Synthesis and Characterization of Erbium-Doped Silica Films Obtained by an Acid–Base-Catalyzed Sol–Gel Process
Abstract
:1. Introduction
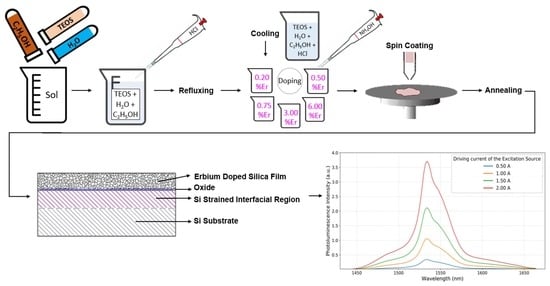
2. Experimental Details
3. Results and Discussion
3.1. Film Thickness and Refractive Index
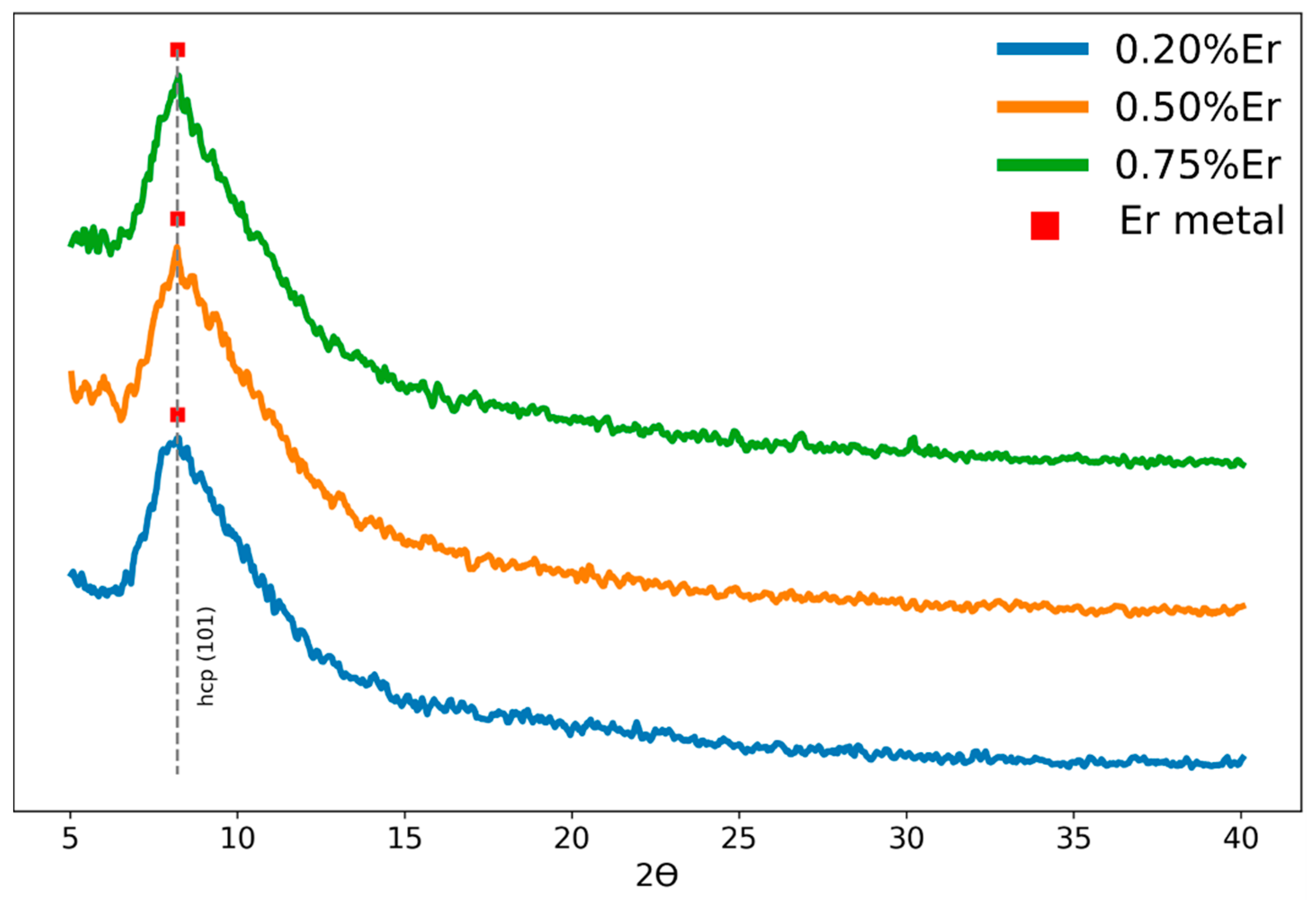
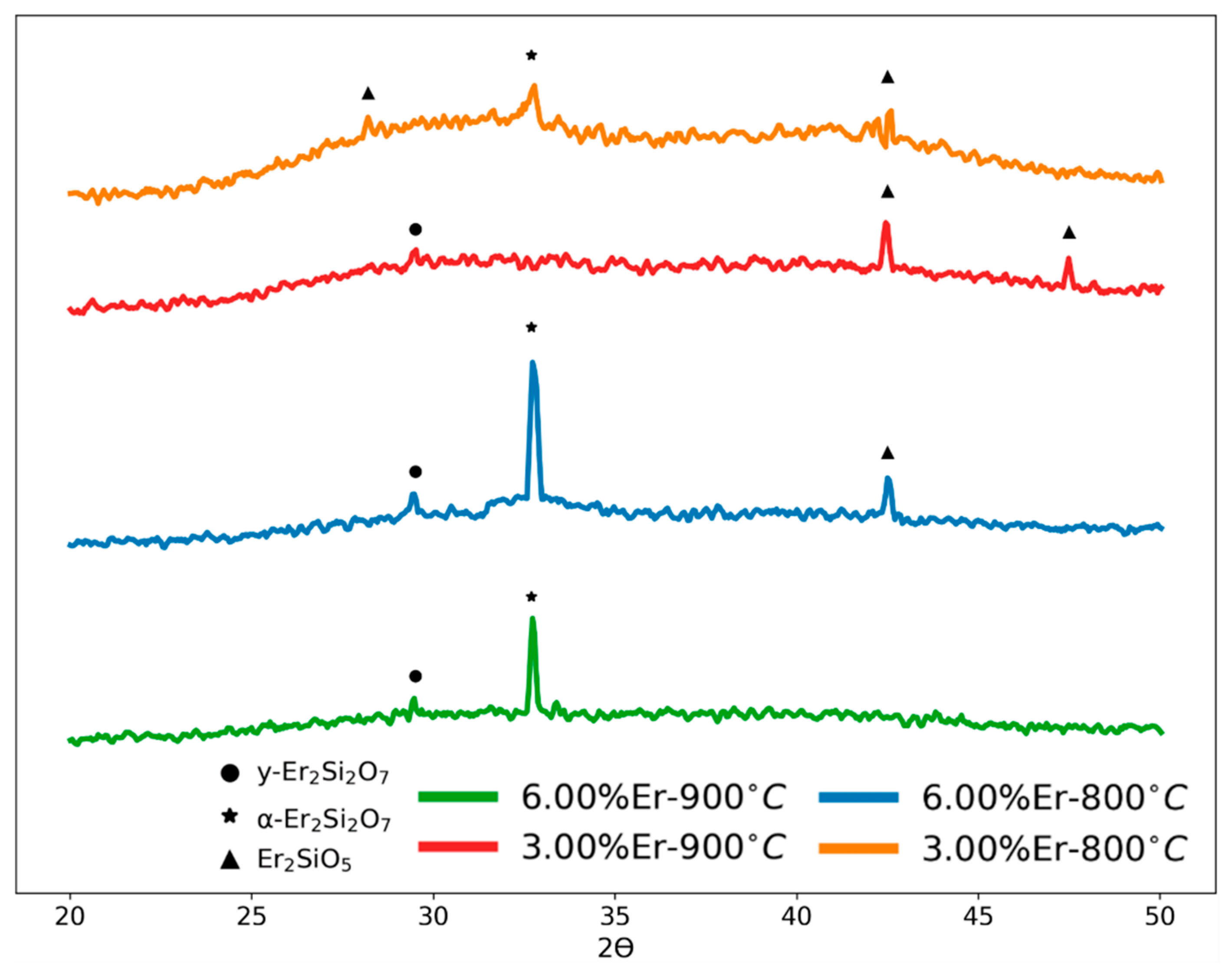
3.2. Structural Analysis (X-ray Diffraction)
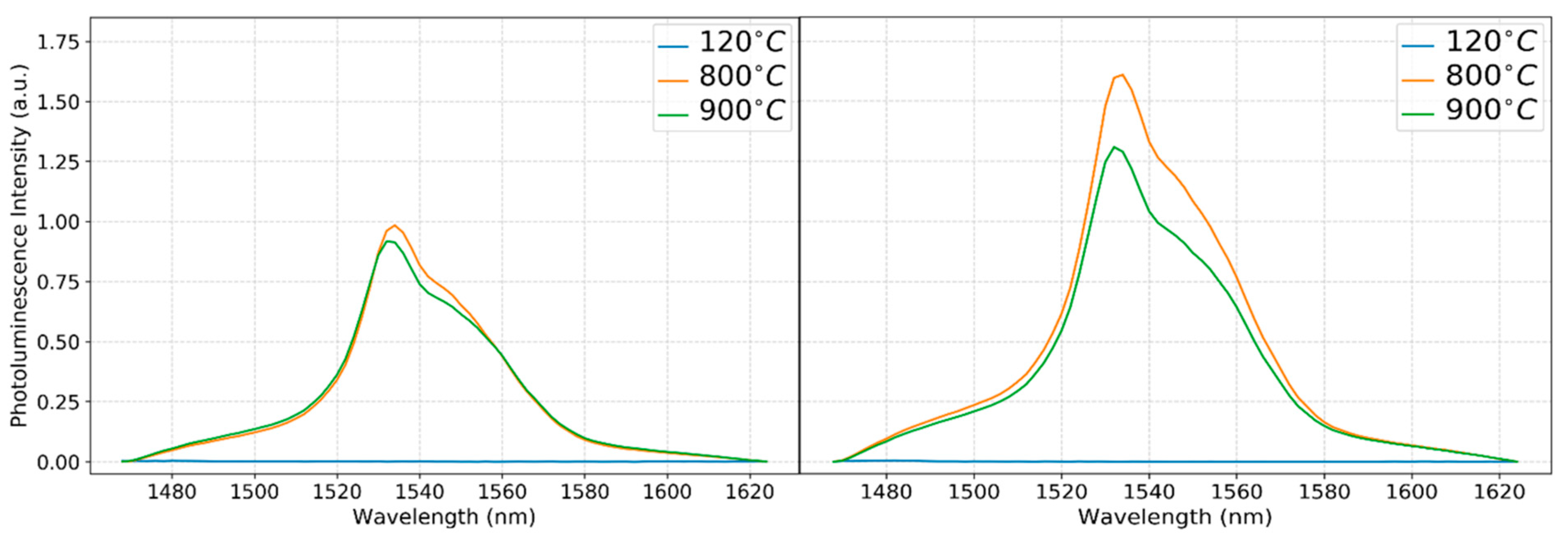
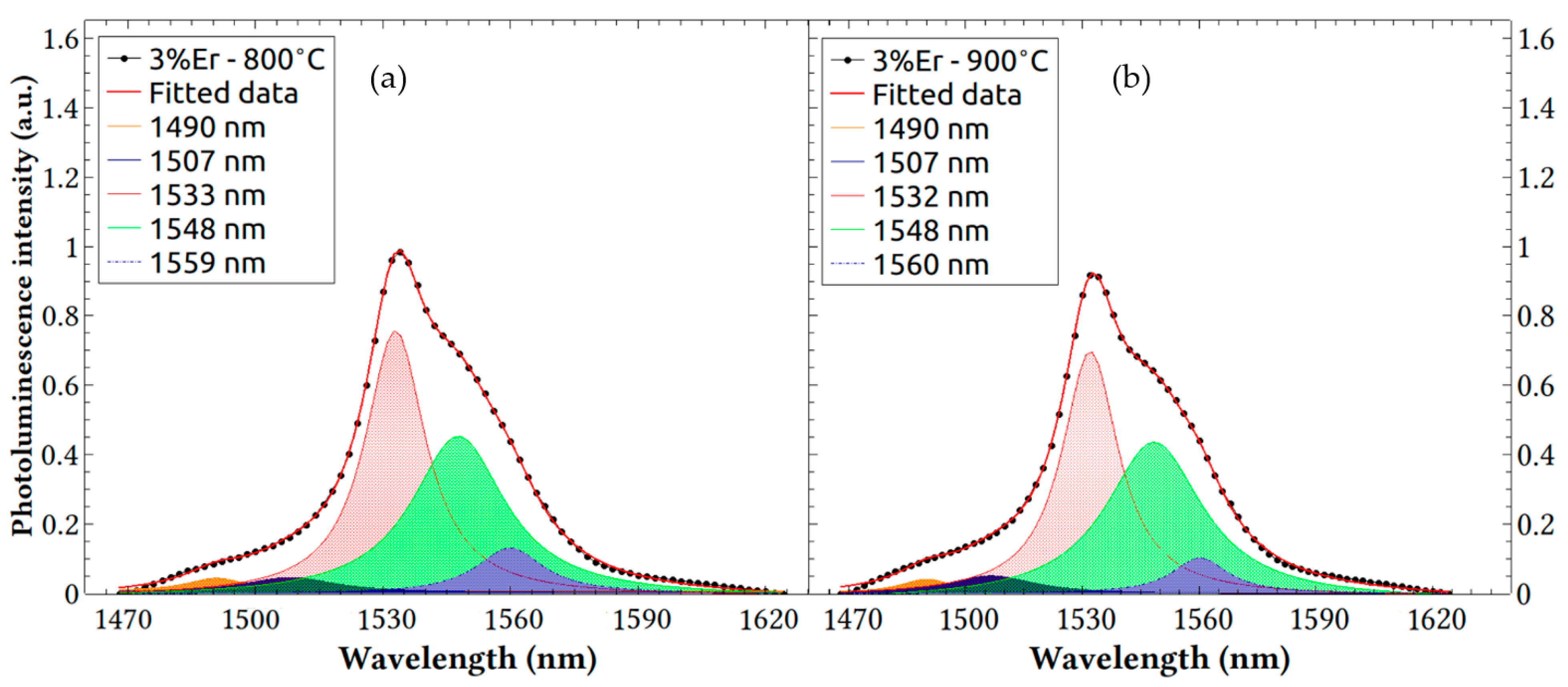
3.3. Photoluminescence Measurements
Erbium Emission at the 4f Transition Band
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, H.; Li, H.; Chen, G.F.R.; Xing, P.; Tan, M.C.; Tan, D.T.H. 3D printed and spiral lithographically patterned erbium-doped polymer micro-waveguide amplifiers. Sci. Rep. 2021, 11, 21292. [Google Scholar] [CrossRef] [PubMed]
