Formamidinium Perovskite Deposition in Ambient Air Environment for Inverted p-i-n Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Device Fabrication
2.3. Characterization
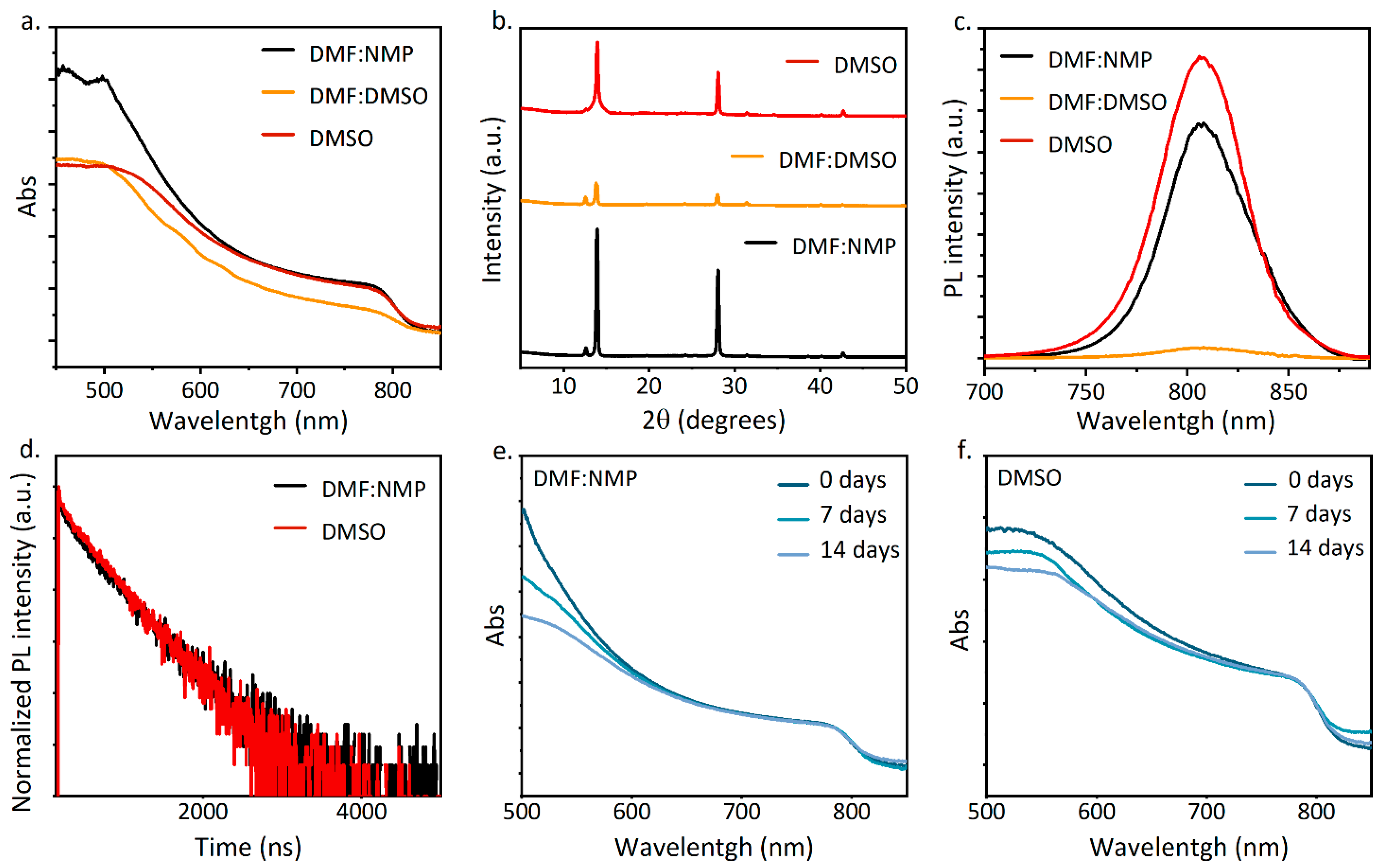
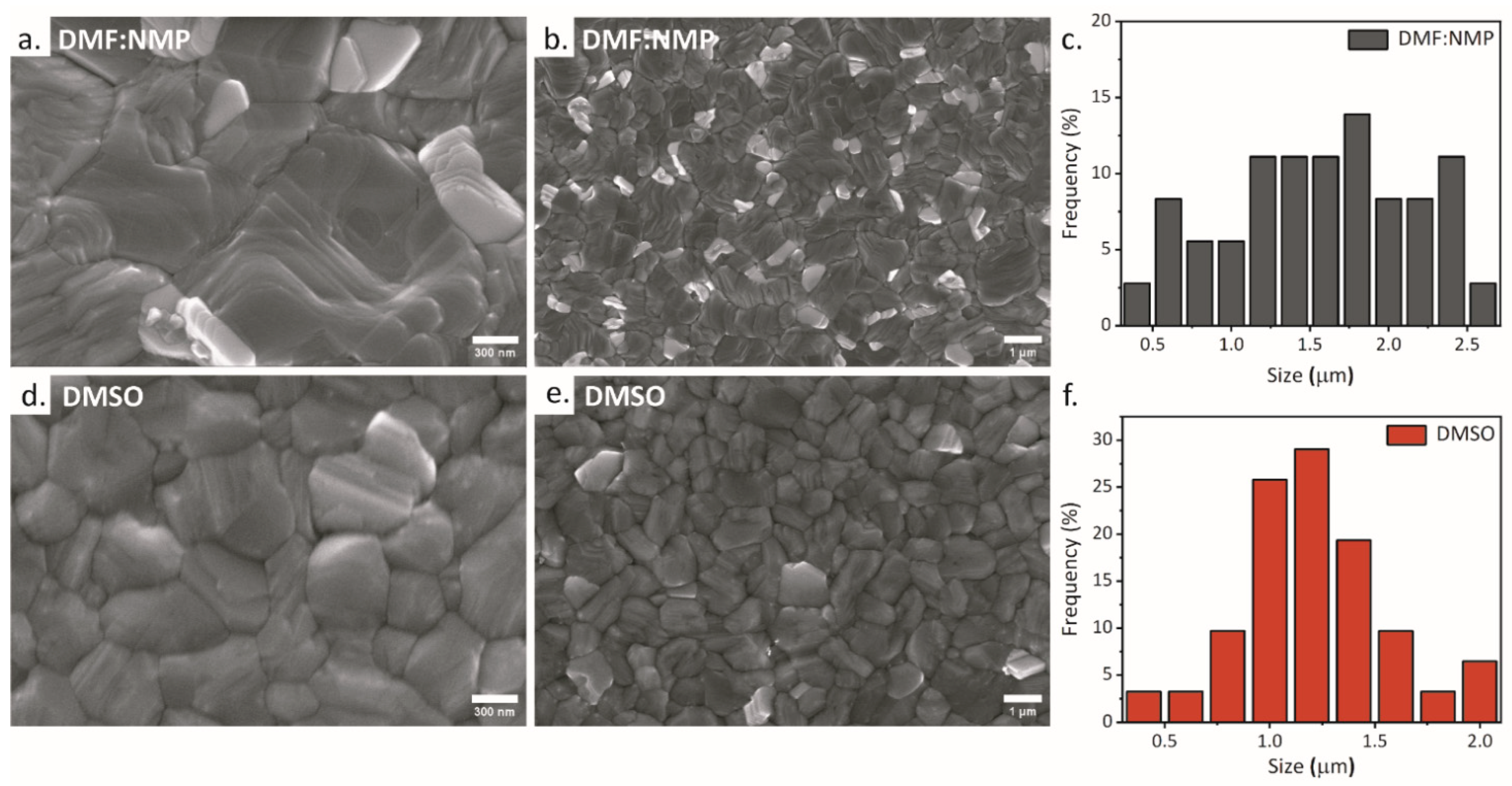
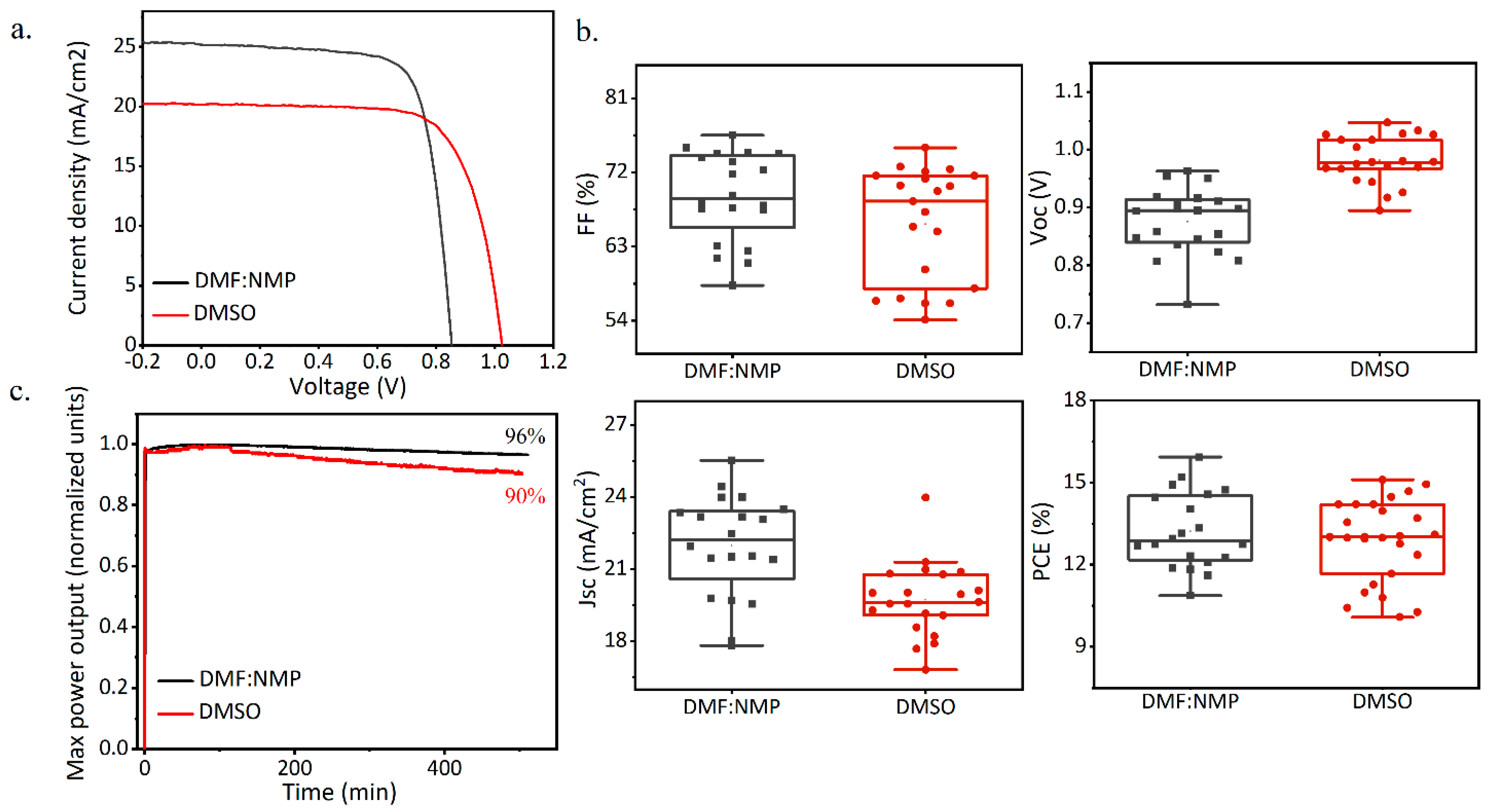
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Ho-Baillie, A.; Snaith, H. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Bist, A.; Pant, B.; Ojha, G.P.; Acharya, J.; Park, M.; Saud, P.S. Novel Materials in Perovskite Solar Cells: Efficiency, Stability, and Future Perspectives. Nanomaterials 2023, 13, 1724. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Rudd, P.N.; Yang, S.; Yuan, Y.; Huang, J. Imperfections and their passivation in halide perovskite solar cells. Chem. Soc. Rev. 2019, 48, 3842–3867. [Google Scholar] [CrossRef] [PubMed]
- Akin, S.; Arora, N.; Zakeeruddin, S.M.; Grätzel, M.; Friend, R.H.; Dar, M.I. New Strategies for Defect Passivation in High-Efficiency Perovskite Solar Cells. Adv. Energy Mater. 2020, 10, 1903090. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Jung, H.S.; Park, N.-G. Perovskite Solar Cells: From Materials to Devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef]
- De Wolf, S.; Holovsky, J.; Moon, S.J.; Löper, P.; Niesen, B.; Ledinsky, M.; Haug, F.J.; Yum, J.H.; Ballif, C. Organometallic Halide Perovskites: Sharp Optical Absorption Edge and Its Relation to Photovoltaic Performance. J. Phys. Chem. Lett. 2014, 20, 1035–1039. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Liu, H.; Xiang, L.; Gao, P.; Wang, D.; Yang, J.; Chen, X.; Li, S.; Shi, Y.; Gao, F.; Zhang, Y. Improvement Strategies for Stability and Efficiency of Perovskite Solar Cells. Nanomaterials 2022, 12, 3295. [Google Scholar] [CrossRef] [PubMed]
