Biochars from Olive Stones as Carbonaceous Support in Pt/TiO2-Carbon Photocatalysts and Application in Hydrogen Production from Aqueous Glycerol Photoreforming
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Carbonaceous Materials, Composites, and Photocatalysts
2.2.1. Synthesis of Biochars from Olive Stones
2.2.2. Synthesis of Activated Carbon (AC)
2.2.3. Pyrolyzed Carbon Functionalized (PyCF)
2.2.4. Mesoporous Carbon Functionalized (MCF)
2.2.5. Synthesis of the TiO2-Biochar Composites
2.2.6. Synthesis of Pt/TiO2-Carbon Photocatalysts
2.3. Characterization Techniques and Procedures
2.4. Glycerol Photoreforming Experiments
3. Results and Discussion
3.1. Synthesis of Carbonaceous Supports and Photocatalyst
3.2. Catalyst Characterization
3.2.1. Textural and Morphological Characterization of Synthesized Solids
3.2.2. Chemical and Structural Characterization of Carbonaceous Materials Synthesized
3.2.3. Optoelectronic and Electric Characterization of the Solids
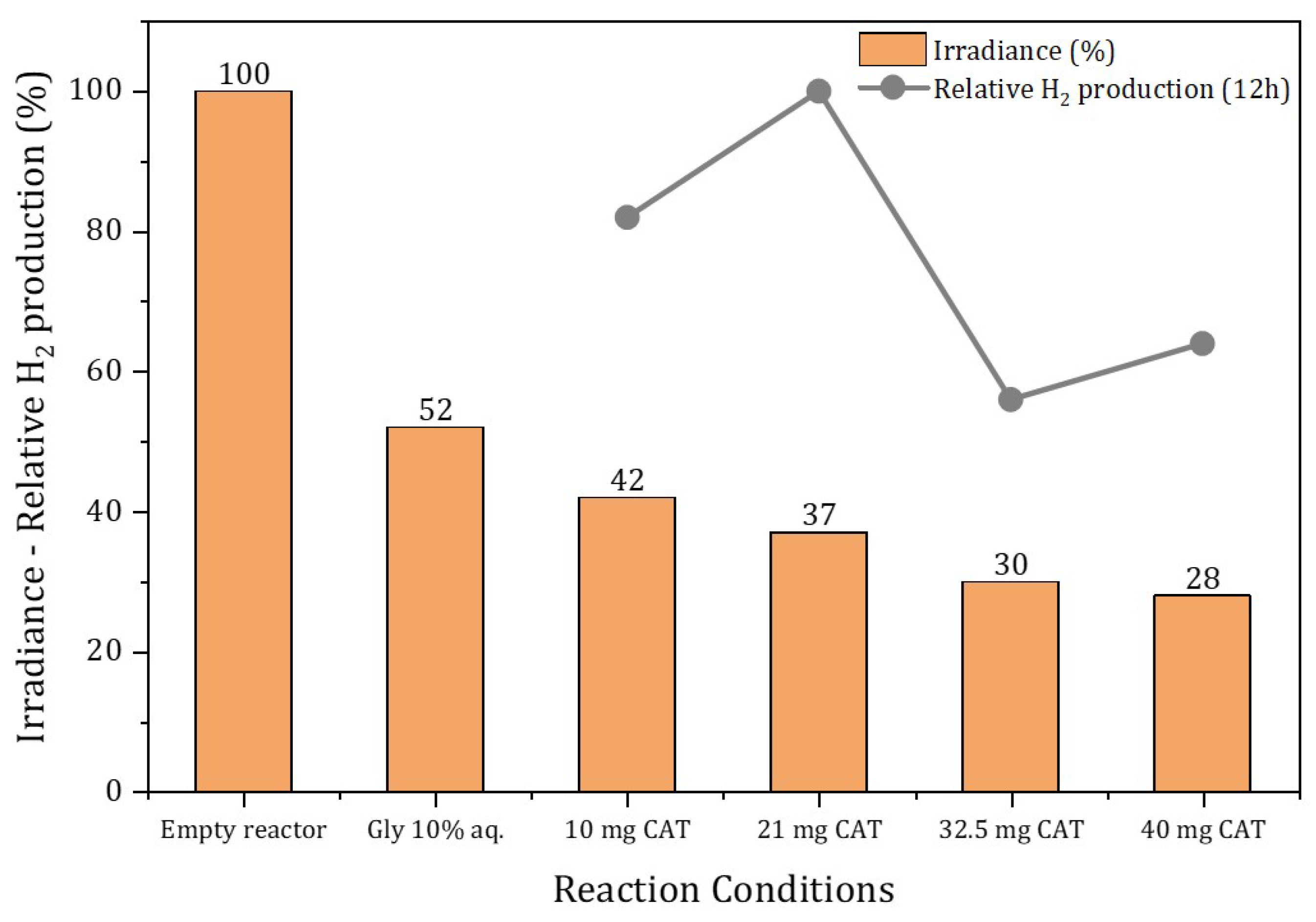
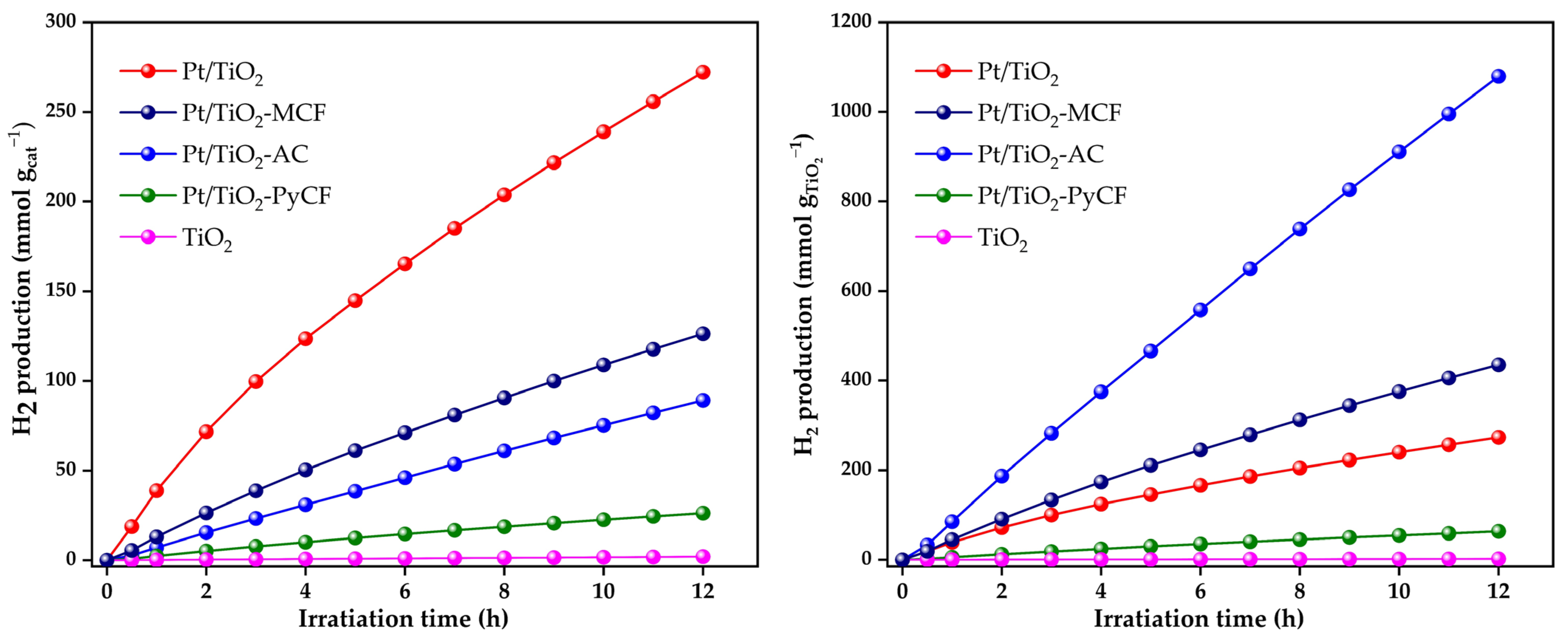
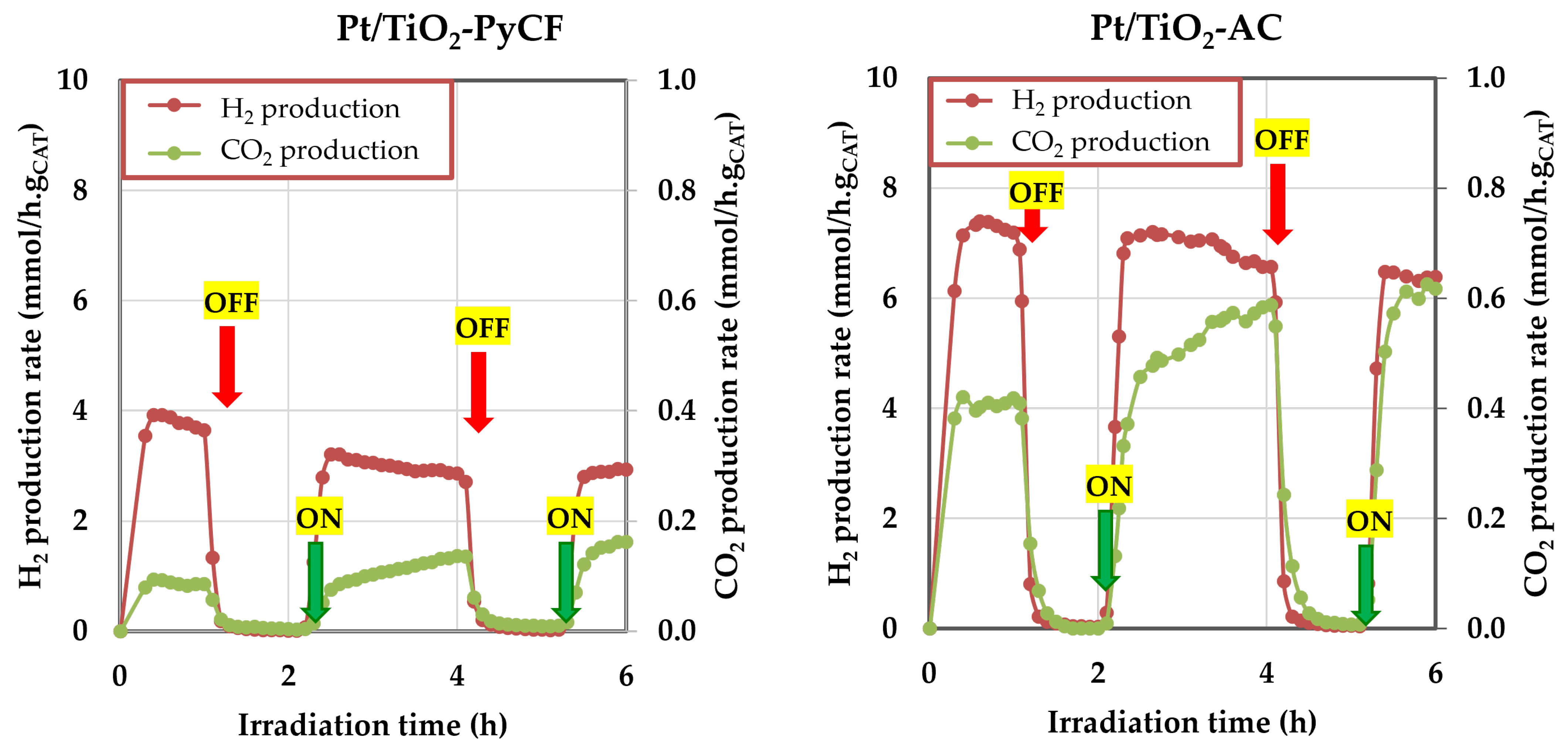
3.3. Glycerol Photoreforming
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An Overview of Hydrogen Production from Biomass. Fuel Process Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Colón, G. Towards the Hydrogen Production by Photocatalysis. Appl. Catal. A Gen. 2016, 518, 48–59. