Microwave-Assisted Synthesis of MoS2/BiVO4 Heterojunction for Photocatalytic Degradation of Tetracycline Hydrochloride
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of MoS2 Nanosphere
2.3. Preparation of MoS2/BiVO4 Heterojunction
2.4. Characterization and Analysis Methods
2.5. Photocatalytic Activity Tests
2.6. PEC Spectra Measurements
3. Results and Discussion
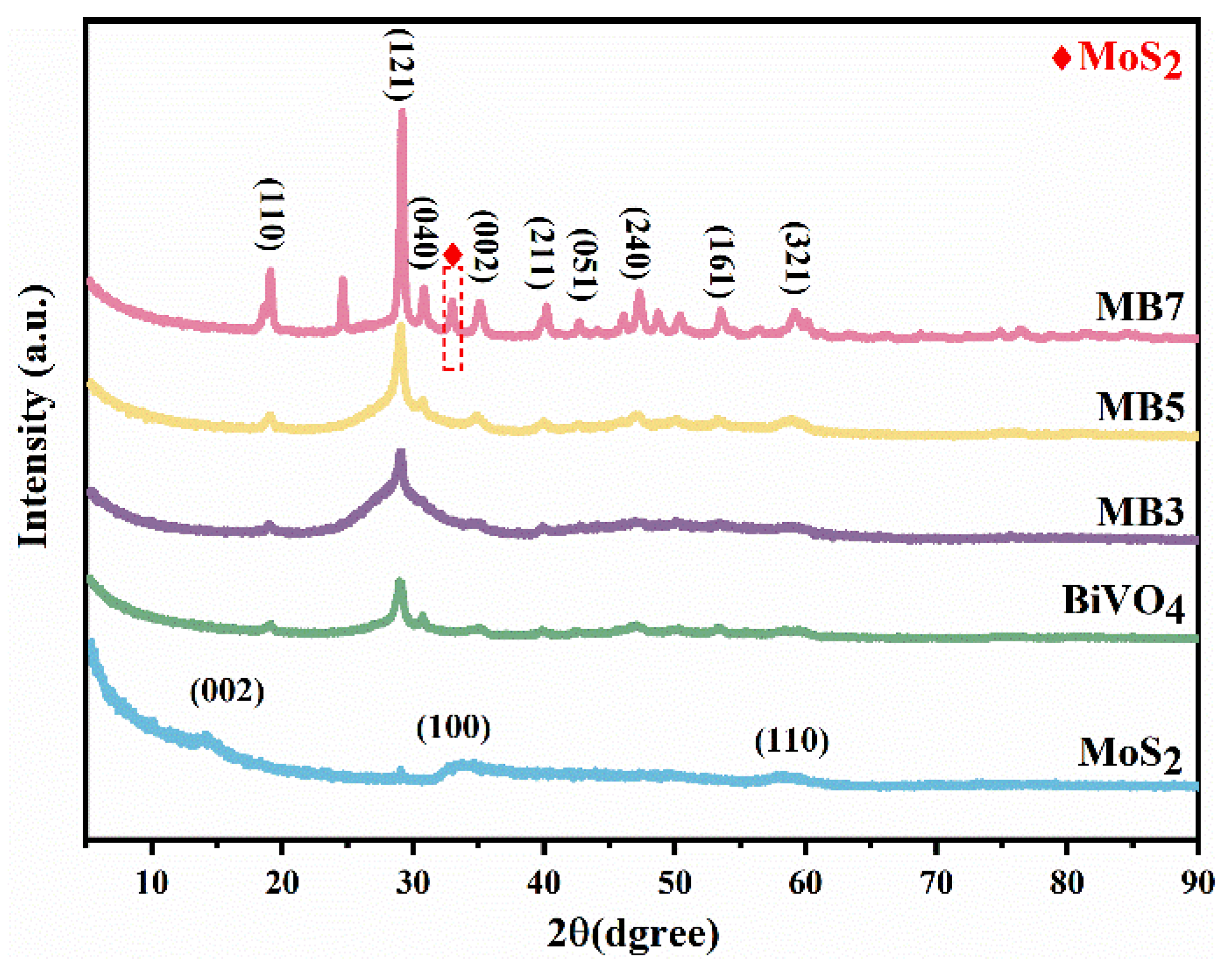
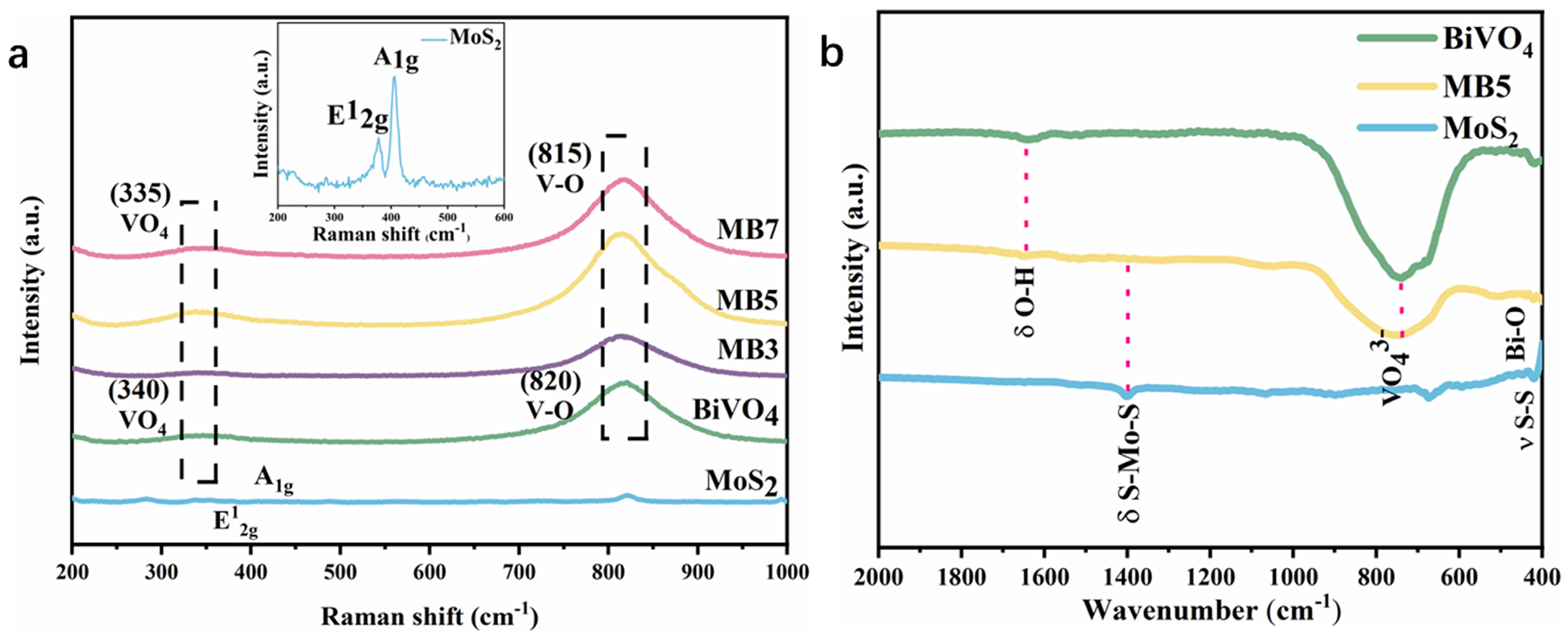
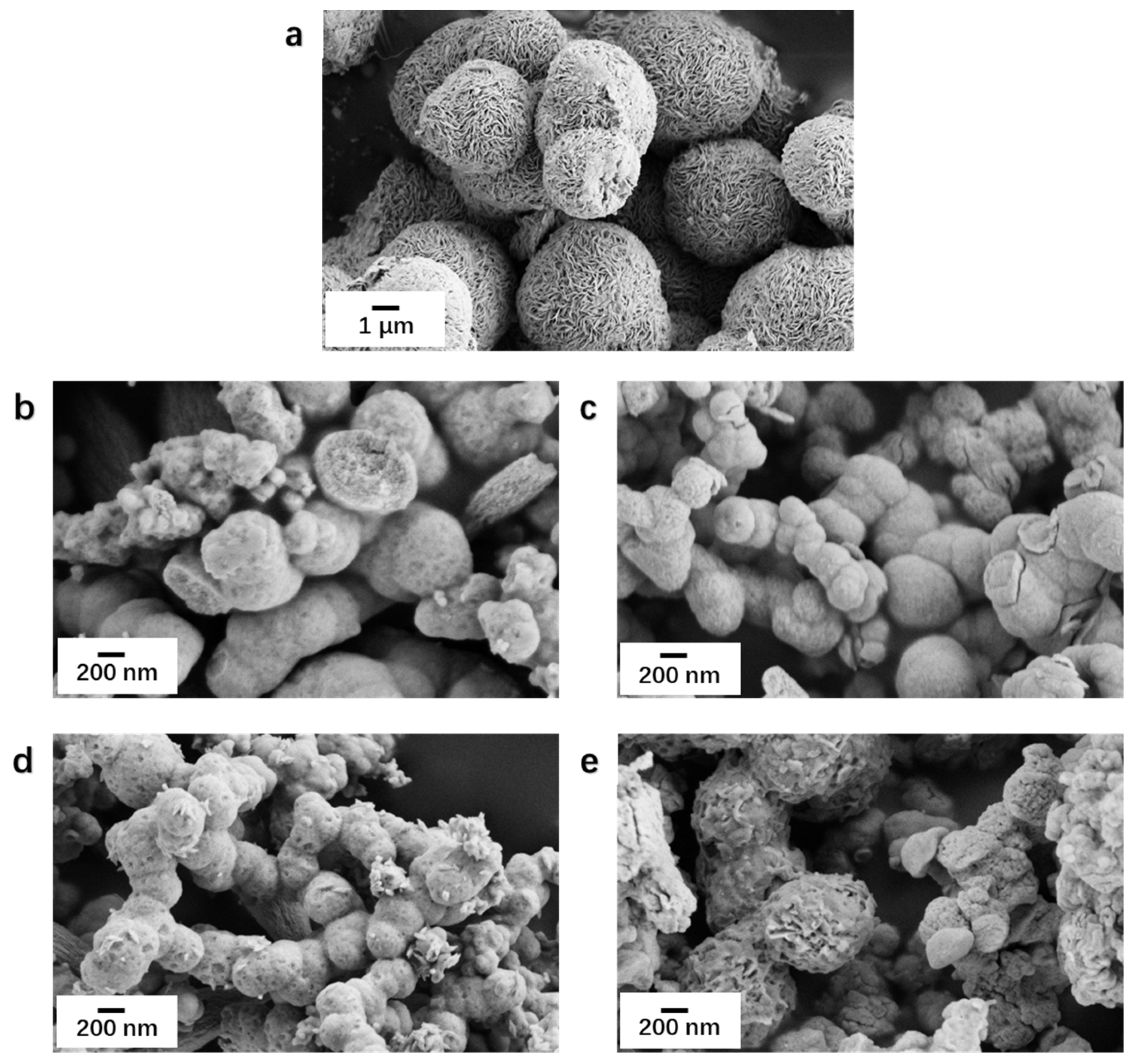
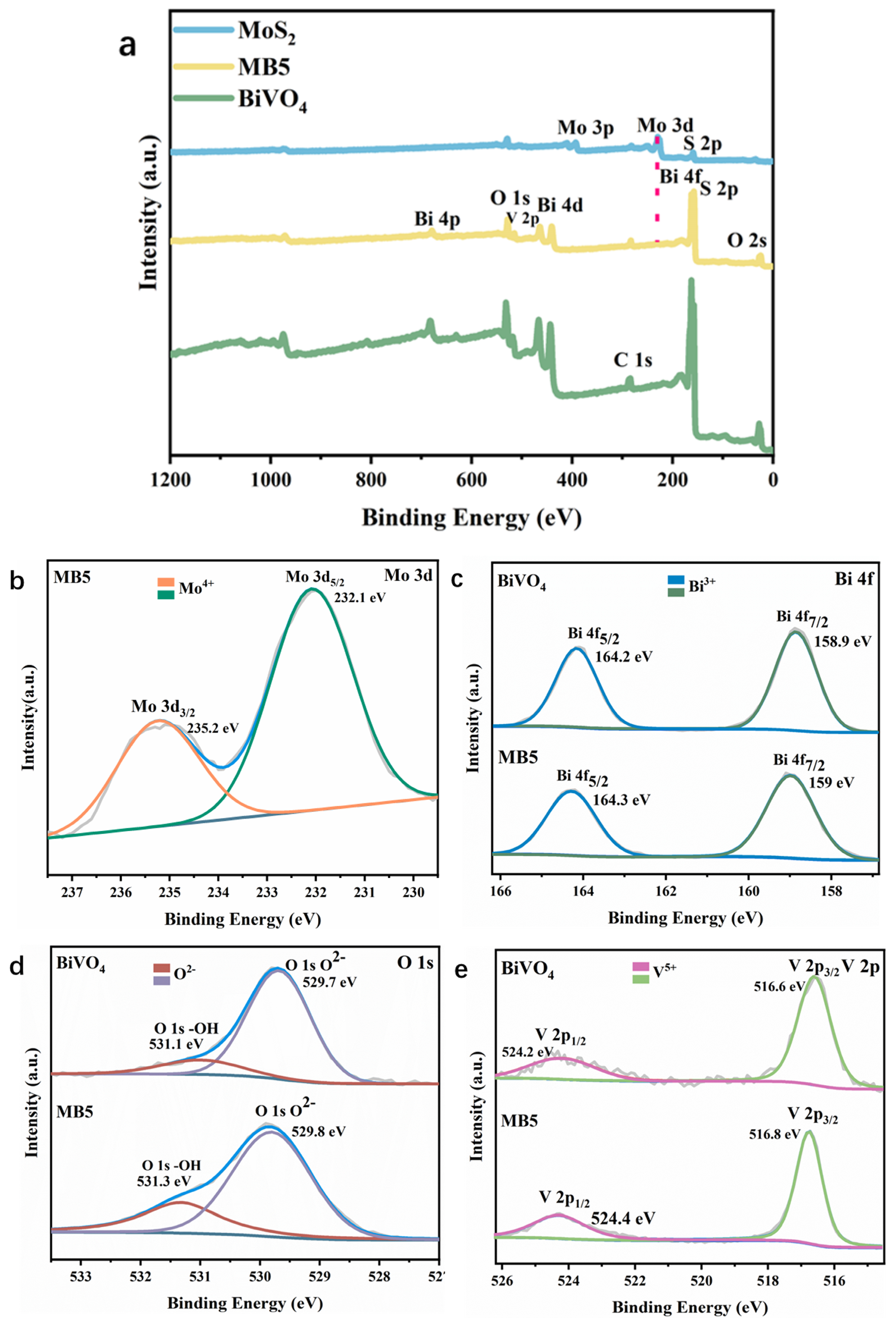
3.1. Characterization
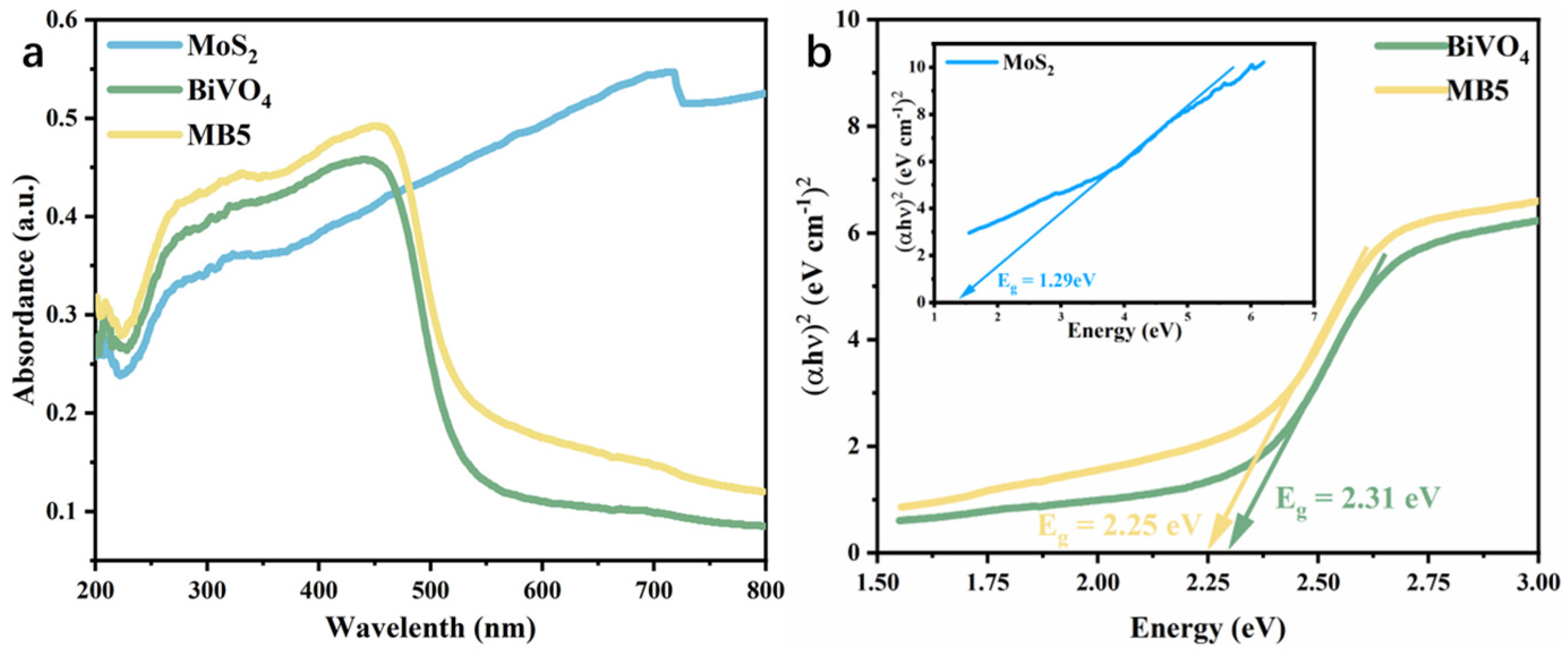
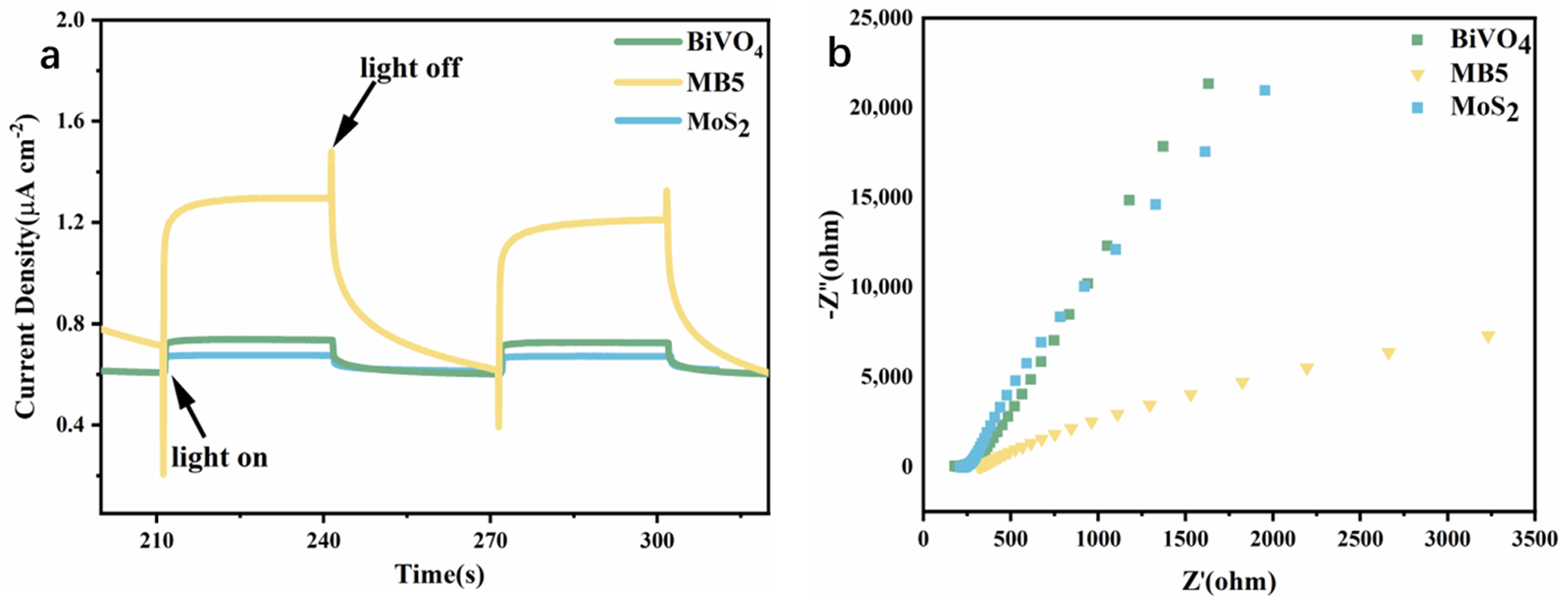
3.2. Light Absorption and Charge Transfer Performance
3.3. Catalytic Capacity of MoS2/BiVO4
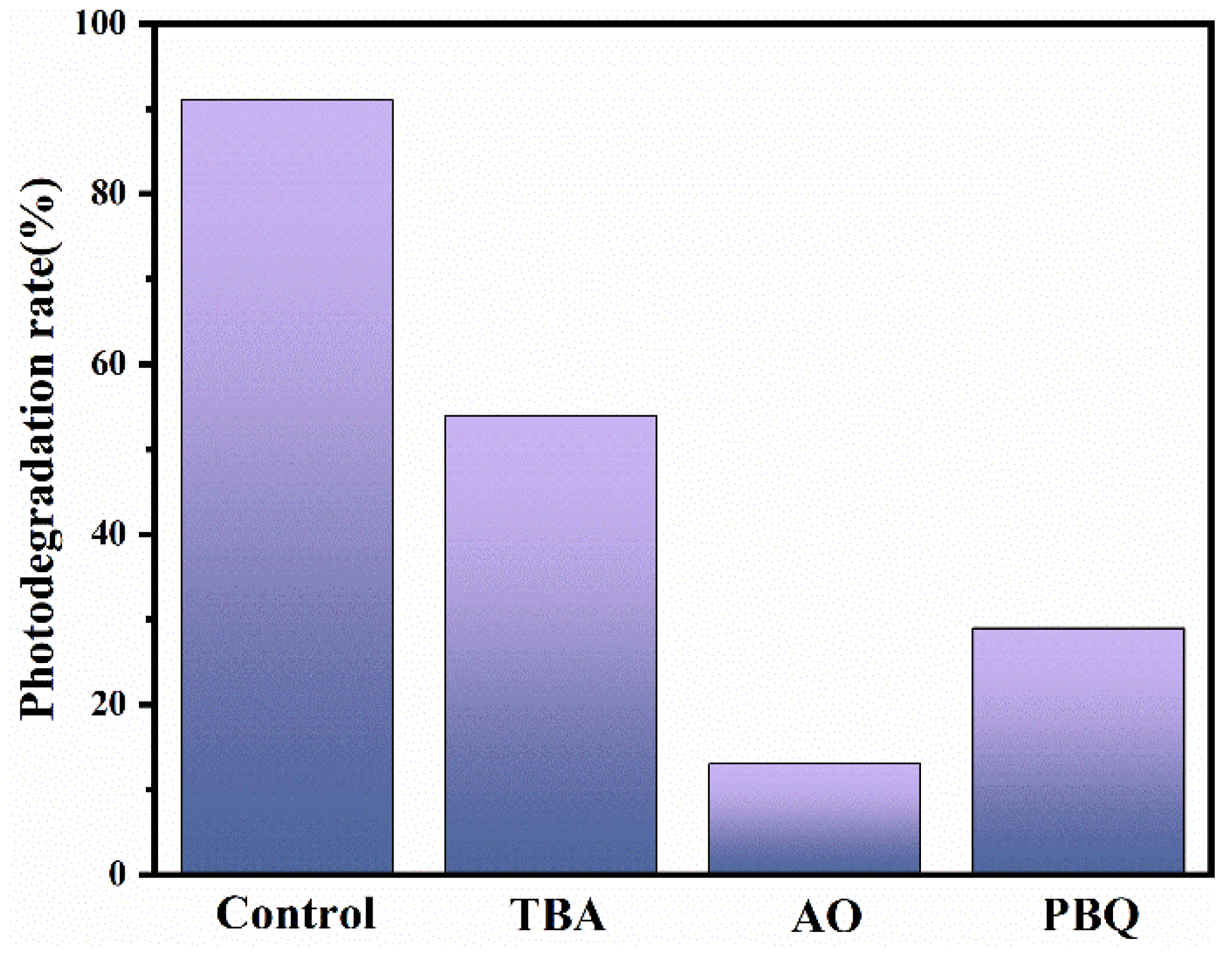
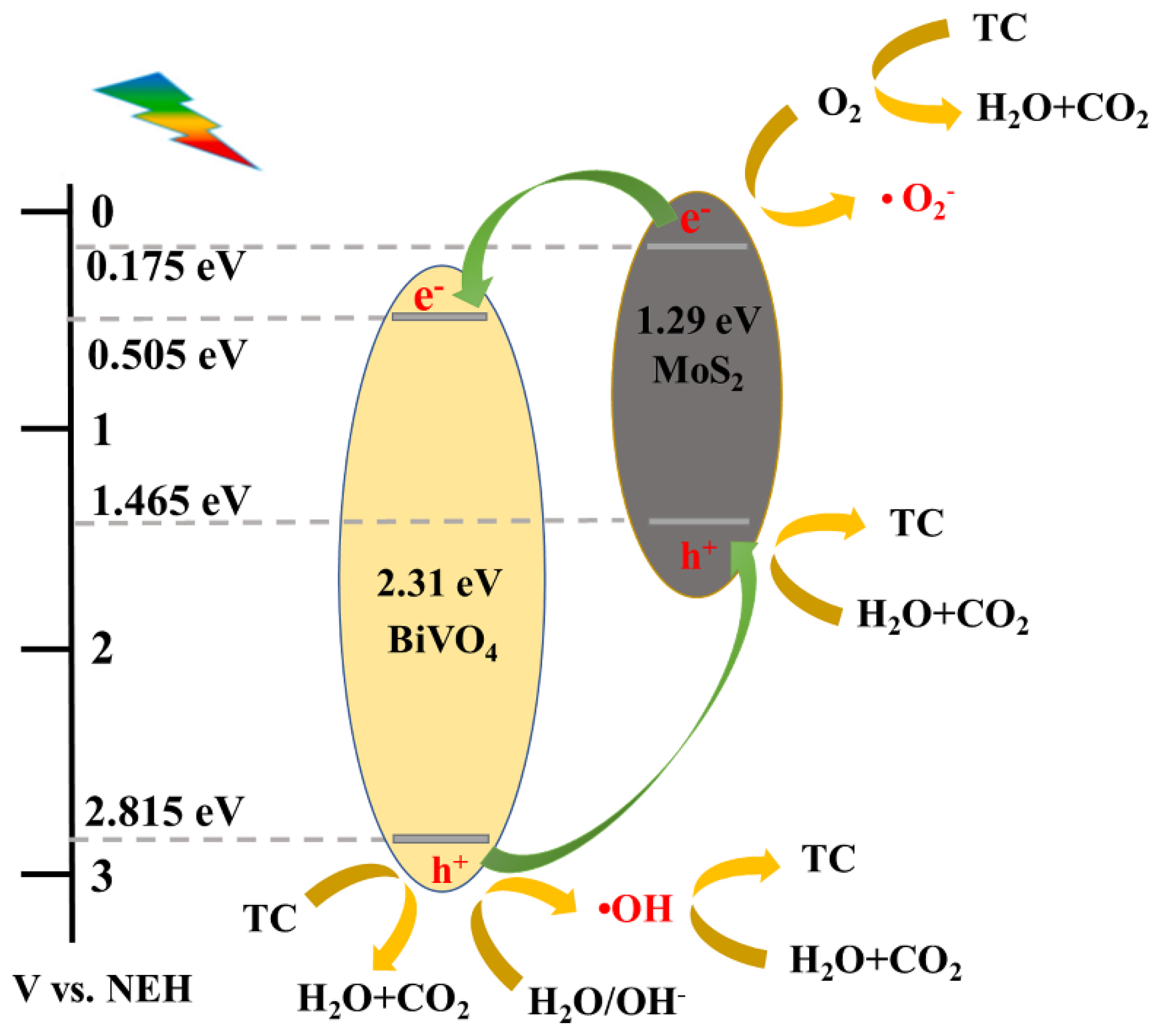
3.4. Photocatalytic Mechanism Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wolf, M.J.; Emerson, J.W.; Esty, D.C.; de Sherbinin, A.; Wendling, Z.A. 2020 Environmental Performance Index; Yale Cener for Environmental Law & Policy: New Haven, CT, USA, 2020. [Google Scholar]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664C, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ji, M.; Zhai, H.; Guo, Y.; Liu, Y. Occurrence of antibiotics and antibiotic resistance genes in WWTP effluent-receiving water bodies and reclaimed wastewater treatment plants. Sci. Total Environ. 2021, 796, 148919. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Liu, K.; Guo, Y.; Ding, C.; Han, J.; Li, P. Global review of macrolide antibiotics in the aquatic environment: Sources, occurrence, fate, ecotoxicity, and risk assessment. J. Hazard. Mater. 2022, 439, 129628. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, T.; Peng, H.; Zheng, X. Efficient Adsorption-Photocatalytic Removal of Tetracycline Hydrochloride over Octahedral MnS. Int. J. Mol. Sci. 2022, 23, 9343. [Google Scholar] [CrossRef]