- Berhe, T.A.; Su, W.-N.; Chen, C.-H.; Pan, C.-J.; Cheng, J.-H.; Chen, H.-M.; Tsai, M.-C.; Chen, L.-Y.; Dubale, A.A.; Hwang, B.-J. Organometal halide perovskite solar cells: Degradation and stability. Energy Environ. Sci. 2016, 9, 323–356. [Google Scholar] [CrossRef]
- Bărar, A.; Maclean, S.A.; Dănilă, O.; Taylor, A.D. Towards High-Efficiency Photon Trapping in Thin-Film Perovskite Solar Cells Using Etched Fractal Metadevices. Materials 2023, 16, 3934. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, M.H.; García-Cámara, B.; López-Fraguas, E.; Vergaz, R. Efficient Light Management in a Monolithic Tandem Perovskite/Silicon Solar Cell by Using a Hybrid Metasurface. Nanomaterials 2019, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Habisreutinger, S.N.; Leijtens, T.; Bruijnaers, B.J.; van Franeker, J.J.; Dequilettes, D.W.; Pathak, S.; Sutton, R.J.; Grancini, G.; Ginger, D.S.; et al. The Importance of Moisture in Hybrid Lead Halide Perovskite Thin Film Fabrication. ACS Nano 2015, 9, 9380–9393. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herza, L.M.; Snaith, H.J. Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Mannino, G.; Deretzis, I.; Smecca, E.; La Magna, A.; Alberti, A.; Ceratti, D.R.; Cahen, D. Temperature-Dependent Optical Band Gap in CsPbBr3, MAPbBr3, and FAPbBr3 Single Crystals. J. Phys. Chem. Lett. 2020, 11, 2490–2496. [Google Scholar] [CrossRef]
- Huang, Y.; Lei, X.; He, T.; Jiang, Y.; Yuan, M. Recent Progress on Formamidinium-Dominated Perovskite Photovoltaics. Adv. Energy Mater. 2022, 12, 2100690. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Zhang, T.; Liu, X.; Wang, X.; Zhao, Y. Advances to High-Performance Black-Phase FAPbI3 Perovskite for Efficient and Stable Photovoltaics. Small Struct. 2021, 2, 2000130. [Google Scholar] [CrossRef]
