Graphene-Oxide-Modified Metal–Organic Frameworks Embedded in Mixed-Matrix Membranes for Highly Efficient CO2/N2 Separation
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Synthesis of MOF-74(Ni)
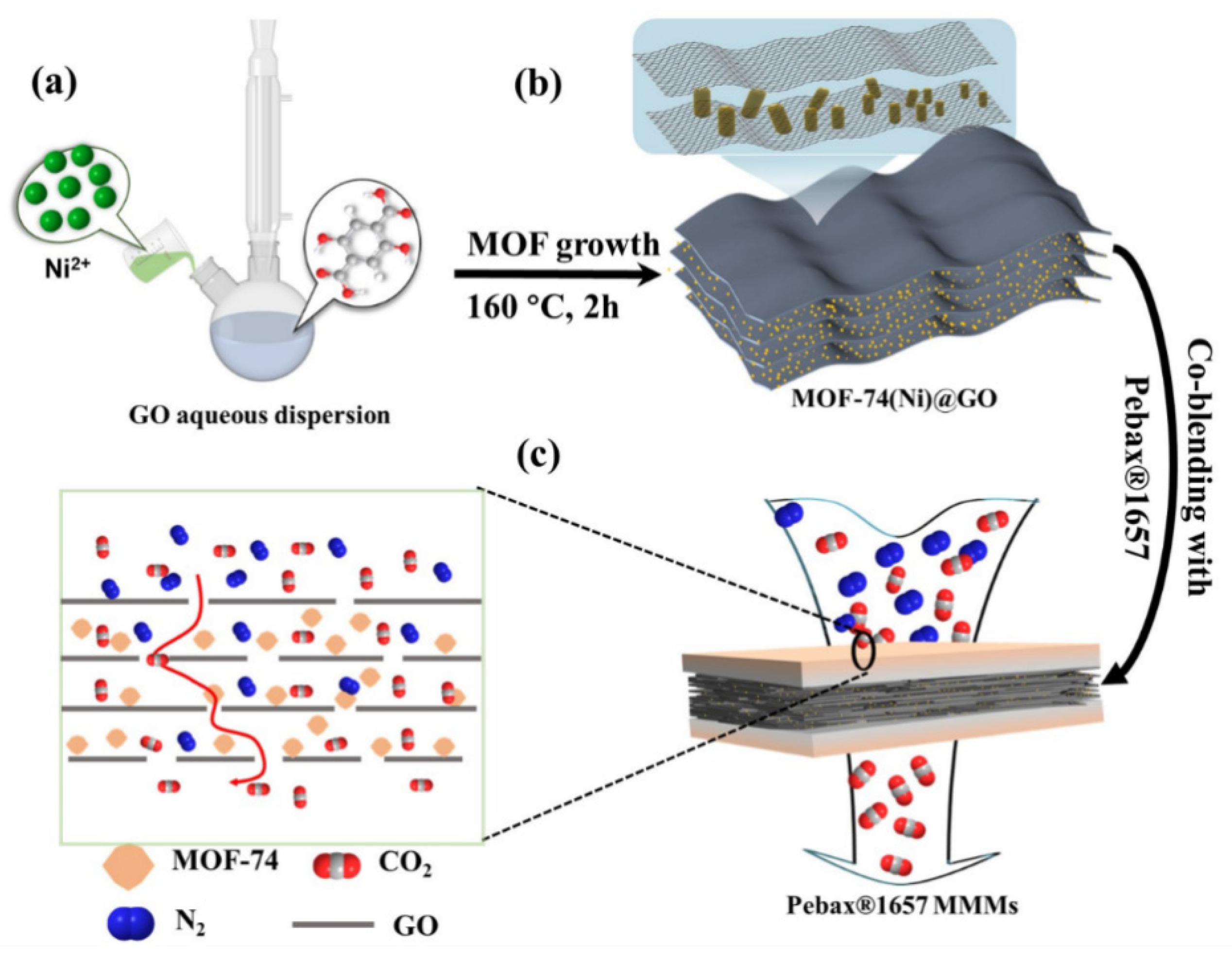
2.3. Synthesis of MOF-74(Ni)@GO
2.4. Fabrication of Composite MMMs
2.4.1. Preparation of Pebax®1657 Polymer Membrane
