Enhancing Visible Light Photocatalytic Degradation of Bisphenol A Using BiOI/Bi2MoO6 Heterostructures
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of Bi2MoO6
2.3. Synthesis of BiOI and BiOI/Bi2MoO6
2.4. Characterization
2.5. Photocatalytic Activity
3. Results
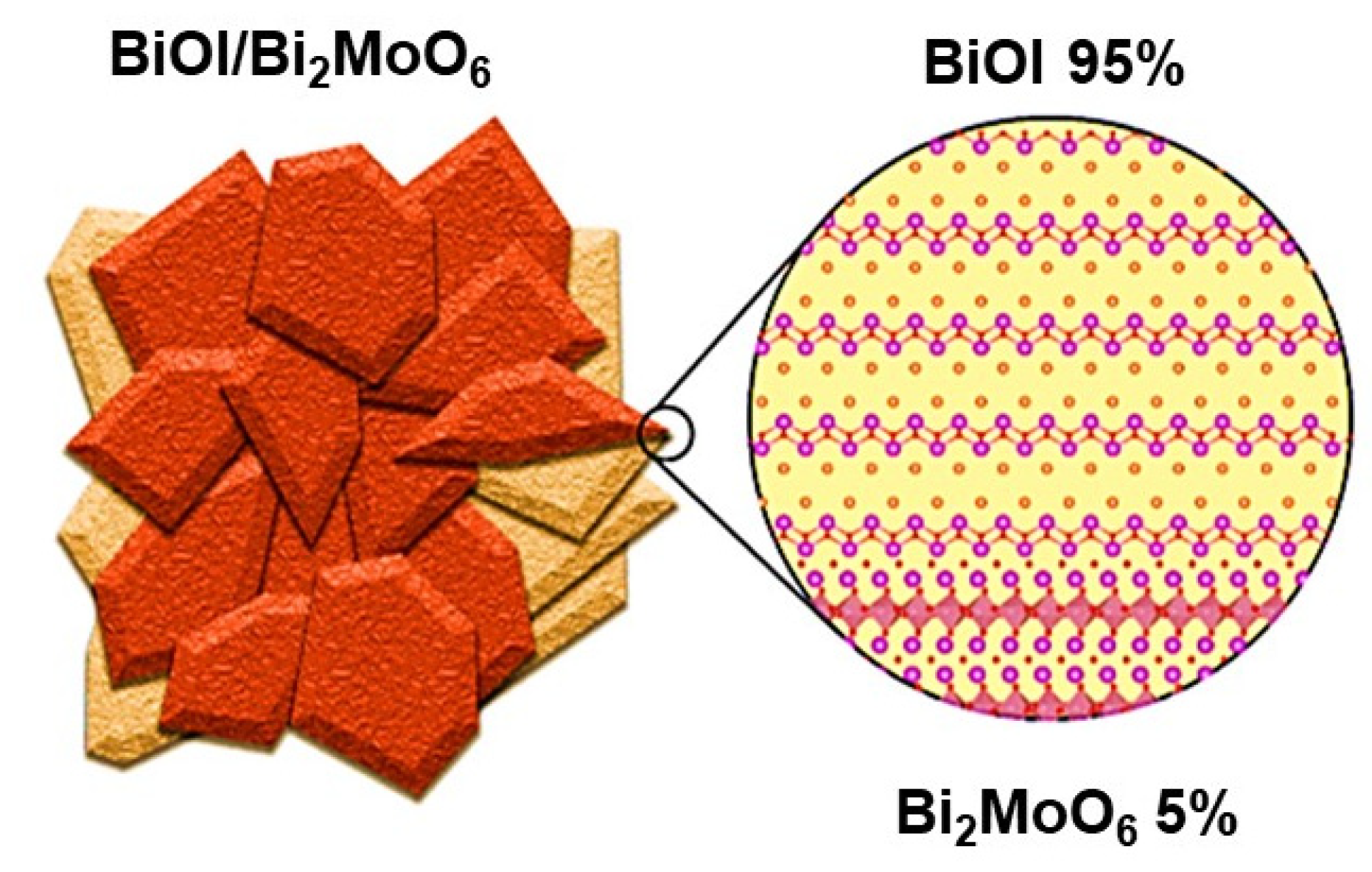
3.1. BiOI/Bi2MoO6 Heterostructure Synergy
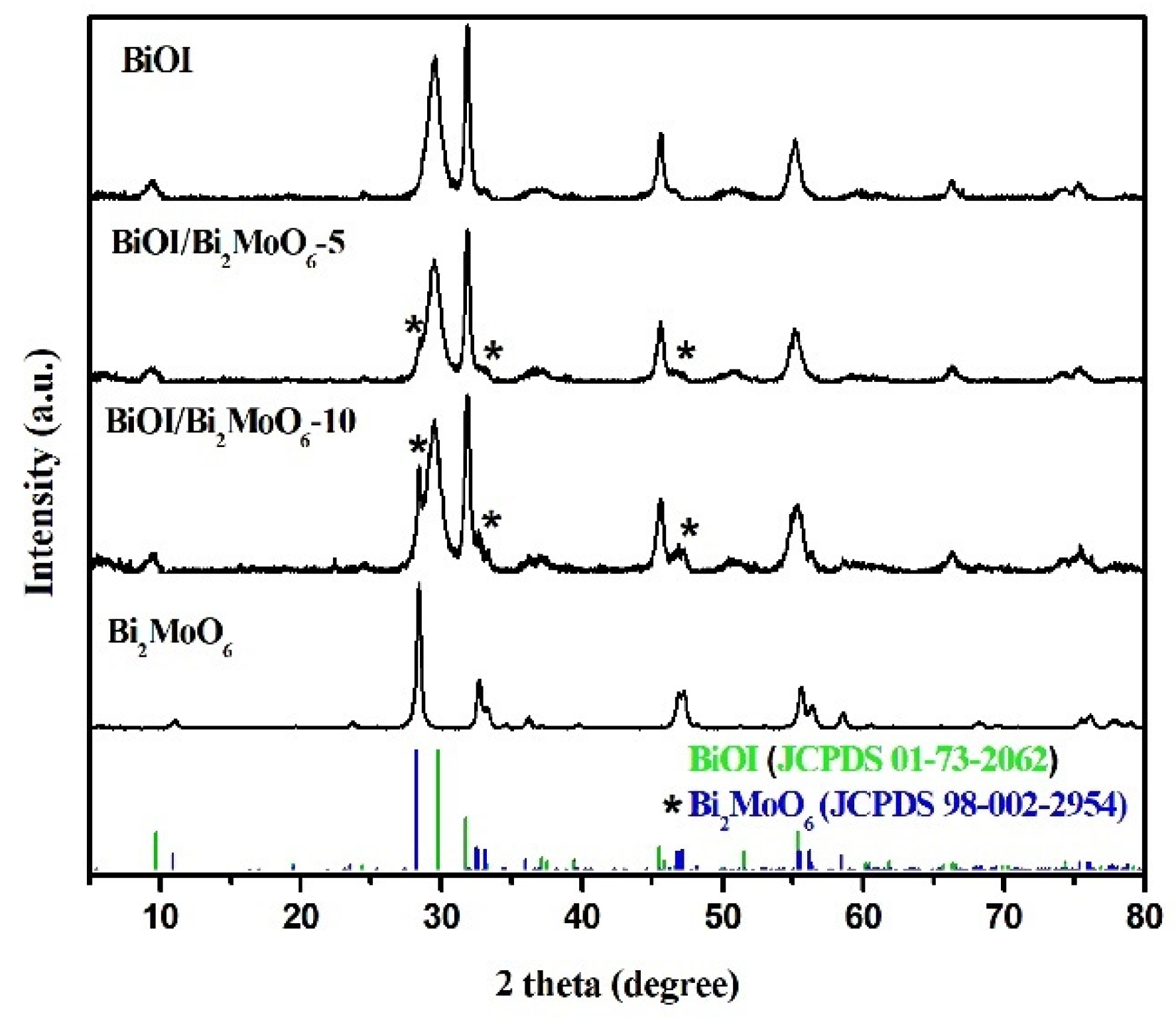
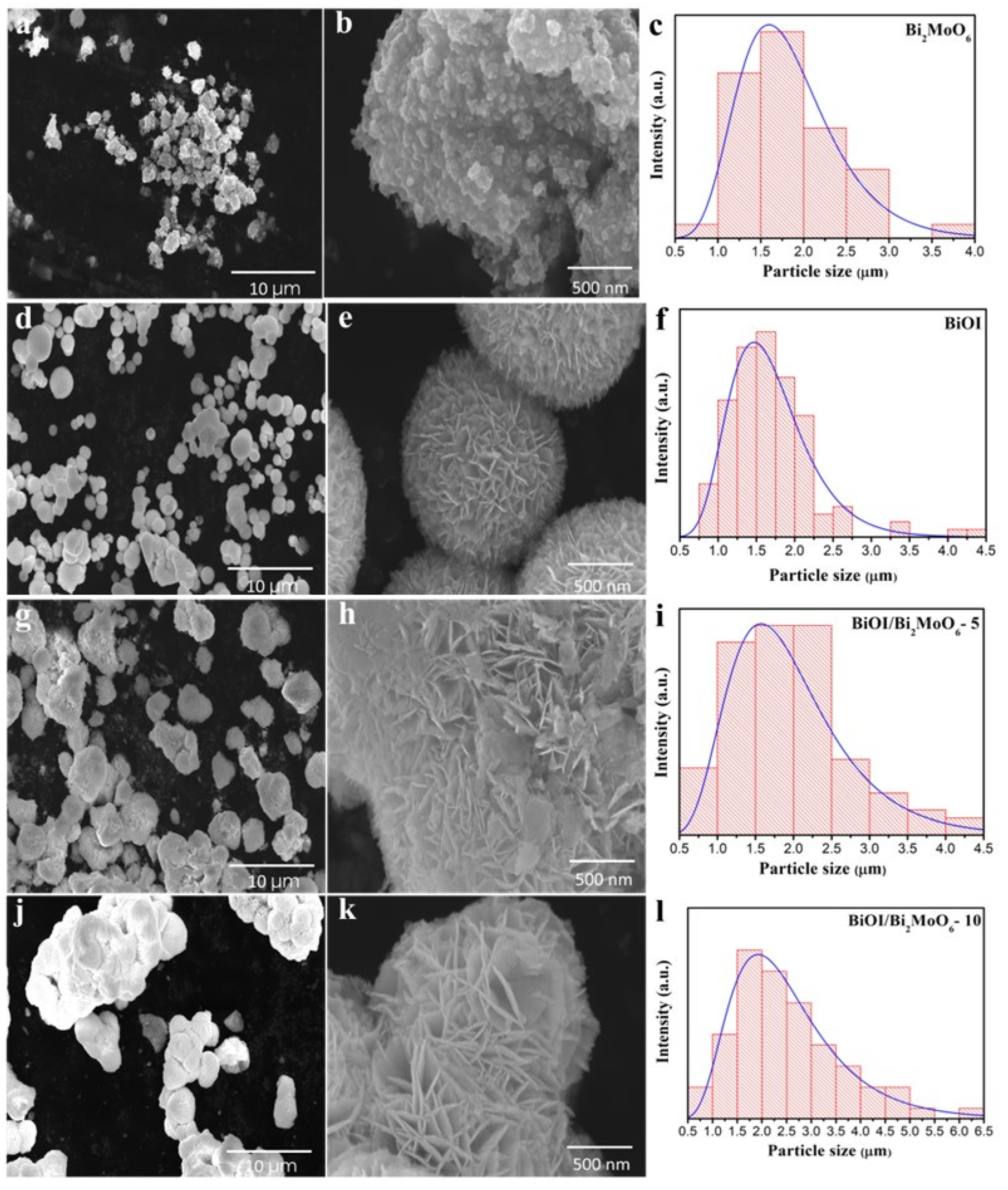
3.1.1. Structural Analysis of BiOI/Bi2MoO6
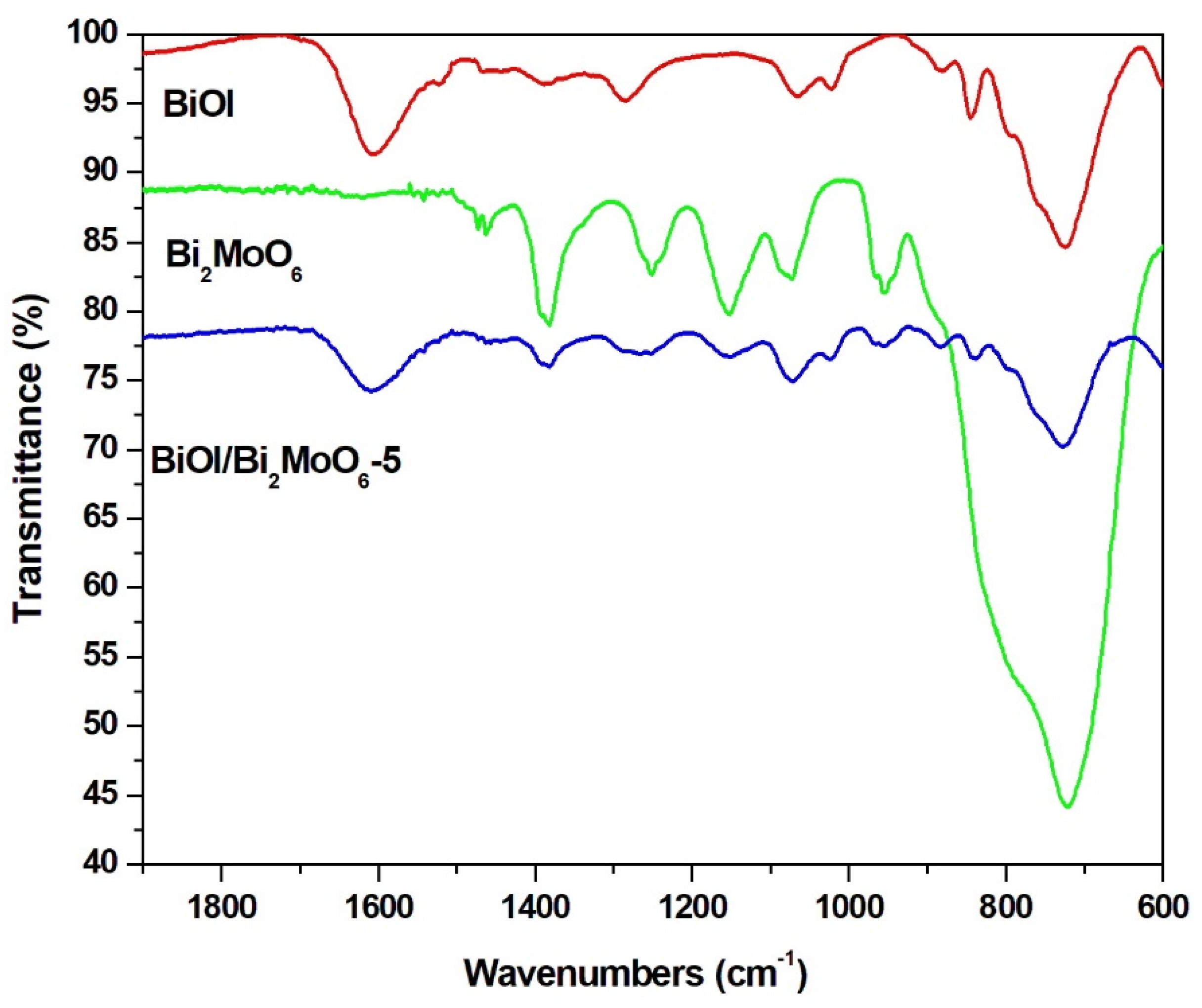
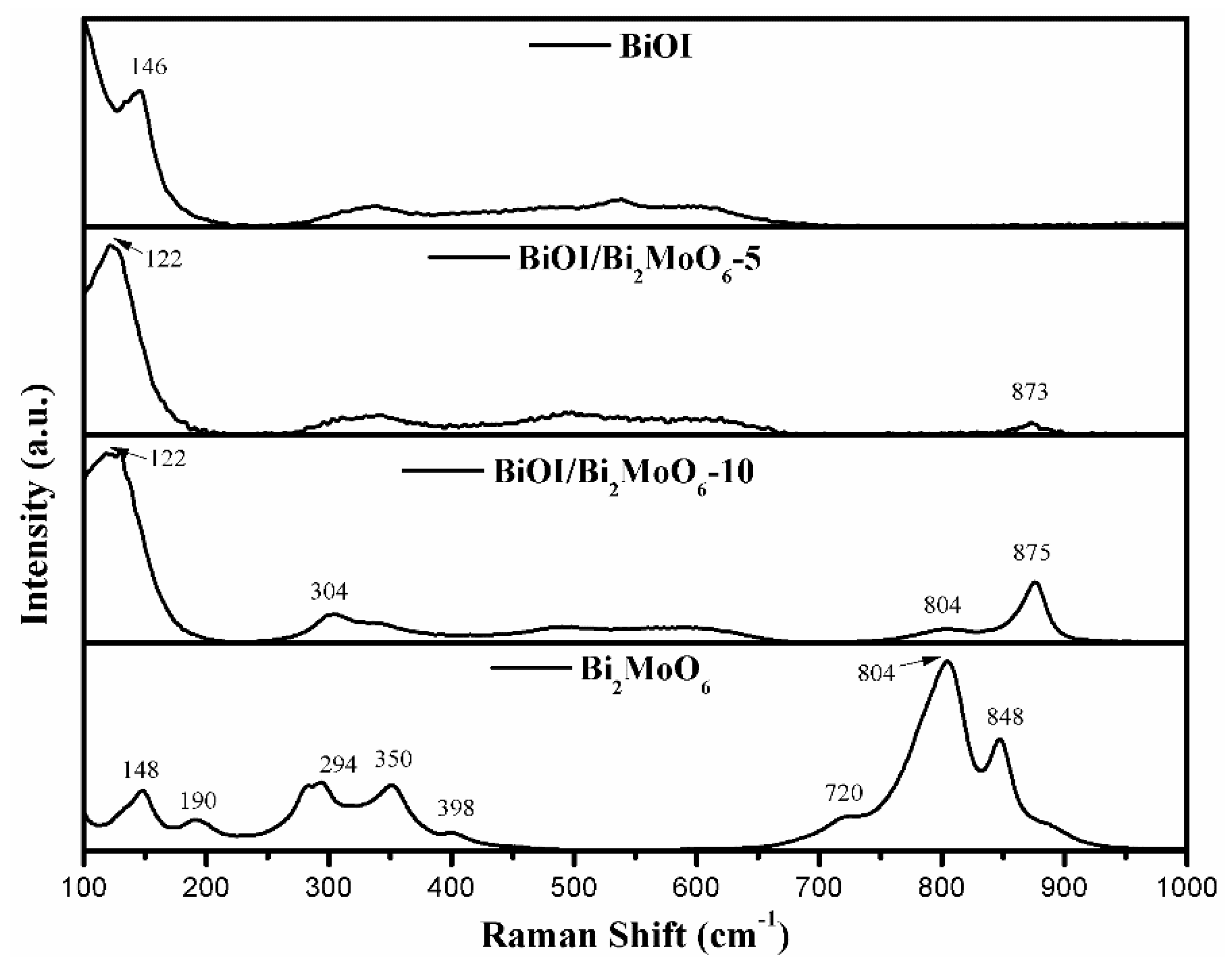
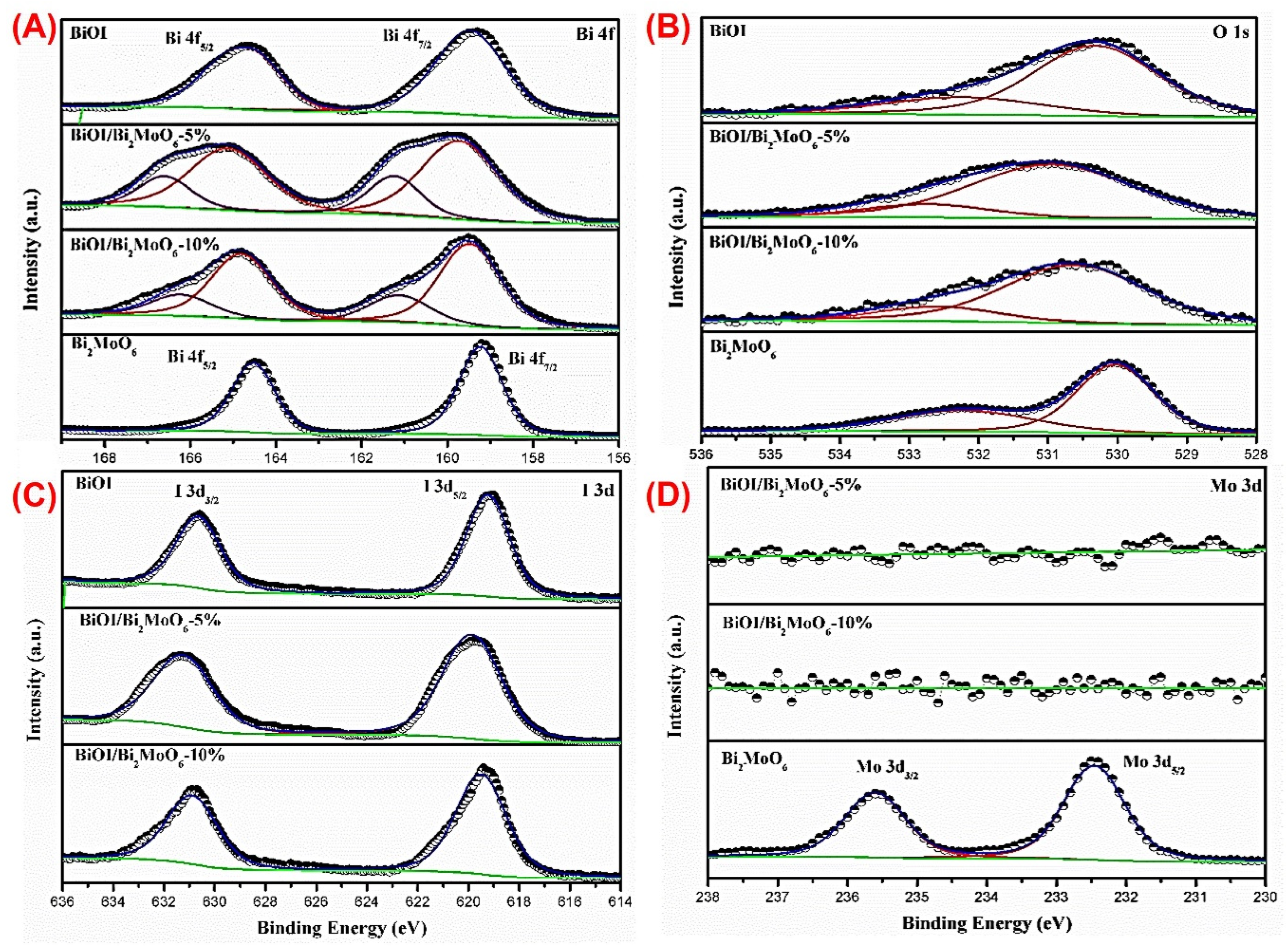
3.1.2. Chemical Species in BiOI/Bi2MoO6
3.2. X-ray Photoelectron Spectroscopy
Optical Properties of the BiOI/Bi2MoO6 Heterostructure Components
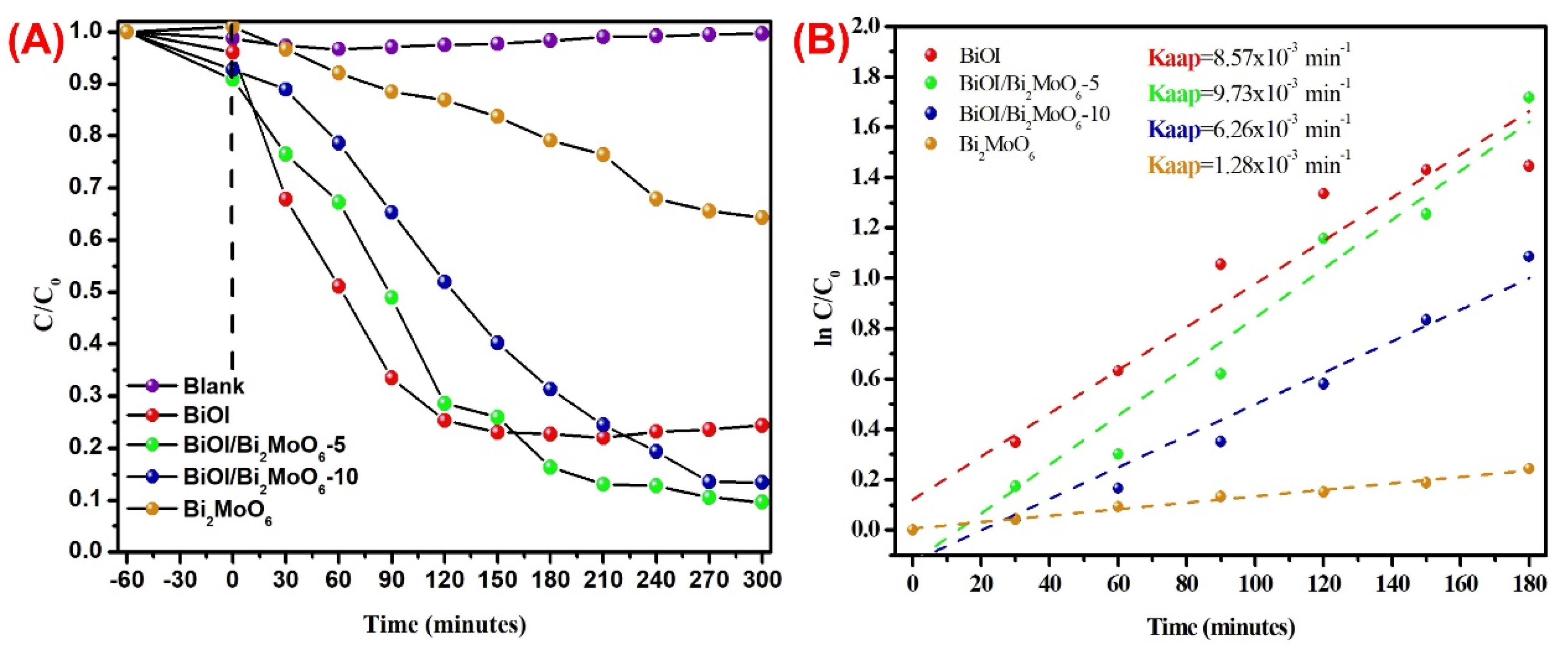
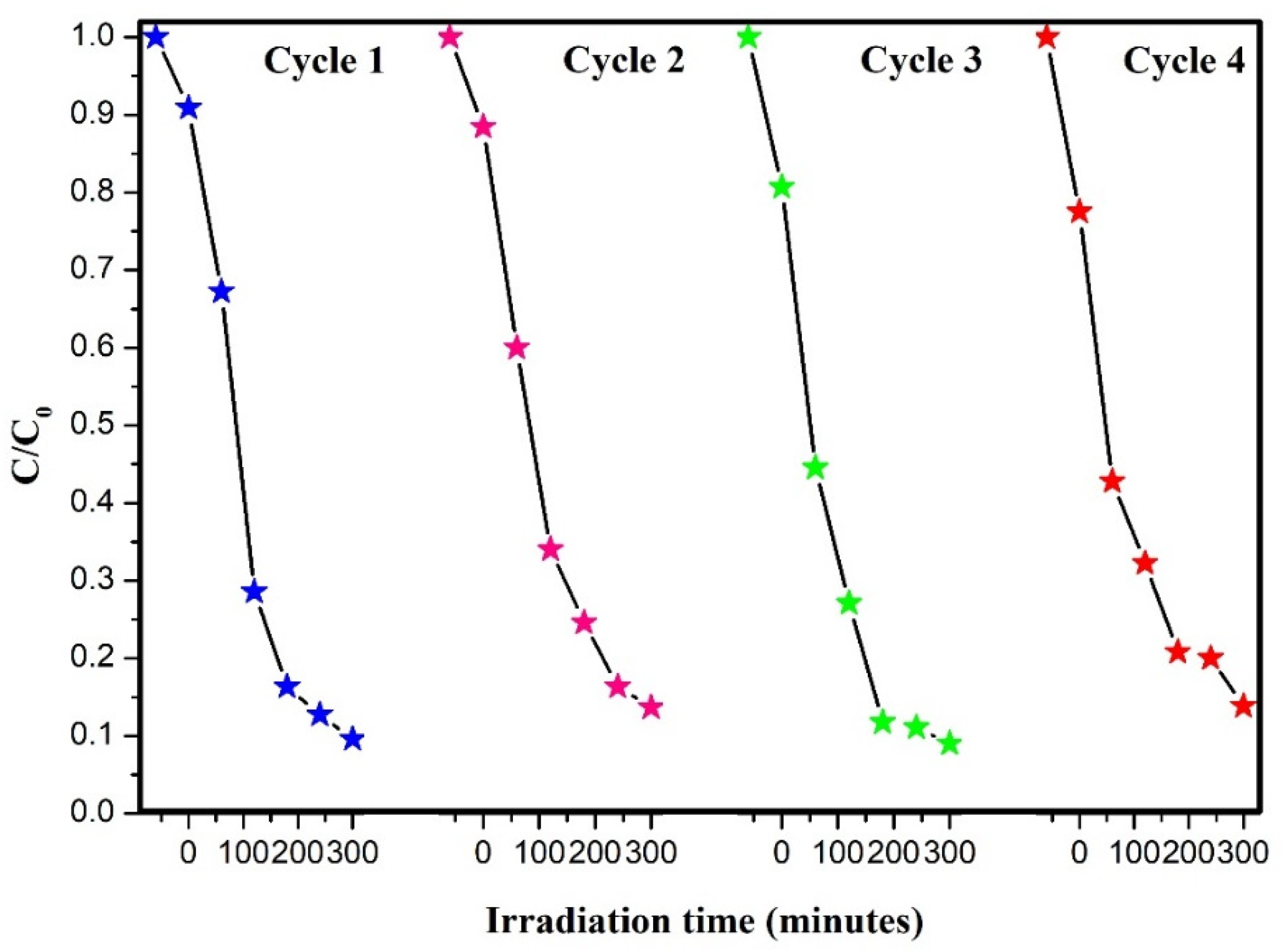
3.3. Heterostructure Synergy to Promote Photocatalytic Degradation
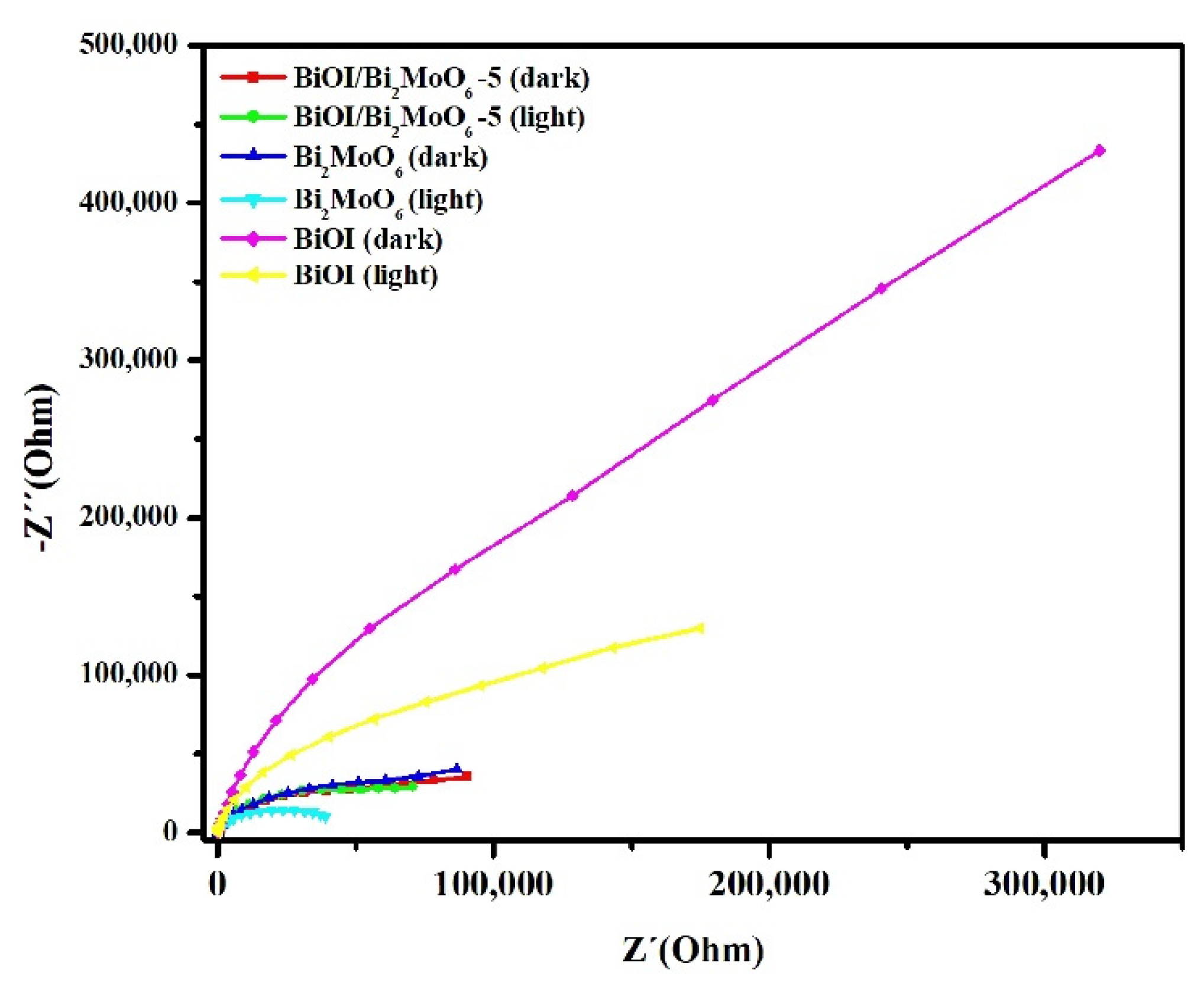
3.4. Mechanistic Insights of the BiOI/Bi2MoO6 Heterostructure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeitoun, M.M.; Mehana, E.S.E. Impact of Water Pollution with Heavy Metals on Fish Health: Overview and Updates. Glob. Vet. 2014, 12, 219–231. [Google Scholar] [CrossRef]