- Chen, L.-C.; Tseng, Z.-L.; Huang, J.-K. A Study of Inverted-Type Perovskite Solar Cells with Various Composition Ratios of (FAPbI3)1−x(MAPbBr3)x. Nanomaterials 2016, 6, 183. [Google Scholar] [CrossRef]
- Weller, M.T.; Weber, O.J.; Frost, J.M.; Walsh, A. Cubic Perovskite Structure of Black Formamidinium Lead Iodide, α-[HC(NH2)2]PbI3, at 298 K. J. Phys. Chem. Lett. 2015, 6, 3209–3212. [Google Scholar] [CrossRef]
- Niu, T.; Chao, L.; Dong, X.; Fu, L.; Chen, Y. Phase-Pure α-FAPbI3 for Perovskite Solar Cells. J. Phys. Chem. Lett. 2022, 13, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, K.; Hu, Q.; Zhu, R.; Gong, Q. Inverted Perovskite Solar Cells: Progresses and Perspectives. Adv. Energy Mater. 2016, 6, 1600457. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Wu, X.; Zhang, S.; Liu, B.; Zhang, D.; Li, B.; Xiao, P.; Xu, F.; Lu, H.; et al. Strain Regulation via Pseudo Halide-Based Ionic Liquid toward Efficient and Stable α-FAPbI3 Inverted Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2300700. [Google Scholar] [CrossRef]
- Li, R.; Ding, J.; Mu, X.; Kang, Y.; Wang, A.; Bi, W.; Zhang, Y.; Cao, J.; Dong, Q. Hyperbranched phthalocyanine enabling black-phase formamidinium perovskite solar cells processing and operating in humidity open air. J. Energy Chem. 2022, 71, 141–149. [Google Scholar] [CrossRef]
- Vidal, R.; Alberola-Borràs, J.-A.; Habisreutinger, S.N.; Gimeno-Molina, J.-L.; Moore, D.T.; Schloemer, T.H.; Mora-Seró, I.; Berry, J.J.; Luther, J.M. Assessing health and environmental impacts of solvents for producing perovskite solar cells. Nat. Sustain. 2021, 4, 277–285. [Google Scholar] [CrossRef]
