Impact of Iodine Electrodeposition on Nanoporous Carbon Electrode Determined by EQCM, XPS and In Situ Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. EQCM Measurements
2.2. Electrodes for In Situ Raman Spectroscopy
2.3. Raman Spectroscopy
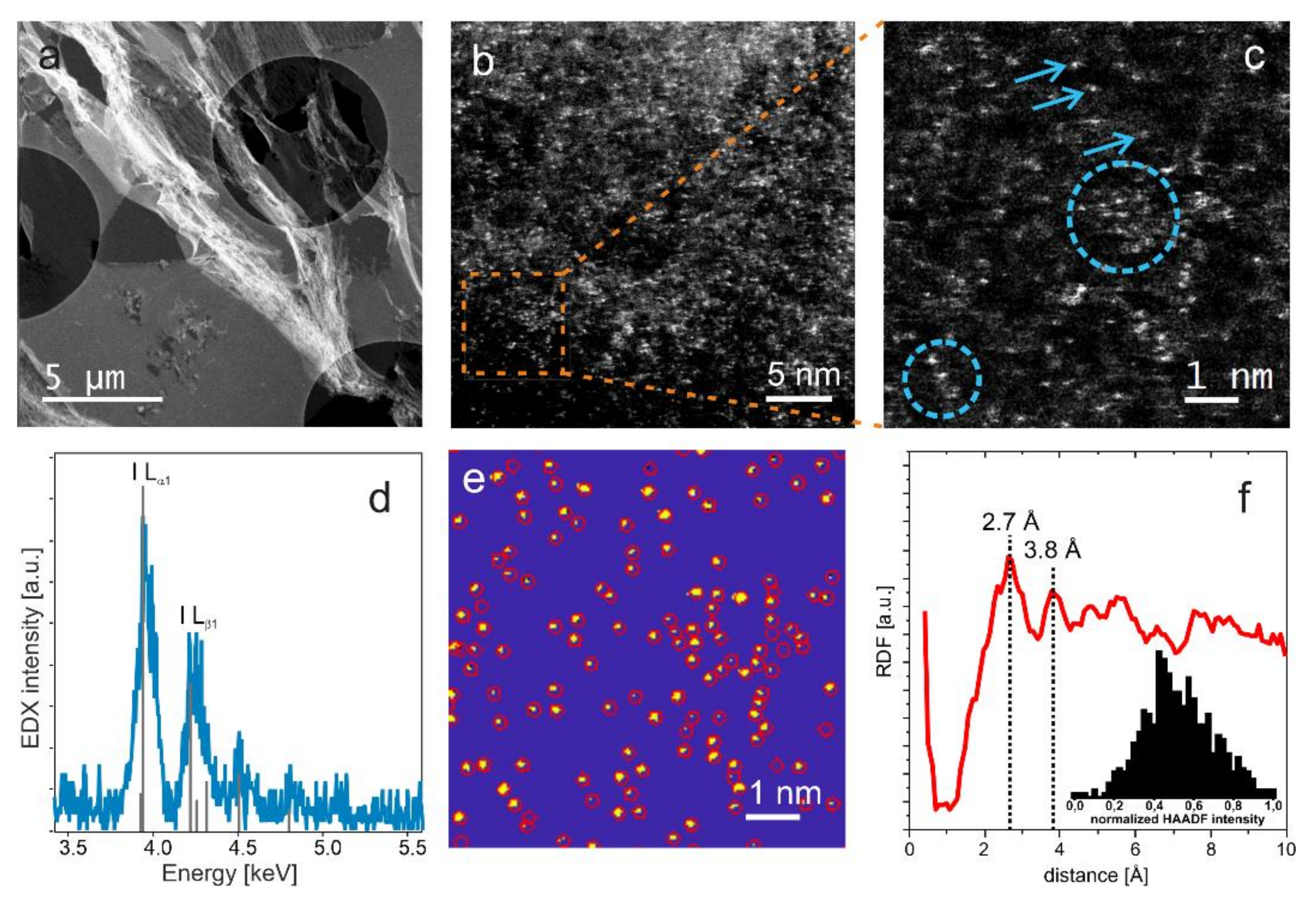
2.4. TEM
2.5. XPS
3. Results and Discussion
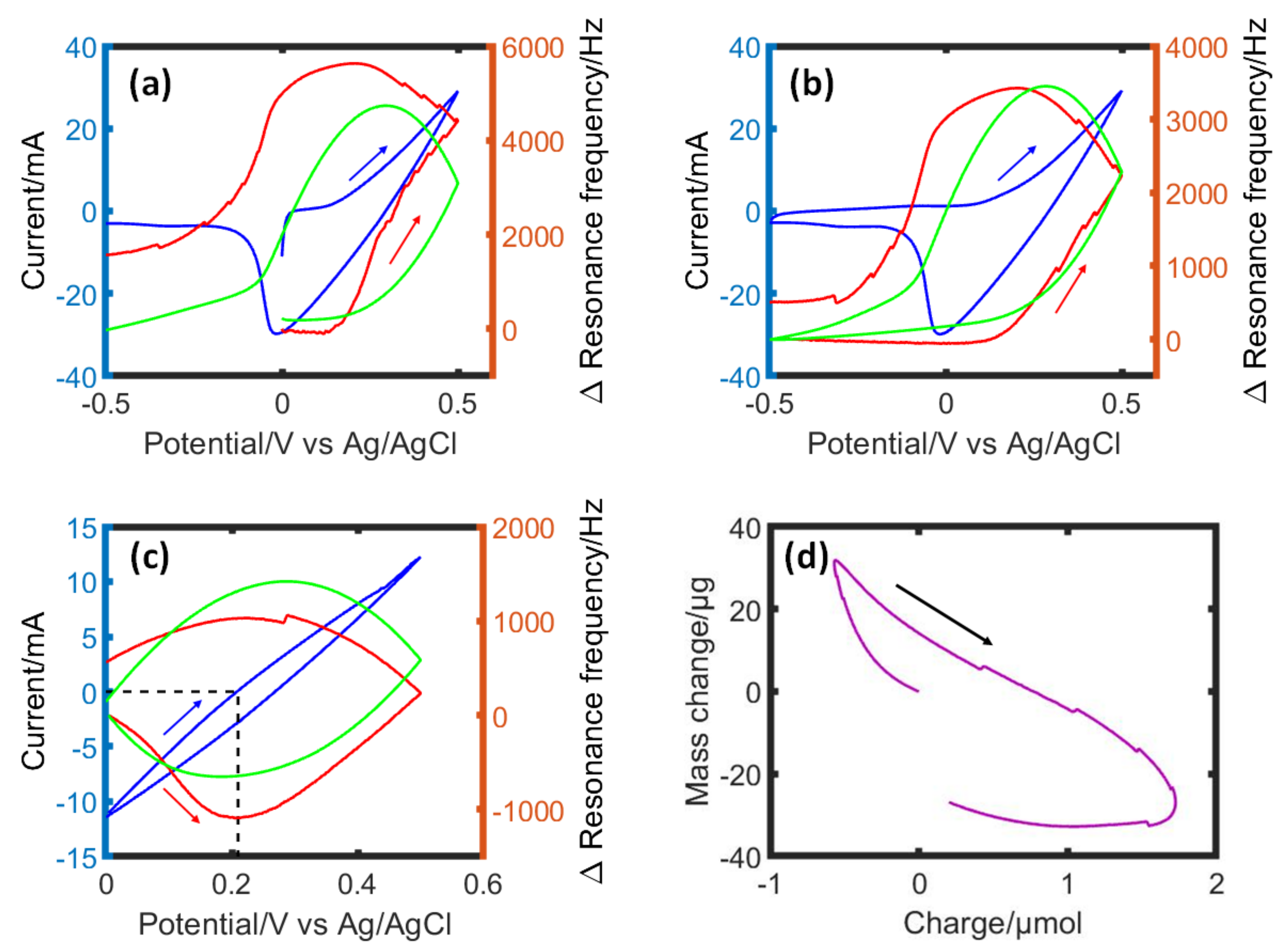
3.1. EQCM Investigation of Iodine Electrodeposition in Porous Carbon Electrode
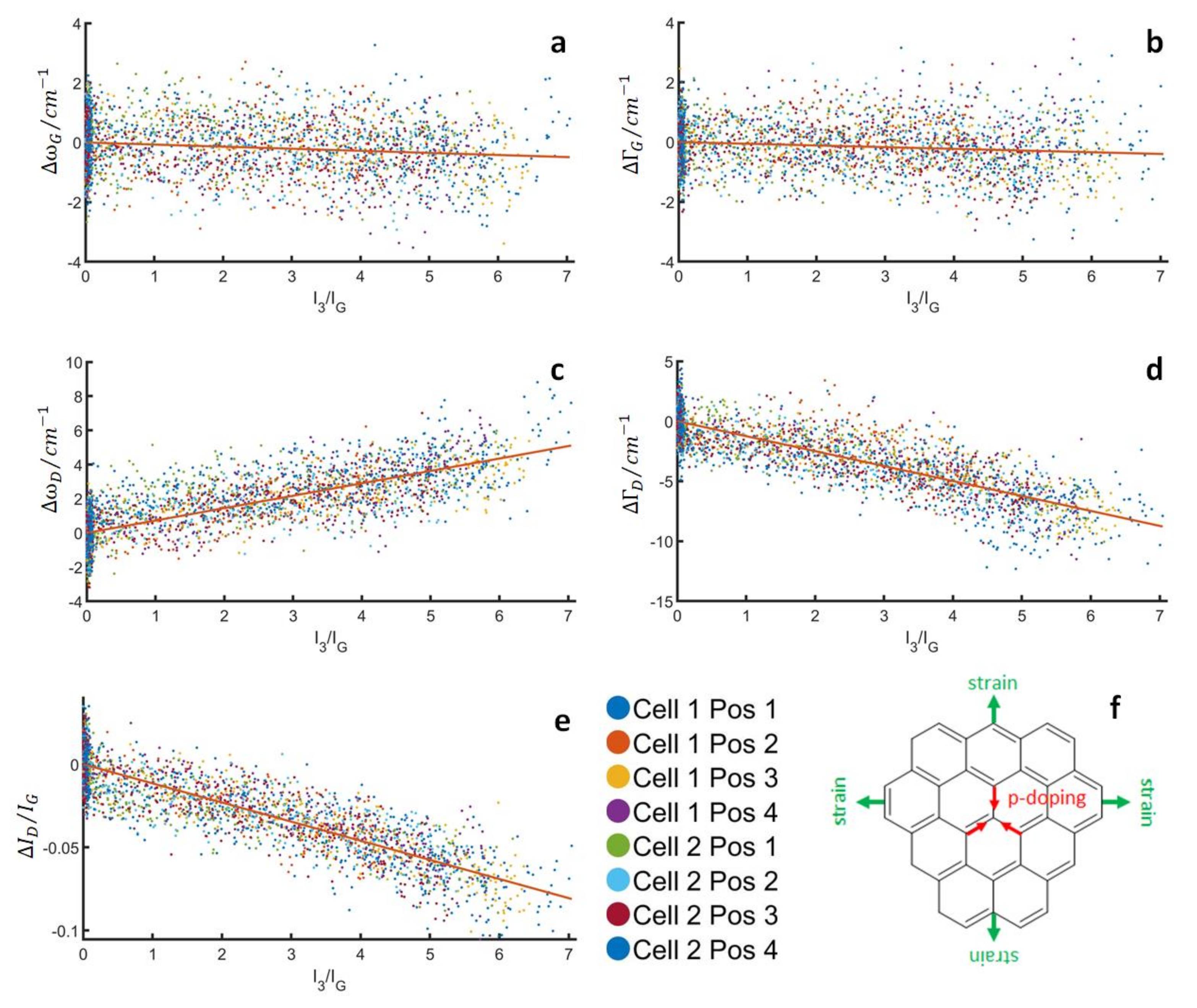
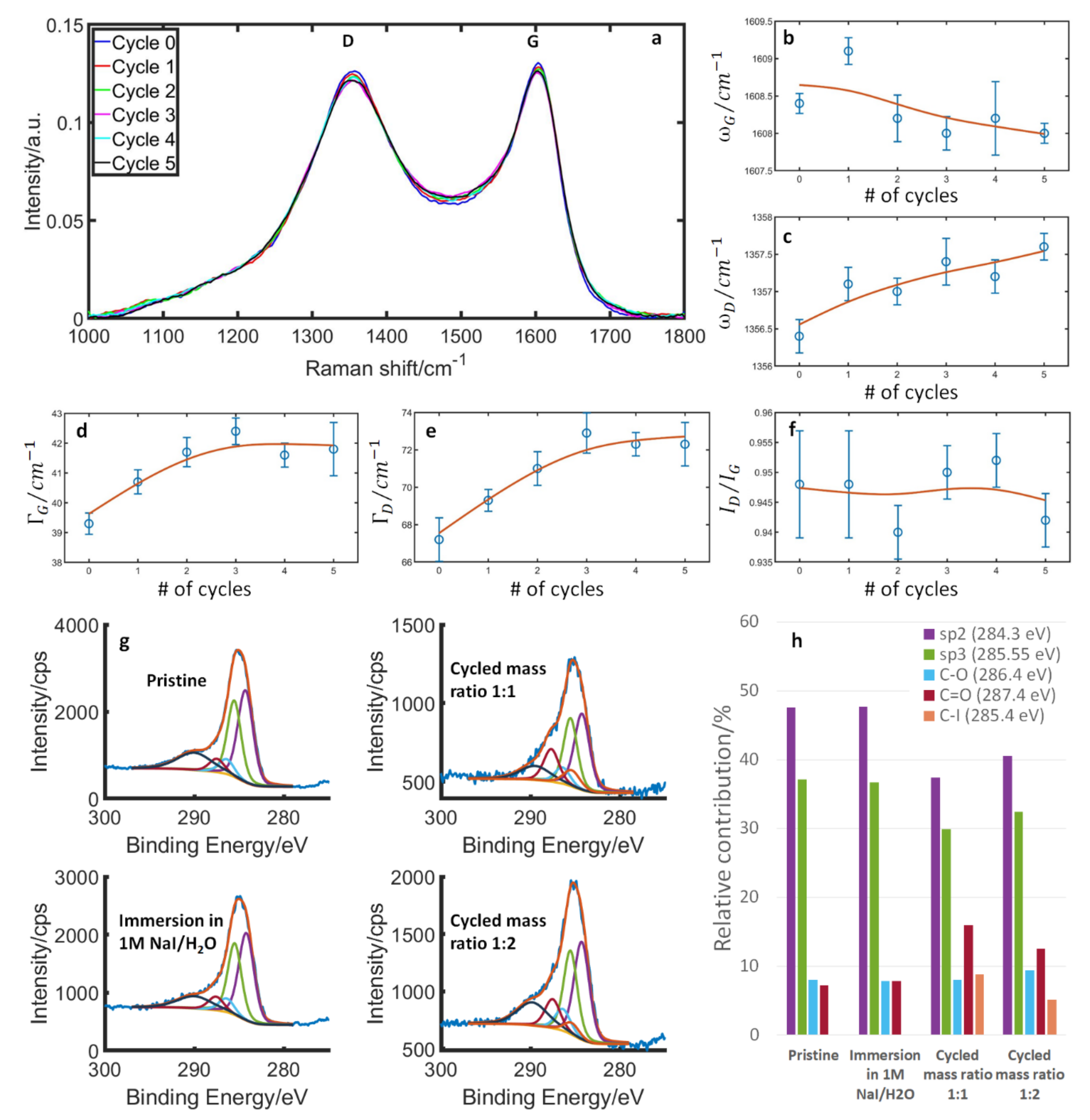
3.2. Structural Parameters of Carbon during Stable Cycling
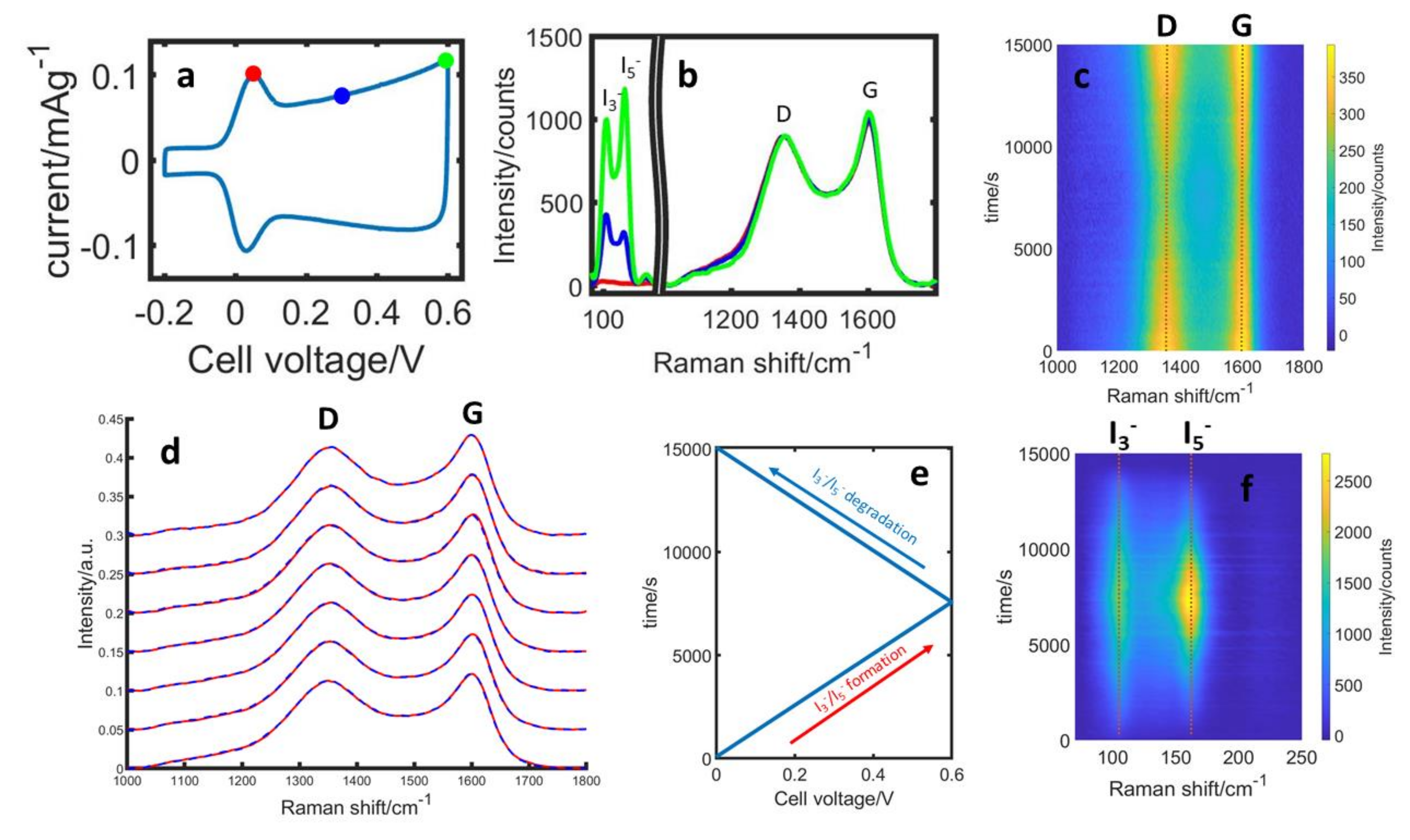
3.3. Structural and Surface Properties during Initial Cycles
3.4. Structural and Surface Properties at Simple Immersion of Carbon in the Electrolyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prehal, C.; Fitzek, H.; Kothleitner, G.; Presser, V.; Gollas, B.; Freunberger, S.A.; Abbas, Q. Persistent and reversible solid iodine electrodeposition in nanoporous carbons. Nat. Commun. 2020, 11, 4838. [Google Scholar] [CrossRef] [PubMed]
- Reber, D.; Kühnel, R.S.; Battaglia, C. High-voltage aqueous supercapacitors based on NaTFSI. Sustain. Energy Fuels 2017, 1, 2155–2161. [Google Scholar] [CrossRef]
- Chen, C.; Li, Z.; Xu, Y.; An, Y.; Wu, L.; Sun, Y.; Liao, H.; Zheng, K.; Zhang, X. High-energy density aqueous zinc-iodine batteries with ultra-long cycle life enabled by the ZnI2 additive. ACS Sustain. Chem. Eng. 2021, 9, 13268–13276. [Google Scholar] [CrossRef]
- Bai, C.; Cai, F.; Wang, L.; Guo, S.; Liu, X.; Yuan, Z. A sustainable aqueous Zn-I2 battery. Nano Res. 2018, 11, 3548–3554. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, T.; Du, Q.; Li, Y.; Yi, H.; Zhou, X.; Li, Z.; Gao, L.; Zhang, X.; Liang, X. A four-electron Zn-I2 aqueous battery enabled by reversible I−/I2/I+ conversion. Nat. Commun. 2021, 12, 170. [Google Scholar] [CrossRef]