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic Hydrogen Production Using Metal Doped TiO2: A Review of Recent Advances. Appl. Catal. B 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Hidalgo-carrillo, J.; Martín, J.; Morales, J.; Espejo, J.C.; Urbano, F.J.; Marinas, A. Hydrogen Photo-Production from Glycerol Using Nickel-Doped TiO2 Catalysts: Effect of Catalyst Pre-Treatment. Energies 2019, 12, 3351. [Google Scholar] [CrossRef]
- Yoong, L.S.; Chong, F.K.; Dutta, B.K. Development of Copper-Doped TiO2 Photocatalyst for Hydrogen Production under Visible Light. Energy 2009, 34, 1652–1661. [Google Scholar] [CrossRef]
- Patil, S.B.; Basavarajappa, P.S.; Ganganagappa, N.; Jyothi, M.S.; Raghu, A.V.; Reddy, K.R. Recent Advances in Non-Metals-Doped TiO2 Nanostructured Photocatalysts for Visible-Light Driven Hydrogen Production, CO2 Reduction and Air Purification. Int. J. Hydrogen Energy 2019, 44, 13022–13039. [Google Scholar] [CrossRef]
- Li, Y.; Ma, G.; Peng, S.; Lu, G.; Li, S. Boron and Nitrogen Co-Doped Titania with Enhanced Visible-Light Photocatalytic Activity for Hydrogen Evolution. Appl. Surf. Sci. 2008, 254, 6831–6836. [Google Scholar] [CrossRef]
- Do, H.H.; Nguyen, D.L.T.; Nguyen, X.C.; Le, T.H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.V.N.; Kim, S.Y.; Le, Q. Van Recent Progress in TiO2-Based Photocatalysts for Hydrogen Evolution Reaction: A Review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Ali, H.M.; Arabpour Roghabadi, F.; Ahmadi, V. Solid-Supported Photocatalysts for Wastewater Treatment: Supports Contribution in the Photocatalysis Process. Sol. Energy 2023, 255, 99–125. [Google Scholar] [CrossRef]
- Zhen, L.; Hu, M.; Xu, Y.; Zhao, D.; Jiang, S.; Fan, K.; Zu, M.; Al-Mamun, M.; Yin, H.; Chen, S.; et al. Photocatalytic Hydrogen Production. In Photo-and Electro-Catalytic Processes; Wiley: Hoboken, NJ, USA, 2022; pp. 415–483. [Google Scholar] [CrossRef]
