BiVO4 As a Sustainable and Emerging Photocatalyst: Synthesis Methodologies, Engineering Properties, and Its Volatile Organic Compounds Degradation Efficiency
Abstract
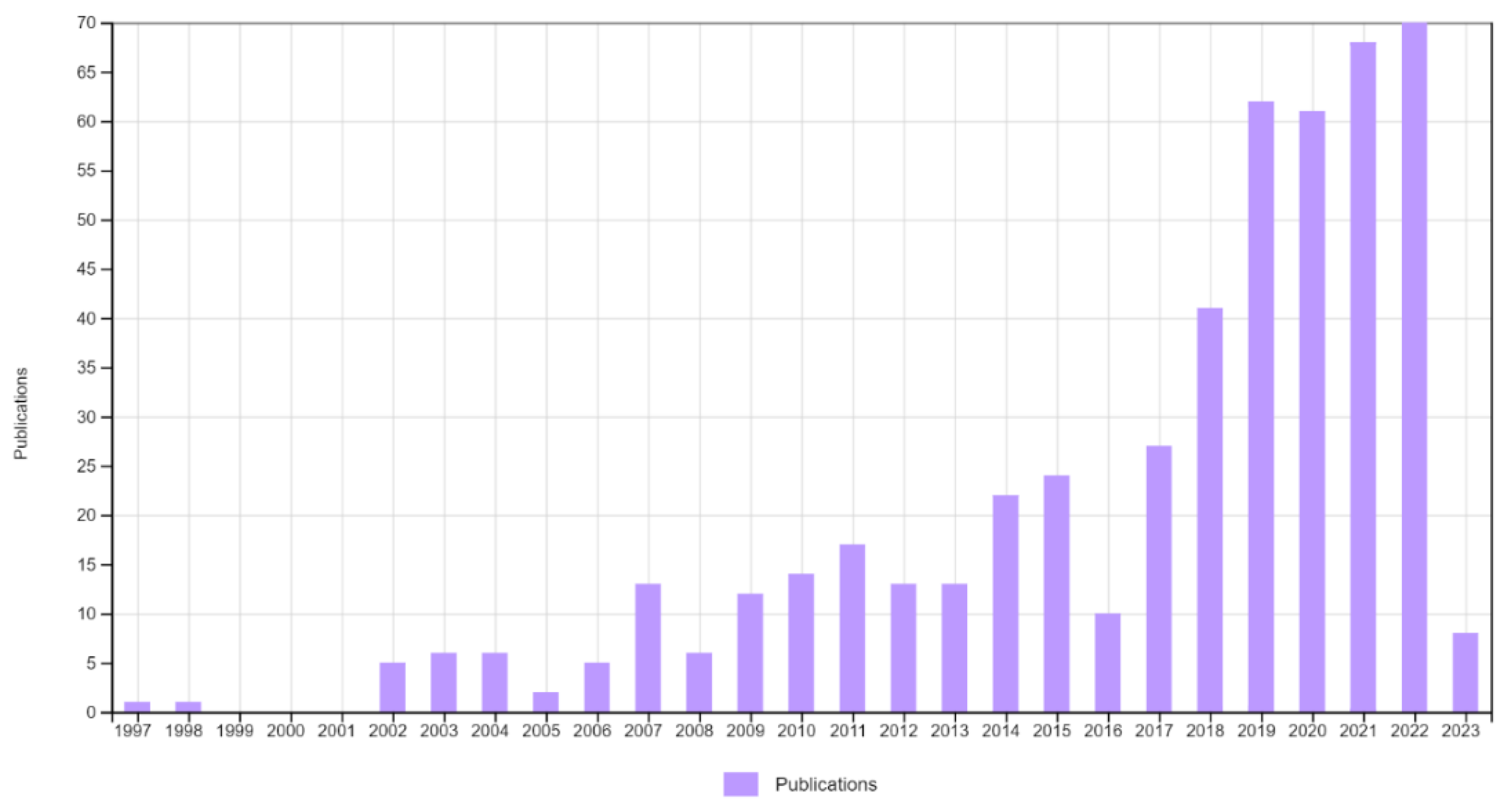
1. Introduction
2. Advanced Oxidation Process and Semiconductor Photocatalysis
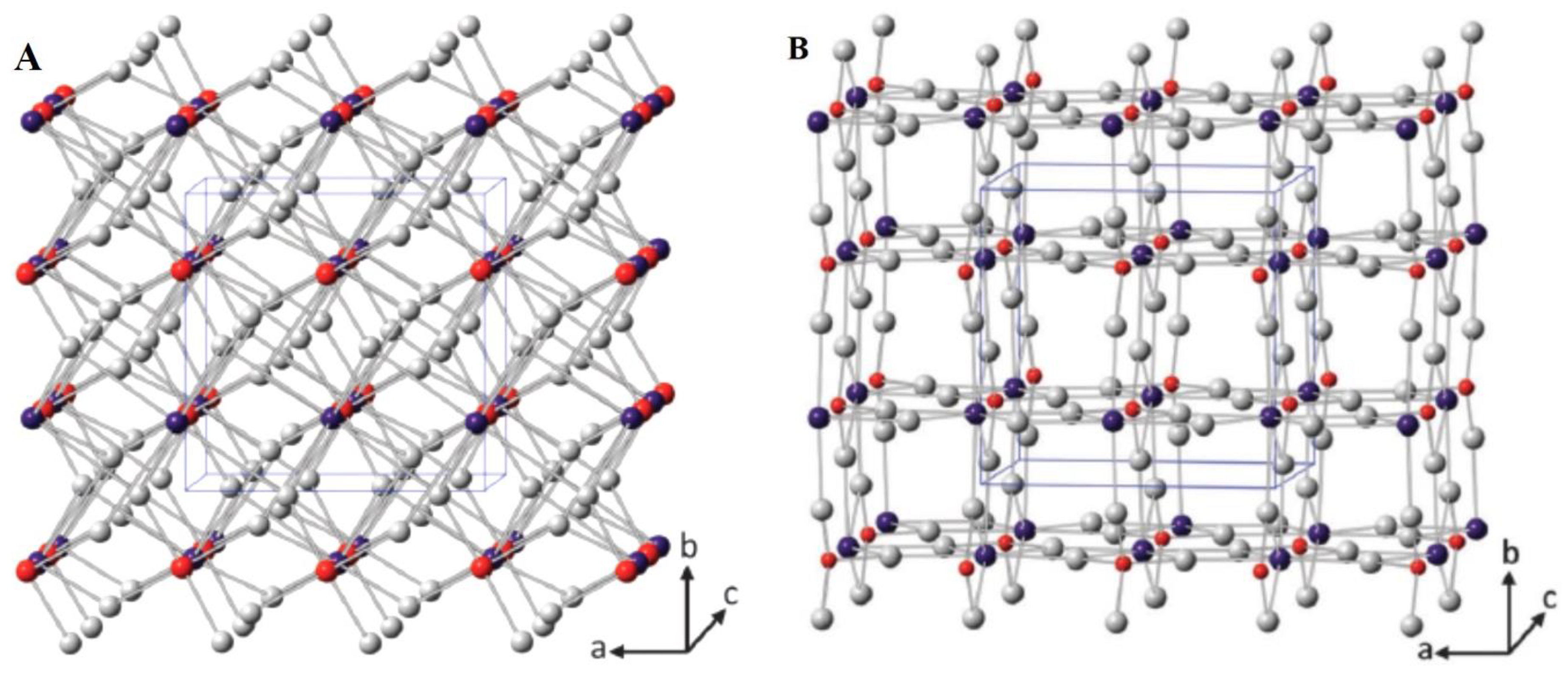
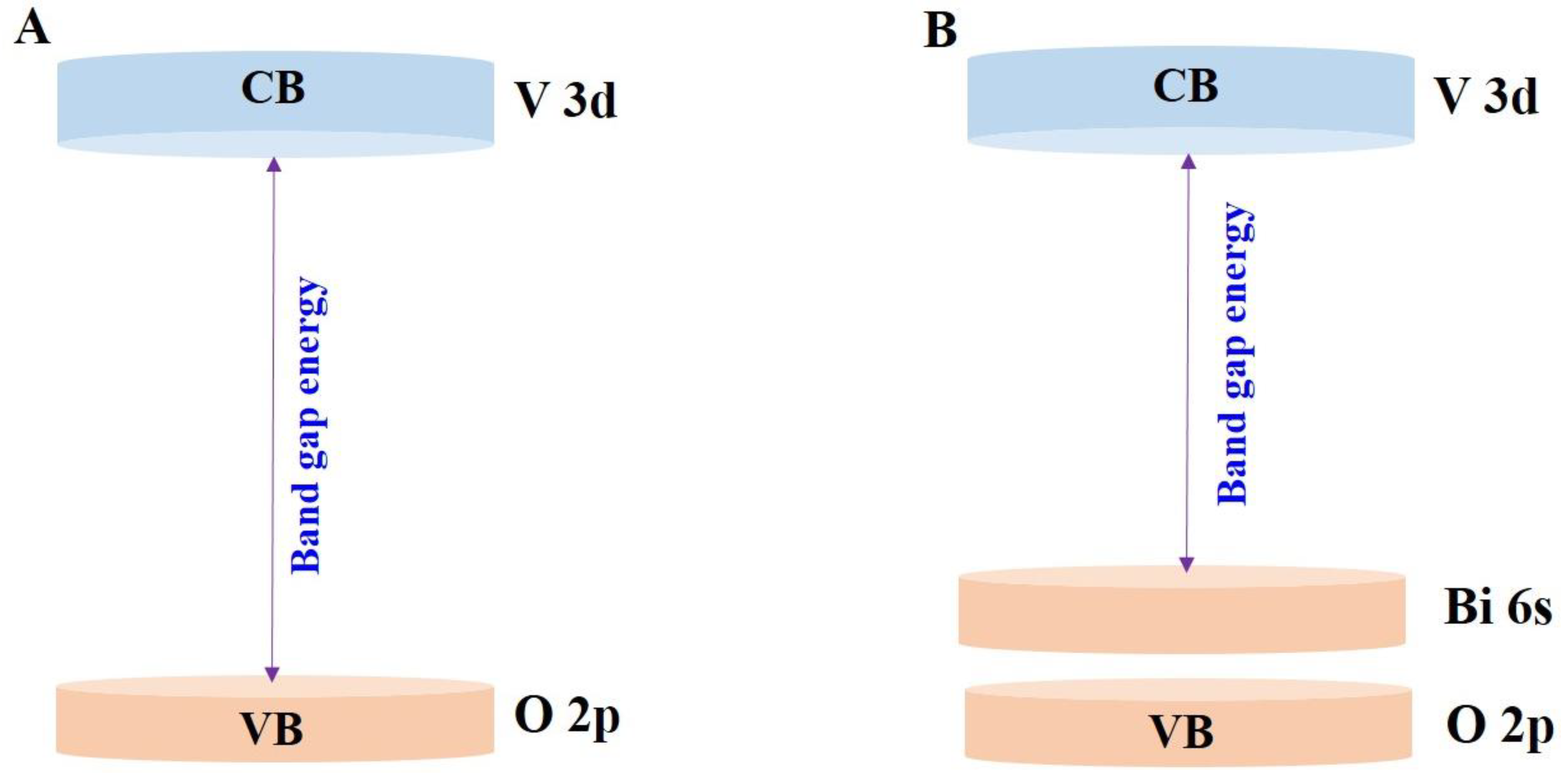
3. Fundamental Aspects of BiVO4 Photocatalyst
4. Synthesis Methodologies of BiVO4 Photocatalyst
4.1. Hydrothermal Method
4.2. Electro-Spinning Method
4.3. Solvothermal Method
4.4. Co-Precipitation Method
4.5. Sol–Gel Method
5. Engineering/Modification Processes of BiVO4 Properties
5.1. Metal/Nonmetal-Doped BiVO4

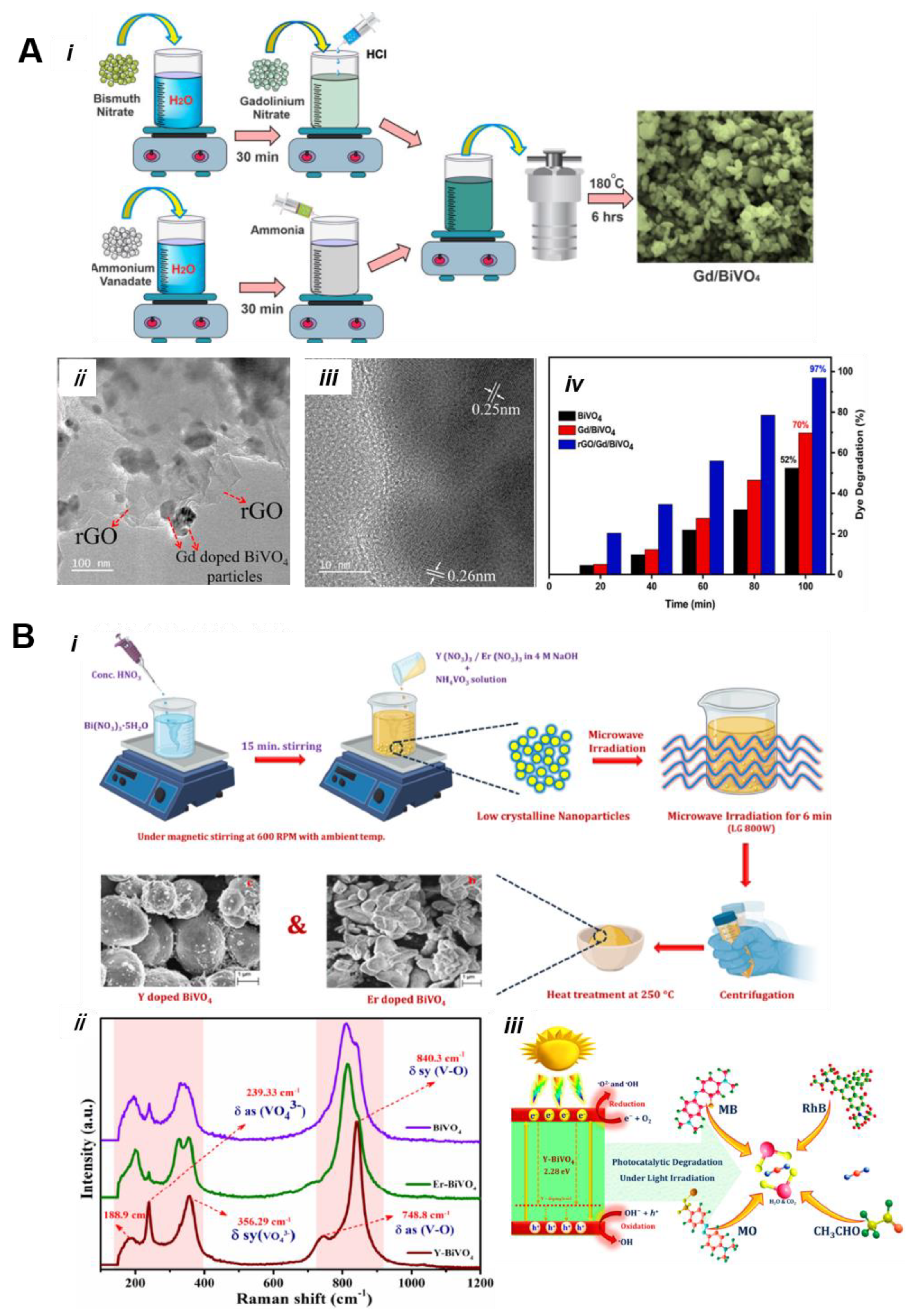
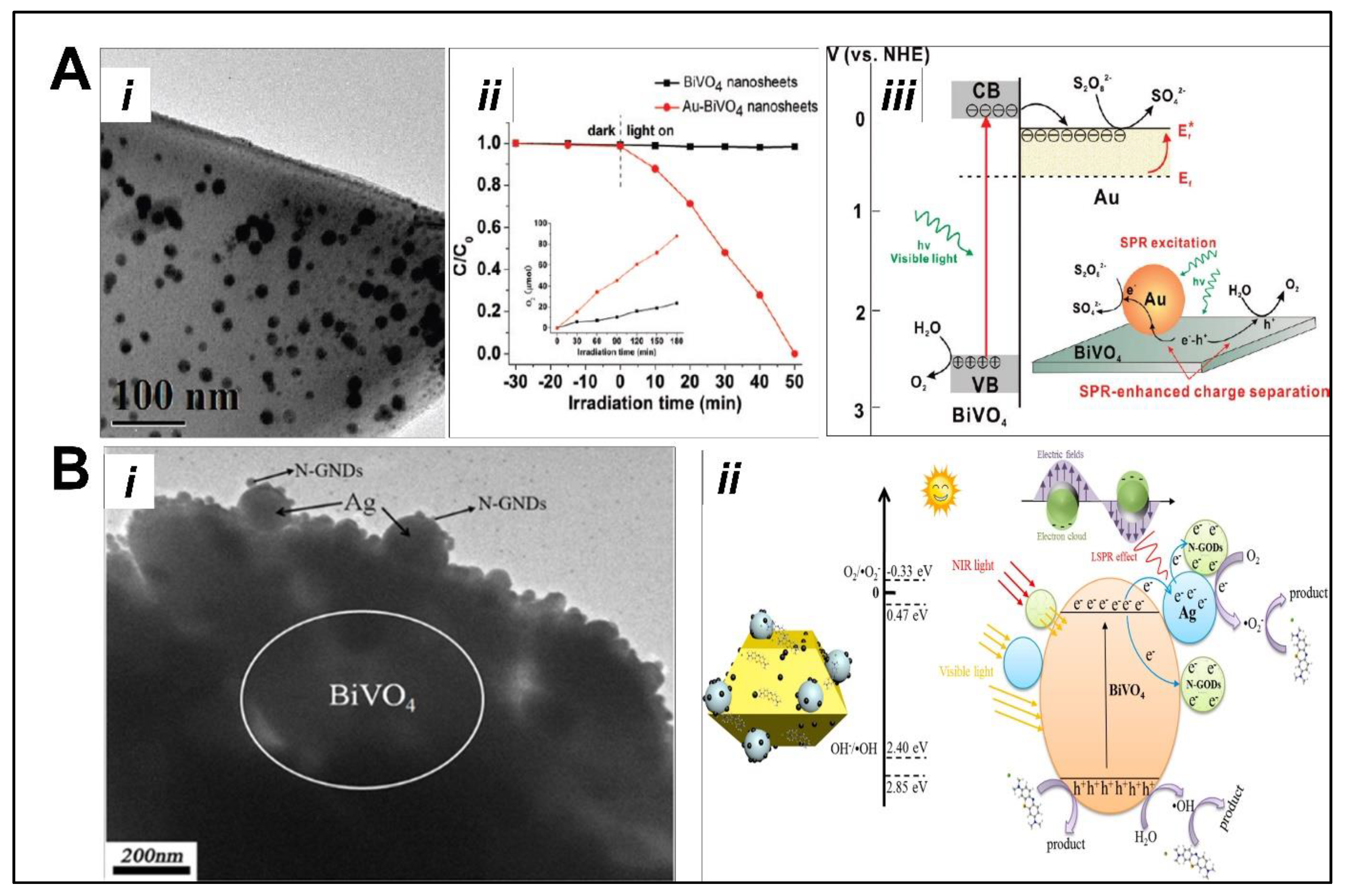
5.2. Noble-Metal-Doped BiVO4 as Photocatalyst
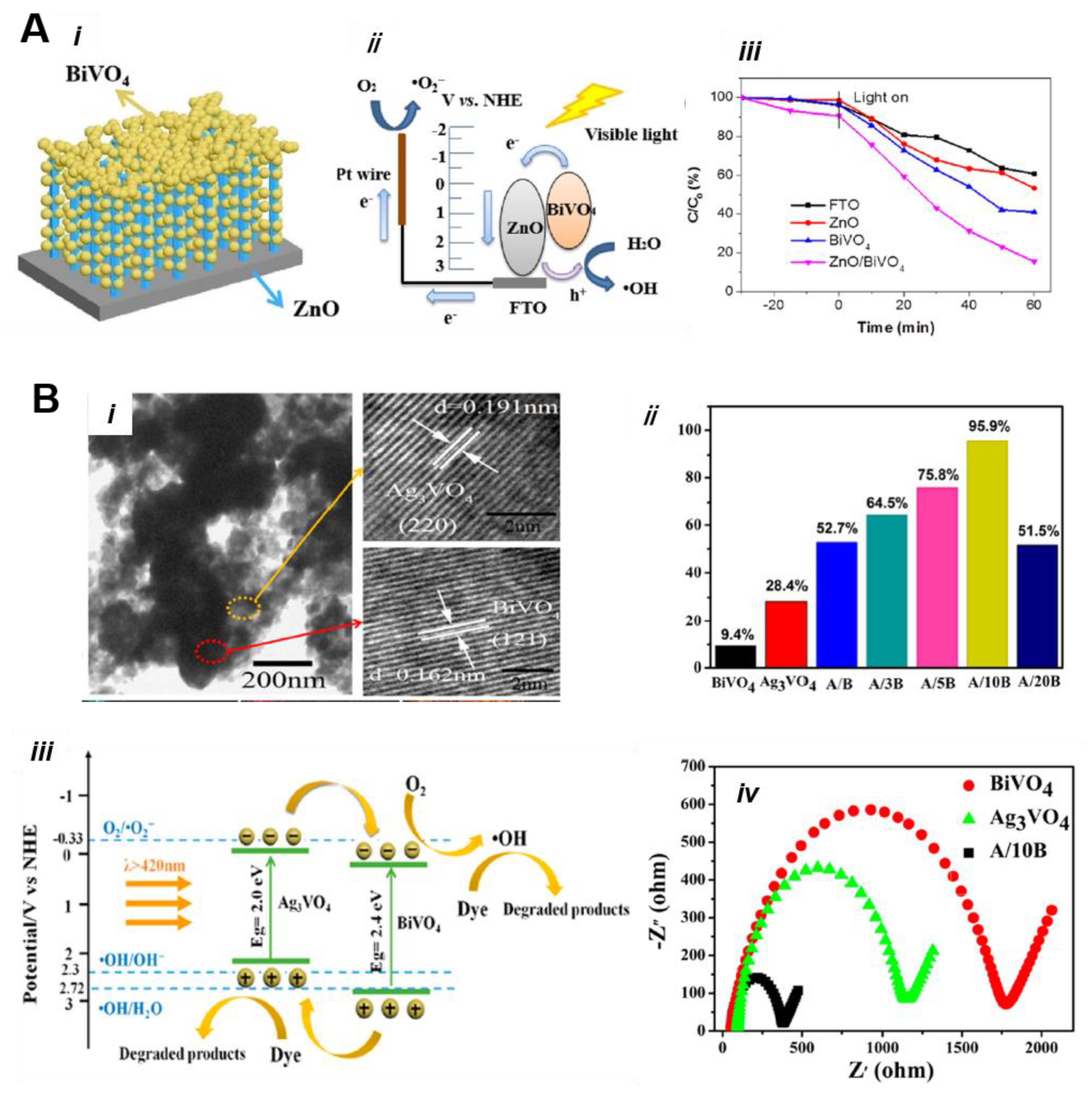
5.3. BiVO4-Based Nanocomposites
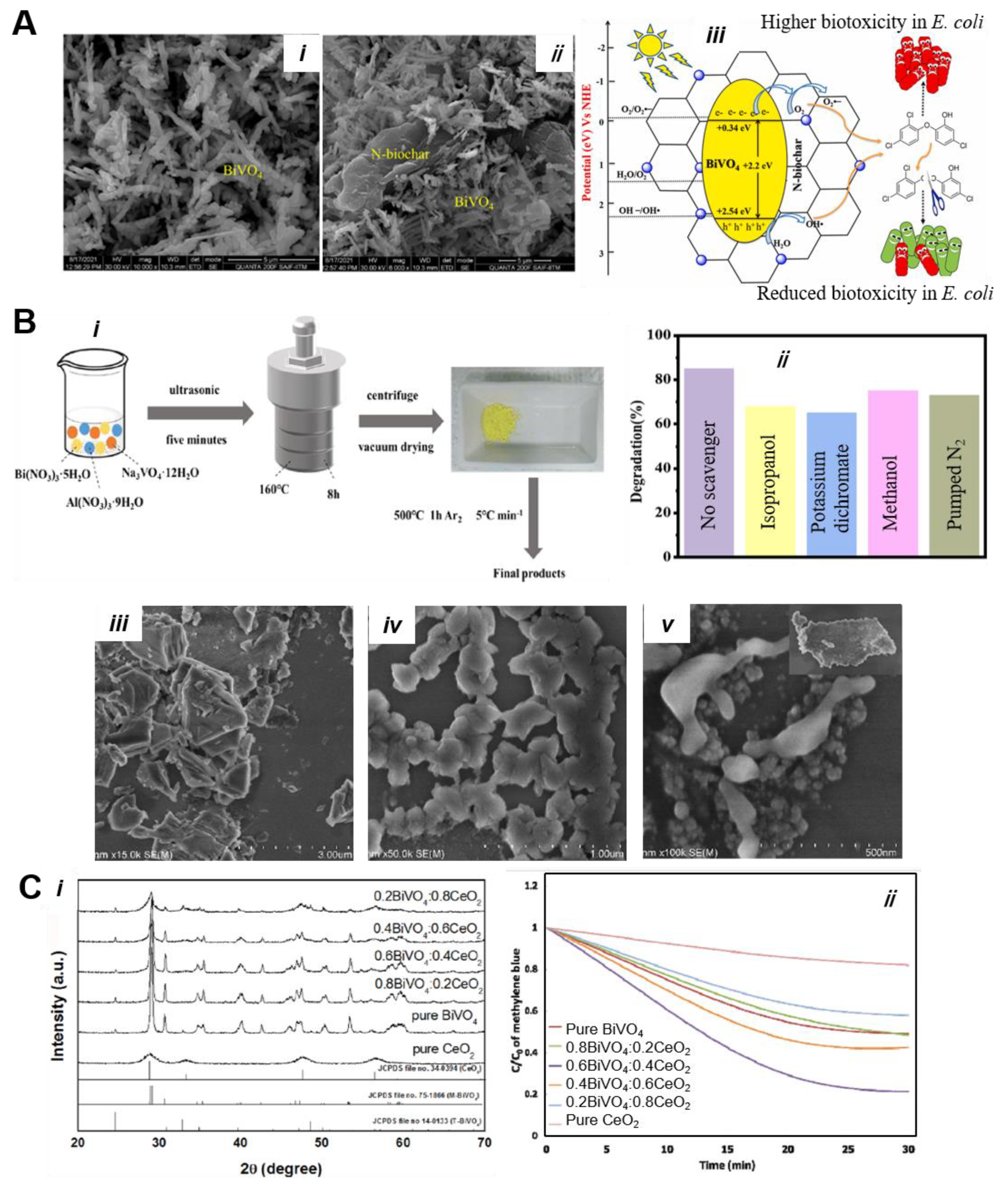
5.3.1. Activated Carbon, Carbon, and Other Adsorbents-Based Composites
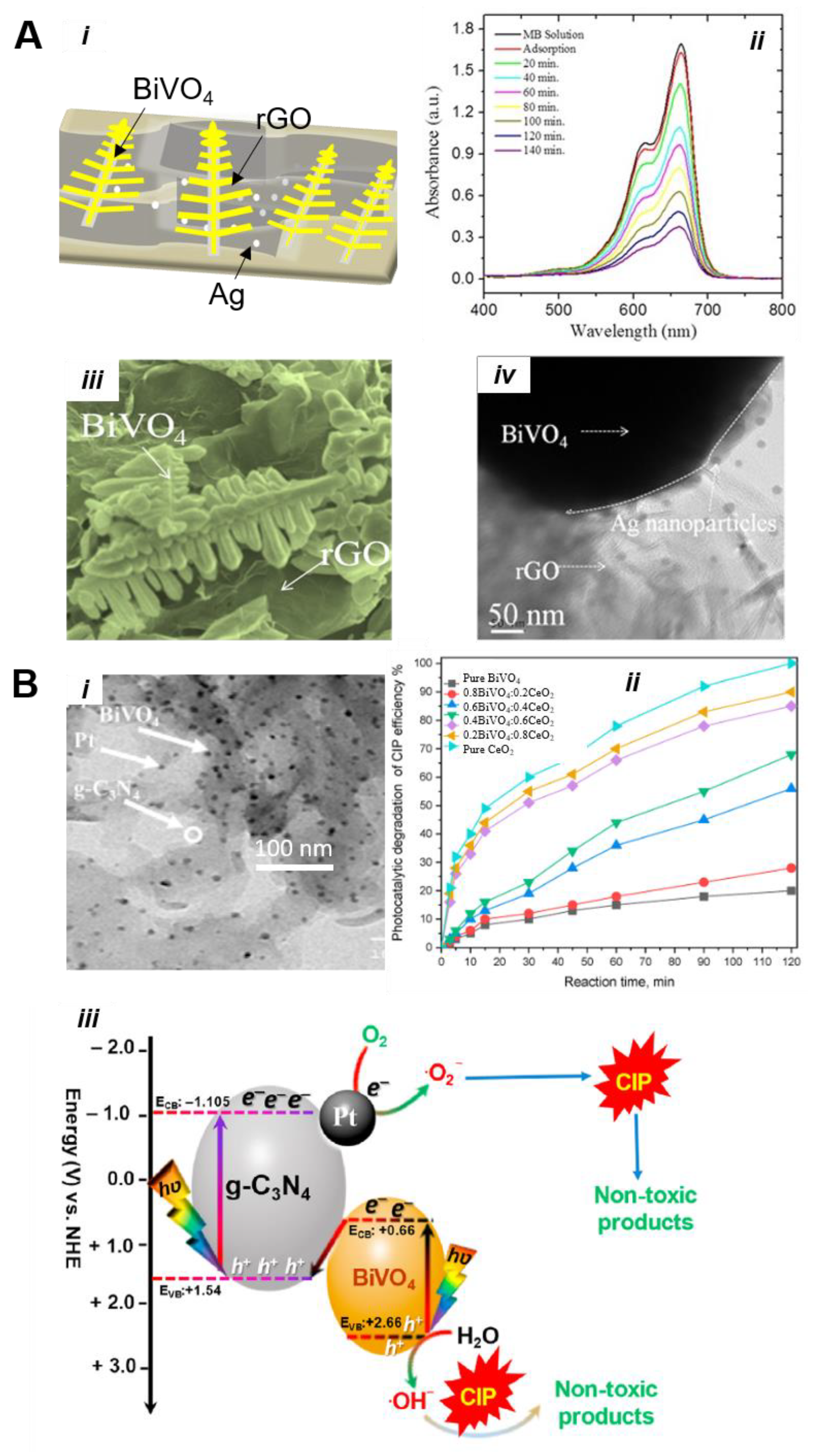
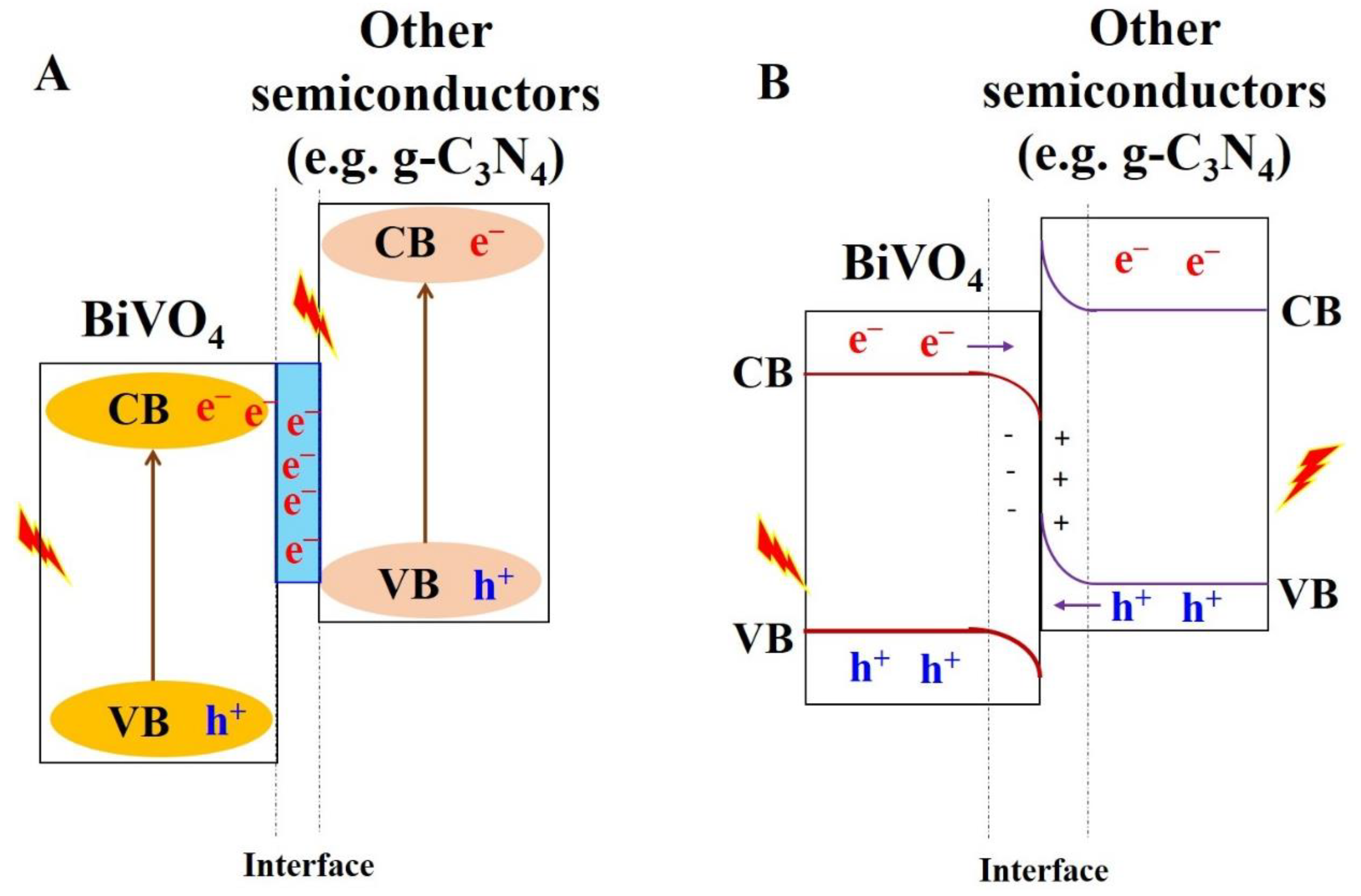
5.3.2. Heterojunction Construction
5.3.3. BiVO4-Based S-Scheme and Z-Scheme Nanocomposite Materials
6. Volatile Organic Compounds Degradation Application
7. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Jing, L.; Zhou, W.; Tian, G.; Fu, H. Surface tuning for oxide-based nanomaterials as efficient photocatalysts. Chem. Soc. Rev. 2013, 42, 9509–9549. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Yu, X.; Zhang, L.; Yu, D. Monodisperse raspberry-like multi hollow polymer/Ag nanocomposite microspheres for rapid catalytic degradation of methylene blue. J. Colloid Interface Sci. 2017, 491, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tao, C.; Xiao, G.; Su, H. Controlled synthesis, and wastewater treatment of Ag2O/TiO2 modified chitosan-based photocatalytic film. RSC Adv. 2017, 7, 11211–11221. [Google Scholar] [CrossRef]
- Huang, L.; Yu, Y.; Fu, C.; Guo, H.; Li, X. Photocatalytic degradation of imidazolium ionic liquids using dye sensitized TiO2/SiO2 composites. RSC Adv. 2017, 7, 732120–732125. [Google Scholar] [CrossRef]
- Rahmawati, F.; Fadillah, I.; Mudjijono, M. Composite of nano-TiO2 with cellulose acetate membrane from nata de coco (Nano-TiO2/CA(NDC)) for methyl orange degradation. J. Mater. Environ. Sci. 2017, 8, 289–297. [Google Scholar]
- You, J.; Guo, Y.; Guo, R.; Liu, X. A review of visible light-active photocatalysts for water disinfection: Features and prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Vijitha, R.; Nagaraja, K.; Hanafiah, M.M.; Rao, K.M.; Venkateswarlu, K.; Lakkaboyana, S.K.; Rao, K.S.V. Fabrication of eco-friendly polyelectrolyte membranes based on sulfonate grafted sodium alginate for drug delivery, toxic metal ion removal and fuel cell applications. Polymers 2021, 13, 3293. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Rahim Pouran, S. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, H. Investigation and assessment of volatile organic compounds in water sources in China. Environ. Monit. Assess. 2011, 173, 825–836. [Google Scholar] [CrossRef]
- Kostopoulou, M.N.; Golfinopoulos, S.K.; Nikolaou, A.D.; Xilourgidis, N.K.; Lekkas, T.D. Volatile organic compounds in the surface waters of Northern Greece. Chemosphere 2000, 40, 527–532. [Google Scholar] [CrossRef]
- Almaie, S.; Vatanpour, V.; Rasoulifard, M.H.; Koyuncu, I. Volatile organic compounds (VOCs) removal by photocatalysts: A review. Chemosphere 2022, 306, 135655. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wu, Y.; Santhamoorthy, M.; Le, T.T.N.; Le, V.T.; Yuan, Y.; Xia, C. Volatile organic compounds in water matrices: Recent progress, challenges, and perspective. Chemosphere 2022, 308, 136182. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, C.; Wang, M.; Zhang, S.; Sun, J.; Dong, S.; Sun, J. Synergistic effect of adsorption and photocatalysis of 3D g-C3N4-agar hybrid aerogels. Appl. Surf. Sci. 2019, 467–468, 286–292. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Hu, B.W.; Hayat, T.; Rabah, S.O.; Alsaedi, A.; Zhuang, L.; Wang, X.K. Efficient elimination of environmental pollutants through sorption reduction and photocatalytic degradation using nanomaterials. Front. Chem. Sci. Eng. 2020, 14, 1124–1135. [Google Scholar] [CrossRef]
- Qu, S.; Wang, W.; Pan, X.; Li, C. Improving the Fenton catalytic performance of FeOCl using an electron mediator. J. Hazard. Mater. 2020, 384, 121494. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Chen, Z.; Wong, P.K.; Liu, J. Bi2WO6 micro/nano-structures: Synthesis, modifications and visible-light-driven photocatalytic applications. Appl. Catal. B Environ. 2011, 106, 1–13. [Google Scholar] [CrossRef]
- Yang, J. Evaluation Procedure of Photocatalysts for VOCs Degradation Based on Density Functional Theory: G-C3N4 Dots/Graphene. Acta Phys.-Chim. Sin. 2021, 37, 2011039. [Google Scholar] [CrossRef]
- Wang, Y.; Long, Y.; Zhang, D. Novel bifunctional V2O5/BiVO4 nanocomposite materials with enhanced antibacterial activity. Taiwan Inst. Chem. Eng. 2016, 68, 387–395. [Google Scholar] [CrossRef]