- Hoyt, R.A.; Remillard, E.M.; Cubuk, E.D.; Vecitis, C.D.; Kaxiras, E. Polyiodide-doped graphene. J. Phys. Chem. C 2017, 121, 609–615. [Google Scholar] [CrossRef]
- Tristant, D.; Puech, P.; Gerber, I.C. Theoretical study of polyiodide formation and stability on monolayer and bilayer graphene. Phys. Chem. Chem. Phys. 2015, 17, 30045–30051. [Google Scholar] [CrossRef]
- Tristant, D.; Puech, P.; Gerber, I.C. Theoretical study of graphene doping mechanism by iodine molecules. J. Phys. Chem. C 2015, 119, 12071–12078. [Google Scholar] [CrossRef]
- Karlicky, F.; Kumara Ramanatha Datta, K.; Otyepka, M.; Zboril, R. Halogenated graphenes: Rapidly growing family of graphene derivatives. ACS Nano 2013, 7, 6434–6464. [Google Scholar] [CrossRef]
- Jung, N.; Kim, N.; Jockusch, S.; Turro, N.J.; Kim, P.; Brus, L. Charge transfer chemical doping of few layer graphenes: Charge distribution and band gap formation. Nano Lett. 2009, 9, 4133–4137. [Google Scholar] [CrossRef]
- Yao, Z.; Nie, H.; Yang, Z.; Zhou, X.; Liu, Z.; Huang, S. Catalyst-free synthesis of iodine-doped graphene via a facile thermal annealing process and its use for electrocatalytic oxygen reduction in an alkaline medium. Chem. Commun. 2012, 48, 1027–1029. [Google Scholar] [CrossRef] [PubMed]
- Marinoiu, A.; Gatto, I.; Raceanu, M.; Varlam, M.; Moise, C.; Pantazi, A.; Enachescu, M. Low cost iodine doped graphene for fuel cell electrodes. Int. J. Hydrog. Energy 2017, 42, 26877–26888. [Google Scholar] [CrossRef]
- Majee, S.; Banerjee, D.; Liu, X.; Zhang, S.L.; Zhang, Z.B. Efficient and thermally stable iodine doping of printed graphene nano-platelets. Carbon 2017, 117, 240–245. [Google Scholar] [CrossRef]
- Jung, N.; Crowther, A.C.; Kim, N.; Kim, P.; Brus, L. Raman enhancement on graphene: Adsorbed and intercalated molecular species. ACS Nano 2010, 4, 7005–7013. [Google Scholar] [CrossRef] [PubMed]