- Li Puma, G.; Bono, A.; Krishnaiah, D.; Collin, J.G. Preparation of Titanium Dioxide Photocatalyst Loaded onto Activated Carbon Support Using Chemical Vapor Deposition: A Review Paper. J. Hazard Mater. 2008, 157, 209–219. [Google Scholar] [CrossRef]
- Ramesh Reddy, N.; Bhargav, U.; Mamatha Kumari, M.; Cheralathan, K.K.; Sakar, M. Review on the Interface Engineering in the Carbonaceous Titania for the Improved Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 7584–7615. [Google Scholar] [CrossRef]
- Imparato, C.; Iervolino, G.; Fantauzzi, M.; Koral, C.; Macyk, W.; Kobielusz, M.; D’Errico, G.; Rea, I.; Di Girolamo, R.; De Stefano, L.; et al. Photocatalytic Hydrogen Evolution by Co-Catalyst-Free TiO2/C Bulk Heterostructures Synthesized under Mild Conditions. RSC Adv. 2020, 10, 12519–12534. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, A.; Casas, J.A.; Carbajo, J.; Faraldos, M.; Bahamonde, A. Influence of TiO2 Optical Parameters in a Slurry Photocatalytic Reactor: Kinetic Modelling. Appl. Catal. B 2017, 200, 164–173. [Google Scholar] [CrossRef]
- Cao, S.; Piao, L. Considerations for a More Accurate Evaluation Method for Photocatalytic Water Splitting. Angew. Chem. 2020, 132, 18468–18476. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate Photocatalysts for Overall Water Splitting. Nat. Rev. Mater. 2017, 2. [Google Scholar] [CrossRef]
- Qureshi, M.; Takanabe, K. Insights on Measuring and Reporting Heterogeneous Photocatalysis: Efficiency Definitions and Setup Examples. Chem. Mater. 2017, 29, 158–167. [Google Scholar] [CrossRef]