2.4.2. Preparation of MMMs
3. Results and Discussion
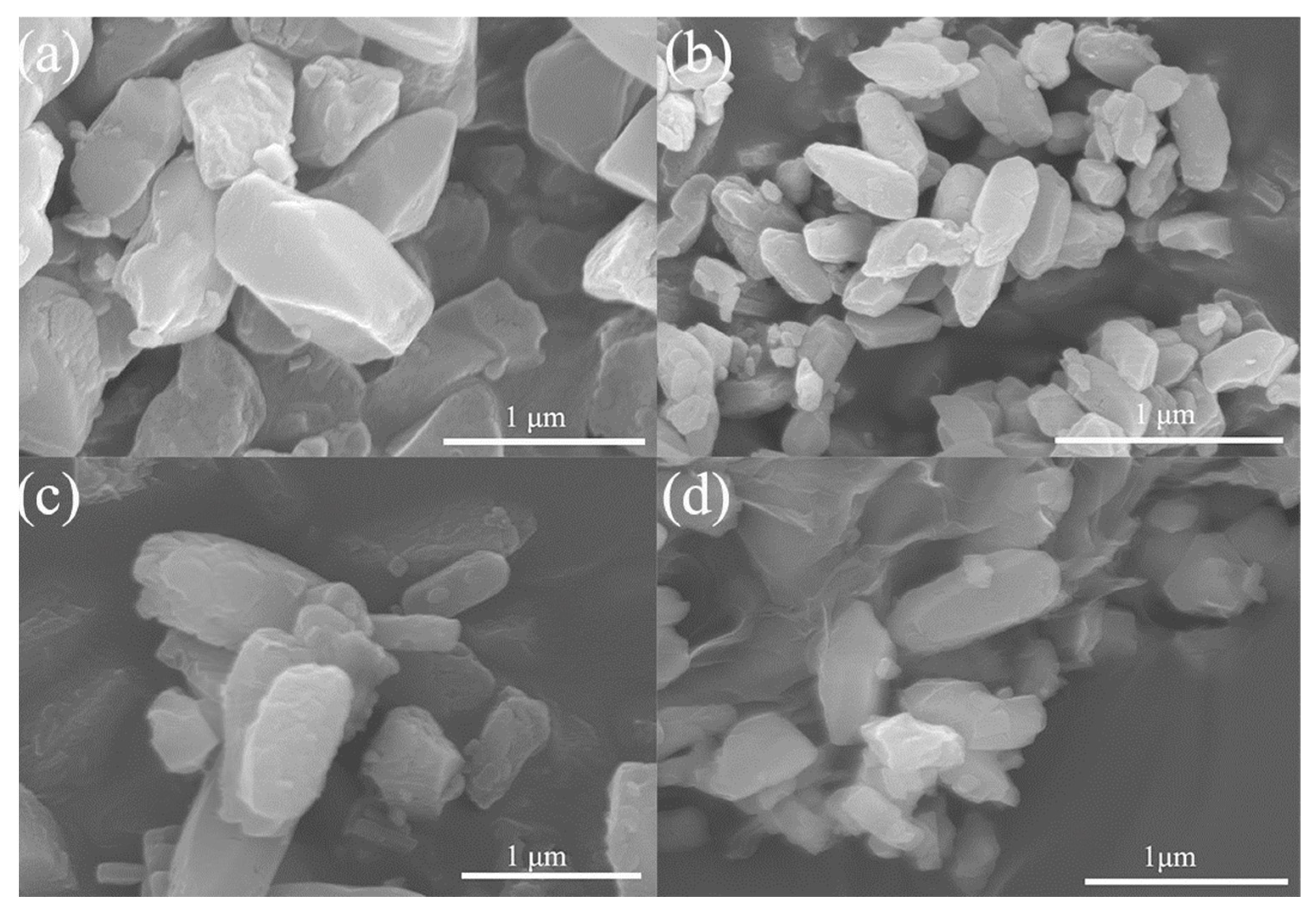
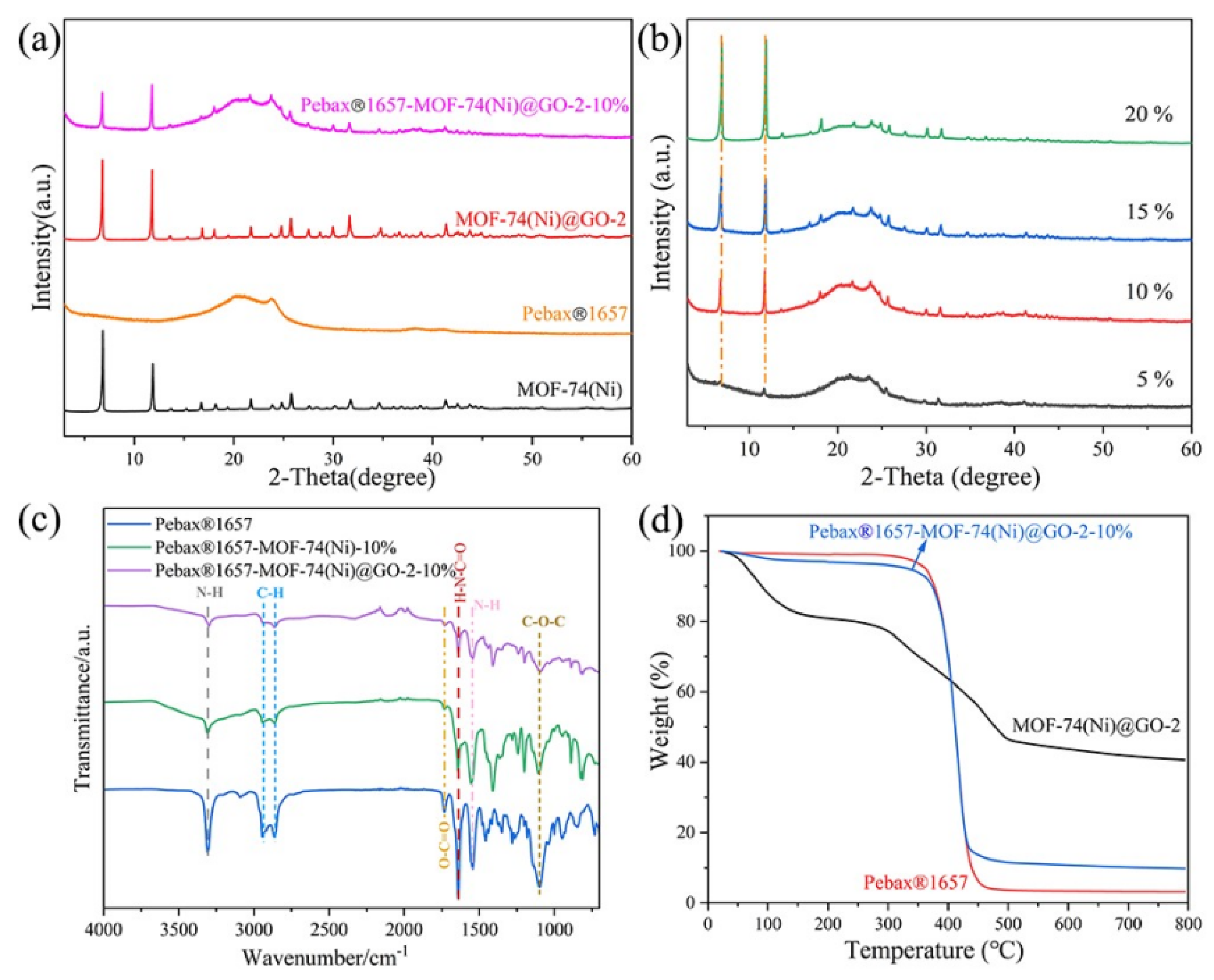
3.1. Characterization of MOF-74(Ni) and MOF-74(Ni)@GO
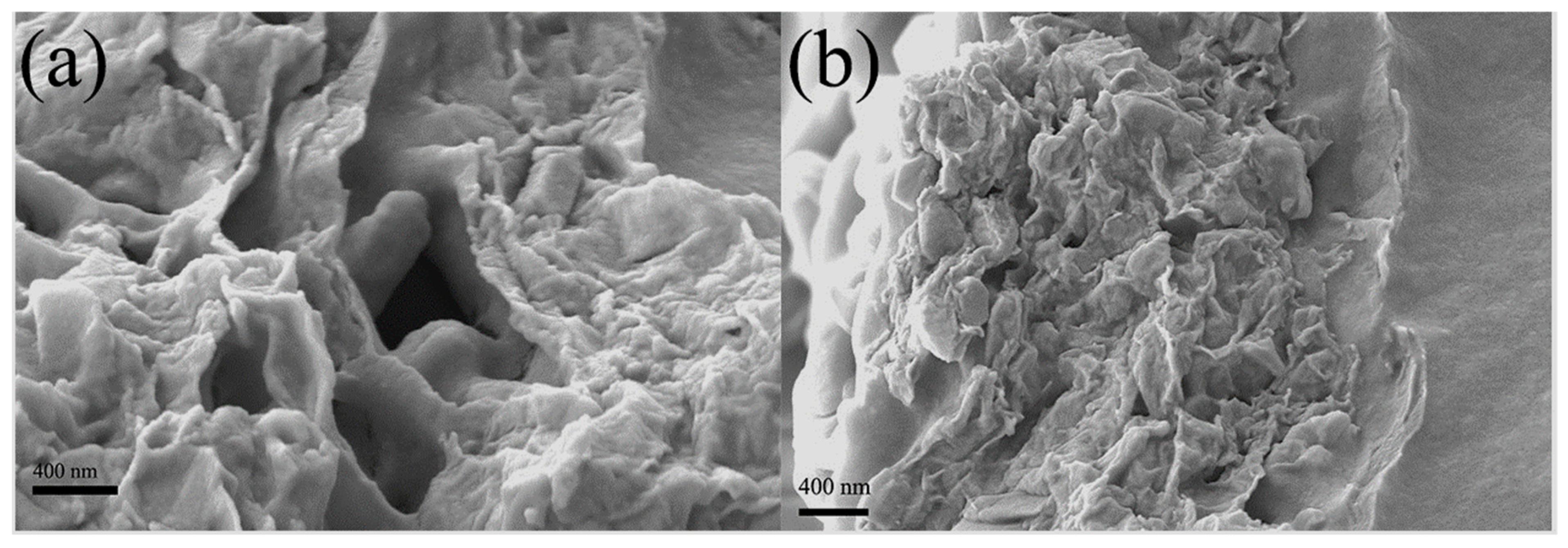
3.2. Characterization of MMMs
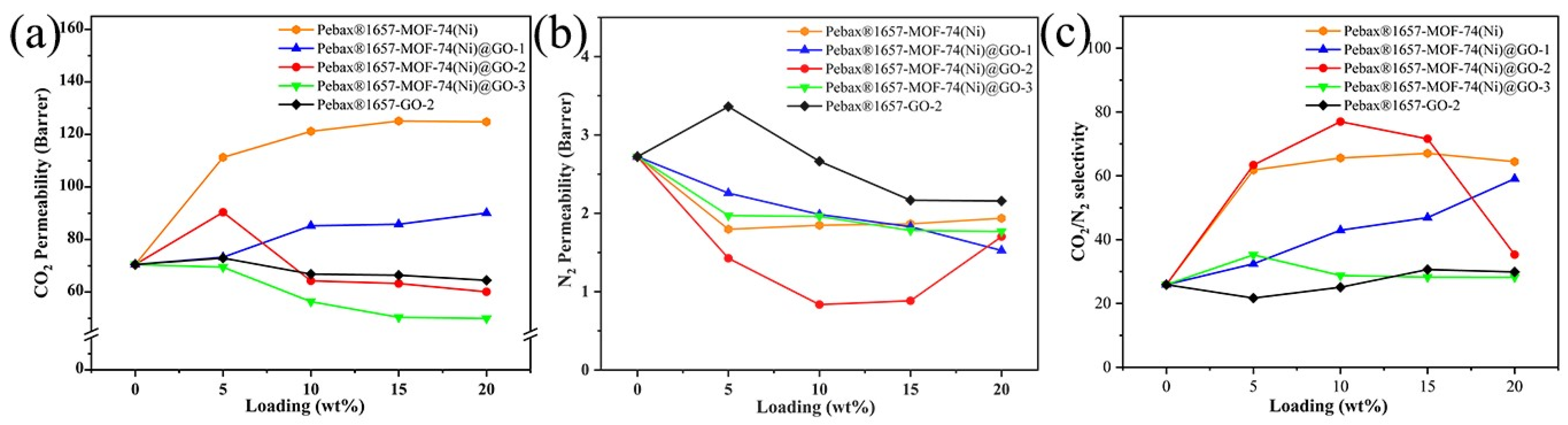
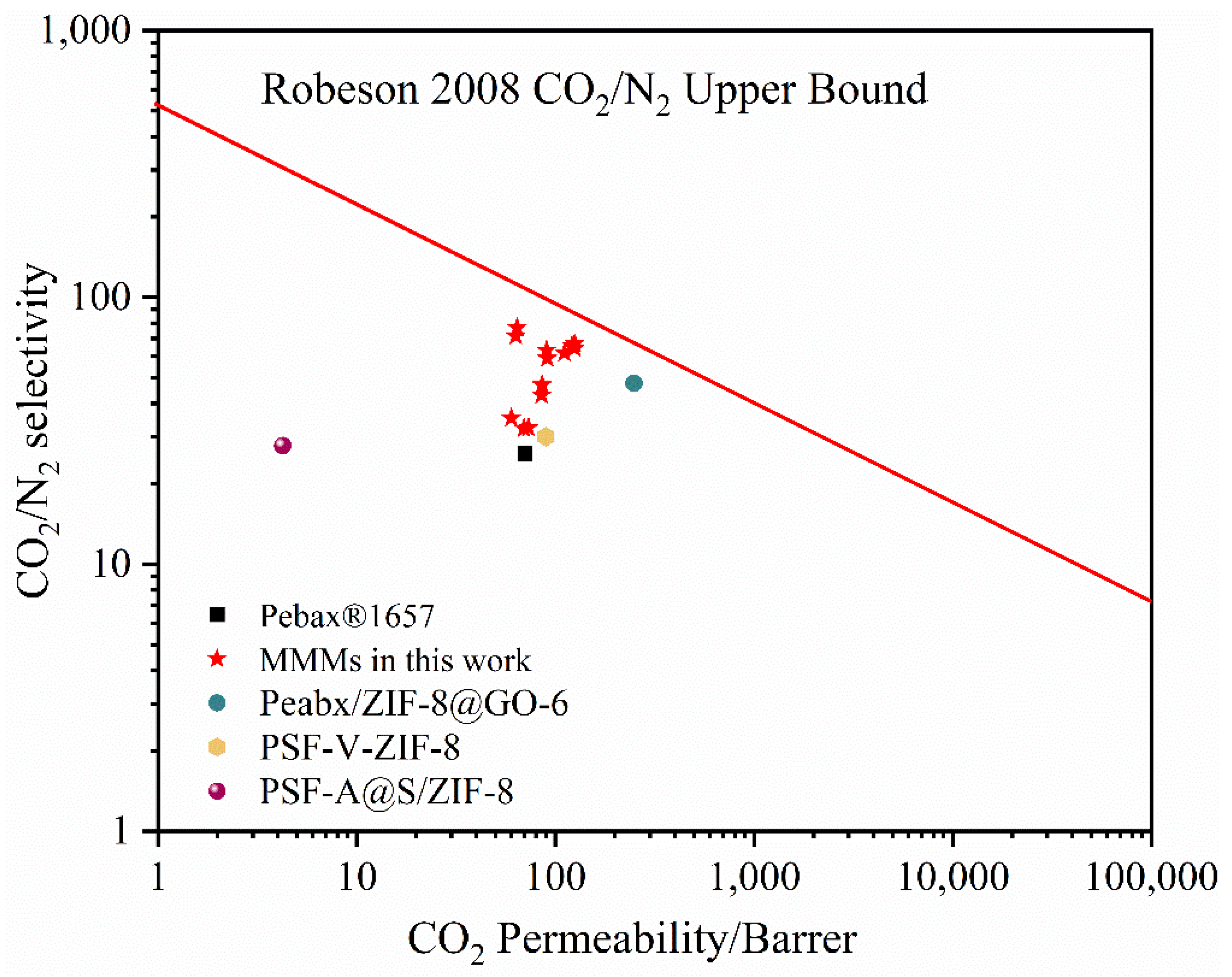
