Ligand Size and Carbon-Chain Length Study of Silver Carboxylates in Focused Electron-Beam-Induced Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Formula and Properties of Silver Carboxylates
2.2. Precursor Delivery
2.3. Deposition Process
2.4. Characterization
3. Results and Discussion
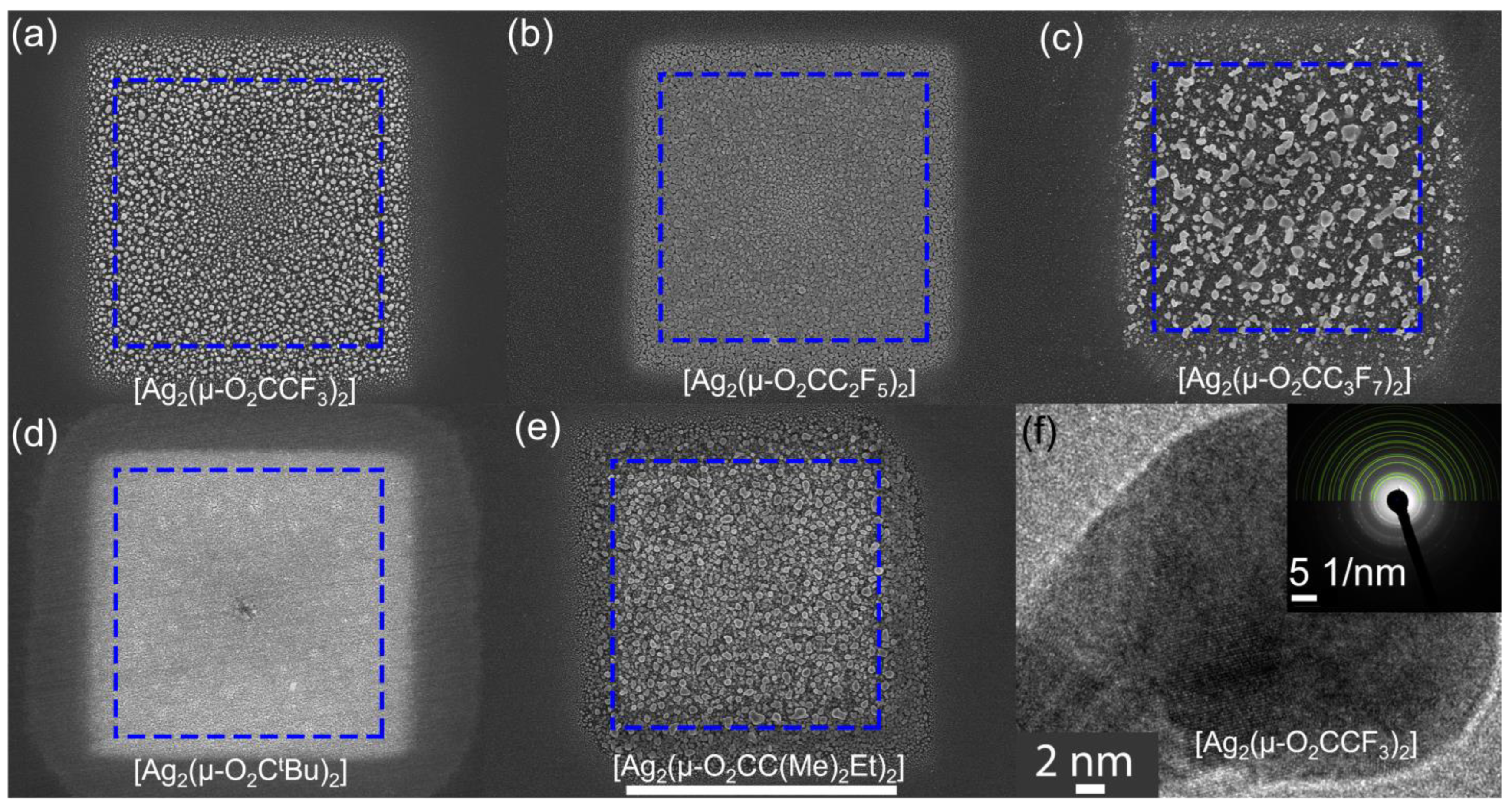
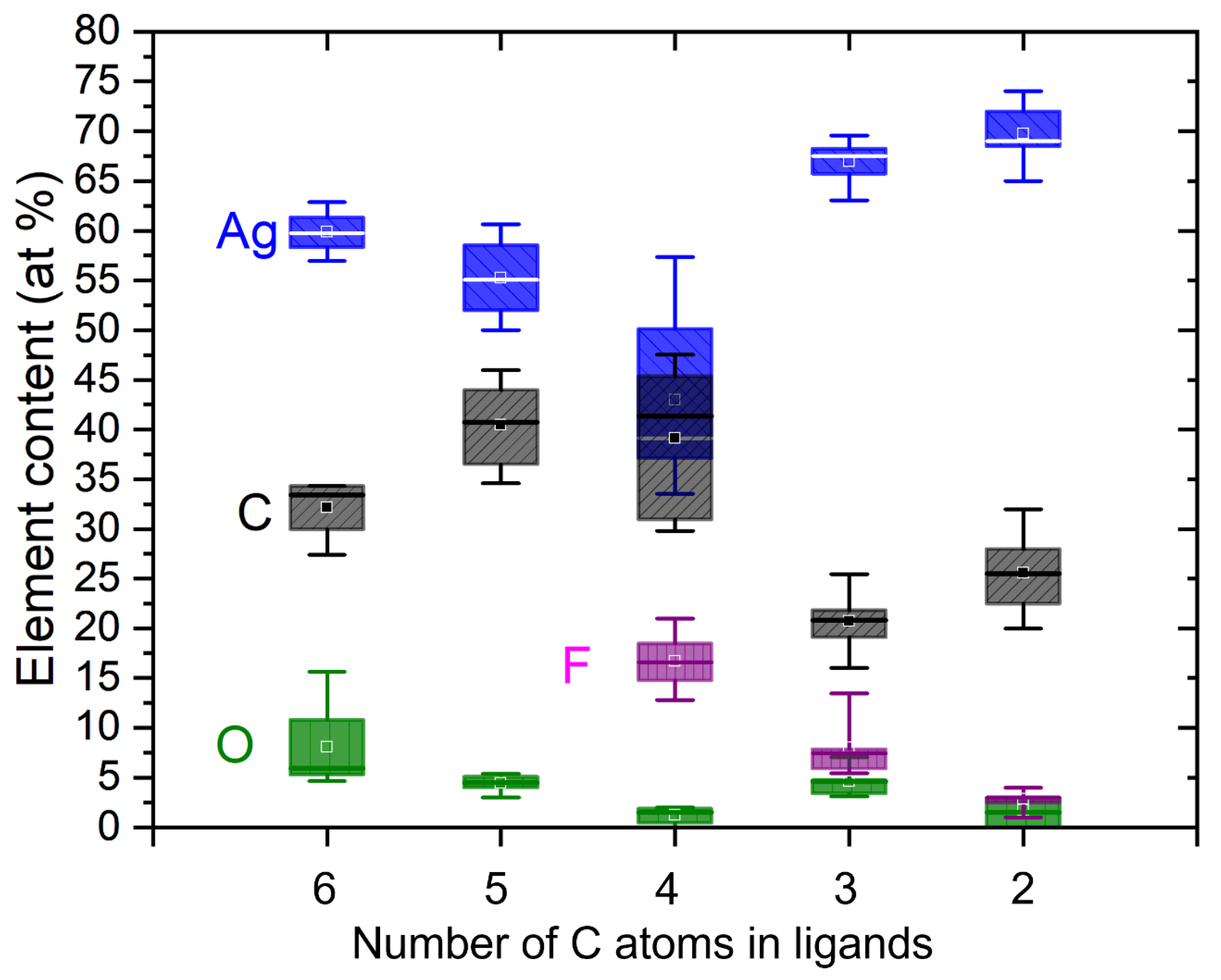
3.1. Morphology and Composition of Square Deposits
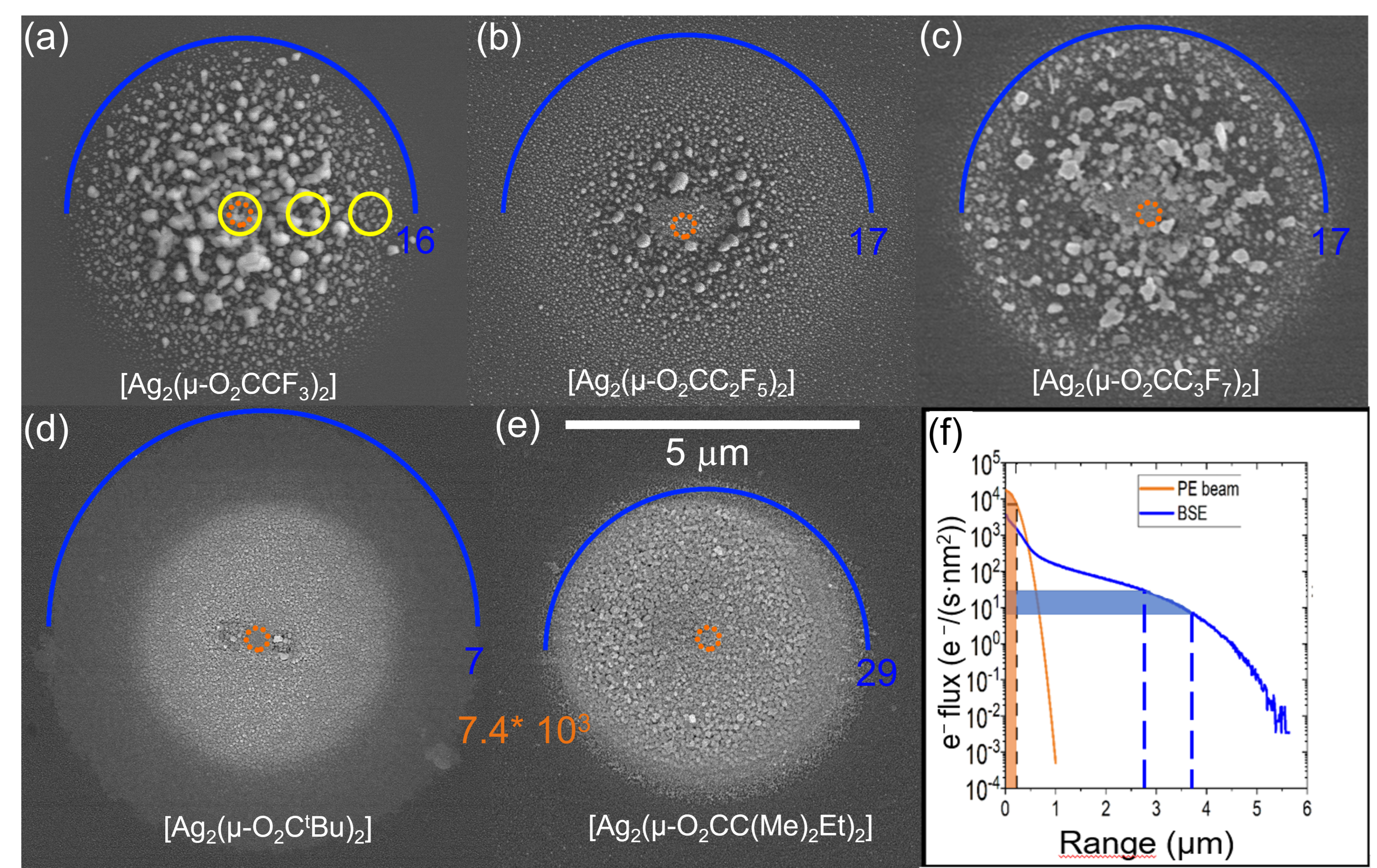
3.2. Morphology and Composition of Spot Deposits
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huth, M.; Porrati, F.; Dobrovolskiy, O.V. Focused electron beam induced deposition meets materials science. Microelectron. Eng. 2018, 185-186, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, C.; Hierro-Rodríguez, A.; Abert, C.; Witte, K.; Skoric, L.; Sanz-Hernández, D.; Finizio, S.; Meng, F.; McVitie, S.; Raabe, J.; et al. Complex free-space magnetic field textures induced by three-dimensional magnetic nanostructures. Nat. Nanotechnol. 2022, 17, 136–142. [Google Scholar] [CrossRef]
- Keller, L.; Al Mamoori, M.K.I.; Pieper, J.; Gspan, C.; Stockem, I.; Schröder, C.; Barth, S.; Winkler, R.; Plank, H.; Pohlit, M.; et al. Direct-write of free-form building blocks for artificial magnetic 3D lattices. Sci. Rep. 2018, 8, 6160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Pacheco, A.; Skoric, L.; De Teresa, J.M.; Pablo-Navarro, J.; Huth, M.; Dobrovolskiy, O.V. Writing 3D Nanomagnets Using Focused Electron Beams. Materials 2020, 13, 3774. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.; Schmidt, F.-P.; Haselmann, U.; Fowlkes, J.D.; Lewis, B.B.; Kothleitner, G.; Rack, P.D.; Plank, H. Direct-Write 3D Nanoprinting of Plasmonic Structures. ACS Appl. Mater. Interfaces 2017, 9, 8233–8240. [Google Scholar] [CrossRef]
- Höflich, K.; Feichtner, T.; Hansjürgen, E.; Haverkamp, C.; Kollmann, H.; Lienau, C.; Silies, M. Resonant behavior of a single plasmonic helix. Optica 2019, 6, 1098–1105. [Google Scholar] [CrossRef]
- Woźniak, P.; De Leon, I.; Höflich, K.; Leuchs, G.; Banzer, P. Interaction of light carrying orbital angular momentum with a chiral dipolar scatterer. Optica 2019, 6, 961–965. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, F.; Das, G.; Candeloro, P.; Patrini, M.; Galli, M.; Bek, A.; Lazzarino, M.; Maksymov, I.; Liberale, C.; Andreani, L.C.; et al. Nanoscale chemical mapping using three-dimensional adiabatic compression of surface plasmon polaritons. Nat. Nanotechnol. 2010, 5, 67–72. [Google Scholar] [CrossRef]