- Liu, K.; Liang, Q.; Qin, M.; Shen, D.; Yin, H.; Ren, Z.; Zhang, Y.; Zhang, H.; Fong, P.W.; Wu, Z.; et al. Zwitterionic-Surfactant-Assisted Room Temperature Coating of Efficient Perovskite Solar Cells. Joule 2020, 4, 2404–2425. [Google Scholar] [CrossRef]
- Al-Ashouri, A.; Magomedov, A.; Roß, M.; Jošt, M.; Talaikis, M.; Chistiakova, G.; Bertram, T.; Márquez, J.A.; Köhnen, E.; Kasparavičius, E.; et al. Conformal monolayer contacts with lossless interfaces for perovskite single junction and monolithic tandem solar cells. Energy Environ. Sci. 2019, 12, 3356–3369. [Google Scholar] [CrossRef]
- Cao, X.; Zhi, L.; Jia, Y.; Li, Y.; Zhao, K.; Cui, X.; Ci, L.; Zhuang, D.; Wei, J. A Review of the Role of Solvents in Formation of High-Quality Solution-Processed Perovskite Films. ACS Appl. Mater. Interfaces 2019, 11, 7639–7654. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.-Y.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly Reproducible Perovskite Solar Cells with Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead(II) Iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef]
- Lee, J.-W.; Dai, Z.; Lee, C.; Lee, H.M.; Han, T.-H.; De Marco, N.; Lin, O.; Choi, C.S.; Dunn, B.; Koh, J.; et al. Tuning Molecular Interactions for Highly Reproducible and Efficient Formamidinium Perovskite Solar Cells via Adduct Approach. J. Am. Chem. Soc. 2018, 140, 6317–6324. [Google Scholar] [CrossRef] [PubMed]
- Gratia, P.; Zimmermann, I.; Schouwink, P.; Yum, J.; Audinot, J.; Sivula, K.; Wirtz, T.; Nazeeruddin, M. The Many Faces of Mixed Ion Perovskites: Unraveling and Understanding the Crystallization Process. ACS Energy Lett. 2017, 2, 2686–2693. [Google Scholar] [CrossRef]