- Kalita, G.; Wakita, K.; Takahashi, M.; Umeno, M. Iodine doping in solid precursor-based CVD growth graphene film. J. Mater. Chem. 2011, 21, 15209–15213. [Google Scholar] [CrossRef]
- Rao, A.M.; Eklund, P.C.; Bandow, S.; Thess, A.; Smalley, R.E. Evidence for charge transfer in doped carbon nanotube bundles from Raman scattering. Nature 1997, 388, 257–259. [Google Scholar] [CrossRef]
- Grigorian, L.; Williams, K.A.; Fang, S.; Sumanasekera, G.U.; Loper, A.L.; Dickey, E.C.; Eklund, P.C. Reversible intercalation of charged iodine chains into carbon nanotube ropes. Phys. Rev. Lett. 1998, 80, 5560. [Google Scholar] [CrossRef]
- Komsa, H.P.; Senga, R.; Suenaga, K.; Krasheninnikov, A.V. Structural distortions and charge density waves in iodine chains encapsulated inside carbon nanotubes. Nano Lett. 2017, 17, 3694–3700. [Google Scholar] [CrossRef]
- Ghosh, S.; Yamijala, S.S.; Pati, S.K.; Rao, C.N.R. The interaction of halogen molecules with SWNTs and graphene. RSC Adv. 2012, 2, 1181–1188. [Google Scholar]
- Allon-Alaluf, M.; Klibanov, L.; Croitoru, N. Iodine doping of amorphous diamond-like carbon films. Diam. Relat. Mater. 1996, 5, 1497–1502. [Google Scholar] [CrossRef]
- Kumari, L.; Subramanyam, S.V. Optical properties and electrical transport in intercalated amorphous carbon. Mater. Res. Bull. 2006, 41, 2000–2006. [Google Scholar] [CrossRef]
- Šimek, P.; Klímová, K.; Sedmidubský, D.; Jankovský, O.; Pumera, M.; Sofer, Z. Towards graphene iodide: Iodination of graphite oxide. Nanoscale 2015, 7, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Przygocki, P.; Abbas, Q.; Béguin, F. Capacitance enhancement of hybrid electrochemical capacitor with asymmetric carbon electrodes configuration in neutral aqueous electrolyte. Electrochim. Acta 2018, 269, 640–648. [Google Scholar] [CrossRef]