- Utke, I.; Jenke, M.G.; Röling, C.; Thiesen, P.H.; Iakovlev, V.; Sirbu, A.; Mereuta, A.; Caliman, A.; Kapon, E. Polarisation stabilisation of vertical cavity surface emitting lasers by minimally invasive focused electron beam triggered chemistry. Nanoscale 2011, 3, 2718–2722. [Google Scholar] [CrossRef]
- Wen, F.; Ye, J.; Liu, N.; Van Dorpe, P.; Nordlander, P.; Halas, N.J. Plasmon Transmutation: Inducing New Modes in Nanoclusters by Adding Dielectric Nanoparticles. Nano Lett. 2012, 12, 5020–5026. [Google Scholar] [CrossRef] [PubMed]
- Gabureac, M.; Bernau, L.; Utke, I.; Boero, G. Granular Co–C nano-Hall sensors by focused-beam-induced deposition. Nanotechnology 2010, 21, 115503. [Google Scholar] [CrossRef] [Green Version]
- Arnold, G.; Winkler, R.; Stermitz, M.; Orthacker, A.; Noh, J.; Fowlkes, J.D.; Kothleitner, G.; Huth, M.; Rack, P.D.; Plank, H. Tunable 3D Nanoresonators for Gas-Sensing Applications. Adv. Funct. Mater. 2018, 28, 1707387. [Google Scholar] [CrossRef]
- Dukic, M.; Winhold, M.; Schwalb, C.H.; Adams, J.D.; Stavrov, V.; Huth, M.; Fantner, G.E. Direct-write nanoscale printing of nanogranular tunnelling strain sensors for sub-micrometre cantilevers. Nat. Commun. 2016, 7, 12487. [Google Scholar] [CrossRef] [Green Version]
- Di Prima, G.; Sachser, R.; Trompenaars, P.; Mulders, H.; Huth, M. Direct-write single electron transistors by focused electron beam induced deposition. Nano Futures 2019, 3, 025001. [Google Scholar] [CrossRef]
- Baranowski, M.; Sachser, R.; Marinković, B.P.; Ivanović, S.D.; Huth, M. Charge Transport inside TiO2 Memristors Prepared via FEBID. Nanomaterials 2022, 12, 4145. [Google Scholar] [CrossRef]
- Qian, W.; Sun, S.; Song, J.; Nguyen, C.; Ducharme, S.; A Turner, J. Focused electron-beam-induced deposition for fabrication of highly durable and sensitive metallic AFM-IR probes. Nanotechnology 2018, 29, 335702. [Google Scholar] [CrossRef] [PubMed]
- Plank, H.; Winkler, R.; Schwalb, C.H.; Hütner, J.; Fowlkes, J.D.; Rack, P.D.; Utke, I.; Huth, M. Focused Electron Beam-Based 3D Nanoprinting for Scanning Probe Microscopy: A Review. Micromachines 2020, 11, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pablo-Navarro, J.; Sangiao, S.; Magén, C.; de Teresa, J.M. Magnetic Functionalization of Scanning Probes by Focused Electron Beam Induced Deposition Technology. Magnetochemistry 2021, 7, 140. [Google Scholar] [CrossRef]