- Lai, Y.; Meng, M.; Yu, Y. One-step synthesis, characterizations and mechanistic study of nanosheets-constructed fluffy ZnO and Ag/ZnO spheres used for Rhodamine B photodegradation. Appl. Catal. B 2010, 100, 491–501. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A. ZnO/NiWO4/Ag2CrO4 nanocomposites with p–n–n heterojunctions: Highly improved activity for degradations of water contaminants under visible light. Sep. Purif. Technol. 2018, 193, 69–80. [Google Scholar] [CrossRef]
- Choi, Y.I.; Jeon, K.H.; Kim, H.S.; Lee, J.H.; Park, S.J.; Roh, J.E.; Khan, M.M.; Sohn, Y. TiO2/BiOX (X = Cl, Br, I) hybrid microspheres for artificial waste water and real sample treatment under visible light irradiation. Sep. Purif. Technol. 2016, 160, 28–42. [Google Scholar] [CrossRef]
- Akhundi, A.; Habibi-Yangjeh, A. Graphitic carbon nitride nanosheets decorated with CuCr2O4 nanoparticles: Novel photocatalysts with high performances in visible light degradation of water pollutants. J. Colloid Interface Sci. 2017, 504, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ravi Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Feizpoor, S.; Habibi-Yangjeh, A. Integration of Ag2WO4 and AgBr with TiO2 to fabricate ternary nanocomposites: Novel plasmonic photocatalysts with remarkable activity under visible light. Mater. Res. Bull. 2018, 99, 93–102. [Google Scholar] [CrossRef]
- Deng, W.; Zhao, H.; Pan, F.; Feng, X.; Jung, B.; Wahab, A.A.; Batchelor, B.; Li, Y. Visible-light-driven photocatalytic degradation of organic water pollutants promoted by sulfate addition. Environ. Sci. Technol. 2017, 51, 13372–13379. [Google Scholar] [CrossRef] [PubMed]
- Wenderich, K.; Mul, G. Methods, Mechanism, and applications of photo deposition in photocatalysis: A review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Malathi, A.; Vasanthakumar, V.; Arunachalam, P.; Madhavan, J.; Ghanem, M.A. A low-cost additive-free facile synthesis of BiFeWO6/BiVO4 nanocomposite with enhanced visible-light induced photocatalytic activity. J. Colloid Interface Sci. 2017, 506, 553–563. [Google Scholar] [CrossRef]
- Qiao, Z.; Yan, T.; Li, W.; Huang, B. In situ anion exchange synthesis of In2S3/In (OH)3 heterostructures for efficient photocatalytic degradation of MO under solar light. New J. Chem. 2017, 41, 3134–3142. [Google Scholar] [CrossRef]
- Malathi, A.; Arunachalam, P.; Grace, A.N.; Madhavan, J.; Al-Mayouf, A.M. A robust visible-light driven BiFeWO6/BiOI nanohybrid with efficient photocatalytic and photoelectrochemical performance. Appl. Surf. Sci. 2017, 412, 85–95. [Google Scholar] [CrossRef]
- Palmai, M.; Zahran, E.M.; Angaramo, S.; Balint, S.; Paszti, Z.; Knecht, M.R.; Bachas, L.G. Pd-decorated m-BiVO4/BiOBr ternary composite with dual heterojunction for enhanced photocatalytic activity. J. Mater. Chem. A 2017, 5, 529–534. [Google Scholar] [CrossRef]
- Chen, L.; Meng, D.; Wu, X.; Wang, A.; Wang, J.; Yu, M.; Liang, Y. Enhanced Visible Light Photocatalytic Performances of Self-Assembled Hierarchically Structured BiVO4/Bi2WO6 Heterojunction Composites with Different Morphologies. RSC Adv. 2016, 6, 52300–52309. [Google Scholar] [CrossRef]
- Huang, H.; Liu, L.; Zhang, Y.; Tian, N. Novel BiIO4/BiVO4 composite photocatalyst with highly improved visible-light-induced photocatalytic performance for rhodamine B degradation and photocurrent generation. RSC Adv. 2015, 5, 1161–1167. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Ren, J.; Sun, S.; Zhang, L. A Novel BiVO4 Hierarchical Nanostructure: Controllable Synthesis, Growth Mechanism, and Application in Photocatalysis. CrystEngComm 2010, 12, 1754–1758. [Google Scholar] [CrossRef]
- Fan, T.; Chen, C.; Tang, Z. Hydrothermal Synthesis of Novel BiFeO3/BiVO4 Heterojunctions with Enhanced Photocatalytic Activities under Visible Light Irradiation. RSC Adv. 2016, 6, 9994–10000. [Google Scholar] [CrossRef]
- Su, C.; Li, R.; Li, C.; Wang, W. Piezo-promoted regeneration of Fe2+ boosts peroxydisulfate activation by Bi2Fe4O9 nanosheets. Appl. Catal. B Environ. 2022, 310, 121330. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Zhang, L.; Xu, H.; Zhu, W. Single-Crystalline BiVO4 Microtubes with Square Cross-Sections: Microstructure, Growth Mechanism, and Photocatalytic Property. J. Phys. Chem. C 2007, 111, 13659–13664. [Google Scholar] [CrossRef]
- Walsh, A.; Yan, Y.; Huda, M.N.; Al-Jassim, M.M.; Wei, S.-H. Band Edge Electronic Structure of BiVO4: Elucidating the Role of the Bi s and V d Orbitals. Chem. Mater. 2009, 21, 547–551. [Google Scholar] [CrossRef]
- Guo, M.; Wang, Y.; He, Q.; Wang, W.; Wang, W.; Fu, Z.; Wang, H. Enhanced Photocatalytic Activity of S-Doped BiVO4 Photocatalysts. RSC Adv. 2015, 5, 58633–58639. [Google Scholar] [CrossRef]
- Hu, Y.; Fan, J.; Pu, C.; Li, H.; Liu, E.; Hu, X. Facile Synthesis of Double Cone-Shaped Ag4V2O7/BiVO4 Nanocomposites with Enhanced Visible Light Photocatalytic Activity for Environmental Purification. J. Photochem. Photobiol. A Chem. 2017, 337, 172–183. [Google Scholar] [CrossRef]
- Lv, D.; Zhang, D.; Pu, X.; Kong, D.; Lu, Z.; Shao, X.; Ma, H.; Dou, J. One-Pot Combustion Synthesis of BiVO4/BiOCl Composites with Enhanced Visible-Light Photocatalytic Properties. Sep. Purif. Technol. 2017, 174, 97–103. [Google Scholar] [CrossRef]