- El-Fadel, M.; Zeinati, M.; El-Jisr, K.; Jamali, D. Industrial-Waste Management in Developing Countries: The Case of Lebanon. J. Environ. Manag. 2001, 61, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Papu-Zamxaka, V.; Harpham, T.; Mathee, A. Environmental Legislation and Contamination: The Gap between Theory and Reality in South Africa. J. Environ. Manag. 2010, 91, 2275–2280. [Google Scholar] [CrossRef]
- González-Arqueros, M.L.; Domínguez-Vázquez, G.; Alfaro-Cuevas-villanueva, R.; Israde-Alcántara, I.; Buenrostro-Delgado, O. Hazardous Solid Waste Confined in Closed Dump of Morelia: An Urgent Environmental Liability to Attend in Developing Countries. Sustainability 2021, 13, 2557. [Google Scholar] [CrossRef]
- Ferronato, N.; Torretta, V. Waste Mismanagement in Developing Countries: A Review of Global Issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 786–789. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Yang, R.; Zhou, G.; Chen, X.; Liu, Y.; Chi, J.; Li, X.; Fang, H.; Li, H.; Li, W. New progress in photocatalytic degradation of bisphenol a as representative endocrine disrupting chemicals. In Current Opinion in Green and Sustainable Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 1936663112. [Google Scholar]
- Kim, B.; Jang, J.; Lee, D.S. Enhanced Photocatalytic Degradation of Bisphenol A by Magnetically Separable Bismuth Oxyiodide Magnetite Nanocomposites under Solar Light Irradiation. Chemosphere 2022, 289, 133040. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Li, F.; Zhu, L. Degradation and Mineralization of Bisphenol a by Mesoporous Bi2WO6 under Simulated Solar Light Irradiation. Environ. Sci. Technol. 2010, 44, 6843–6848. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Kim, K.H.; Kavitha, B.; Kumar, V.; Raza, N.; Kalagara, S. Photocatalytic Degradation of Bisphenol A in Aqueous Media: A Review. J. Environ. Manag. 2018, 213, 189–205. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, L.; Jia, Y.; Zhang, Y.; Dong, Q.; Huang, C. A Study on Environmental Bisphenol A Pollution in Plastics Industry Areas. Water. Air. Soil Pollut. 2017, 228, 98. [Google Scholar] [CrossRef]
- Russo, G.; Barbato, F.; Mita, D.G.; Grumetto, L. Occurrence of Bisphenol A and Its Analogues in Some Foodstuff Marketed in Europe. Food Chem. Toxicol. 2019, 131, 110575. [Google Scholar] [CrossRef]
- Mengting, Z.; Kurniawan, T.A.; Yanping, Y.; Avtar, R.; Othman, M.H.D. 2D Graphene Oxide (GO) Doped p-n Type BiOI/Bi2WO6 as a Novel Composite for Photodegradation of Bisphenol A (BPA) in Aqueous Solutions under UV-Vis Irradiation. Mater. Sci. Eng. C 2020, 108, 110420. [Google Scholar] [CrossRef] [PubMed]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic Degradation Pathway of Methylene Blue in Water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Vadivel, S.; Vanitha, M.; Muthukrishnaraj, A.; Balasubramanian, N. Graphene Oxide–BiOBr Composite Material as Highly Efficient Photocatalyst for Degradation of Methylene Blue and Rhodamine-B Dyes. J. Water Process Eng. 2014, 1, 17–26. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic Degradation of Dyes Using Semiconductor Photocatalysts to Clean Industrial Water Pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Guan, Y.; Liu, Y.; Lv, Q.; Wu, J. Bismuth-Based Photocatalyst for Photocatalytic Oxidation of Flue Gas Mercury Removal: A Review. J. Hazard. Mater. 2021, 418, 126280. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 Photocatalysis: A Brief Review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Zhou, G.; Tian, Z.; Sun, H.; Zhang, J.; Zhao, H.; Li, P.; Sun, H. Understanding the Photocatalytic Mechanisms of the BiOI/Bi2MoO6 and BiOCl/Bi2MoO6 Heterostructures: First-Principles Study. J. Phys. Chem. Solids 2020, 146, 109577. [Google Scholar] [CrossRef]
- Yi, J.; Mo, H.; Zhang, B.; Song, J.; Liu, D.; Zhuo, G. CeO2/Bi2MoO6 Heterostructured Microspheres with Synergistic Effect for Accelerating Photo-generated Charge Separation. Sep. Purif. Technol. 2019, 211, 474–480. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Jia, S.; Jiang, D.; Zhan, Q. Z-Scheme Bi2MoO6 Nanoplate-Decorated Flower-like Bi12SiO20 for Efficient Photocatalytic Degradation of Organic Pollutants. J. Mater. Sci. 2021, 56, 15241–15257. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, Y.; Li, Y.; Ma, H.; Zhao, J. PH-Dependent Synthesis of Iodine-Deficient Bismuth Oxyiodide Microstructures: Visible-Light Photocatalytic Activity. J. Colloid Interface Sci. 2018, 510, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Contreras, D.; Melin, V.; Pérez-González, G.; Henríquez, A.; González, L. Advances and challenges in BiOX (X: Cl, Br, I)-based materials for harvesting sunlight. In Green Photocatalysts; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9783030156084. [Google Scholar]
- Yan, T.; Sun, M.; Liu, H.; Wu, T.; Liu, X.; Yan, Q.; Xu, W.; Du, B. Fabrication of Hierarchical BiOI/Bi2MoO6 Heterojunction for Degradation of Bisphenol A and Dye under Visible Light Irradiation. J. Alloys Compd. 2015, 634, 223–231. [Google Scholar] [CrossRef]
- Chen, F.; Niu, C.; Yang, Q.; Li, X.; Zeng, G. Facile Synthesis of Visible-Light-Active BiOI Modified Bi2MoO6 Photocatalysts with Highly Enhanced Photocatalytic Activity. Ceram. Int. 2016, 42, 2515–2525. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, G.; Kong, J.; Guo, Y.; Xian, Z.; Dai, Y.; Wang, J.; Gong, T.; Sun, C.; Xian, Q. Sheet-on-Sheet TiO2/Bi2MoO6 Heterostructure for Enhanced Photocatalytic Amoxicillin Degradation. J. Hazard. Mater. 2022, 421, 126634. [Google Scholar] [CrossRef]