- Abbas, Q.; Fitzek, H.; Schröttner, H.; Dsoke, S.; Gollas, B. Immobilization of polyiodide redox species in porous carbon for battery-like electrodes in eco-friendly hybrid electrochemical capacitors. Nanomaterials 2019, 9, 1413. [Google Scholar] [CrossRef]
- Abbas, Q.; Fitzek, H.; Pavlenko, V.; Gollas, B. Towards an optimized hybrid electrochemical capacitor in iodide based aqueous redox-electrolyte: Shift of equilibrium potential by electrodes mass-balancing. Electrochim. Acta 2020, 337, 135785. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Lucazeau, G. Effect of pressure and temperature on Raman spectra of solids: Anharmonicity. J. Raman Spectrosc. 2003, 34, 478–496. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Rodil, S.E.; Robertson, J. Interpretation of infrared and Raman spectra of amorphous carbon nitrides. Phys. Rev. B 2003, 67, 155306. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond–like carbon, and nanodiamond. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Mohiuddin, T.M.G.; Lombardo, A.; Nair, R.R.; Bonetti, A.; Savini, G.; Jalil, R.; Ferrari, A.C. Uniaxial strain in graphene by Raman spectroscopy: G peak splitting, Grüneisen parameters, and sample orientation. Phys. Rev. B 2009, 79, 205433. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Yoon, D.; Son, Y.W.; Cheong, H. Strain-dependent splitting of the double-resonance Raman scattering band in graphene. Phys. Rev. Lett. 2011, 106, 155502. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Cong, C.; Zhang, J.; Xiong, Q.; Yu, T. The origin of sub-bands in the Raman D-band of graphene. Carbon 2012, 50, 4252–4258. [Google Scholar] [CrossRef]
- Chacón-Torres, J.C.; Wirtz, L.; Pichler, T. Raman spectroscopy of graphite intercalation compounds: Charge transfer, strain, and electron–phonon coupling in graphene layers. Phys. Status Solidi (b) 2014, 251, 2337–2355. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, X.; Lin, Y.; Wang, F. A ternary phase diagram for amorphous carbon. Carbon 2015, 94, 202–213. [Google Scholar] [CrossRef]