- Yang, M.; Cui, C.; Liu, L.; Dai, L.; Bai, W.; Zhai, J.; Jiang, S.; Wang, W.; Ren, E.; Cheng, C.; et al. Porous activated carbons derived from bamboo pulp black liquor for effective adsorption removal of tetracycline hydrochloride and malachite green from water. Water Sci. Technol. 2022, 86, 244–260. [Google Scholar] [CrossRef]
- Zhou, Y.; You, S.; Zhang, J.; Wu, M.; Yan, X.; Zhang, C.; Liu, Y.; Qi, W.; Su, R.; He, Z. Copper ions binding regulation for the high-efficiency biodegradation of ciprofloxacin and tetracycline-HCl by low-cost permeabilized-cells. Bioresour. Technol. 2022, 344, 126297. [Google Scholar] [CrossRef]
- Tian, T.; Zhu, X.; Song, Z.; Li, X.; Zhang, W.; Mao, Y.; Chen, S.; Wu, J.; Ouyang, G. The potential of a natural iron ore residue application in the efficient removal of tetracycline hydrochloride from an aqueous solution: Insight into the degradation mechanism. Environ. Sci. Pollut. Res. Int. 2022, 29, 76782–76792. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Kudo, A.; Ueda, K.; Kato, H.; Mikami, I. Photocatalytic O2 evolution under visible light irradiation on BiVO4 in aqueous AgNO3 solution. Catal. Lett. 1998, 53, 229–230. [Google Scholar] [CrossRef]
- Shi, H.; Guo, H.; Wang, S.; Zhang, G.; Hu, Y.; Jiang, W.; Liu, G. Visible Light Photoanode Material for Photoelectrochemical Water Splitting: A Review of Bismuth Vanadate. Energy Fuels 2022, 36, 11404–11427. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.; Jia, Y.; Cheng, H.; Wang, L.; Wang, Q. Recent advances in bismuth vanadate-based photocatalysts for photoelectrochemical water splitting. Chin. Chem. Lett. 2021, 32, 1869–1878. [Google Scholar] [CrossRef]
- Zhong, X.; Li, Y.; Wu, H.; Xie, R. Recent progress in BiVO4-based heterojunction nanomaterials for photocatalytic applications. Mater. Sci. Eng. B 2023, 289, 116278. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Tao, F.; Dong, Y.; Wang, L.; Hong, T. Facile construction of Z-scheme g-C3N4/BiVO4 heterojunctions for boosting visible-light photocatalytic activity. Mater. Sci. Eng. B 2022, 279, 115676. [Google Scholar] [CrossRef]