- Madhu, A.; Srinatha, N. Structural and spectroscopic studies on the concentration dependent erbium doped lithium bismuth boro tellurite glasses for optical fiber applications. Infrared Phys. Technol. 2020, 107, 103300. [Google Scholar] [CrossRef]
- Arafin, S.; Coldren, L.A. Advanced InP photonic integrated circuits for communication and sensing. IEEE J. Sel. Top. Quantum Electron. 2017, 24, 1–12. [Google Scholar] [CrossRef]
- Bhemarajam, J.; Babu, M.M.; Prasad, P.S.; Prasad, M. Spectroscopic studies on Er3+ ions incorporated bismuth borolead lithium glasses for solid state lasers and fiber amplifiers. Opt. Mater. 2021, 113, 110818. [Google Scholar]
- Gupta, S.K.; Kadam, R.M.; Pujari, P.K. Lanthanide spectroscopy in probing structure-property correlation in multi-site photoluminescent phosphors. Coord. Chem. Rev. 2020, 420, 213405. [Google Scholar]
- Mahajan, S.V. Ultra-Small Rare-Earth Oxide Nanocrystals: Development, Film Assembly, Optical and Dielectric Studies; Vanderbilt University: Nashville, TN, USA, 2010; p. 3445632. [Google Scholar]