- Smith, M.W.; Dallmeyer, I.; Johnson, T.J.; Brauer, C.S.; McEwen, J.S.; Espinal, J.F.; Garcia-Perez, M. Structural analysis of char by Raman spectroscopy: Improving band assignments through computational calculations from first principles. Carbon 2016, 100, 678–692. [Google Scholar] [CrossRef]
- Lünsdorf, N.K.; Lünsdorf, J.O. Evaluating Raman spectra of carbonaceous matter by automated, iterative curve-fitting. Int. J. Coal Geol. 2016, 160, 51–62. [Google Scholar] [CrossRef]
- Piscanec, S.; Lazzeri, M.; Mauri, F.; Ferrari, A.C.; Robertson, J. Kohn anomalies and electron-phonon interactions in graphite. Phys. Rev. Lett. 2004, 93, 185503. [Google Scholar] [CrossRef]
- Kavan, L.; Dunsch, L. Spectroelectrochemistry of carbon nanostructures. ChemPhysChem 2007, 8, 974–998. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Y.; Kim, P.; Pinczuk, A. Electric field effect tuning of electron-phonon coupling in graphene. Phys. Rev. Lett. 2007, 98, 166802. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Pisana, S.; Chakraborty, B.; Piscanec, S.; Saha, S.K.; Waghmare, U.V.; Sood, A.K. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nat. Nanotechnol. 2008, 3, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kalbac, M.; Farhat, H.; Kavan, L.; Kong, J.; Dresselhaus, M.S. Competition between the spring force constant and the phonon energy renormalization in electrochemically doped semiconducting single-walled carbon nanotubes. Nano Lett. 2008, 8, 3532–3537. [Google Scholar] [CrossRef] [PubMed]
- Kalbac, M.; Kavan, L. The influence of doping on the Raman intensity of the D band in single walled carbon nanotubes. Carbon 2010, 48, 832–838. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Zhu, D. Chemical doping of graphene. J. Mater. Chem. 2011, 21, 3335–3345. [Google Scholar] [CrossRef]