- Wang, Z.; Hisatomi, T.; Li, R.; Sayama, K.; Liu, G.; Domen, K.; Li, C.; Wang, L. Efficiency Accreditation and Testing Protocols for Particulate Photocatalysts toward Solar Fuel Production. Joule 2021, 5, 344–359. [Google Scholar] [CrossRef]
- Serpone, N.; Salinaro, A. Terminology, Relative Photonic Efficiencies and Quantum Yields in Heterogeneous Photocatalysis. Part I: Suggested Protocol. Pure Appl. Chem. 1999, 71, 303–320. [Google Scholar] [CrossRef]
- Escamilla, J.C.; Hidalgo-Carrillo, J.; Martín-Gómez, J.; Estévez-Toledano, R.C.; Montes, V.; Cosano, D.; Urbano, F.J.; Marinas, A. Hydrogen Production through Glycerol Photoreforming on TiO2/Mesoporous Carbon: Influence of the Synthetic Method. Materials 2020, 13, 3800. [Google Scholar] [CrossRef]
- Escamilla-Mejía, J.C.; Hidalgo-Carrillo, J.; Martín-Gómez, J.; López-Tenllado, F.J.; Estévez-Toledano, R.C.; Marinas, A.; Urbano, F.J. Pt Preferential Incorporation onto TiO2 IN TiO2-Carbon Composites for Hydrogen Production from Glycerol Photoreforming. Catal. Today 2023, 413, 113943. [Google Scholar] [CrossRef]
- Yakout, S.M.; Sharaf El-Deen, G. Characterization of Activated Carbon Prepared by Phosphoric Acid Activation of Olive Stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Montes, V.; Caballero, A.; Bautista, F.M. Sulfonated Carbons from Olive Stones as Catalysts in the Microwave-Assisted Etherification of Glycerol with Tert-Butyl Alcohol. Mol. Catal. 2020, 488, 110921. [Google Scholar] [CrossRef]