- Guo, W.; Luo, H.; Jiang, Z.; Shangguan, W. In-situ pressure-induced BiVO4/Bi0.6Y0.4VO4 S-scheme heterojunction for enhanced photocatalytic overall water splitting activity. Chin. J. Catal. 2022, 43, 316–328. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Y.; Liu, D.; Ding, H.; Mamba, B.B.; Kuvarega, A.T.; Gui, J. Fabrication of a La-doped BiVO4@CN step-scheme heterojunction for effective tetracycline degradation with dual-enhanced molecular oxygen activation. Sep. Purif. Technol. 2021, 277, 119224. [Google Scholar] [CrossRef]
- Hu, C.; Tian, M.; Wu, L.; Chen, L. Enhanced photocatalytic degradation of paraben preservative over designed g-C3N4/BiVO4 S-scheme system and toxicity assessment. Ecotoxicol. Environ. Saf. 2022, 231, 113175. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, B.; Guo, H.; Wang, H.; Shi, Q.; Xu, M.; Yang, M.; Luo, X.; Wang, L. Reclaimable MoS2 Sponge Absorbent for Drinking Water Purification Driven by Solar Energy. Environ. Sci. Technol. 2022, 56, 11718–11728. [Google Scholar] [CrossRef]
- Liu, H.; Wu, R.; Zhang, H.; Ma, M. Microwave Hydrothermal Synthesis of 1T@2H−MoS2 as an Excellent Photocatalyst. Chem-catchem 2019, 12, 893–902. [Google Scholar] [CrossRef]
- Li, H.; Yu, K.; Lei, X.; Guo, B.; Fu, H.; Zhu, Z. Hydrothermal Synthesis of Novel MoS2/BiVO4 Hetero-Nanoflowers with Enhanced Photocatalytic Activity and a Mechanism Investigation. J. Phys. Chem. C 2015, 119, 22681–22689. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Jang, W.Y.; Rao, M.C.; Arumugam, M.; Shim, J. Novel BiVO4-nanosheet-supported MoS2-nanoflake-heterostructure with synergistic enhanced photocatalytic removal of tetracycline under visible light irradiation. Chemosphere 2022, 305, 135465. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Ng, Y.H.; Tang, Y.; Li, Y.; Chen, W.; Wang, J.; Li, X.; Li, L. Visible-light-driven photoelectrocatalytic activation of chloride by nanoporous MoS2@BiVO4 photoanode for enhanced degradation of bisphenol A. Chemosphere 2021, 263, 128279. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Tu, W.; Shen, S.; Lin, Z.; Gao, N.; Zhong, W. BiVO4@MoS2 core-shell heterojunction with improved photocatalytic activity for discoloration of Rhodamine B. Appl. Surf. Sci. 2020, 528, 146949. [Google Scholar] [CrossRef]