- Bret, T.; Hofmann, T.; Edinger, K. Industrial perspective on focused electron beam-induced processes. Appl. Phys. A Mater. Sci. Process. 2014, 117, 1607–1614. [Google Scholar] [CrossRef]
- Barth, S.; Huth, M.; Jungwirth, F. Precursors for direct-write nanofabrication with electrons. J. Mater. Chem. C 2020, 8, 15884–15919. [Google Scholar] [CrossRef]
- Utke, I.; Swiderek, P.; Höflich, K.; Madajska, K.; Jurczyk, J.; Martinović, P.; Szymańska, I. Coordination and organometallic precursors of group 10 and 11: Focused electron beam induced deposition of metals and insight gained from chemical vapour deposition, atomic layer deposition, and fundamental surface and gas phase studies. Co-ord. Chem. Rev. 2022, 458, 213851. [Google Scholar] [CrossRef]
- Utke, I.; Hoffmann, P.; Melngailis, J. Gas-assisted focused electron beam and ion beam processing and fabrication. J. Vac. Sci. Technol. B 2008, 26, 1197–1276. [Google Scholar] [CrossRef] [Green Version]
- Geier, B.; Gspan, C.; Winkler, R.; Schmied, R.; Fowlkes, J.D.; Fitzek, H.; Rauch, S.; Rattenberger, J.; Rack, P.D.; Plank, H. Rapid and Highly Compact Purification for Focused Electron Beam Induced Deposits: A Low Temperature Approach Using Electron Stimulated H2O Reactions. J. Phys. Chem. C 2014, 118, 14009–14016. [Google Scholar] [CrossRef]
- Shawrav, M.M.; Taus, P.; Wanzenboeck, H.D.; Schinnerl, M.; Stöger-Pollach, M.; Schwarz, S.; Steiger-Thirsfeld, A.; Bertagnolli, E. Highly conductive and pure gold nanostructures grown by electron beam induced deposition. Sci. Rep. 2016, 6, 34003. [Google Scholar] [CrossRef] [Green Version]
- Plank, H.; Noh, J.H.; Fowlkes, J.D.; Lester, K.; Lewis, B.B.; Rack, P.D. Electron-Beam-Assisted Oxygen Purification at Low Temperatures for Electron-Beam-Induced Pt Deposits: Towards Pure and High-Fidelity Nanostructures. ACS Appl. Mater. Interfaces 2014, 6, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.; Stanford, M.; Fowlkes, J.; Lester, K.; Plank, H.; Rack, P. Electron-stimulated purification of platinum nanostructures grown via focused electron beam induced deposition. Beilstein J. Nanotechnol 2015, 6, 907–918. [Google Scholar] [CrossRef] [Green Version]