- Liu, T.; Zong, Y.; Zhou, Y.; Yang, M.; Li, Z.; Game, O.S.; Zhu, K.; Zhu, R.; Gong, Q.; Padture, N.P. High-Performance Formamidinium-Based Perovskite Solar Cells via Microstructure-Mediated δ-to-α Phase Transformation. Chem. Mater. 2017, 29, 3246–3250. [Google Scholar] [CrossRef]
- Jiang, S.; Luan, Y.; Jang, J.I.; Baikie, T.; Huang, X.; Li, R.; Saouma, F.O.; Wang, Z.; White, T.J.; Fang, J. Phase Transitions of Formamidinium Lead Iodide Perovskite under Pressure. J. Am. Chem. Soc. 2018, 140, 13952–13957. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, L.; Qiu, J.; Yan, Z.; Tai, K.; Yu, W.; Jiang, X. Fabrication of efficient formamidinium perovskite solar cells under ambient air via intermediate-modulated crystallization. Sol. Energy 2019, 187, 147–155. [Google Scholar] [CrossRef]
- Tan, W.; Cheng, Y.; McNeill, C. Direct assessment of structural order and evidence for stacking faults in layered hybrid perovskite films from X-ray scattering measurements. J. Mater. Chem. A 2020, 8, 12790–12798. [Google Scholar] [CrossRef]
- Akin, S.; Akman, E.; Sonmezoglu, S. FAPbI3-Based Perovskite Solar Cells Employing Hexyl-Based Ionic Liquid with an Efficiency Over 20% and Excellent Long-Term Stability. Adv. Funct. Mater. 2020, 30, 2002964. [Google Scholar] [CrossRef]
- Akman, E.; Shalan, A.; Sadegh, F.; Akin, S. Moisture-Resistant FAPbI3 Perovskite Solar Cell with 22.25 % Power Conversion Efficiency through Pentafluorobenzyl Phosphonic Acid Passivation. ChemSusChem 2021, 14, 1176–1180. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, T.; Endo, M.; Wakamiya, A.; Kanemitsu, Y. Photocarrier Recombination Dynamics in Perovskite CH3NH3PbI3 for Solar Cell Applications. J. Am. Chem. Soc. 2014, 136, 11610–11613. [Google Scholar] [CrossRef]