- Ju, P.; Hao, L.; Zhang, Y.; Sun, J.; Dou, K.; Lu, Z.; Liao, D.; Zhai, X.; Sun, C. Facile fabrication of a novel spindlelike MoS2/BiVO4 Z-scheme heterostructure with superior visible-light-driven photocatalytic disinfection performance. Sep. Purif. Technol. 2022, 299, 121706. [Google Scholar] [CrossRef]
- Liu, X.; Xu, L.; Zhou, G.; Liu, Q.; Song, M.; Han, S.; Esakkimuthu, S.; Vinh, J.; Barati, B.; Lu, Z. Greatly improved photocatalytic performance of BiVO4/MoS2 heterojunction with enhanced hole transfer and attack capability by ultrasonic agitation and in-situ hydrothermal method. J. Taiwan Chem. Eng. 2020, 117, 48–55. [Google Scholar] [CrossRef]
- Acevedo, L.; Usón, S.; Uche, J. Exergy transfer analysis of microwave heating systems. Energy 2014, 68, 349–363. [Google Scholar] [CrossRef]
- Selvam, S.M.; Paramasivan, B. Microwave assisted carbonization and activation of biochar for energy-environment nexus: A review. Chemosphere 2022, 286, 131631. [Google Scholar] [CrossRef]
- Acevedo, L.; Ferreira, G.; López-Sabirón, A.M. Exergy transfer principles of microwavable materials under electromagnetic effects. Mater. Today Commun. 2021, 27, 102313. [Google Scholar] [CrossRef]
- Lu, H.; Ju, T.; She, H.; Wang, L.; Wang, Q. Microwave-assisted synthesis and characterization of BiOI/BiF3 p–n heterojunctions and its enhanced photocatalytic properties. J. Mater. Sci. Mater. Electron. 2020, 31, 13787–13795. [Google Scholar] [CrossRef]
- Hu, H.; Wang, T.; Peng, L.; Ling, X.; He, Y.; Sun, M.; Yang, M.; Deng, C. Microwave-assisted synthesis of Z-scheme CdS/BiOBr heterojunction for improved visible-light photocatalytic degradation of organic dyes. Appl. Phys. A 2022, 128, 452. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, H.; Li, D.; Zou, Z.; Xia, D. Microwave-assisted synthesis of La(OH)3/BiOCl n-n heterojunctions with high oxygen vacancies and its enhanced photocatalytic properties. Chem. Phys. Lett. 2019, 736, 136805. [Google Scholar] [CrossRef]