- Puydinger dos Santos, M.V.; Velo, M.F.; Domingos, R.D.; Zhang, Y.; Maeder, X.; Guerra-Nuñez, C.; Best, P.J.; Béron, F.; Pirota, K.F.; Moshkalev, S.; et al. Annealing-Based Electrical Tuning of Cobalt–Carbon Deposits Grown by Fo-cused-Electron-Beam-Induced Deposition. ACS Appl. Mater. Interfaces 2016, 8, 32496–32503. [Google Scholar] [CrossRef] [PubMed]
- dos Santos MV, P.; Szkudlarek, A.; Rydosz, A.; Guerra-Nuñez, C.; Béron, F.; Pirota, K.R.; Moshkalev, S.; Diniz, J.A.; Utke, I. Comparative study of post-growth annealing of Cu(hfac)2, Co2(CO)8 and Me2Au(acac) metal precursors deposited by FEBID. Beilstein J. Nanotechnol. 2018, 9, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkudlarek, A.; Vaz, A.R.; Zhang, Y.; Rudkowski, A.; Kapusta, C.; Erni, R.; Moshkalev, S.; Utke, I. Formation of pure Cu nanocrystals upon post-growth annealing of Cu–C material obtained from focused electron beam induced deposition: Comparison of different methods. Beilstein J. Nanotechnol. 2015, 6, 1508–1517. [Google Scholar] [CrossRef]
- Begun, E.; Dobrovolskiy, O.V.; Kompaniiets, M.; Sachser, R.; Gspan, C.; Plank, H.; Huth, M. Post-growth purification of Co nanostructures prepared by focused electron beam induced deposition. Nanotechnology 2015, 26, 075301. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskiy, O.V.; Kompaniiets, M.; Sachser, R.; Porrati, F.; Gspan, C.; Plank, H.; Huth, M. Tunable magnetism on the lateral mesoscale by post-processing of Co/Pt heterostructures. Beilstein J. Nanotechnol. 2015, 6, 1082–1090. [Google Scholar] [CrossRef]
- Jurczyk, J.; Brewer, C.R.; Hawkins, O.M.; Polyakov, M.N.; Kapusta, C.; McElwee-White, L.; Utke, I. Focused Electron Beam-Induced Deposition and Post-Growth Purification Using the Heteroleptic Ru Complex (η3-C3H5)Ru(CO)3Br. ACS Appl. Mater. Interfaces 2019, 11, 28164–28171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, L.; Jurczyk, J.; Madajska, K.; Edwards, T.E.J.; Szymańska, I.; Hoffmann, P.; Utke, I. High-Purity Copper Structures from a Perfluorinated Copper Carboxylate Using Focused Electron Beam Induced Deposition and Post-Purification. ACS Appl. Electron. Mater. 2020, 2, 1989–1996. [Google Scholar] [CrossRef]
- Höflich, K.; Yang, R.B.; Berger, A.; Leuchs, G.; Christiansen, S. The Direct Writing of Plasmonic Gold Nanostructures by Electron-Beam-Induced Deposition. Adv. Mater. 2011, 23, 2657–2661. [Google Scholar] [CrossRef]