- Yang, C.-K.; Naveenraj, S.; Lee, G.-J.; Wu, J.J. Microwave-Assisted Synthesis of BiOBr Microspheres for Photocatalytic Degradation of Tartaric Acids in Aqueous Solution. Top. Catal. 2015, 58, 1100–1111. [Google Scholar] [CrossRef]
- Nava Núñez, M.Y.; Martínez-de la Cruz, A.; López-Cuellar, E. Preparation of BiOI Microspheres in 2-Propanol/Ethylene Glycol by Microwave Method with High Visible-Light Photocatalytic Activity. Res. Chem. Intermed. 2019, 45, 1475–1492. [Google Scholar] [CrossRef]
- Li, W.T.; Zheng, Y.F.; Yin, H.Y.; Song, X.C. Heterojunction BiOI/Bi2MoO6 Nanocomposite with Much Enhanced Photocatalytic Activity. J. Nanopart. Res. 2015, 17, 271. [Google Scholar] [CrossRef]
- Silva Ribeiro, C.; Azário Lansarin, M. Facile Solvo-Hydrothermal Synthesis of Bi2MoO6 for the Photocatalytic Reduction of CO2 into Ethanol in Water under Visible Light. React. Kinet. Mech. Catal. 2019, 127, 1059–1071. [Google Scholar] [CrossRef]
- Putri, A.A.; Kato, S.; Kishi, N.; Soga, T. Study of Annealing Temperature Effect on the Photovoltaic Performance of BiOI-Based Materials. Appl. Sci. 2019, 9, 3342. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Na, Y.; Pradhan, D.; Min, B.K.; Sohn, Y. Adsorption and UV/Visible Photocatalytic Performance of BiOI for Methyl Orange, Rhodamine B and Methylene Blue: Ag and Ti-Loading Effects. CrystEngComm 2014, 16, 3155–3167. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, L.; Wang, D.; Yang, F.; Ye, J. Synthesis of Bismuth Molybdate Photocatalysts for CO2 Photo-Reduction. J. CO2 Util. 2019, 29, 196–204. [Google Scholar] [CrossRef]
