nm | Staphylococcus, Streptococcus, Micrococcus, Escherichia Coli, Enterobacter and Pseudomonas | Antibacterial activity increased with decreased particle size | [62] |
| ZnO nanoparticle | Bio-mediated solution combustion | 11–26 nm of crystallite size | Pseudomonas aeruginosa and Staphylococcus aureus | ZnO nano-flower exhibited excellent photocatalytic activity over the ZnO with other morphology | [59] |
| ZnO nanoparticle | Bio-mediated method using Aloe vera and Hibiscus sabdariffa plant extracts | 9–18 nm | Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Staphylococcus aureus | More efficient bactericidal activity was found in nanoparticles via biosynthesis than those from chemical synthesis | [68] |
| Ag-decorated ZnO nanoparticle | Chemical reduction | 30–50 nm | Staphylococcus aureus and Escherichia coli | The hybrid of nanoparticles improved the antibacterial performance | [69] |
| ZnO/graphene oxide composites | Covalent bonding | 170 nm of ZnO | Escherichia coli, Salmonella typhimurium, Bacillus subtilis and Enterococcus faecalis | The composite action of materials improved antibacterial properties | [2] |
| Chitosan-stabilized ZnO nanoparticles | Microwave heating | 50–70 nm | Staphylococcus aureus and Escherichia coli | Chitosan-stabilized ZnO nanoparticles exhibited excellent antibacterial activity | [70] |
| ZnO nanoparticles | Sol-gel | Less than 50 nm | Candida albicans | Effective antifungal effect was observed | [75] |
| ZnO nanoparticles | Homogeneous precipitation method | ~30 nm | Candida albicans | The antifungal activity was positively correlated with the ZnO concentration | [80] |
| ZnO nanoparticles | Bio-mediated method using Citrus hystrix leaf extract | 26−69 ± 0.5 nm | Candida albicans and Aspergillus niger | Exhibition of good antifungal activity at a higher concentration of 5 μg/mL | [76] |
| ZnO nanoparticles | Bio-mediated method using Azadirachta indica leaf extract | 29.024 nm | Aspergillus niger | Maximum suppression of lychee fruit rot was obtained at the dose of 1.0 mg/mL | [72] |
| HDPE/ZnO-MMT nanocomposites | A combination of simple ion exchange and reduction reactions | ~20 nm | Aspergillus niger | Significant antifungal properties were found at 5 wt% of loading | [79] |
| 5%Pd-doped nano-ZnO | Thermal decomposition method | 35 nm | Aspergilus niger and Candida albicans | Pd-doped nano-ZnO increased the antifungal activity over pure nano-ZnO | [73] |
| TiO2/ZnO nanocomposites | Chemical vapor deposition | 50−100 nm | Candida albicans biofilms | TiO2/ZnO nanocomposites enhanced antifungal activity more than single component | [74] |
| Nanomaterials | Synthetic Method | Particle Size | Type of Cancer Cells | Anti-Cancer Outcomes | Ref. |
|---|---|---|---|---|---|
| ZnO nanoparticles | / | 14.13 ± 0.92 nm | Huh7 liver cancer cells | Effectively inhibited liver cancer cells’ growth and proliferation | [89] |
| ZnO nanoparticles | Co-precipitation | 21.59 ± 4.89 nm | HepG2 liver cancer cells | The HepG2 cell viability was significantly decreased to 39% at dose of 15 μg/mL | [90] |
| ZnO nanoparticles | Bio-mediated method using H. officinalis plant extracts | 10−100 nm | HepG2 and Huh7 liver cancer cells | IC50 values for HepG2 and Huh-7 cells were 40 μg/mL at 72 h and 15 μg/mL at 48 h, respectively | [91] |
| ZnO/CeO2 nanocomposites | Combustion | 31.9 nm | HepG2 liver cancer cells | The anticancer effect of ZnO/CeO2 nanocomposites was better than that of single component | [92] |
| ZnO/CdS nanocomposites | Chemical synthesis | / | MCF-7 breast cancer cells | The anticancer effect of ZnO/CdS nanocomposites was better than that of ZnO alone | [93] |
| ZnO/chitosan nanocomposite | Solid-state synthesis | 25−31.67 nm | MCF-7 breast cancer cells | ZnO/chitosan nanocomposites exhibited excellent anti-tumor activity compared with ZnO alone | [94] |
| ZnO-Fe3O4 magnetic nanocomposite | Ex situ conjugation | 44.05 ± 1.2 nm | MDA-MB-231 breast cancer cells | ZnO-Fe3O4 showed preferential toxicity to breast cancer cells but low ctoxicity towards noncancerous cells | [96] |