- Claudino, C.H.; Kuznetsova, M.; Rodrigues, B.S.; Chen, C.; Wang, Z.; Sardela, M.; Souza, J.S. Facile one-pot microwave-assisted synthesis of tungsten-doped BiVO4/WO3 heterojunctions with enhanced photocatalytic activity. Mater. Res. Bull. 2020, 125, 110783. [Google Scholar] [CrossRef]
- Sriram, B.; Baby, J.N.; Hsu, Y.F.; Wang, S.F.; George, M. In Situ Synthesis of a Bismuth Vanadate/Molybdenum Disulfide Composite: An Electrochemical Tool for 3-Nitro-l-Tyrosine Analysis. Inorg. Chem. 2022, 61, 14046–14057. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cong, S.; He, Y.; Zou, D.; Liu, Y.; Yao, B.; Sun, W. Study on the mechanism of degradation of tetracycline hydrochloride by microwave-activated sodium persulfate. Water Sci. Technol. 2020, 82, 1961–1970. [Google Scholar] [CrossRef]
- Fardush Tanha, J.; Farhad, S.F.U.; Honey, U.; Tanvir, N.I.; Hasan, T.; Shahriyar Nishat, S.; Kabir, A.; Ahmed, S.; Hakim, M.; Khan, M.N.I.; et al. A DFT+U look into experimentally synthesized monoclinic scheelite BiVO4. J. Appl. Phys. 2021, 130, 235107. [Google Scholar] [CrossRef]
- Li, Q.; Huang, L.; Dai, W.; Zhang, Z. Controlling 1T/2H heterophase junctions in the MoS2 microsphere for the highly efficient photocatalytic hydrogen evolution. Catal. Sci. Technol. 2021, 11, 7914–7921. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Wei, Z.; Yang, S.; He, H.; Sun, C. Fabrication of a novel p–n heterojunction photocatalyst n-BiVO4@p-MoS2 with core–shell structure and its excellent visible-light photocatalytic reduction and oxidation activities. Appl. Catal. B 2016, 185, 242–252. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Ma, C.; Zhao, F.; Chen, K. Morphology Effect of Bismuth Vanadate on Electrochemical Sensing for the Detection of Paracetamol. Nanomaterials 2022, 12, 1173. [Google Scholar] [CrossRef]
- Lutsko, J.F. How crystals form: A theory of nucleation pathways. Sci. Adv. 2019, 5, 7399. [Google Scholar] [CrossRef]