- Majeed, I.; Ali, H.; Idrees, A.; Arif, A.; Ashraf, W.; Rasul, S.; Khan, M.A.; Nadeem, M.A.; Nadeem, M.A. Un-derstanding the Role of Metal Supported on TiO2 in Photoreforming of Oxygenates. Energy Adv. 2022, 1, 842–867. [Google Scholar] [CrossRef]
- Han, M.L.; Wei, X.L.; Zhang, J.C.; Liu, Y.; Tang, X.; Li, P.; Liu, Z.Y. Influence of Structural Damage on Evaluation of Microscopic Pore Structure in Marine Continental Transitional Shale of the Southern North China Basin: A Method Based on the Low-Temperature N2 Adsorption Experiment. Pet. Sci. 2022, 19, 100–115. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure. Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Dalto, F.; Kuźniarska-Biernacka, I.; Pereira, C.; Mesquita, E.; Soares, O.S.G.P.; Pereira, M.F.R.; Rosa, M.J.; Mestre, A.S.; Carvalho, A.P.; Freire, C. Solar Light-Induced Methylene Blue Removal over TiO2/AC Composites and Photocatalytic Regeneration. Nanomaterials 2021, 11, 3016. [Google Scholar] [CrossRef]
- Serra, T.; Colomer, J.; Casamitjana, X.J. Aggregation and breakup of particles in a shear flow. Colloid Interface Sci. 1997, 187, 466–473. [Google Scholar] [CrossRef]
- Park, M.H.; Yun, Y.S.; Cho, S.Y.; Kim, N.R.; Jin, H.J. Waste Coffee Grounds-Derived Nanoporous Carbon Nanosheets for Supercapacitors. Carbon Lett. 2016, 19, 66–71. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash. Part 1. Phase-Mineral and Chemical Composition and Classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Panda, A.K.; Raghavan, C.M.; Singh, A.; Prabhakar, M.N.; Mohandas, E.; Kuppusami, P.; Song, J. Microstructure, Optical and Dielectric Properties of Cerium Oxide Thin Films Prepared by Pulsed Laser Deposition. J. Mater. Sci. Mater. Electron. 