- Kamble, G.S.; Ling, Y.-C. Solvothermal Synthesis of Facet-Dependent BiVO4 Photocatalyst with Enhanced Visible-Light-Driven Photocatalytic Degradation of Organic Pollutant: Assessment of Toxicity by Zebrafish Embryo. Sci. Rep. 2020, 10, 12993. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and Its Photocatalytic and Photophysical Properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. Chem. Eng. 2022, 6, 8. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 Photocatalysis: A Brief Review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Zhong, X.; Li, Y.; Wu, H.; Xie, R. Recent Progress in BiVO4-Based Heterojunction Nanomaterials for Photocatalytic Applications. Mater. Sci. Eng. B 2023, 289, 116278. [Google Scholar] [CrossRef]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective Preparation of Monoclinic and Tetragonal BiVO4 with Scheelite Structure and Their Photocatalytic Properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef]
- Nagabhushana, G.P.; Nagaraju, G.; Chandrappa, G.T. Synthesis of Bismuth Vanadate: Its Application in H2 Evolution and Sunlight-Driven Photodegradation. J. Mater. Chem. A Mater. 2013, 1, 388–394. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Zhou, L.; Xu, H. Efficient Methylene Blue Removal Over Hydrothermally Synthesized Starlike BiVO4. Ind. Eng. Chem. Res. 2009, 48, 1735–1739. [Google Scholar] [CrossRef]
- Lei, B.-X.; Zhang, P.; Wang, S.-N.; Li, Y.; Huang, G.-L.; Sun, Z.-F. Additive-Free Hydrothermal Synthesis of Novel Bismuth Vanadium Oxide Dendritic Structures as Highly Efficient Visible-Light Photocatalysts. Mater. Sci. Semicond. Process. 2015, 30, 429–434. [Google Scholar] [CrossRef]
- Wang, B.; Guo, L.; He, T. Fabrication of an Olive-like BiVO4 Hierarchical Architecture with Enhanced Visible-Light Photocatalytic Activity. RSC Adv. 2016, 6, 30115–30124. [Google Scholar] [CrossRef]
- Obregón, S.; Caballero, A.; Colón, G. Hydrothermal Synthesis of BiVO4: Structural and Morphological Influence on the Photocatalytic Activity. Appl. Catal. B 2012, 117, 59–66. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Y.-S.; Xiao, H.-M.; Fu, S.-Y. Novel Core–Shell Structured BiVO4 Hollow Spheres with an Ultra-High Surface Area as Visible-Light-Driven Catalyst. CrystEngComm 2014, 16, 6059–6065. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, L.; Dai, H.; Zhao, Z.; Zhang, R.; Liu, Y. Surfactant-Assisted Hydrothermal Fabrication and Visible-Light-Driven Photocatalytic Degradation of Methylene Blue over Multiple Morphological BiVO4 Single-Crystallites. Mater. Chem. Phys. 2011, 125, 59–65. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, D.; Jiao, X. Monoclinic Structured BiVO4 Nanosheets: Hydrothermal Preparation, Formation Mechanism, and Coloristic and Photocatalytic Properties. J. Phys. Chem. B 2006, 110, 2668–2673. [Google Scholar] [CrossRef]
- Naing, H.H.; Wang, K.; Li, Y.; Mishra, A.K.; Zhang, G. Sepiolite Supported BiVO4 Nanocomposites for Efficient Photocatalytic Degradation of Organic Pollutants: Insight into the Interface Effect towards Separation of Photogenerated Charges. Sci. Total Environ. 2020, 722, 137825. [Google Scholar] [CrossRef]
- Chen, Z.; Mi, N.; Huang, L.; Wang, W.; Li, C.; Teng, Y.; Gu, C. Snow-like BiVO4 with rich oxygen defects for efficient visible light photocatalytic degradation of ciprofloxacin. Sci. Total Environ. 2022, 808, 152083. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, J.; Yan, X.; Zheng, Z.; Xue, Q. Preparation of Porous BiVO4 Fibers by Electrospinning and Their Photocatalytic Performance under Visible Light. RSC Adv. 2013, 3, 20606–20612. [Google Scholar] [CrossRef]
- Liu, G.; Liu, S.; Lu, Q.; Sun, H.; Xu, F.; Zhao, G. Synthesis of Monoclinic BiVO4 Microribbons by Sol–Gel Combined with Electrospinning Process and Photocatalytic Degradation Performances. J. Sol-Gel Sci. Technol. 2014, 70, 24–32. [Google Scholar] [CrossRef]
- Chen, L.; Meng, D.; Wu, X.; Wang, J.; Wang, Y.; Liang, Y. Shape-Controlled Synthesis of Novel Self-Assembled BiVO4 Hierarchical Structures with Enhanced Visible Light Photocatalytic Performances. Mater. Lett. 2016, 176, 143–146. [Google Scholar] [CrossRef]
- Suwanchawalit, C.; Buddee, S.; Wongnawa, S. Triton X-100 Induced Cuboid-like BiVO4 Microsphere with High Photocatalytic Performance. J. Environ. Sci. 2017, 55, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.-F.; Chen, C.-C.; Chang, Y.-K.; Lu, C.-S.; Wu, R.-J. Efficient Photocatalytic Degradation of Thiobencarb over BiVO4 Driven by Visible Light: Parameter and Reaction Pathway Investigations. Sep. Purif. Technol. 2014, 122, 78–86. [Google Scholar] [CrossRef]
- Tseng, T.K.; Lin, Y.S.; Chen, Y.J.; Chu, H. A Review of Photocatalysts Prepared by Sol-Gel Method for VOCs Removal. Int. J. Mol. Sci. 2010, 11, 2336–2361. [Google Scholar] [CrossRef]
- Wang, M.; Che, Y.; Niu, C.; Dang, M.; Dong, D. Lanthanum and boron co-doped BiVO4 with enhanced visible light photocatalytic activity for degradation of methyl orange. J. Rare Earths 2013, 31, 878–884. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M. Cube-like Cu/Cu2O/BiVO4/Bi7VO13 composite nanoparticles: Facile sol-gel synthesis for photocatalytic desulfurization of thiophene under visible light. J. Alloy. Comp. 2020, 823, 153786. [Google Scholar] [CrossRef]