- Montoya-Zamora, J.M.; Martínez-de la Cruz, A.; López-Cuéllar, E.; Pérez González, F.A. BiOBr Photocatalyst with High Activity for NOx Elimination. Adv. Powder Technol. 2020, 31, 3618–3627. [Google Scholar] [CrossRef]
- Cao, J.; Xu, B.; Luo, B.; Haili Lin, H.; Chen, S. Novel BiOI/BiOBr Heterojunction Photocatalysts with Enhanced Visible Light Photocatalytic Properties. Catal. Commun. 2011, 13, 63–68. [Google Scholar] [CrossRef]
- Nava-Núñez, M.Y.; Jimenez-Relinque, E.; Grande, M.; Martínez-de la Cruz, A.; Castellote, M. Photocatalytic BiOX Mortars under Visible Light Irradiation: Compatibility, Nox Efficiency and Nitrate Selectivity. Catalysts 2020, 10, 226. [Google Scholar] [CrossRef] [Green Version]



| Sample | Binding Energy (eV) | |||||||
|---|---|---|---|---|---|---|---|---|
| Relation Bi:I | Bi 4f7/2 | Bi 4f5/2 | I 3d5/2 | I 3d3/2 | Mo 3d5/2 | Mo 3d3/2 | O 1s | |
| BiOI | 0.89 | 159.3 | 164.7 | 619.2 | 630.7 | - | - | 530.3 |
| BiOI/Bi2MoO6-5 | 0.87 | 159.8 161.3 | 165.2 166.6 | 619.8 | 631.3 | - | - | 531.3 |
| BiOI/Bi2MoO6-10 | 0.84 | 159.5 161.2 | 164.8 166.3 | 619.5 | 630.9 | - | - | 530.7 |
| Bi2MoO6 | - | 159.2 | 164.5 | - | - | 232.5 | 235.6 | 530.3 |