2019, 30, 16548–16553. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Jarunsak, N.; Chuangchai, T.; Buasri, A. The Photocatalytic Reduction of Hexavalent Chromium by Controllable Mesoporous Anatase TiO2 Nanoparticles. Adv. Mat. Sci. Eng. 2014, 2014, 348427. [Google Scholar] [CrossRef]
- Hidalgo-Carrillo, J.; Martín-Gómez, J.; Herrera-Beurnio, M.C.; Estévez, R.C.; Urbano, F.J.; Marinas, A. Olive Leaves as Biotemplates for Enhanced Solar-Light Harvesting by a Titania-Based Solid. Nanomaterials 2020, 10, 1057. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Raman Spectra of Titanium Dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Aramendía, M.A.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Synthesis, Characterization and Photocatalytic Activity of Different Metal-Doped Titania Systems. Appl Catal. A Gen. 2006, 306, 120–127. [Google Scholar] [CrossRef]
- Ulyanova, E.V.; Molchanov, A.N.; Prokhorov, I.Y.; Grinyov, V.G. Fine Structure of Raman Spectra in Coals of Different Rank. Int. J. Coal Geol. 2014, 121, 37–43. [Google Scholar] [CrossRef]
- Penki, T.R.; Shanmughasundaram, D.; Kishore, B.; Munichandraiah, N. High Rate Capability of Coconut Kernel Derived Carbon as an Anode Material for Lithium-Ion Batteries. Adv. Mater. Lett. 2014, 5, 184–190. [Google Scholar] [CrossRef]
- Barabash, V.M.; Abiev, R.S.; Kulov, N.N. Theory and Practice of Mixing: A Review. Theor. Found. Chem. Eng. 2018, 52, 473–487. [Google Scholar] [CrossRef]