- Drisya, K.T.; Solís-López, M.; Ríos-Ramírez, J.J.; Durán-Álvarez, J.C.; Rousseau, A.; Velumani, S.; Asomoza, R.; Kassiba, A.; Jantrania, A.; Castaneda, H. Electronic and optical competence of TiO2/BiVO4 nanocomposites in the photocatalytic processes. Sci. Rep. 2020, 10, 13507. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A Review on BiVO4 Photocatalyst: Activity Enhancement Methods for Solar Photocatalytic Applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar] [CrossRef]
- Villa, K.; Novotný, F.; Zelenka, J.; Browne, M.P.; Ruml, T.; Pumera, M. Visible-Light-Driven Single-Component BiVO4 Micromotors with the Autonomous Ability for Capturing Microorganisms. ACS Nano 2019, 13, 8135–8145. [Google Scholar] [CrossRef]
- Kim, T.W.; Ping, Y.; Galli, G.A.; Choi, K.S. Simultaneous Enhancements in Photon Absorption and Charge Transport of Bismuth Vanadate Photoanodes for Solar Water Splitting. Nat. Commun. 2015, 6, 8769. [Google Scholar] [CrossRef] [PubMed]
- Laraib, I.; Carneiro, M.A.; Janotti, A. Effects of Doping on the Crystal Structure of BiVO4. J. Phys. Chem. C 2019, 123, 26752–26757. [Google Scholar] [CrossRef]
- Patil, S.S.; Mali, M.G.; Hassan, M.A.; Patil, D.R.; Kolekar, S.S.; Ryu, S.W. One-Pot in Situ Hydrothermal Growth of BiVO4/Ag/RGO Hybrid Architectures for Solar Water Splitting and Environmental Remediation. Sci. Rep. 2017, 7, 8404. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Ye, J. Synthesis of Bismuth Vanadate Nanoplates with Exposed {001} Facets and Enhanced Visible-Light Photocatalytic Properties. Chem. Comm. 2010, 46, 1893–1895. [Google Scholar] [CrossRef]
- Rodrigues, B.S.; Branco, C.M.; Corio, P.; Souza, J.S. Controlling Bismuth Vanadate Morphology and Crystalline Structure through Optimization of Microwave-Assisted Synthesis Conditions. Cryst. Growth Des. 2020, 20, 3673–3685. [Google Scholar] [CrossRef]
- Li, R.; Zhang, F.; Wang, D.; Yang, J.; Li, M.; Zhu, J.; Zhou, X.; Han, H.; Li, C. Spatial Separation of Photogenerated Electrons and Holes among {010} and {110} Crystal Facets of BiVO4. Nat. Commun. 2013, 4, 1432. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, R.; Mu, L.; Li, C. Significance of Crystal Morphology Controlling in Semiconductor-Based Photocatalysis: A Case Study on BiVO4 Photocatalyst. Cryst. Growth Des. 2017, 17, 2923–2928. [Google Scholar] [CrossRef]
- Yin, X.; Sun, X.; Mao, Y.; Wang, R.; Li, D.; Xie, W.; Liu, Z.; Liu, Z. Synergistically enhanced photocatalytic degradation of tetracycline hydrochloride by Z-scheme heterojunction MT-BiVO4 microsphere/P-doped g-C3N4 nanosheet composite. J. Environ. Chem. Eng. 2023, 11, 109412. [Google Scholar] [CrossRef]
- Sun, H.; Hua, W.; Li, Y.; Wang, J.-G. Promoting Photoelectrochemical Activity and Stability of WO3/BiVO4 Heterojunctions by Coating a Tannin Nickel Iron Complex. ACS Sustain. Chem. Eng. 2020, 8, 12637–12645. [Google Scholar] [CrossRef]
- Qin, C.; Tang, X.; Chen, J.; Liao, H.; Zhong, J.; Li, J. In-Situ Fabrication of Bi/BiVO4 Heterojunctions with N-Doping for Efficient Elimination of Contaminants. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126224. [Google Scholar] [CrossRef]
- Kahng, S.; Kim, J.H. Heterojunction Photoanode of SnO2 and Mo-Doped BiVO4 for Boosting Photoelectrochemical Performance and Tetracycline Hydrochloride Degradation. Chemosphere 2022, 291, 132800. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Han, N.; Liao, D.; Bai, S.; Yang, X.; Luo, R.; Li, D.; Chen, A. rGO Decorated W Doped BiVO4 Novel Material for Sensing Detection of Trimethylamine. Sens. Actuators B Chem. 2019, 298, 126749. [Google Scholar] [CrossRef]
- Liu, B.; Yan, X.; Yan, H.; Yao, Y.; Cai, Y.; Wei, J.; Chen, S.; Xu, X.; Li, L. Preparation and Characterization of Mo Doped in BiVO4 with Enhanced Photocatalytic Properties. Materials 2017, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deng, M.; Ren, F.; Wu, Y.; Wang, Y. Effects of Mo/W Codoping on the Visible-Light Photocatalytic Activity of Monoclinic BiVO4 within the GGA + U Framework. RSC Adv. 2016, 6, 12290–12297. [Google Scholar] [CrossRef]
- Yao, W.; Iwai, H.; Ye, J. Effects of Molybdenum Substitution on the Photocatalytic Behavior of BiVO4. Dalton Trans. 2008, 11, 1426–1430. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, F.; Chi, X.; Tian, Y.; Zhu, Z.; Guan, R.; Song, J. A Mesoporous Nanofibrous BiVO4-Ni/AgVO3 Z-Scheme Heterojunction Photocatalyst with Enhanced Photocatalytic Reduction of Cr6+ and Degradation of RhB under Visible Light. Appl. Surf. Sci. 2022, 603, 154416. [Google Scholar] [CrossRef]
- Regmi, C.; Kshetri, Y.K.; Kim, T.-H.; Pandey, R.P.; Ray, S.K.; Lee, S.W. Fabrication of Ni-Doped BiVO4 Semiconductors with Enhanced Visible-Light Photocatalytic Performances for Wastewater Treatment. Appl. Surf. Sci. 2017, 413, 253–265. [Google Scholar] [CrossRef]
- Bashir, S.; Jamil, A.; Khan, M.S.; Alazmi, A.; Abuilaiwi, F.A.; Shahid, M. Gd-Doped BiVO4 Microstructure and Its Composite with a Flat Carbonaceous Matrix to Boost Photocatalytic Performance. J. Alloy. Compd. 2022, 913, 165214. [Google Scholar] [CrossRef]
- Seabold, J.A.; Choi, K.-S. Efficient and Stable Photo-Oxidation of Water by a Bismuth Vanadate Photoanode Coupled with an Iron Oxyhydroxide Oxygen Evolution Catalyst. J. Am. Chem. Soc. 2012, 134, 2186–2192. [Google Scholar] [CrossRef]