| Samples | X (eV) | Eg (eV) | ECB (eV) | EVB (eV) |
|---|---|---|---|---|
| BiOI | 5.99 | 1.9 | 0.54 | 2.44 |
| Bi2MoO6-5 | 5.5 | 2.55 | −0.275 | 2.275 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez, M.Y.N.; Rehlaender, M.Á.; Martínez-de la Cruz, A.; Susarrey-Arce, A.; Cuevas-Muñiz, F.M.; Sánchez-Domínguez, M.; Lara-Ceniceros, T.E.; Bonilla-Cruz, J.; Zapata, A.A.; Hurtado, P.C.; et al. Enhancing Visible Light Photocatalytic Degradation of Bisphenol A Using BiOI/Bi2MoO6 Heterostructures. Nanomaterials 2023, 13, 1503. https://doi.org/10.3390/nano13091503
Núñez MYN, Rehlaender MÁ, Martínez-de la Cruz A, Susarrey-Arce A, Cuevas-Muñiz FM, Sánchez-Domínguez M, Lara-Ceniceros TE, Bonilla-Cruz J, Zapata AA, Hurtado PC, et al. Enhancing Visible Light Photocatalytic Degradation of Bisphenol A Using BiOI/Bi2MoO6 Heterostructures. Nanomaterials. 2023; 13(9):1503. https://doi.org/10.3390/nano13091503
Chicago/Turabian StyleNúñez, Magaly Y. Nava, Moisés Ávila Rehlaender, Azael Martínez-de la Cruz, Arturo Susarrey-Arce, Francisco Mherande Cuevas-Muñiz, Margarita Sánchez-Domínguez, Tania E. Lara-Ceniceros, José Bonilla-Cruz, Alejandro Arizpe Zapata, Patricia Cerda Hurtado, and et al. 2023. "Enhancing Visible Light Photocatalytic Degradation of Bisphenol A Using BiOI/Bi2MoO6 Heterostructures" Nanomaterials 13, no. 9: 1503. https://doi.org/10.3390/nano13091503