- Lee, J.E.; Ahn, G.; Shim, J.; Lee, Y.S.; Ryu, S. Optical separation of mechanical strain from charge doping in graphene. Nat. Commun. 2012, 3, 1024. [Google Scholar] [CrossRef]
- Kalbac, M.; Kong, J.; Dresselhaus, M.S. Raman spectroscopy as a tool to address individual graphene layers in few-layer graphene. J. Phys. Chem. C 2012, 116, 19046–19050. [Google Scholar] [CrossRef]
- Bouša, M.; Frank, O.; Jirka, I.; Kavan, L. In situ raman spectroelectrochemistry of graphene oxide. Phys. Status Solidi (b) 2013, 250, 2662–2667. [Google Scholar] [CrossRef]
- Zhong, J.H.; Liu, J.Y.; Li, Q.; Li, M.G.; Zeng, Z.C.; Hu, S.; Ren, B. Interfacial capacitance of graphene: Correlated differential capacitance and in situ electrochemical Raman spectroscopy study. Electrochim. Acta 2013, 110, 754–761. [Google Scholar] [CrossRef]
- Bruna, M.; Ott, A.K.; Ijaäs, M.; Yoon, D.; Sassi, U.; Ferrari, A.C. Doping dependence of the Raman spectrum of defected graphene. ACS Nano 2014, 8, 7432–7441. [Google Scholar] [CrossRef]
- Ott, A.; Verzhbitskiy, I.A.; Clough, J.; Eckmann, A.; Georgiou, T.; Casiraghi, C. Tunable D peak in gated graphene. Nano Res. 2014, 7, 338–344. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Y.; Xu, Z.; Wang, S.; Kobayashi, H.; Wang, J. The Effects of Oxygen Functional Groups on Graphene Oxide on the Efficient Adsorption of Radioactive Iodine. Materials 2020, 13, 5770. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, S.; Fulvio, P.F.; Andreoli, E. Carbon black reborn: Structure and chemistry for renewable energy harnessing. Carbon 2020, 162, 604–649. [Google Scholar] [CrossRef]
- Davis, A.; Tran, T. Gold dissolution in iodide electrolytes. Hydrometallurgy 1991, 26, 163–177. [Google Scholar] [CrossRef]