| DOX-FA-ZnO NS | EDC/NHS coupling and physical absorption | 160 nm | MDA-MB-231 breast cancer cells | The combined therapy based on DOX-FA-ZnO NS possessed maximum death rate of breast cancer cells compared with single therapy | [8] |
| ZnONPs(Cp/Gem) | Mixing | ~20 nm | A549 lung cancer cells | The effect of ZnONPs(Cp/Gem) on lung cancer cells was higher than that of single component | [98] |
| DM-ZnO-I3C nanoemulsion | Ultrasonication | 239.6 ± 6.13 nm | A549 lung cancer cells | DM-ZnO-I3C-NE presented more toxicity in lung cancer cells than free DM-ZnO or I3C | [99] |
| ZnO−ISO nanocomposite | Self-assembly | ~100 nm | A549 lung cancer cells | The antitumor effect of ZnO-ISO composite was better than the existing drugs of Nintedanib and Crizotinib on the market | [100] |
| HA-ZnO-DOX | EDC/NHS coupling | ~3 nm of ZnO | A549 lung cancer cells | HA-ZnO-DOX exhibited a good synergistic antitumor effect to lung cancer cells | [101] |
| Nanomaterials | Synthetic Method | Particle Size | Inflammatory Indicators | Anti-Inflammatory Outcomes | Ref. |
|---|---|---|---|---|---|
| ZnO nanoparticles | Combustion method | 21.4–27.2 nm | Phospholipase A2 | Presenting high inhibition for Phospholipase A2 | [106] |
| ZnO nanoparticles | Bio-mediated method using Andrographis paniculata leaf extract | The sizes of 96–115 and 57 ± 0.3 nm with spherical and hexagonal shapes, respectively | Inhibiting protein denaturation | Presenting significant inhibitory effects with IC50 of 66.78 μg/mL | [107] |
| ZnO nanoparticles | / | 23.0 nm | IFN-γ, IL-1β, TNF-α and NF-κB | Inhibiting the expression of the inflammatory factor | [104] |
| ZnO nanoparticles | Bio-mediated method using kalanchoe pinnata leaf extract | 24 nm | TNF-α, IL-1β, IL-6 and COX-2 | Blocking the production and release of inflammatory mediators | [108] |
| ZnO nanoparticles | Bio-mediated method using Polygala tenuifolia root leaf extract | 33.03−73.48 nm | iNOS, COX-2, IL-1β, IL-6 and TNF-α. | Suppressing both mRNA and protein expressions of iNOS, COX-2, IL-1β, IL-6 and TNF-α. | [109] |
| ZnO nanoparticles | Bio-mediated method using Heritiera fomes and Sonneratia apetala extracts | 40–50 nm | Inhibiting protein denaturation | Presenting significant inhibitory effects with IC50 of 63.25 μg/mL | [110] |
| ZnO nanoparticles | Bio-mediated method using pelargonium odoratissimum aqueous leaf extract | 21.6 nm | Human red blood cells’ membrane stabilization | Promoting the stabilization of the red blood cells’ membrane | [111] |
| ZnO nanoparticles | Bio-mediated method using Trianthema portulacastrum Linn. | 10–20 nm | Human red blood cells’ membrane stabilization, protein denaturation and proteinase inhibitor activity | Good membrane stabilization efficiency against human red blood cell membrane, effectively preventing albumin denaturation and obvious proteinase inhibitory activity | [112] |
| ZnO nanoparticles | Bio-mediated method using acacia caesia bark extract | 32.32 nm | COX | Inhibiting the expression of the COX | [113] |
| TNTs/ZnO | Electrodeposition | Inner diameter 50 nm | Macrophage | TNTs/ZnO can effectively inhibit the macrophage proliferation and adhesion | [114] |
| Nanomaterials | Synthetic Method | Particle Size | Labeling Model | Bio-Imaging Outcomes | Ref. |
|---|---|---|---|---|---|
| AET–ZnO nanoparticles | Double-phase reaction | 30 nm | Hela cells | Hela cells were successfully labeled and imaged via blue emission | [141] |
| ZnO-TiO2 QDs | Sol-gel method | ~50 nm | Mung bean seedling plant cells | Good bio-imaging capability on plant cells | [150] |
| ZnO@Polymer core–shell nanoparticles | Sol-gel method | 3–4 nm | Human hepatoma cells (QGY 7763) | The ZnO QDs penetrated into the living cells and exhibited bright fluorescence imaging | [143] |
| CoFe2O4-ZnO core–shell nanoparticles | Wet chemical synthesis | 11.6 ± 1.8 nm | MCF-7 (human breast cancer cells) | The cells containing the core–shell nanoparticles were visible in blue, green and red emission | [144] |