- Botman, A.; Mulders, J.J.L.; Hagen, C.W. Creating pure nanostructures from electron-beam-induced deposition using puri-fication techniques: A technology perspective. Nanotechnology 2009, 20, 372001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, L.; Jurczyk, J.; Madajska, K.; Szymańska, I.B.; Hoffmann, P.; Utke, I. Room Temperature Direct Electron Beam Lithography in a Condensed Copper Carboxylate. Micromachines 2021, 12, 580. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.; Madajska, K.; Szymańska, I.B.; Hoflich, K.; Polyakov, M.N.; Jurczyk, J.; Guerra-Nuñez, C.; Utke, I. Gas-assisted silver deposition with a focused electron beam. Beilstein J. Nanotechnol. 2018, 9, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Höflich, K.; Jurczyk, J.M.; Madajska, K.; Götz, M.; Berger, L.; Guerra-Nuñez, C.; Haverkamp, C.; Szymanska, I.; Utke, I. Towards the third dimension in direct electron beam writing of silver. Beilstein J. Nanotechnol. 2018, 9, 842–849. [Google Scholar] [CrossRef] [Green Version]
- Höflich, K.; Jurczyk, J.; Zhang, Y.; dos Santos, M.V.P.; Götz, M.; Guerra-Nuñez, C.; Best, J.P.; Kapusta, C.; Utke, I. Direct Electron Beam Writing of Silver-Based Nanostructures. ACS Appl. Mater. Interfaces 2017, 9, 24071–24077. [Google Scholar] [CrossRef] [Green Version]
- Szymańska, I.B. Influence of the gas phase composition on the properties of bimetallic Ag/Cu nanomaterials obtained via chemical vapor deposition. Polyhedron 2013, 65, 82–88. [Google Scholar] [CrossRef]
- Szymańska, I.B. Gaseous phase studies of new copper(II) carboxylate complexes with tert-butylamine as potential precursors for chemical vapor deposition (CVD). Polyhedron 2013, 50, 200–207. [Google Scholar] [CrossRef]
- Piszczek, P.; Szymańska, I.B.; Talik, E.; Heimann, J. Deposition of Thin Copper Layers using Copper(II) Carboxylate Complexes with tert-Butylamine as New CVD Precursors. Chem. Vap. Depos. 2013, 19, 251–259. [Google Scholar] [CrossRef]
- Szymańska, I.B.; Piszczek, P.; Szłyk, E. Gas phase studies of new copper(I) carboxylates compounds with vinylsilanes and their application in Chemical Vapor Deposition (CVD). Polyhedron 2009, 28, 721–728. [Google Scholar] [CrossRef]