- Labhane, P.K.; Sonawane, G.H.; Sonawane, S.H. Influence of Rare-Earth Metal on the Zinc Oxide Nanostructures: Application in the Photocatalytic Degradation of Methylene Blue and p-Nitro Phenol. Green Process. Synth. 2018, 7, 360–371. [Google Scholar] [CrossRef]
- Moscow, S.; Kavinkumar, V.; Sriramkumar, M.; Jothivenkatachalam, K.; Saravanan, P.; Rajamohan, N.; Vasseghian, Y.; Rajasimman, M. Impact of Erbium (Er) and Yttrium (Y) Doping on BiVO4 Crystal Structure towards the Enhancement of Photoelectrochemical Water Splitting and Photocatalytic Performance. Chemosphere 2022, 299, 134343. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.; Sharmin, F.; Mamun, M.A.A.; Hasan, S.; Hakim, M.A.; Basith, M.A. Effect of Gd and Y Co-Doping in BiVO4 Photocatalyst for Enhanced Degradation of Methylene Blue Dye. J. Alloy. Compd. 2022, 895, 162639. [Google Scholar] [CrossRef]
- Sudrajat, H.; Hartuti, S. Increased photocatalytic activity caused by B doping into BiVO4. Res. Chem. Intermed. 2019, 45, 2179–2195. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Q.; Che, Y.; Zhang, L.; Zhang, D. Characterization and Photocatalytic Properties of N-Doped BiVO4 Synthesized via a Sol–Gel Method. J Alloy. Compd. 2013, 548, 70–76. [Google Scholar] [CrossRef]
- Li, J.-Q.; Guo, Z.-Y.; Liu, H.; Du, J.; Zhu, Z.-F. Two-Step Hydrothermal Process for Synthesis of F-Doped BiVO4 Spheres with Enhanced Photocatalytic Activity. J Alloy. Compd. 2013, 581, 40–45. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, H.; Liu, J.; Dong, D.; Che, Y.; Yang, C. Enhanced Visible-Light-Driven Photocatalytic Activity of B-Doped BiVO4 Synthesized Using a Corn Stem Template. Mater. Sci. Semicond. Process. 2015, 30, 307–313. [Google Scholar] [CrossRef]
- Cao, S.W.; Yin, Z.; Barber, J.; Boey, F.Y.C.; Loo, S.C.J.; Xue, C. Preparation of Au-BiVO4 Heterogeneous Nanostructures as Highly Efficient Visible-Light Photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 418–423. [Google Scholar] [CrossRef]
- Venkata Reddy, C.; Neelakanta Reddy, I.; Ravindranadh, K.; Raghava Reddy, K.; Shim, J.; Cheolho, B. Au-Doped BiVO4 Nanostructure-Based Photoanode with Enhanced Photoelectrochemical Solar Water Splitting and Electrochemical Energy Storage Ability. Appl. Surf. Sci. 2021, 545, 149030. [Google Scholar] [CrossRef]
- Ma, C.; Din, S.T.U.; Seo, W.C.; Lee, J.; Kim, Y.; Jung, H.; Yang, W. BiVO4 Ternary Photocatalyst Co-Modified with N-Doped Graphene Nanodots and Ag Nanoparticles for Improved Photocatalytic Oxidation: A Significant Enhancement in Photoinduced Carrier Separation and Broad-Spectrum Light Absorption. Sep. Purif. Technol. 2021, 264, 118423. [Google Scholar] [CrossRef]
- Yalçın, E.; Dükkancı, M. Ternary CuS@Ag/BiVO4 Composite for Enhanced Photo-Catalytic and Sono-Photocatalytic Performance under Visible Light. J. Solid State Chem. 2022, 313, 123319. [Google Scholar] [CrossRef]
- Yang, L.; Chen, H.; Xu, Y.; Qian, R.; Chen, Q.; Fang, Y. Synergetic Effects by Co2+ and PO43− on Mo-Doped BiVO4 for an Improved Photoanodic H2O2 Evolution. Chem. Eng. Sci. 2022, 251, 117435. [Google Scholar] [CrossRef]
- Resasco, J.; Zhang, H.; Kornienko, N.; Becknell, N.; Lee, H.; Guo, J.; Briseno, A.L.; Yang, P. TiO2/BiVO4 Nanowire Heterostructure Photoanodes Based on Type II Band Alignment. ACS Cent. Sci. 2016, 2, 80–88. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Tang, Y.; Ng, Y.H.; Li, L.; Jiang, F.; Wang, J.; Chen, W.; Li, L. Understanding Photoelectrocatalytic Degradation of Tetracycline over Three-Dimensional Coral-like ZnO/BiVO4 Nanocomposite. Mater. Chem. Phys. 2021, 271, 124871. [Google Scholar] [CrossRef]
- Hemmatpour, P.; Nezamzadeh-Ejhieh, A. A Z-Scheme CdS/BiVO4 Photocatalysis towards Eriochrome Black T: An Experimental Design and Mechanism Study. Chemosphere 2022, 307, 135925. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Wen, Y.; Lin, K.; Li, X. 2D/3D WO3/BiVO4 Heterostructures for Efficient Photoelectrocatalytic Water Splitting. Int. J. Hydrogen Energy 2021, 46, 27506–27515. [Google Scholar] [CrossRef]
- Liaqat, M.; Khalid, N.R.; Tahir, M.B.; Znaidia, S.; Alrobei, H.; Alzaid, M. Visible Light Induced Photocatalytic Activity of MnO2/BiVO4 for the Degradation of Organic Dye and Tetracycline. Ceram. Int. 2022, 49, 10455–10461. [Google Scholar] [CrossRef]
- Gao, L.; Long, X.; Wei, S.; Wang, C.; Wang, T.; Li, F.; Hu, Y.; Ma, J.; Jin, J. Facile Growth of AgVO3 Nanoparticles on Mo-Doped BiVO4 Film for Enhanced Photoelectrochemical Water Oxidation. Chem. Eng. J 2019, 378, 122193. [Google Scholar] [CrossRef]
- Iwase, A.; Yoshino, S.; Takayama, T.; Ng, Y.H.; Amal, R.; Kudo, A. Water Splitting and CO2 Reduction under Visible Light Irradiation Using Z-Scheme Systems Consisting of Metal Sulfides, CoOx-Loaded BiVO4, and a Reduced Graphene Oxide Electron Mediator. J. Am. Chem. Soc. 2016, 138, 10260–10264. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Chen, W.; Huang, M.; Xu, J.; Lv, J. Construction of a Switchable G-C3N4/BiVO4 Heterojunction from the Z-Scheme to the Type II by Incorporation of Pyromellitic Diimide. Cryst. Growth Des. 2022, 22, 1645–1653. [Google Scholar] [CrossRef]