- Doherty, T.A.S.; Nagane, S.; Kubicki, D.J.; Jung, Y.-K.; Johnstone, D.N.; Iqbal, A.N.; Guo, D.; Frohna, K.; Danaie, M.; Tennyson, E.M.; et al. Stabilized tilted-octahedra halide perovskites inhibit local formation of performance-limiting phases. Science 2021, 374, 1598–1605. [Google Scholar] [CrossRef]
- Salim, K.M.M.; Masi, S.; Gualdrón-Reyes, A.F.; Sánchez, R.S.; Barea, E.M.; Kreĉmarová, M.; Sánchez-Royo, J.F.; Mora-Seró, I. Boosting Long-Term Stability of Pure Formamidinium Perovskite Solar Cells by Ambient Air Additive Assisted Fabrication. ACS Energy Lett. 2021, 6, 3511–3521. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yang, Z.; Yang, D.; Zhang, X.; Cui, D.; Liu, Y.; Wei, Q.; Fan, H.; Liu, S. Modulating crystal grain size and optoelectronic properties of perovskite films for solar cells by reaction temperature. Nanoscale 2016, 8, 3816–3822. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zheng, X.; Bai, Y.; Wang, Q.; Zhao, J.; Huang, J. Surfactant-controlled ink drying enables high-speed deposition of perovskite films for efficient photovoltaic modules. Nat. Energy 2018, 3, 560–566. [Google Scholar] [CrossRef]
- Way, A.; Luke, J.; Evans, A.D.; Li, Z.; Kim, J.-S.; Durrant, J.R.; Lee, H.K.H.; Tsoi, W.C. Fluorine doped tin oxide as an alternative of indium tin oxide for bottom electrode of semi-transparent organic photovoltaic devices. AIP Adv. 2019, 9, 085220. [Google Scholar] [CrossRef]
- Rahighi, R.; Gholipour, S.; Amin, M.A.; Ansari, M.Z. Hole-Transport Material Engineering in Highly Durable Carbon-Based Perovskite Photovoltaic Devices. Nanomaterials 2023, 13, 1417. [Google Scholar] [CrossRef] [PubMed]