- Verma, A.; Bisen, D.P.; Brahme, N.; Sahu, I.P.; Singh, A.K. Yttrium aluminum garnet based novel and advanced phosphor synthesized by combustion route activated by Dy, Eu, and Tb rare earth metals. J. Mater. Sci. Electron. 2023, 34, 644. [Google Scholar] [CrossRef]
- Ullah, S.; Fayeza; Khan, A.A.; Jan, A.; Aain, S.Q.; Neto, E.P.; Serge-Correales, Y.E.; Parveen, R.; Wender, H.; Rodrigues-Filho, U.P.; et al. Enhanced photoactivity of BiVO4/Ag/Ag2O Z-scheme photocatalyst for efficient environmental remediation under natural sunlight and low-cost LED illumination. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124946. [Google Scholar] [CrossRef]
- van Nguyen, T.; Tekalgne, M.; Nguyen, T.P.; Wang, W.; Hong, S.H.; Cho, J.H.; van Le, Q.; Jang, H.W.; Ahn, S.H.; Kim, S.Y. Control of the morphologies of molybdenum disulfide for hydrogen evolution reaction. Int. J. Energy Res. 2022, 46, 11479–11491. [Google Scholar] [CrossRef]
- Liu, J.J.; Jiang, Z.W.; Hsu, S.W. Investigation of the Performance of Heterogeneous MOF-Silver Nanocube Nanocomposites as CO2 Reduction Photocatalysts by In Situ Raman Spectroscopy. ACS Appl. Mater. Interfaces 2023, 15, 6716–6725. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, Y.; Fan, X.; Li, G.; Hao, Q.; Qiu, T. Mixed-dimensional van der Waals heterojunction-enhanced Raman scattering. Nano Res. 2021, 15, 637–643. [Google Scholar] [CrossRef]
- Chawla, H.; Garg, S.; Rohilla, J.; Szamosvölgyi, Á.; Efremova, A.; Szenti, I.; Ingole, P.P.; Sápi, A.; Kónya, Z.; Chandra, A. Visible LED-light driven photocatalytic degradation of organochlorine pesticides (2,4-D & 2,4-DP) by Curcuma longa mediated bismuth vanadate. J. Clean. Prod. 2022, 367, 132923. [Google Scholar] [CrossRef]
- Kite, S.V.; Kadam, A.N.; Sathe, D.J.; Patil, S.; Mali, S.S.; Hong, C.K.; Lee, S.W.; Garadkar, K.M. Nanostructured TiO2 Sensitized with MoS2 Nanoflowers for Enhanced Photodegradation Efficiency toward Methyl Orange. ACS Omega 2021, 6, 17071–17085. [Google Scholar] [CrossRef]