- Zhao, D.; Zong, W.; Fan, Z.; Xiong, S.; Du, M.; Wu, T.; Fang, Y.-W.; Ji, F.; Xu, X. Synthesis of Carbon-Doped BiVO4@multi-Walled Carbon Nanotubes with High Visible-Light Absorption Behavior, and Evaluation of Their Photocatalytic Properties. CrystEngComm 2016, 18, 9007–9015. [Google Scholar] [CrossRef]
- Wei, X.; Xu, X.; Yang, X.; Liu, Z.; Naraginti, S.; Sen, L.; Weidi, S.; Buwei, L. Novel Assembly of BiVO4@N-Biochar Nanocomposite for Efficient Detoxification of Triclosan. Chemosphere 2022, 298, 134292. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, Y.; Zhang, R.; Li, S.; Zhang, J.; Xie, T.; Wang, D.; Lin, Y. Investigation on Al3+and Al2O3 Coexist in BiVO4 for Efficient Methylene Blue Degradation: Insight into Surface States and Charge Separation. Langmuir 2021, 37, 7617–7624. [Google Scholar] [CrossRef]
- Wetchakun, N.; Chaiwichain, S.; Inceesungvorn, B.; Pingmuang, K.; Phanichphant, S.; Minett, A.I.; Chen, J. BiVO4/CeO2 Nanocomposites with High Visible-Light-Induced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2012, 4, 3718–3723. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wan, S.; Li, C.; Zhang, K. Graphene oxide as a hole extraction layer loaded on BiVO4 photoanode for highly efficient photoelectrochemical water splitting. Rare Met. 2022, 41, 3795–3802. [Google Scholar] [CrossRef]
- Alhaddad, M.; Amin, M.S. Removal of Ciprofloxacin Applying Pt@BiVO4-g-C3N4 Nanocomposite under Visible Light. Opt. Mater. 2022, 124, 111976. [Google Scholar] [CrossRef]
- Dabodiya, T.S.; Selvarasu, P.; Murugan, A.V. Tetragonal to Monoclinic Crystalline Phases Change of BiVO4 via Microwave-Hydrothermal Reaction: In Correlation with Visible-Light-Driven Photocatalytic Performance. Inorg. Chem. 2019, 58, 5096–5110. [Google Scholar] [CrossRef]
- Patil, S.S.; Lee, J.; Kim, T.; Nagappagari, L.R.; Lee, K. Controlled Synthesis and Structural Modulation to Boost Intrinsic Photocatalytic Activity of BiVO4. CrystEngComm 2022, 24, 2686–2696. [Google Scholar] [CrossRef]
- Yan, M.; Wu, Y.; Yan, Y.; Yan, X.; Zhu, F.; Hua, Y.; Shi, W. Synthesis and Characterization of Novel BiVO4/Ag3VO4 Heterojunction with Enhanced Visible-Light-Driven Photocatalytic Degradation of Dyes. ACS Sustain. Chem. Eng. 2016, 4, 757–766. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Z.; Zhang, J.; Tian, B. Facet-Heterojunction-Based Z-Scheme BiVO4{010} Microplates Decorated with AgBr-Ag Nanoparticles for the Photocatalytic Inactivation of Bacteria and the Decomposition of Organic Contaminants. ACS Appl. Nano Mater. 2020, 3, 8604–8617. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, X.; Xia, Y.; Zhang, A.; Shi, J.; Gao, L.; Wei, H.; Chen, W. Z-scheme BiVO4/g-C3N4 heterojunction: An efficient, stable and heterogeneous catalyst with highly enhanced photocatalytic activity towards Malachite Green assisted by H2O2 under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126445. [Google Scholar] [CrossRef]
- Hu, C.; Tian, M.; Wu, L.; Chen, L. Enhanced photocatalytic degradation of paraben preservative over designed g-C3N4/BiVO4 S-scheme system and toxicity assessment. Ecotoxicol. Environ. Saf. 2022, 231, 113175. [Google Scholar] [CrossRef]
- Huang, C.-M.; Pan, G.-T.; Peng, P.-Y.; Yang, T.C.-K. In Situ DRIFT Study of Photocatalytic Degradation of Gaseous Isopropanol over BiVO4 under Indoor Illumination. J. Mol. Catal. A Chem. 2010, 327, 38–44. [Google Scholar] [CrossRef]
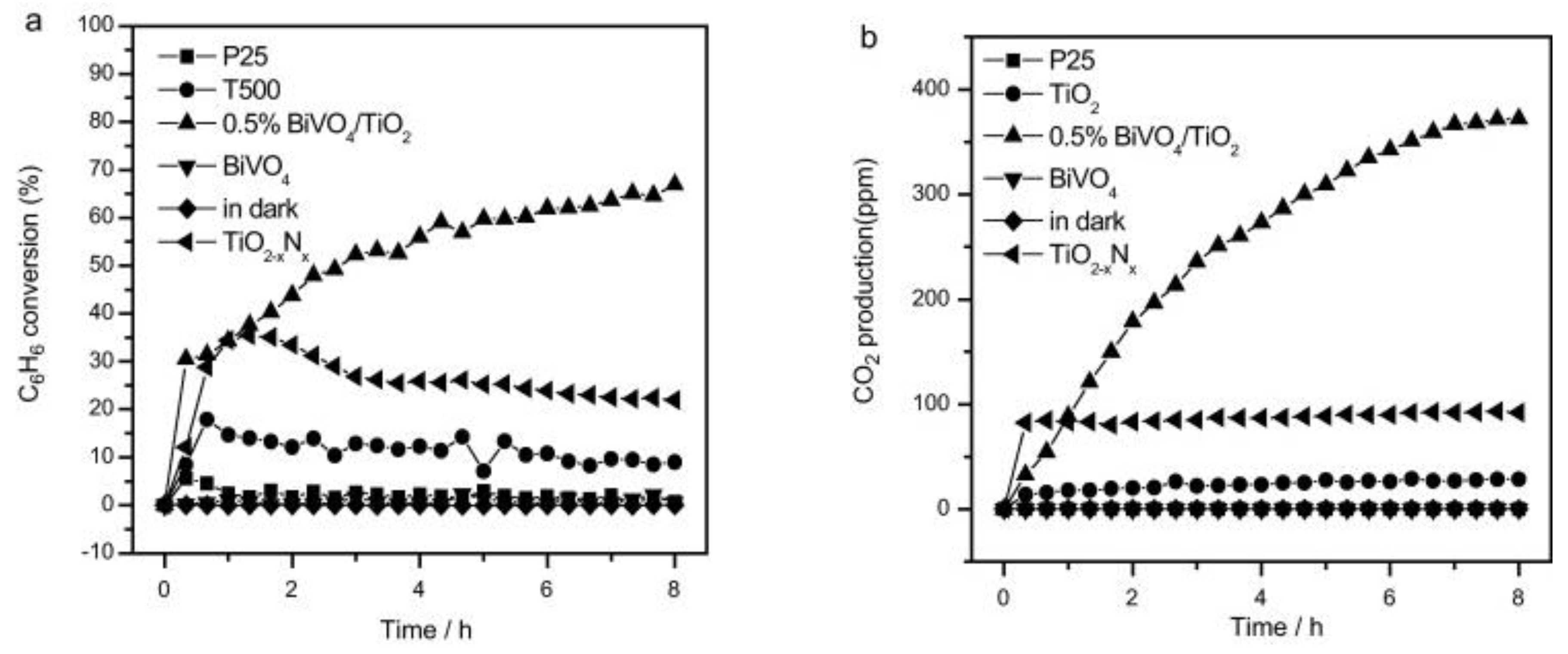
- Hu, Y.; Li, D.; Zheng, Y.; Chen, W.; He, Y.; Shao, Y.; Fu, X.; Xiao, G. BiVO4/TiO2 Nanocrystalline Heterostructure: A Wide Spectrum Responsive Photocatalyst towards the Highly Efficient Decomposition of Gaseous Benzene. Appl. Catal. B 2011, 104, 30–36. [Google Scholar] [CrossRef]
- Zhao, W.R.; Zeng, W.Y.; Xi, H.P.; Yu, X.X. Photocatalytic Degradation of Gas-Phase Toluene over CuO Loaded BiVO4 Hollow Nanospheres under Visible-Light Irradiation. Wuli Huaxue Xuebao/Acta Phys.-Chim. Sin. 2014, 30, 761–767. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Zhao, Q.; Ke, J.; Zhang, D. Novel V2O5/BiVO4/TiO2 Nanocomposites with High Visible-Light-Induced Photocatalytic Activity for the Degradation of Toluene. J. Phys. Chem. C 2014, 118, 10113–10121. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Zhao, Q.; Tadé, M.O.; Liu, S. Quantum-Sized BiVO4 Modified TiO2 Microflower Composite Heterostructures: Efficient Production of Hydroxyl Radicals towards Visible Light-Driven Degradation of Gaseous Toluene. J. Mater. Chem. A Mater. 2015, 3, 21655–21663. [Google Scholar] [CrossRef]
- Shi, Q.; Zhao, W.; Xie, L.; Chen, J.; Zhang, M.; Li, Y. Enhanced Visible-Light Driven Photocatalytic Mineralization of Indoor Toluene via a BiVO4/Reduced Graphene Oxide/Bi2O3 All-Solid-State Z-Scheme System. J. Alloy. Compd. 2016, 662, 108–117. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Wei, Z.; Ye, S.; Dionysiou, D.D. Synthesis of BiVO4/P25 Composites for the Photocatalytic Degradation of Ethylene under Visible Light. Chem. Eng. J. 2017, 314, 443–452. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Zhao, Q.; Tadé, M.O.; Liu, S. Construction of p-n heterojunction β-Bi2O3/BiVO4 nanocomposite with improved photoinduced charge transfer property and enhanced activity in degradation of ortho-dichlorobenzene. Appl. Catal. B 2017, 219, 259–268. [Google Scholar] [CrossRef]