- Degani, M.; An, Q.; Albaladejo-Siguan, M.; Hofstetter, Y.J.; Cho, C.; Paulus, F.; Grancini, G.; Vaynzof, Y. 23.7% Efficient inverted perovskite solar cells by dual interfacial modification. Sci. Adv. 2021, 7, eabj7930. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Wang, Q.; Shao, Y.; Yuan, Y.; Xiao, Z.; Huang, J. Non-wetting surface-driven high-aspect-ratio crystalline grain growth for efficient hybrid perovskite solar cells. Nat. Commun. 2015, 6, 7747. [Google Scholar] [CrossRef]
- Xia, Y.; Yan, G.; Lin, J. Review on Tailoring PEDOT:PSS Layer for Improved Device Stability of Perovskite Solar Cells. Nanomaterials 2021, 11, 3119. [Google Scholar] [CrossRef]
- Zuo, C.; Ding, L. Modified PEDOT Layer Makes a 1.52 V Voc for Perovskite/PCBM Solar Cells. Adv. Energy Mater. 2017, 7, 1601193. [Google Scholar] [CrossRef]
- Xiong, Q.; Tian, H.; Zhang, J.; Han, L.; Lu, C.; Shen, B.; Zhang, Y.; Zheng, Y.; Lu, C.; Zeng, Z.; et al. CuSCN modified PEDOT:PSS to improve the efficiency of low temperature processed perovskite solar cells. Org. Electron. 2018, 61, 151–156. [Google Scholar] [CrossRef]
- Shibayama, N.; Fukumoto, S.; Sugita, H.; Kanda, H.; Ito, S. Influence of transparent conductive oxide layer on the inverted perovskite solar cell using PEDOT: PSS for hole transport layer. Mater. Res. Bull. 2018, 28, 433–438. [Google Scholar] [CrossRef]
- Al-Ashouri, A.; Köhnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Márquez, J.A.; Vilches, A.B.M.; Kasparavicius, E.; Smith, J.A.; et al. Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- De Quilettes, D.W.; Vorpahl, S.M.; Stranks, S.D.; Nagaoka, H.; Eperon, G.E.; Ziffer, M.E.; Snaith, H.J.; Ginger, D.S. Impact of microstructure on local carrier lifetime in perovskite solar cells. Science 2015, 348, 6235. [Google Scholar] [CrossRef] [PubMed]