- Sanwald, K.E.; Berto, T.F.; Eisenreich, W.; Gutiérrez, O.Y.; Lercher, J.A. Catalytic Routes and Oxidation Mechanisms in Photoreforming of Polyols. J. Catal. 2016, 344, 806–816. [Google Scholar] [CrossRef]

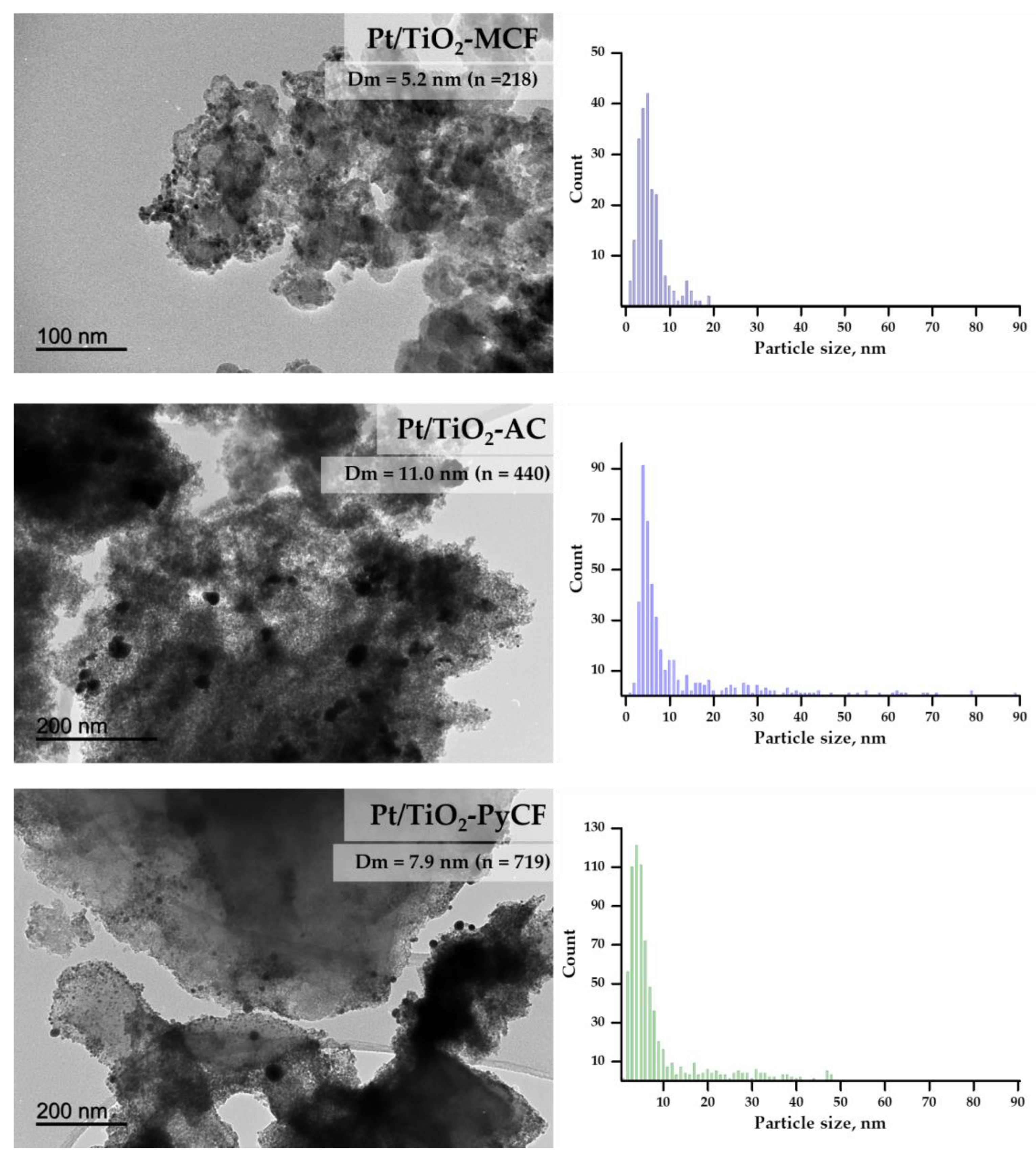


| Sample | a SBET (m2/g) | b Vtotal (10−2 cm3/g) | c Vmic (10−2 cm3/g) | Average Pore Size (nm) | Micropore Volume (%) | Mesopore Volume (%) |
|---|---|---|---|---|---|---|
| Pt/TiO2-MCF | 173 | 46 | 0 | 11.0 | 0 | 100 |
| TiO2-MCF | 172 | 115 | 0 | 26.8 | 0 | 100 |
| Pt/TiO2-AC | 616 | 48 | 8 | 3.1 | 18 | 82 |
| TiO2-AC | 864 | 71 | 7 | 3.3 | 11 | 89 |
| Pt/TiO2-PyCF | 552 | 28 | 18 | 2.1 | 66 | 34 |
| TiO2-PyCF | 602 | 28 | 23 | 1.5 | 85 | 15 |
| Pt/TiO2 P25 | 63 | 46 | 0 | 29.0 | 0 | 100 |
| TiO2 P25 | 56 | 65 | 0 | 45.9 | 0 | 100 |
| Catalyst | Conditions | a Z-Ave (nm) | b D (4,3) Vol. Weighed Mean (µm) |
|---|---|---|---|
| Pt/TiO2-MCF | As synthesized Recovered after use (12 h) After stirring without light (12 h) | 366 ± 16 290 ± 18 297 ± 4 | |
| Pt/TiO2-AC | As synthesized Recovered after use (12 h) After stirring without light (12 h) | 10.41 ± 1.14 4.54 ± 0.04 6.53 ± 0.37 | |
| Pt/TiO2-PyCF | As synthesized Recovered after use (12 h) After stirring without light (12 h) | 36.68 ± 3.03 16.79 ± 0.50 20.29 ± 1.98 | |
| Pt/TiO2 | As synthesized Recovered after use (12 h) After stirring without light (12 h) | 326 ± 23 229 ± 10 - |
| Catalyst | a Pt (wt.%) | a TiO2 (wt.%) | Pt Particle Size (TEM, nm) | b pHIEP |
|---|---|---|---|---|
| Pt/TiO2-MCF | 0.83 | 29.0 | 5.2 | 4.5 |
| Pt/TiO2-AC | 0.69 | 41.4 | 11.0 | 5.9 |
| Pt/TiO2-PyCF | 0.49 | 8.3 | 7.9 | 5.7 |