- Peng, D.; Zou, H.; Xu, C.; Wang, X.; Yao, X. Er doped BaBi4Ti4O15 multifunctional ferroelectrics: Up-conversion photoluminescence, dielectric and ferroelectric properties. J. Alloy. Compd. 2013, 552, 463–468. [Google Scholar] [CrossRef]
- Iacob, M.; Airinei, A.; Asandulesa, M.; Dascalu, M.; Tudorachi, N.; Hernandez, L.; Cazacu, M. Silicone elastomers filled with rare earth oxides. Mater. Res. Express 2020, 7, 035703. [Google Scholar] [CrossRef]
- Hossain, M.K.; Ahmed, M.H.; Khan, M.I.; Miah, M.S.; Hossain, S. Recent progress of rare earth oxides for sensor, detector, and electronic device applications: A review. ACS Appl. Electron. Mater. 2021, 3, 4255–4283. [Google Scholar] [CrossRef]
- Steinkemper, H.; Fischer, S.; Hermle, M.; Goldschmidt, J.C. Stark level analysis of the spectral line shape of electronic transitions in rare earth ions embedded in host crystals. New J. Phys. 2013, 15, 053033. [Google Scholar] [CrossRef]
- Atwood, D.A. (Ed.) The Rare Earth Elements: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Trupke, T.; Zhao, J.; Wang, A.; Corkish, R.; Green, M.A. Very efficient light emission from bulk crystalline silicon. Appl. Phys. Lett. 2003, 82, 2996–2998. [Google Scholar] [CrossRef]
- Kenyon, A.J. Semicond. Erbium in silicon. Sci. Technol. 2005, 20, R65. [Google Scholar]
- Lockwood, D.J.; Pavesi, L. Silicon Photonics; Springer: Berlin, Germany, 2004; pp. 1–50. [Google Scholar]
- Abedrabbo, S.; Lahlouh, B.; Shet, S.; Fiory, A.T. Room-temperature silicon band-edge photoluminescence enhanced by spin-coated sol–gel films. Scr. Mater. 2011, 65, 767–770. [Google Scholar] [CrossRef]
- Abedrabbo, S.; Fiory, A.T.; Ravindra, N.M. Evidence of Silicon Band-Edge Emission Enhancement When Interfaced with SiO2: Er Films. JOM 2017, 69, 241–246. [Google Scholar] [CrossRef]
- Abedrabbo, S.; Fiory, A.T.; Ravindra, N.M. Processing for Highly Emissive CZ-Silicon by Depositing Stressed Sol–Gel Films. JOM 2014, 66, 643–648. [Google Scholar] [CrossRef]
- Abedrabbo, S.; Ravindra, N.M.; Fiory, A.T. Engineered Stresses for a Functional Si Light Emitter at Bandgap: An Overview. JOM 2019, 71, 4857–4866. [Google Scholar] [CrossRef]
- Abedrabbo, S.; Lahlouh, B.; Fiory, A.T. Analytical study of thermal annealing behaviour of erbium emission in Er2O3-sol–gel silica films. J. Phys. D Appl. Phys. 2011, 44, 315401. [Google Scholar] [CrossRef]
- Abedrabbo, S.; Lahlouh, B.; Fiory, A.T.; Ravindra, N.M. Optical polarizability of erbium-oxygen complexes in sol-gel-based silica films. J. Phys. D Appl. Phys. 2021, 54, 135101. [Google Scholar] [CrossRef]
- Profex. Available online: https://www.profex-xrd.org/?page_id=139 (accessed on 22 November 2022).