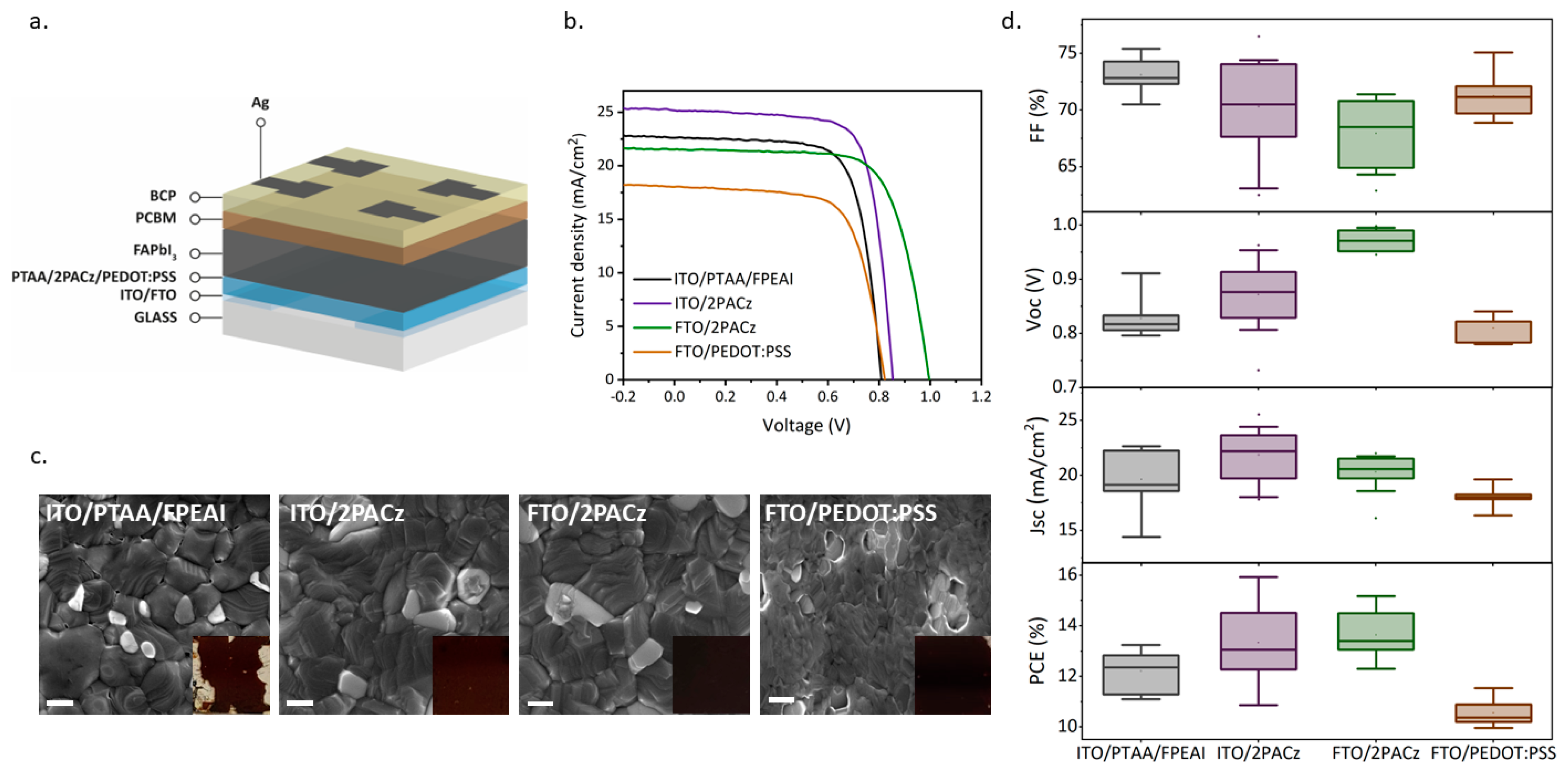
| FF (%) | Voc (V) | Jsc (mA/cm2) | PCE (%) | |
|---|---|---|---|---|
| ITO/PTAA/FPEAI | 73.1 ± 1.6 | 0.83 ± 0.03 | 19.6 ± 2.8 | 12.2 ± 0.8 |
| best device | 72.5 | 0.809 | 22.61 | 13.3 |
| ITO/2PACz | 70.3 ± 4.0 | 0.87 ± 0.06 | 21.7 ± 2.2 | 13.3 ± 1.3 |
| best device | 73.8 | 0.845 | 25.53 | 15.9 |
| FTO/2PACz | 67.9 ± 3.6 | 0.97 ± 0.02 | 20.3 ± 1.6 | 13.6 ± 0.9 |
| best device | 70.8 | 0.995 | 21.54 | 15.2 |
| FTO/PEDOT:PSS | 71.2 ± 2.0 | 0.81 ± 0.02 | 18.1 ± 0.90 | 10.5 ± 0.5 |
| best device | 75.1 | 0.841 | 18.28 | 11.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanni, N.; Pò, R.; Biagini, P.; Bravetti, G.; Carallo, S.; Giuri, A.; Rizzo, A. Formamidinium Perovskite Deposition in Ambient Air Environment for Inverted p-i-n Solar Cells. Nanomaterials 2024, 14, 107. https://doi.org/10.3390/nano14010107
Vanni N, Pò R, Biagini P, Bravetti G, Carallo S, Giuri A, Rizzo A. Formamidinium Perovskite Deposition in Ambient Air Environment for Inverted p-i-n Solar Cells. Nanomaterials. 2024; 14(1):107. https://doi.org/10.3390/nano14010107
Chicago/Turabian StyleVanni, Nadir, Riccardo Pò, Paolo Biagini, Gianluca Bravetti, Sonia Carallo, Antonella Giuri, and Aurora Rizzo. 2024. "Formamidinium Perovskite Deposition in Ambient Air Environment for Inverted p-i-n Solar Cells" Nanomaterials 14, no. 1: 107. https://doi.org/10.3390/nano14010107
APA StyleVanni, N., Pò, R., Biagini, P., Bravetti, G., Carallo, S., Giuri, A., & Rizzo, A. (2024). Formamidinium Perovskite Deposition in Ambient Air Environment for Inverted p-i-n Solar Cells. Nanomaterials, 14(1), 107. https://doi.org/10.3390/nano14010107