| ZnO/HPEI nanocomposites | Chemical synthesis | 3 nm of ZnO | COS-7 cells | The water-soluble ZnO/HPEI nanocomposites could easily be endocytosed by the COS-7 cells without transfection reagent and exhibited excellent biological imaging behavior | [145] |
| ZnO/SiO2 core–shell nanoparticles | Wet chemical synthesis | 60 nm of ZnO core and 7–10 nm of the width shell | NIH 3 t3 fibroblast cells | This nanoparticle showed high visible florescence even at lower concentrations | [146] |
| ZnO@alizarin nanoparticles | Solvothermal synthesis | 47.4 nm of ZnO | Amyloid oligomers and plaques | Effective imaging properties | [148] |
| Graphene/FA-ZnO nanocomposite | Co-precipitation and conjugation method | 22.4–35.4 nm of diameter and 84.1–154 nm of length. | Swiss albino mice implanted with Ehrlich Tumor | The position of fluorescence in the tumor confirmed that the nanocomposite was prepared for in vivo tumor targeting. | [149] |
| Nanomaterials | Synthetic Method | Particle Size | Evaluation Indicators | Outcomes | Ref. |
|---|---|---|---|---|---|
| CS-ZnO PE | Stirring | ~55 nm of ZnO | Antimicrobial efficacy | Completely inactivated and prevented the food pathogens’ growth | [160] |
| CS-ZnO | Stirring | 50 ± 10 nm of ZnO | Light-blocking effect, radical scavenging effects, antimicrobial effect | UV light blocking was the most obvious, DPPH and ABTS radical scavenging effects was obvious and antimicrobial effect against E. coli and S. aureus was powerful | [161] |
| CS-ZnO@gal complex | Solution casting | 19.2 nm of ZnO@gal | TS, EAB, solubility, swelling, antibacterial and antioxidant effect, WVP and OP | Improved the TS and EAB, reduced the solubility and swelling, enhanced the antibacterial and antioxidant effect and decreased WVP and OP | [152] |
| PC3-5Z film | Solvent casting | ~55 nm of ZnO | Mechanical properties, water resistance, antimicrobial activity, biodegradability | All these properties of PC3-5Z film were better than those of plain PU films | [162] |
| PLA/ACNC/ZnO films | Solution casting | 50 nm of ZnO | UV blocking, mechanical strength, oxygen and water vapor barrier, antibacterial activity | Improved the UV barrier with a slight decrease in transparency, mechanical and barrier properties. The tensile strength, oxygen barrier and water vapor barrier increased compared with those of pure PLA film | [163] |
| PLA/ZnO | Melt mixing | 100−500 nm of ZnO | Mechanical and barrier properties, antimicrobial property | Good mechanical properties, a slight increase in water vapor and excellent antimicrobial property against E. Coli | [157] |
| PSNZJ | Solvent casting | / | Water barrier, UV barrier, mechanical and antimicrobial properties | This film enhanced the water barrier, UV barrier, mechanical and antimicrobial properties. | [164] |
| SPI/MPE/ZnO composite film | Solution casting | 30 ± 10 nm of ZnO | Mechanical strength, water vapor transmission, UV-blocking, antioxidant and antibacterial properties | The composite film exhibited excellent UV-blocking, antioxidant and antibacterial properties against Escherichia coli and Staphylococcus aureus | [165] |
| ZnO/polycarbonate nanocomposite | Blade coating | 15–20 nm of ZnO | Antibacterial properties, UV-blocking properties and hydrophobicity | Good UV-blocking capability, enhanced bacteriostatic action against S. aureus and E. coli and enhanced hydrophobic character. | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Li, H.; Zhang, T.; Song, B.; Wang, X.; Gu, Z. Recent Advances in ZnO Nanomaterial-Mediated Biological Applications and Action Mechanisms. Nanomaterials 2023, 13, 1500. https://doi.org/10.3390/nano13091500
Xie J, Li H, Zhang T, Song B, Wang X, Gu Z. Recent Advances in ZnO Nanomaterial-Mediated Biological Applications and Action Mechanisms. Nanomaterials. 2023; 13(9):1500. https://doi.org/10.3390/nano13091500
Chicago/Turabian StyleXie, Jiani, Huilun Li, Tairan Zhang, Bokai Song, Xinhui Wang, and Zhanjun Gu. 2023. "Recent Advances in ZnO Nanomaterial-Mediated Biological Applications and Action Mechanisms" Nanomaterials 13, no. 9: 1500. https://doi.org/10.3390/nano13091500