- Sharp, S.B.; Gellene, G.I. Ab initio calculations of the ground electronic states of polyiodide anions. J. Phys. Chem. A 1997, 101, 2192–2197. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Für Physic 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Levi, M.D.; Salitra, G.; Levy, N.; Aurbach, D.; Maier, J. Application of a quartz-crystal microbalance to measure ionic fluxes in microporous carbons for energy storage. Nat. Mater. 2009, 8, 872–875. [Google Scholar] [CrossRef]
- Hillman, A.R. The EQCM: Electrogravimetry with a light touch. J. Solid State Electrochem. 2011, 15, 1647–1660. [Google Scholar] [CrossRef]
- Prehal, C.; Koczwara, C.; Jäckel, N.; Schreiber, A.; Burian, M.; Amenitsch, H.; Paris, O. Quantification of ion confinement and desolvation in nanoporous carbon supercapacitors with modelling and in situ X-ray scattering. Nat. Energy 2017, 2, 16215. [Google Scholar] [CrossRef]
- Merlet, C.; Péan, C.; Rotenberg, B.; Madden, P.A.; Daffos, B.; Taberna, P.L.; Salanne, M. Highly confined ions store charge more efficiently in supercapacitors. Nat. Commun. 2013, 4, 2701. [Google Scholar] [CrossRef]
- Reimer, L. Transmission Electron Microscopy: Physics of Image Formation and Microanalysis; Springer: Berlin/Heidelberg, Germany, 2013; Volume 36. [Google Scholar]
- Knez, D.; Schnedlitz, M.; Lasserus, M.; Schiffmann, A.; Ernst, W.E.; Hofer, F. Modelling electron beam induced dynamics in metallic nanoclusters. Ultramicroscopy 2018, 192, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Furnival, T.; Knez, D.; Schmidt, E.; Leary, R.K.; Kothleitner, G.; Hofer, F.; Bristowe, P.D.; Midgley, P.A. Adatom dynamics and the surface reconstruction of Si(110) revealed using time-resolved electron microscopy. Appl. Phys. Lett. 2018, 113, 183104. [Google Scholar] [CrossRef]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Sumanasekera, G.U.; Allen, J.L.; Fang, S.L.; Loper, A.L.; Rao, A.M.; Eklund, P.C. Electrochemical oxidation of single wall carbon nanotube bundles in sulfuric acid. J. Phys. Chem. B 1999, 103, 4292–4297. [Google Scholar] [CrossRef]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy. J. Raman Spectrosc. 