- Sun, R.; Shi, Q.; Zhang, M.; Xie, L.; Chen, J.; Yang, X.; Chen, M.; Zhao, W. Enhanced Photocatalytic Oxidation of Toluene with a Coral-like Direct Z-Scheme BiVO4/g-C3N4 Photocatalyst. J. Alloys. Compd. 2017, 714, 619–626. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, C.; Lu, J.; Xiao, J.; Lei, Y.; Yu, Z. BiVO4/α-Fe2O3 Catalytic Degradation of Gaseous Benzene: Preparation, Characterization and Photocatalytic Properties. Appl. Surf. Sci. 2018, 427, 141–147. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Fu, J.; Ba, M.; Sun, F.; Zhang, P.; Zou, J. Hydrothermal Synthesis of BiVO4/TiO2 Composites and Their Application for Degradation of Gaseous Benzene under Visible Light Irradiation. Appl. Surf. Sci. 2018, 436, 319–326. [Google Scholar] [CrossRef]
- Yang, J.; Shi, Q.; Zhang, R.; Xie, M.; Jiang, X.; Wang, F.; Cheng, X.; Han, W. BiVO4 Quantum Tubes Loaded on Reduced Graphene Oxide Aerogel as Efficient Photocatalyst for Gaseous Formaldehyde Degradation. Carbon 2018, 138, 118–124. [Google Scholar] [CrossRef]
- Li, Y.; Xing, X.; Pei, J.; Li, R.; Wen, Y.; Cui, S.; Liu, T. Automobile Exhaust Gas Purification Material Based on Physical Adsorption of Tourmaline Powder and Visible Light Catalytic Decomposition of g-C3N4/BiVO4. Ceram. Int. 2020, 46, 12637–12647. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Chen, Z.; Murugananthan, M.; Chen, Y.; Zhang, Y. Stabilized Oxygen Vacancies over Heterojunction for Highly Efficient and Exceptionally Durable VOCs Photocatalytic Degradation. Appl. Catal. B 2020, 273, 119061. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, M.; Zhang, Z.; Li, Y.; Qu, Y.; Liu, Z.; Yang, J.; Xie, M.; Han, W. Energy and Separation Optimization of Photogenerated Charge in BiVO4 Quantum Dots by Piezo-Potential for Efficient Gaseous Pollutant Degradation. Nano Energy 2020, 69, 104448. [Google Scholar] [CrossRef]
- Zhu, Z.; Lin, Y.-C.; Chung, C.-L.; Wu, R.-J.; Huang, C.-L. A Novel Composite of Triangular Silver Nanoplates on BiVO4 for Gaseous Formaldehyde Degradation. Appl. Surf. Sci. 2021, 543, 148784. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Q.; Ma, Y.; Zhu, R.; Fan, Y.; Huang, J.; Niu, H.; Dong, Y.; Li, D.; Zhang, Y.; et al. Highly Efficient Photocatalytic Hydrogen Evolution and Simultaneous Formaldehyde Degradation over Z-Scheme ZnIn2S4-NiO/BiVO4 Hierarchical Heterojunction under Visible Light Irradiation. Chem. Eng. J. 2021, 423, 130164. [Google Scholar] [CrossRef]
- Shi, L.; Xue, J.; Xiao, W.; Wang, P.; Long, M.; Bi, Q. Efficient Degradation of VOCs Using Semi-Coke Activated Carbon Loaded Ternary Z-Scheme Heterojunction Photocatalyst BiVO4–BiPO4–g-C3N4 under Visible Light Irradiation. Phys. Chem. Chem. Phys. 2022, 24, 22987–22997. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Sun, G.; Tian, B.; Zhang, J. Facet-Heterojunction-Based Photothermocatalyst CdS-Au-{010}BiVO4{110}-MnOx with Excellent Synergetic Effect for Toluene Degradation. Chem. Eng. J. 2022, 442, 135835. [Google Scholar] [CrossRef]

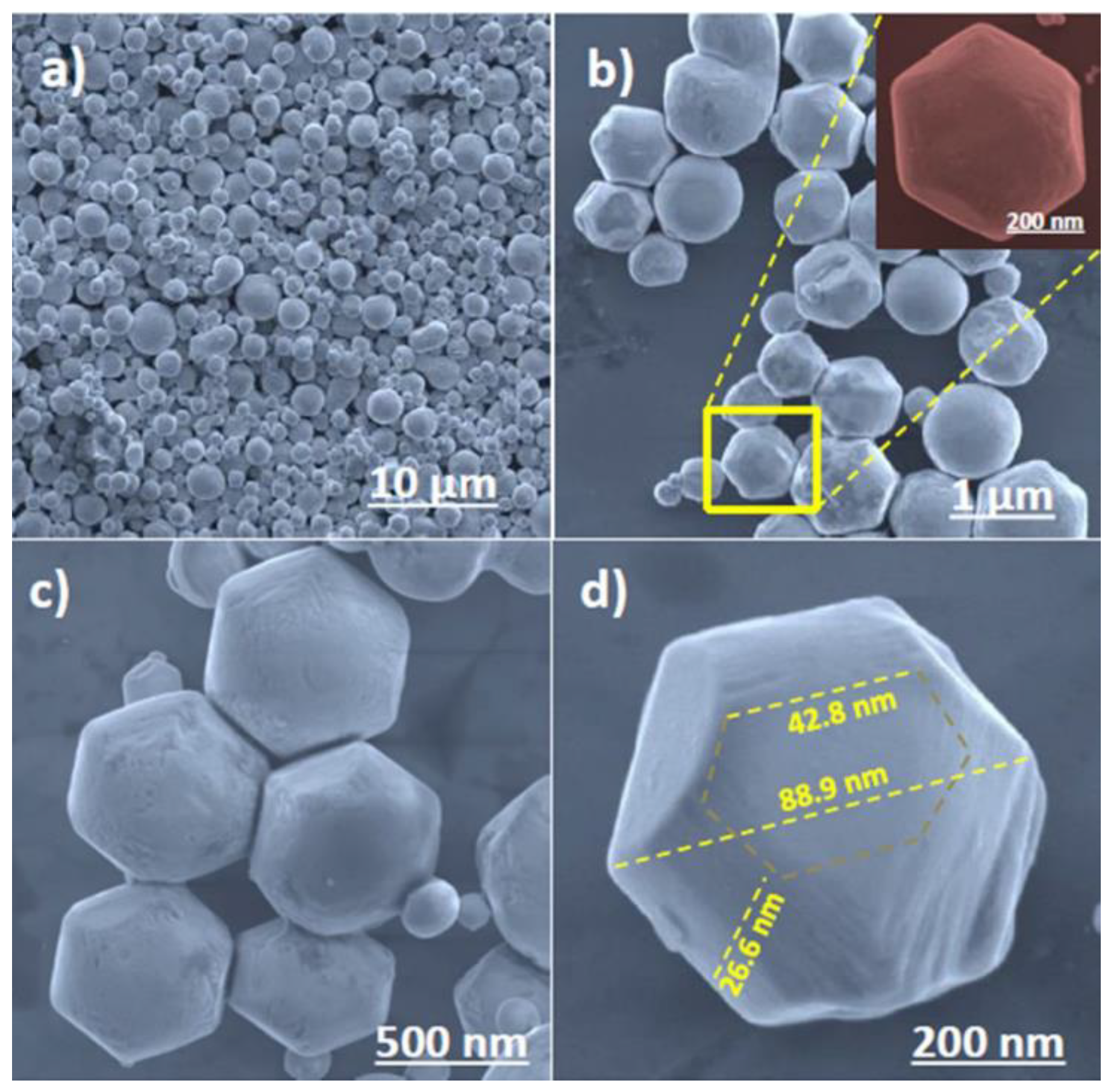
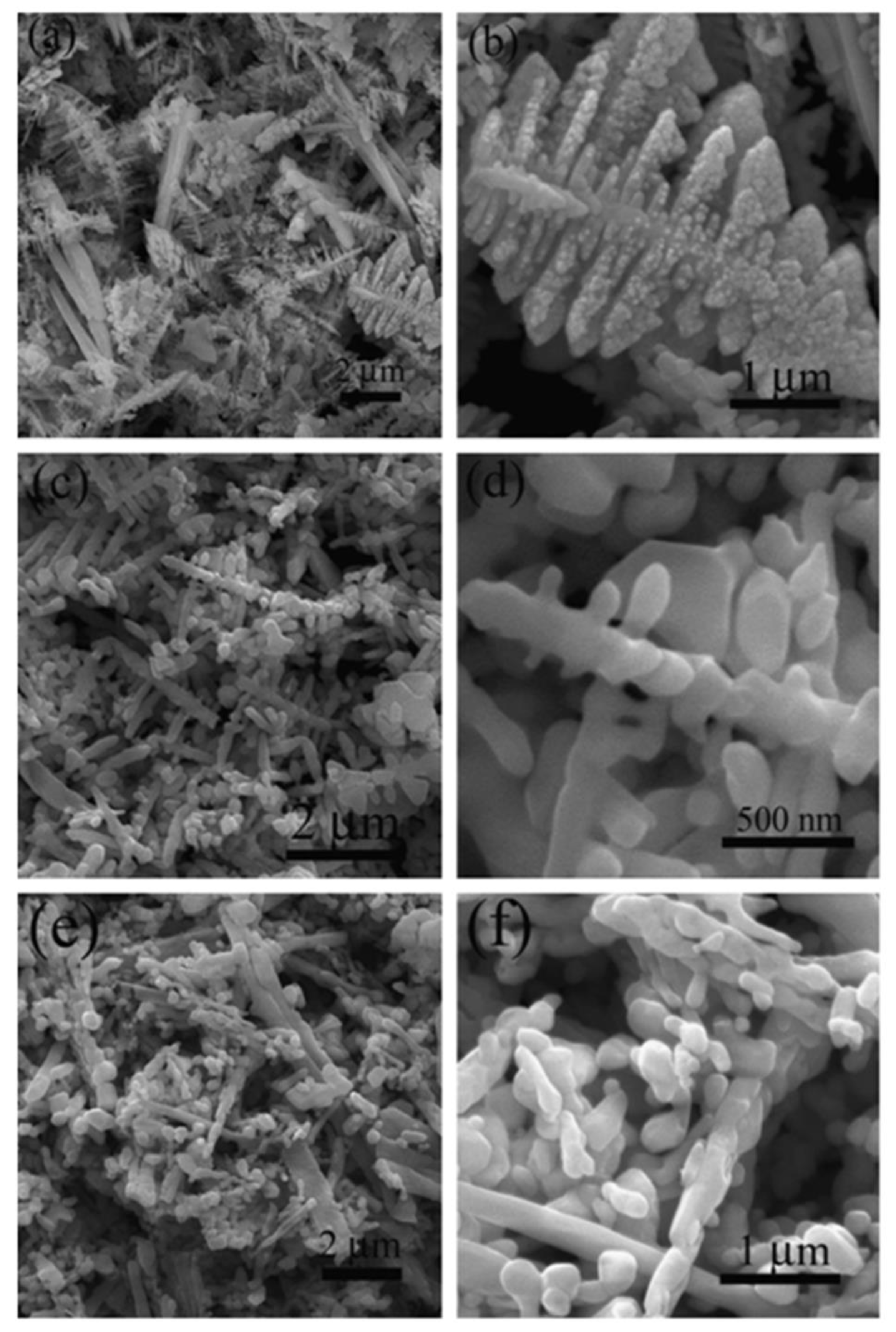
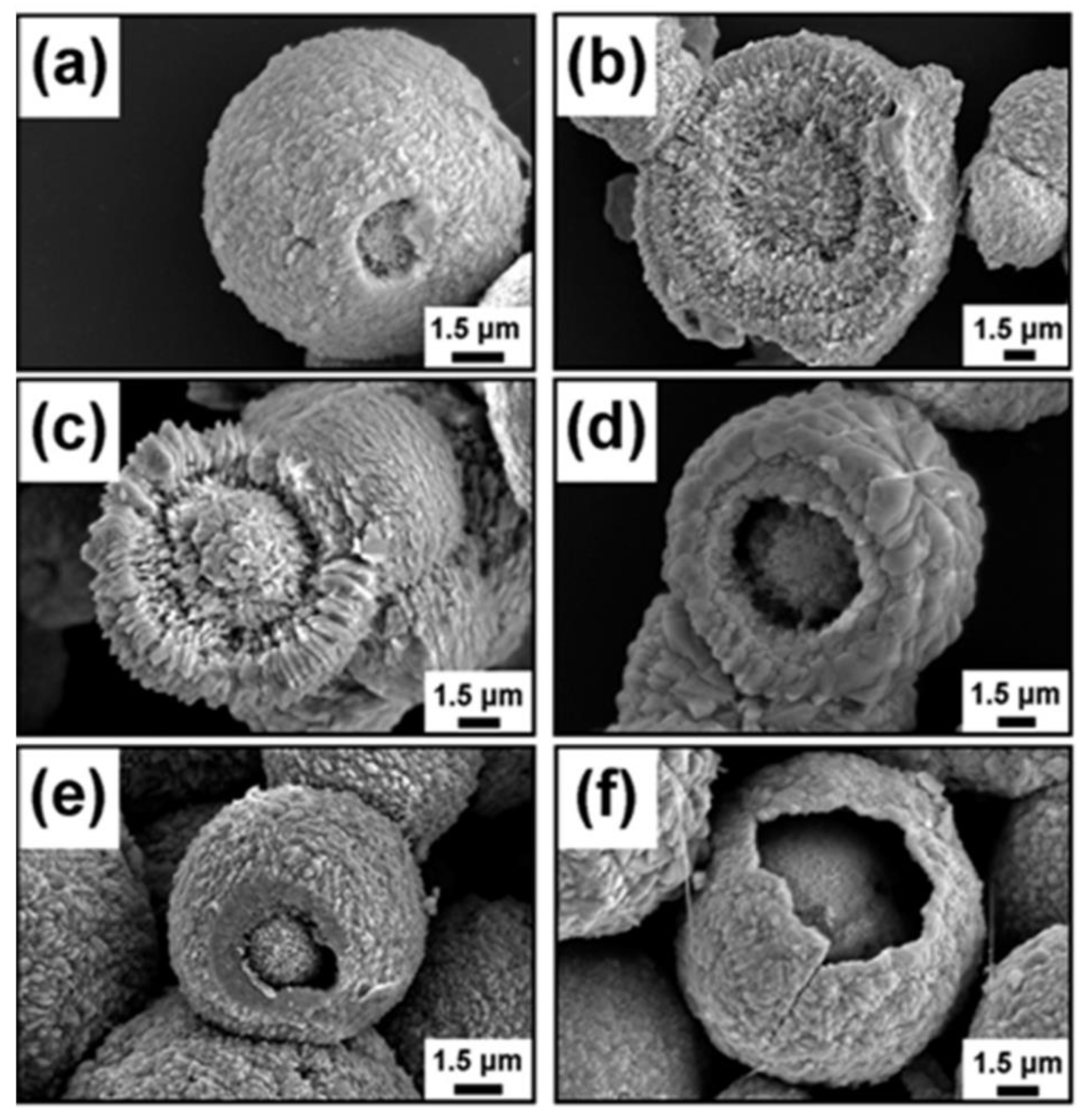

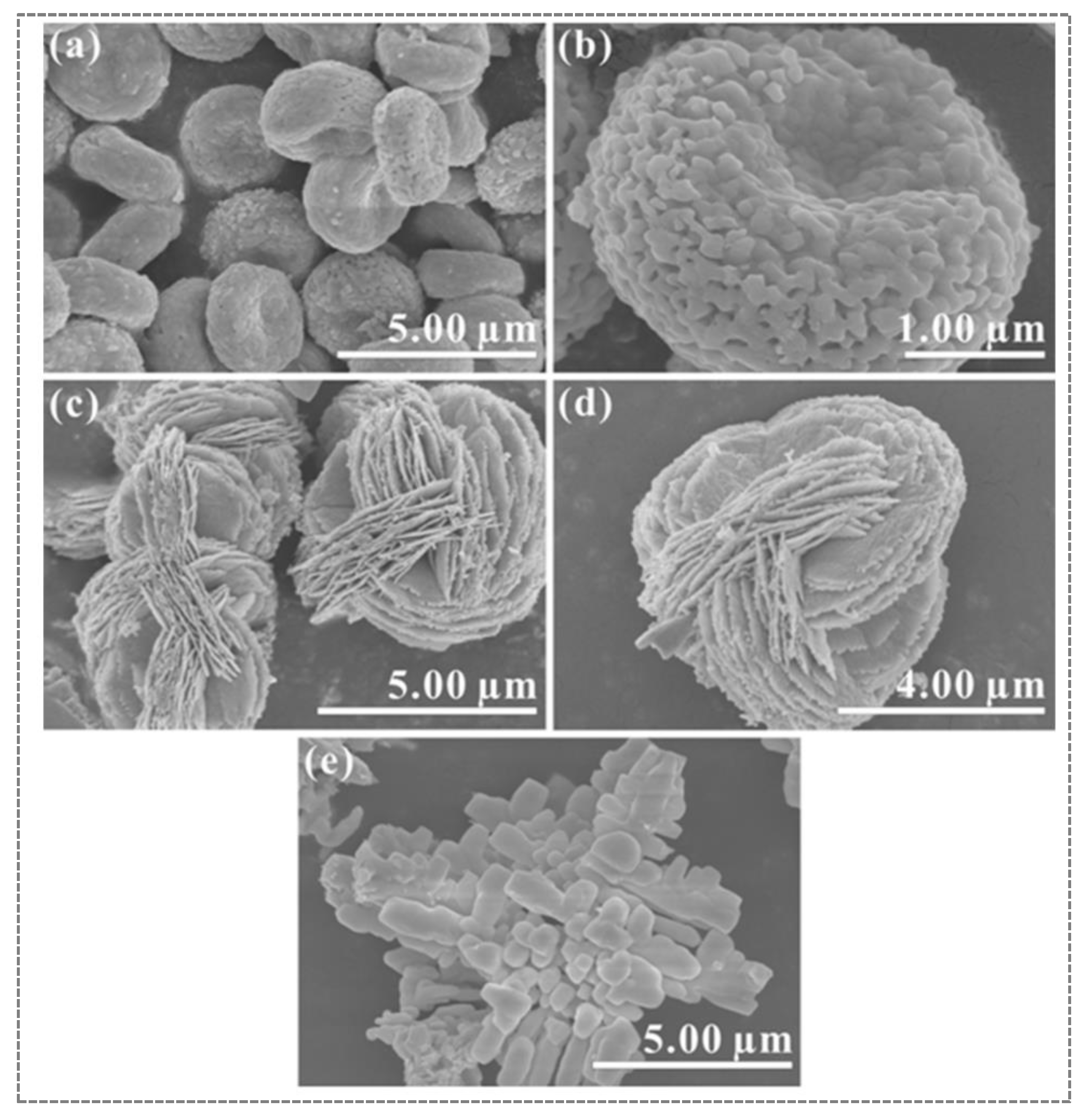



| S. No | Semiconductor | Bandgap Energy (Eg), eV | VB Position | CB Position |
|---|---|---|---|---|
| 1. | TiO2 | 3.2 | +3.1 | −0.1 |
| 2. | ZnO | 3.2 | +3.0 | −0.2 |
| 3. | WO3 | 2.8 | +3.0 | +0.4 |
| 4. | ZrO2 | 5.2 | +5.0 | +0.2 |
| 5. | SnO2 | 3.8 | +4.1 | +0.3 |
| 6. | SrTiO3 | 3.2 | +3.1 | −0.1 |
| 7. | CuO | 2.1 | +2.36 | +0.26 |
| 8. | ZnS | 3.7 | +1.4 | −2.3 |
| 9. | CdS | 2.5 | +2.1 | −0.4 |
| 10. | BiVO4 | 2.3–2.4 | +2.7 | +0.3 |
| 11. | Bi2MoO6 | 2.63 | +2.9 | +0.27 |
| 12. | Bi2WO6 | 2.77 | +3.05 | +0.28 |
| 13. | BiOF | 3.64 | +4.24 | +0.6 |
| 14. | BiOCl | 3.22 | +3.4 | 0.18 |
| 15. | BiOBr | 2.64 | +3.0 | +0.36 |
| 16. | BiOI | 1.77 | +2.32 | +0.55 |
| 17. | Bi2O3 | 2.1–2.8 | +2.48 | +0.38 |
| Method of Synthesis | Morphology | Photocatalytic Activity of Pollutant(s) and Degradation Efficiency | Refs. |
|---|---|---|---|
| Surfactant- and template-free hydrothermal method | Truncated square, 18-sided | Pollutant: MB Dye (20 ppm) Light source: 1000 W xenon lamp % degradation: 91% after 60 min | [42] |
| Hydrothermal method using EDTA as a chelating agent | 2D star-like crystals | Pollutant: MB Dye (15 ppm) Light source: 500-W xenon lamp % degradation: 99.3% after 25 min | [51] |
| Additive-free hydrothermal method | Dendritic structure of BiVO4 | Pollutant: RhB Dye (10 ppm) Light source: 500 W xenon lamp % degradation: 91% after 210 min | [52] |
| Template-free hydrothermal method | Olive-like BiVO4 | Pollutant: MB Dye (10 μM) Light source: 300 W xenon lamp % degradation: 84.1–95.7% (Different pH value) after 180 min | [53] |
| Surfactant-free hydrothermal method | Octahedral | Pollutant: MB Dye (10 ppm) Light source: low power xenon lamp. % degradation: 50–60% after 120 min | [54] |
| Surfactant- and template-free hydrothermal method | Plate morphology and biscuit morphology | Pollutant: RhB Dye (10−5 mol/L) Light source: 500 W xenon lamp % degradation: 99% after 270 min | [55] |