- Gao, Y.; Shen, H.; Cao, J.; Li, D.; Yang, D. Control of the formation and luminescent properties of polymorphic erbium silicates on silicon. Opt. Mater. Express 2019, 9, 1716–1727. [Google Scholar] [CrossRef]
- Kai, Z.; Lingxia, L.; Menglong, W.; Weijia, L. Charge compensation in rare earth doped BaTiO3-based ceramics sintered in reducing atmosphere. Ceram. Int. 2020, 46, 25881–25887. [Google Scholar]
- Wolf, S.; Tauber, R.N. Silicon Processing for the VLSI Era, 2nd ed.; Lattice Press: Sunset Beach, CA, USA, 1990; Chapter 4; Volume 1, p. 277. [Google Scholar]
- Rietveld, H. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.K.; Khan, P.; Mukherjee, S.; Mukhopadhyay, A.K.; Ghosh, J.; Muraleedharan, K. Study of short range structure of amorphous Silica from PDF using Ag radiation in laboratory XRD system, RAMAN and NEXAFS. J. Non Cryst. Solids 2018, 488, 1–9. [Google Scholar] [CrossRef]
- Samudrala, G.K.; Vohra, Y.K. Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2013; Volume 43. [Google Scholar]
- Vasconcelos, H.C.; Pinto, A.S. Fluorescence Properties of Rare-Earth-Doped Sol-Gel Glasses; IntechOpen: London, UK, 2017. [Google Scholar]
- El Hamzaoui, H.; Bouwmans, G.; Capoen, B.; Chen, N.; Douay, M.; Bouazaoui, M. Sol–gel silica glass-cladding semiconductor-core optical fiber. Mater. Today Commun. 2017, 11, 179–183. [Google Scholar] [CrossRef]
- Dai, S.; Yu, C.; Zhou, G.; Zhang, J.; Wang, G.; Hu, L. Concentration quenching in erbium-doped tellurite glasses. J. Lumin. 2006, 117, 39–45. [Google Scholar] [CrossRef]
- Jaba, N.; Mansour, H.B.; Kanoun, A.; Brenier, A.; Champagnon, B. Spectral broadening and luminescence quenching of 1.53 μm emission in Er3+-doped zinc tellurite glass. J. Lumin. 2009, 129, 270–276. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wang, W.; Ye, F.; Yue, S.; Guo, H.; Gao, G.; Zhao, Y.; Fang, Q.; Nguyen, C.; et al. 3D-printed silica with nanoscale resolution. Nat. Mater. 2021, 20, 1506–1511. [Google Scholar] [CrossRef]
- Tanabe, S. Glass and rare-earth elements: A personal perspective. Int. J. Appl. Glass Sci. 2015, 6, 305–328. [Google Scholar] [CrossRef]
- Duchna, M.; Cieślik, I. Rare Earth Elements in New Advanced Engineering Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Yagodkin, D.; Greben, K.; Eljarrat, A.; Kovalchuk, S.; Ghorbani-Asl, M.; Jain, M.; Kretschmer, S.; Severin, N.; Rabe, J.P.; Krasheninnikov, A.V.; et al. Extrinsic localized excitons in patterned 2D semiconductors. Adv. Funct. Mater. 2022, 32, 2203060. [Google Scholar] [CrossRef]
- Desurvire, E.; Simpson, J.R. Evaluation of 4 I 15/2 and 4 I 13/2 Stark-level energies in erbium-doped aluminosilicate glass fibers. Opt. Lett. 1990, 15, 547–549. [Google Scholar] [CrossRef]
- Polman, A.; Custer, J.S.; Snokes, E.; van den Hoven, G.N. Optical doping of silicon with erbium by ion implantation. Nucl. Instr. Meth. Phys. Res. B 1993, 80–81, 653–658. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Zhang, D.; Su, W.; Zhang, N.; Liu, Z.; Wang, Z.; Yang, L. Broadband luminescence at 1.5 μm of Er P co-doped high silica glass by nanoporous doping technology. J. Non-Cryst. Solids 2022, 575, 121206. [Google Scholar] [CrossRef]