- Szłyk, E.; Piszczek, P.; Grodzicki, A.; Chaberski, M.; Goliński, A.; Szatkowski, J.; Błaszczyk, T. CVD of AgI Complexes with Tertiary Phosphines and Perfluorinated Carboxylates—A New Class of Silver Precursors. Chem. Vap. Depos. 2001, 7, 111–116. [Google Scholar] [CrossRef]
- Martinović, P.; Rohdenburg, M.; Butrymowicz, A.; Sarigül, S.; Huth, P.; Denecke, R.; Szymańska, I.B.; Swiderek, P. Electron-Induced Decomposition of Different Silver(I) Complexes: Implications for the Design of Precursors for Focused Electron Beam Induced Deposition. Nanomaterials 2022, 12, 1687. [Google Scholar] [CrossRef]
- Blakeslee, A.E.; Hoard, J.L. The Structure of Silver Perfluorobutyrate1. J. Am. Chem. Soc. 1956, 78, 3029–3033. [Google Scholar] [CrossRef]
- Kuzmina, N.; Paramonov, S.; Ivanov, R.; Kezko, V.; Polamo, K.; Troyanov, S. Silver pivalate as a new volatile precursor for thin film deposition. J. Phys. IV France 1999, 9, Pr8-923–Pr8-928. [Google Scholar] [CrossRef]
- Szymańska, I.; Piszczek, P.; Szczęsny, R.; Szłyk, E. Thermal and MS studies of silver(I) 2,2-dimethylbutyrate complexes with tertiary phosphines and their ap-plication for CVD of silver films. Polyhedron 2007, 26, 2440–2448. [Google Scholar] [CrossRef]
- Szłyk, E.; Łakomska, I.; Grodzicki, A. Thermal and spectroscopic studies of the Ag(I) salts with fluorinated carboxylic and sulfonic acid residues. Thermochim. Acta 1993, 223, 207–212. [Google Scholar] [CrossRef]
- Jurczyk, J.; Glessi, C.; Madajska, K.; Berger, L.; Nyrud, J.I.K.; Szymańska, I.; Kapusta, C.; Tilset, M.; Utke, I. Vacuum versus ambient pressure inert gas thermogravimetry: A study of silver carboxylates. J. Therm. Anal. Calorim. 2021, 147, 2187–2195. [Google Scholar] [CrossRef]
- Friedli, V.; Utke, I. GIS Simulator; EMPA (Thun, Switzerland): 2015. Available online: https://www.empa.ch/web/s206/febip-codes (accessed on 20 December 2017).
- Friedli, V.; Utke, I. Optimized molecule supply from nozzle-based gas injection systems for focused electron- and ion-beam induced deposition and etching: Simulation and experiment. J. Phys. D: Appl. Phys. 2009, 42, 125305. [Google Scholar] [CrossRef]
- Hodoroaba, V.-D.; Kim, K.J.; Unger, W.E.S. Energy dispersive electron probe microanalysis (ED-EPMA) of elemental com-position and thickness of Fe-Ni alloy films. Surf. Interface Anal. 2012, 44, 1459–1461. [Google Scholar] [CrossRef]