- Wang, J.; Jin, J.; Wang, X.; Yang, S.; Zhao, Y.; Wu, Y.; Dong, S.; Sun, J.; Sun, J. Facile fabrication of novel BiVO4/Bi2S3/MoS2 n-p heterojunction with enhanced photocatalytic activities towards pollutant degradation under natural sunlight. J. Colloid Interface Sci. 2017, 505, 805–815. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Y.; Sun, H.; Liu, G.; Liu, S.; Yang, X. Defect-rich 2D reticulated MoS2 monolayers: Facile hydrothermal preparation and marvellous photoelectric properties. J. Taiwan Chem. Eng. 2019, 101, 221–230. [Google Scholar] [CrossRef]
- Dong, P.; Xi, X.; Zhang, X.; Hou, G.; Guan, R. Template-Free Synthesis of Monoclinic BiVO4 with Porous Structure and Its High Photocatalytic Activity. Materials 2016, 9, 685. [Google Scholar] [CrossRef]
- Zhong, Y.; Yu, L.; Chen, Z.F.; He, H.; Ye, F.; Cheng, G.; Zhang, Q. Microwave-Assisted Synthesis of Fe3O4 Nanocrystals with Predominantly Exposed Facets and Their Heterogeneous UVA/Fenton Catalytic Activity. ACS Appl. Mater. Interfaces 2017, 9, 29203–29212. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Du, L.; Lei, L.; Chen, Y.; Wang, G.; Wang, Z.; Zhou, W.; Tao, J.; Li, R.; et al. Peroxymonosulfate activation by surface-modified bismuth vanadate for ciprofloxacin abatement under visible light: Insights into the generation of singlet oxygen. Chem. Eng. J. 2022, 444, 136373. [Google Scholar] [CrossRef]
- Zhang, X.; Suo, H.; Zhang, R.; Niu, S.; Zhao, X.q.; Zheng, J.; Guo, C. Photocatalytic activity of 3D flower-like MoS2 hemispheres. Mater. Res. Bull. 2018, 100, 249–253. [Google Scholar] [CrossRef]
- Geosciences, D.O. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar]
- Pearson, R.G. Absolute Electronegativity and Hardness: Applications to Organic. J. Org. Chem. 1989, 54, 1423–1430. [Google Scholar] [CrossRef]
- Li, J.; Jin, B.; Jiao, Z. Rationally embedded zinc oxide nanospheres serving as electron transport channels in bismuth vanadate/zinc oxide heterostructures for improved photoelectrochemical efficiency. J. Colloid Interface Sci. 2021, 592, 127–134. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Ma, X.; Han, X.; Xing, C.; Qi, X.; He, R.; Arbiol, J.; Pan, H.; Zhao, J.; et al. Selective Ethylene Glycol Oxidation to Formate on Nickel Selenide with Simultaneous Evolution of Hydrogen. Adv. Sci. 2023, 10, 2300841. [Google Scholar] [CrossRef]