| Pt/TiO2 | 0.44 | 99.5 | 4.4 | 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escamilla-Mejía, J.C.; Hidalgo-Carrillo, J.; Martín-Gómez, J.; López-Tenllado, F.J.; Estévez, R.; Marinas, A.; Urbano, F.J. Biochars from Olive Stones as Carbonaceous Support in Pt/TiO2-Carbon Photocatalysts and Application in Hydrogen Production from Aqueous Glycerol Photoreforming. Nanomaterials 2023, 13, 1511. https://doi.org/10.3390/nano13091511
Escamilla-Mejía JC, Hidalgo-Carrillo J, Martín-Gómez J, López-Tenllado FJ, Estévez R, Marinas A, Urbano FJ. Biochars from Olive Stones as Carbonaceous Support in Pt/TiO2-Carbon Photocatalysts and Application in Hydrogen Production from Aqueous Glycerol Photoreforming. Nanomaterials. 2023; 13(9):1511. https://doi.org/10.3390/nano13091511
Chicago/Turabian StyleEscamilla-Mejía, Juan Carlos, Jesús Hidalgo-Carrillo, Juan Martín-Gómez, Francisco J. López-Tenllado, Rafael Estévez, Alberto Marinas, and Francisco J. Urbano. 2023. "Biochars from Olive Stones as Carbonaceous Support in Pt/TiO2-Carbon Photocatalysts and Application in Hydrogen Production from Aqueous Glycerol Photoreforming" Nanomaterials 13, no. 9: 1511. https://doi.org/10.3390/nano13091511
APA StyleEscamilla-Mejía, J. C., Hidalgo-Carrillo, J., Martín-Gómez, J., López-Tenllado, F. J., Estévez, R., Marinas, A., & Urbano, F. J. (2023). Biochars from Olive Stones as Carbonaceous Support in Pt/TiO2-Carbon Photocatalysts and Application in Hydrogen Production from Aqueous Glycerol Photoreforming. Nanomaterials, 13(9), 1511. https://doi.org/10.3390/nano13091511