- StrataGEM. SamX: Rue Galilée, 78280 Guyancourt, France Information. Available online: http://www.samx.com/microanalysis/products/stratagem_us.html (accessed on 3 June 2017).
- Jurczyk, J. Study of novel precursors for Focused Electron Beam Induced Deposition of Metal Nanowires. In Faculty of Physics and Applied Computer Sciences; AGH University of Science and Technology: Kraków, Poland, 2021. [Google Scholar]
- Bresin, M.; Botman, A.; Randolph, S.J.; Straw, M.; Hastings, J.T. Liquid Phase Electron-Beam-Induced Deposition on Bulk Substrates Using Environmental Scanning Electron Microscopy. Microsc. Microanal. 2014, 20, 376–384. [Google Scholar] [CrossRef]
- Toth, M.; Lobo, C.; Friedli, V.; Szkudlarek, A.; Utke, I. Continuum models of focused electron beam induced processing. Beilstein J. Nanotechnol. 2015, 6, 1518–1540. [Google Scholar] [CrossRef]
- Gommes, C.J. Ostwald ripening of confined nanoparticles: Chemomechanical coupling in nanopores. Nanoscale 2019, 11, 7386–7393. [Google Scholar] [CrossRef]
- Demers, H.; Poirier-Demers, N.; Couture, A.R.; Joly, D.; Guilmain, M.; de Jonge, N.; Drouin, D. Three-dimensional electron microscopy simulation with the CASINO Monte Carlo software. Scanning 2011, 33, 135–146. [Google Scholar] [CrossRef]
- Sanz-Hernández, D.; Fernández-Pacheco, A. Modelling focused electron beam induced deposition beyond Langmuir adsorption. Beilstein J. Nanotechnol. 2017, 8, 2151–2161. [Google Scholar] [CrossRef] [Green Version]
- Sheng, H.; Zheng, H.; Jia, S.; Chan, M.K.Y.; Rajh, T.; Wang, J.; Wen, J. Atomistic manipulation of reversible oxidation and reduction in Ag with an electron beam. Nanoscale 2019, 11, 10756–10762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, P.; Dwir, B.; Leifer, K.; Kapon, E.; Doppelt, P. Focused electron beam induced deposition of gold. J. Vac. Sci. Technol. B 2000, 18, 3168–3171. [Google Scholar] [CrossRef]
- Qi, W.H.; Wang, M.P. Size and shape dependent lattice parameters of metallic nanoparticles. J. Nanoparticle Res. 2005, 7, 51–57. [Google Scholar] [CrossRef]

| Precursor | GIS (°C) | Q (1014 Molecules/s) | J (1015 Molecules/(s·cm2)) | M (g/mol) |
|---|---|---|---|---|
| [Ag2(µ-O2CCF3)2] | 170–185 °C | 2.8–2.9 | 1.1–1.2 | 221 |
| [Ag2(µ-O2CC2F5)2] | 150–180 °C | 3.8–5.2 | 1.4–2.0 | 271 |
| [Ag2(µ-O2CC3F7)2] | 175–190 °C | 4.4–6.0 | 1.7–2.4 | 321 |
| [Ag2(µ-O2CtBu)2] | 170–190 °C | 2.2–4.9 | 0.9–2.0 | 209 |