- Chen, P.; Xu, W.; Gao, Y.; Warner, J.H.; Castell, M.R. Epitaxial Growth of Monolayer MoS2 on SrTiO3 Single Crystal Substrates for Applications in Nanoelectronics. ACS Appl. Nano Mater. 2018, 1, 6976–6988. [Google Scholar] [CrossRef]
- Desurvire, E. Erbium Doped Fiber Amplifiers: Principles and Applications; Wiley: New York, NY, USA, 1994; Chapter 4. [Google Scholar]
- Abedrabbo, S.; Mohammed, Q.; Fiory, A.T. Processing for optically active erbium in silicon by film co-deposition and ion-beam mixing. Appl. Surf. Sci. 2009, 255, 4503–4511. [Google Scholar] [CrossRef]
- Abedrabbo, S.; Fiory, A. Ambient erbium luminescence in silicon and silicon-germanium films. Emerg. Mater. Res. 2012, 1, 17–24. [Google Scholar] [CrossRef]
- Abedrabbo, S.; Arafah, D.E.; Salem, S. Ion beam mixing of silicon-germanium thin films. J. Elect. Mat. 2005, 34, 468–473. [Google Scholar] [CrossRef]
- Abedrabbo, S.; Arafah, D.E.; Gokce, O.; Wielunski, L.S.; Gharaibeh, M.; Celik, O.; Ravindra, N.M. Ion beam mixing for processing of nanostructure materials. J. Elect. Mat. 2006, 35, 834–839. [Google Scholar] [CrossRef]




| Coefficient | Value |
|---|---|
| A | 0.215 ± 0.00619 |
| B | 0.16674 ± 0.001414 |
| E | 0.943 ± 00048588 |
| Sample | 3%—800 °C | 3%—900 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak NO. | P1 | P2 | P3 | P4 | P5 | P1 | P2 | P3 | P4 | P5 |
| AUFC | 1.926 | 1.876 | 17.87 | 15.69 | 3.535 | 1.051 | 2.297 | 21.85 | 19.61 | 2.823 |
| Peak position (nm) | 1490 | 1507 | 1533 | 1548 | 1559 | 1490 | 1507 | 1532 | 1548 | 1560 |
| FWMH (nm) | 17.19 | 27.004 | 17.23 | 26.76 | 19.36 | 16.55 | 27.52 | 17.67 | 30.41 | 17.31 |
| Peak intensity (a.u.) | 0.0440 | 0.0463 | 0.758 | 0.452 | 0.131 | 0.0404 | 0.0532 | 0.707 | 0.457 | 0.104 |
| Sample | 6%—800 °C | 6%—900 °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak NO. | P1 | P2 | P3 | P4 | P5 | P1 | P2 | P3 | P4 | P5 |
| AUFC | 2.003 | 4.078 | 35.56 | 30.82 | 8.955 | 1.553 | 3.357 | 28.31 | 30.71 | 4.637 |
| Peak position (nm) | 1491 | 1507 | 1532 | 1548 | 1560 | 1490 | 1506 | 1532 | 1548 | 1560 |
| FWMH (nm) | 17.39 | 28.39 | 17.98 | 27.06 | 20.47 | 16.11 | 26.96 | 18.06 | 31.32 | 17.67 |
| Peak intensity (a.u.) | 0.073 | 0.091 | 1.259 | 0.724 | 0.278 | 0.061 | 0.0792 | 0.998 | 0.634 | 0.167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, A.; Benchafia, E.M.; Choi, D.; Abedrabbo, S. Synthesis and Characterization of Erbium-Doped Silica Films Obtained by an Acid–Base-Catalyzed Sol–Gel Process. Nanomaterials 2023, 13, 1508. https://doi.org/10.3390/nano13091508
Abdullah A, Benchafia EM, Choi D, Abedrabbo S. Synthesis and Characterization of Erbium-Doped Silica Films Obtained by an Acid–Base-Catalyzed Sol–Gel Process. Nanomaterials. 2023; 13(9):1508. https://doi.org/10.3390/nano13091508
Chicago/Turabian StyleAbdullah, Ali, El Mostafa Benchafia, Daniel Choi, and Sufian Abedrabbo. 2023. "Synthesis and Characterization of Erbium-Doped Silica Films Obtained by an Acid–Base-Catalyzed Sol–Gel Process" Nanomaterials 13, no. 9: 1508. https://doi.org/10.3390/nano13091508
APA StyleAbdullah, A., Benchafia, E. M., Choi, D., & Abedrabbo, S. (2023). Synthesis and Characterization of Erbium-Doped Silica Films Obtained by an Acid–Base-Catalyzed Sol–Gel Process. Nanomaterials, 13(9), 1508. https://doi.org/10.3390/nano13091508