2007, 38, 728–736. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Ryu, S.; Liu, L.; Berciaud, S.; Yu, Y.J.; Liu, H.; Kim, P.; Brus, L.E. Atmospheric oxygen binding and hole doping in deformed graphene on a SiO2 substrate. Nano Lett. 2010, 10, 4944–4951. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of graphite oxide and reduced graphene oxide obtained from different graphite precursors and oxidized by different methods using Raman spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzek, H.; Sterrer, M.; Knez, D.; Schranger, H.; Sarapulova, A.; Dsoke, S.; Schroettner, H.; Kothleitner, G.; Gollas, B.; Abbas, Q. Impact of Iodine Electrodeposition on Nanoporous Carbon Electrode Determined by EQCM, XPS and In Situ Raman Spectroscopy. Nanomaterials 2023, 13, 1545. https://doi.org/10.3390/nano13091545
Fitzek H, Sterrer M, Knez D, Schranger H, Sarapulova A, Dsoke S, Schroettner H, Kothleitner G, Gollas B, Abbas Q. Impact of Iodine Electrodeposition on Nanoporous Carbon Electrode Determined by EQCM, XPS and In Situ Raman Spectroscopy. Nanomaterials. 2023; 13(9):1545. https://doi.org/10.3390/nano13091545
Chicago/Turabian StyleFitzek, Harald, Martin Sterrer, Daniel Knez, Horst Schranger, Angelina Sarapulova, Sonia Dsoke, Hartmuth Schroettner, Gerald Kothleitner, Bernhard Gollas, and Qamar Abbas. 2023. "Impact of Iodine Electrodeposition on Nanoporous Carbon Electrode Determined by EQCM, XPS and In Situ Raman Spectroscopy" Nanomaterials 13, no. 9: 1545. https://doi.org/10.3390/nano13091545
APA StyleFitzek, H., Sterrer, M., Knez, D., Schranger, H., Sarapulova, A., Dsoke, S., Schroettner, H., Kothleitner, G., Gollas, B., & Abbas, Q. (2023). Impact of Iodine Electrodeposition on Nanoporous Carbon Electrode Determined by EQCM, XPS and In Situ Raman Spectroscopy. Nanomaterials, 13(9), 1545. https://doi.org/10.3390/nano13091545