| [Ag2(µ-O2CC(Me)2Et)2] | 145–175 °C | 0.2–11.0 | 0.1–4.5 | 222 |
| Precursor | Precursor Stoichiometry Ag:O:C:F:H | Atomic Ratio of Best Purity Ag:O:C:F | Best Ag (at.%) | Atomic Ratio Average Ag:O:C:F | Average Ag (at.%) |
|---|---|---|---|---|---|
| [Ag2(µ-O2CCF3)2] | 1:2:2:3:0 | 1:0.02:0.29:0.03 | >74 | 1:0.03:0.36:0.04 | >70 |
| [Ag2(µ-O2CC2F5)2] | 1:2:3:5:0 | 1:0.04:0.27:0.11 | >70 | 1:0.08:0.41:0.10 | >66 |
| [Ag2(µ-O2CC3F7)2] | 1:2:4:7:0 | 1:0:0.52:0.22 | >57 | 1:0.02:0.91:0.40 | >43 |
| [Ag2(µ-O2CtBu)2] | 1:2:5:0:9 | 1:0.08:0.56:0 | >61 | 1:0.07:0.75:0 | >55 |
| [Ag2(µ-O2CC(Me)2Et)2] | 1:2:6:0:11 | 1:0.08:0.51:0 | >63 | 1:0.12:0.60:0 | >58 |
| Ag:O:C:F | Centre | Halo | Periphery |
|---|---|---|---|
| [Ag2(µ-O2CCF3)2] | 73:0:27:0 | 76:0:24:0 | 56:0:43:1 |
| [Ag2(µ-O2CC2F5)2] | 45:1:51:3 | 46:0:51:3 | 35:1:58:6 |
| [Ag2(µ-O2CC3F7)2] | 50:0:49:1 | 60:0:39:1 | 58:0:41:1 |
| [Ag2(µ-O2CtBu)2] | 38:7:55:0 | 65:2:33:0 | 43:12:45:0 |
| [Ag2(µ-O2CC(Me)2Et)2] | 42:7:51:0 * | 70:7:23:0 * | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurczyk, J.; Höflich, K.; Madajska, K.; Berger, L.; Brockhuis, L.; Edwards, T.E.J.; Kapusta, C.; Szymańska, I.B.; Utke, I. Ligand Size and Carbon-Chain Length Study of Silver Carboxylates in Focused Electron-Beam-Induced Deposition. Nanomaterials 2023, 13, 1516. https://doi.org/10.3390/nano13091516
Jurczyk J, Höflich K, Madajska K, Berger L, Brockhuis L, Edwards TEJ, Kapusta C, Szymańska IB, Utke I. Ligand Size and Carbon-Chain Length Study of Silver Carboxylates in Focused Electron-Beam-Induced Deposition. Nanomaterials. 2023; 13(9):1516. https://doi.org/10.3390/nano13091516
Chicago/Turabian StyleJurczyk, Jakub, Katja Höflich, Katarzyna Madajska, Luisa Berger, Leo Brockhuis, Thomas Edward James Edwards, Czesław Kapusta, Iwona B. Szymańska, and Ivo Utke. 2023. "Ligand Size and Carbon-Chain Length Study of Silver Carboxylates in Focused Electron-Beam-Induced Deposition" Nanomaterials 13, no. 9: 1516. https://doi.org/10.3390/nano13091516
APA StyleJurczyk, J., Höflich, K., Madajska, K., Berger, L., Brockhuis, L., Edwards, T. E. J., Kapusta, C., Szymańska, I. B., & Utke, I. (2023). Ligand Size and Carbon-Chain Length Study of Silver Carboxylates in Focused Electron-Beam-Induced Deposition. Nanomaterials, 13(9), 1516. https://doi.org/10.3390/nano13091516