- Hu, C.; Xiong, C.; Lin, Y.L.; Zhu, Y.; Wang, Q.; Xu, L.; Huang, D. Degradation of 2-phenylbenzimidazole 5-sulfonic acid by UV/chlorine advanced oxidation technology: Kinetic model, degradation byproducts and reaction pathways. J. Hazard. Mater. 2022, 431, 128574. [Google Scholar] [CrossRef]
- Yang, W.; Ding, K.; Chen, G.; Wang, H.; Deng, X. Synergistic Multisystem Photocatalytic Degradation of Anionic and Cationic Dyes Using Graphitic Phase Carbon Nitride. Molecules 2023, 28, 2796. [Google Scholar] [CrossRef]
- Xia, L.; Liang, W.; Chen, G.; Li, W.; Gao, M. Catalytic Ozonation of Quinoline Utilizing Manganese-Based Catalyst with Abundant Oxygen Vacancies. Catal. Lett. 2021, 152, 1669–1677. [Google Scholar] [CrossRef]
- Masoumi, Z.; Tayebi, M.; Kolaei, M.; Lee, B.-K. Unified surface modification by double heterojunction of MoS2 nanosheets and BiVO4 nanoparticles to enhance the photoelectrochemical water splitting of hematite photoanode. J. Alloys Compd. 2022, 890, 161802. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Shi, Q.; Zhu, W.; Zhang, Y.; Su, W.; Lu, Z.; Yan, J.; Chen, K.; Wang, Q.; Li, J. Microwave-Assisted Synthesis of MoS2/BiVO4 Heterojunction for Photocatalytic Degradation of Tetracycline Hydrochloride. Nanomaterials 2023, 13, 1522. https://doi.org/10.3390/nano13091522
Cheng C, Shi Q, Zhu W, Zhang Y, Su W, Lu Z, Yan J, Chen K, Wang Q, Li J. Microwave-Assisted Synthesis of MoS2/BiVO4 Heterojunction for Photocatalytic Degradation of Tetracycline Hydrochloride. Nanomaterials. 2023; 13(9):1522. https://doi.org/10.3390/nano13091522
Chicago/Turabian StyleCheng, Cixin, Qin Shi, Weiwei Zhu, Yuheng Zhang, Wanyi Su, Zizheng Lu, Jun Yan, Kao Chen, Qi Wang, and Junshan Li. 2023. "Microwave-Assisted Synthesis of MoS2/BiVO4 Heterojunction for Photocatalytic Degradation of Tetracycline Hydrochloride" Nanomaterials 13, no. 9: 1522. https://doi.org/10.3390/nano13091522