| Hydrothermal method in the presence of triblock copolymer P123 as a surfactant | Polyhedral, rod-like, tubular, leaf-like, and spherical | Pollutant: MB Dye 1.0 × 10−5 mol/L) Light source: 300 W xenon lamp % degradation: 90% after 120 min | [56] |
| Hydrothermal route using of SDBS as an anionic surfactant | 2D single-crystal nanosheets | Pollutant: RhB Dye (2.09 10−4 mol dm−3) Light source: Sunlight % degradation: - 95% after 100 min | [57] |
| Hydrothermal process | Fibrous or needle-like sepiolite distributed peanut-shape monoclinic BiVO4 surface | Pollutant: TCs (5 ppm); MB Dye (10 ppm) Light source: LED lamp % degradation: TCs 78%; MB Dye 96% after 240 min | [58] |
| CTAB-assisted hydrothermal method | Snow-like | Pollutant: CIP (10 ppm) Light source: 500 W xenon lamp % degradation: 98.5% after 70 min | [59] |
| Electro-spinning method | 1D nanofibers | Pollutant: RhB Dye (10 ppm) Light source: 300 W xenon-illuminator % degradation: 100% after 120 min | [60] |
| Electro-spinning method | 1D micro-ribbons | Pollutant: RhB Dye (20 ppm) Light source: 500 W xenon lamp % degradation: 93.3% after 300 min | [61] |
| Solvothermal method through adjusting the solution pH | Red blood cell, flower-like microsphere, and dendrite morphologies | Pollutant: MB Dye (10 ppm) Light source: 500 W xenon lamp % degradation: dendrite-like < flower-like microsphere < red-blood-cell-like morphology after 180 min | [62] |
| Coprecipitation (500 °C for 5 h) | Cuboids | Pollutant: IC Dye (50 mL) Light source: fluorescence light 18 W % degradation: ~90% after 300 min | [63] |
| Precipitation (450 °C for 15 min) | Polyhedral | Pollutant: TBC (5 ppm) Light source: two visible lamps (15 W) % degradation: 97% after 300 min | [64] |
| Sol–gel method | Spherical structures | Pollutant: MO (15 ppm) Light source: 250 W halogen lamp % degradation: 98% after 50 min | [65] |
| Pechini sol–gel method | Rectangular cube-like, plate-like microstructures, plate-like nanostructures, nanorods, quasi-spherical structures | Pollutant: Thiophen (800 ppm) Light source: 400 W Osram lamp % degradation: 92% after 150 min | [66] |
| Modified one-step sol–gel method | Spherical | Pollutant: AB-113 (40 ppm) Light source: 1.6 kW xenon arc ozone-free lamp % degradation: (~99%) after 120 min | [67] |
| S. No. | Materials | VOCs Degraded | VOCs Concentration | Degradation of VOCs (%) | Degradation Time of VOCs (h) | Ref. |
|---|---|---|---|---|---|---|
| 1 | BiVO4 | Isopropanol | 160 ppm | 88 | 12 | [122] |
| 2 | BiVO4/TiO2 | Benzene | 260 ppm | 84 | 8 | [123] |
| 3 | BiVO4/CuO | Toluene | 75 μL/L | 85 | 6 | [124] |
| 4 | V2O5/BiVO4/TiO2 | Toluene | 120 ppm | 91 | 6 | [125] |
| 5 | Quantum-sized BiVO4/TiO2 microflower | Toluene | - | 89 | 6 | [126] |
| 6 | BiVO4/RGO/Bi2O3 | Toluene | 25 ppm | 95.6 | 6 | [127] |
| 7 | BiVO4/P25 | Ethylene | 0.15 ppm | 11.2 | 6 | [128] |
| 8 | β-Bi2O3/BiVO4 | o-DCB | - | 70 | 6 | [129] |
| 9 | Coral-like Z-scheme BiVO4/g-C3N4 | Toluene | 25 ppm | 68.2 | 8 | [130] |
| 10 | BiVO4/α-Fe2O3 | Benzene | 100 ppm | 66.87 | 3.5 | [131] |
| 11 | BiVO4/TiO2 | Benzene | 260 ppm | 41 | 8 | [132] |
| 12 | BiVO4 quantum tubes/rGO | HCHO | 50 ppm | 60 | 15 min | [133] |
| 13 | g-C3N4/BiVO4/tourmaline powder | Automobile exhaust gas (HC, NO, CO) | 300–400 ppm (HC), 2.5–4% (NO), and 45–65 ppm (CO) | 6.9 (HC) 7.2 (NO) 46.7 (CO) | 1 | [134] |
| 14 | Oxygen vacancies (OVs) introduced BiVO4/WO3/TiO2 nanotubes | Toluene | 100 ppm | 100 | 1 | [135] |
| 15 | BiVO4 quantum dots/ZnO nanorod | HCHO | 50 ppm | 100 | 1 | [136] |
| 16 | Ag/BiVO4 | HCHO | 10 ppm | 84 | 5 | [137] |
| 17 | ZnIn2S4-NiO/BiVO4 | HCHO | 1.5 mol/L | 17 mmol/h | 3 | [138] |
| 18 | semi-coke activated carbon/ BiVO4–BiPO4–g-C3N4 | Toluene | 200 ppm | 85.6 | 2 | [139] |
| 19 | CdS-Au-{010}BiVO4{110}-MnOx | Toluene | 4723 mg/m3 | 97.2 | 100 min | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamble, G.S.; Natarajan, T.S.; Patil, S.S.; Thomas, M.; Chougale, R.K.; Sanadi, P.D.; Siddharth, U.S.; Ling, Y.-C. BiVO4 As a Sustainable and Emerging Photocatalyst: Synthesis Methodologies, Engineering Properties, and Its Volatile Organic Compounds Degradation Efficiency. Nanomaterials 2023, 13, 1528. https://doi.org/10.3390/nano13091528
Kamble GS, Natarajan TS, Patil SS, Thomas M, Chougale RK, Sanadi PD, Siddharth US, Ling Y-C. BiVO4 As a Sustainable and Emerging Photocatalyst: Synthesis Methodologies, Engineering Properties, and Its Volatile Organic Compounds Degradation Efficiency. Nanomaterials. 2023; 13(9):1528. https://doi.org/10.3390/nano13091528
Chicago/Turabian StyleKamble, Ganesh S., Thillai Sivakumar Natarajan, Santosh S. Patil, Molly Thomas, Rajvardhan K. Chougale, Prashant D. Sanadi, Umesh S. Siddharth, and Yong-Chein Ling. 2023. "BiVO4 As a Sustainable and Emerging Photocatalyst: Synthesis Methodologies, Engineering Properties, and Its Volatile Organic Compounds Degradation Efficiency" Nanomaterials 13, no. 9: 1528. https://doi.org/10.3390/nano13091528
APA StyleKamble, G. S., Natarajan, T. S., Patil, S. S., Thomas, M., Chougale, R. K., Sanadi, P. D., Siddharth, U. S., & Ling, Y.-C. (2023). BiVO4 As a Sustainable and Emerging Photocatalyst: Synthesis Methodologies, Engineering Properties, and Its Volatile Organic Compounds Degradation Efficiency. Nanomaterials, 13(9), 1528. https://doi.org/10.3390/nano13091528