3.3. Characterization of Gas Separation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.A.; Luo, J.Z.; Zhong, Z.Y.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Zainuddin, M.I.F.; Ahmad, A.L. Mixed matrix membrane development progress and prospect of using 2D nanosheet filler for CO2 separation and capture. J. CO2 Util. 2022, 62, 102094. [Google Scholar] [CrossRef]
- Nanda, S.; Reddy, S.N.; Mitra, S.K.; Kozinski, J.A. The progressive routes for carbon capture and sequestration. Energy Sci. Eng. 2016, 4, 99–122. [Google Scholar] [CrossRef]
- Mao, H.Y.; Tang, J.; Day, G.S.; Peng, Y.C.; Wang, H.Z.; Xiao, X.; Yang, Y.F.; Jiang, Y.W.; Chen, S.; Halat, D.M.; et al. A scalable solid-state nanoporous network with atomic-level interaction design for carbon dioxide capture. Sci. Adv. 2022, 8, eabo6849. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Ruan, X.H.; Bai, F.; Yu, M.; Li, H.; Zhao, Z.C.; He, G.H. High solvent resistance PTFPMS/PEI hollow fiber composite membrane for gas separation. Appl. Surf. Sci. 2016, 360, 164–173. [Google Scholar] [CrossRef]
- Mao, H.Y.; Tang, J.; Chen, J.; Wan, J.Y.; Hou, K.P.; Peng, Y.C.; Halat, D.M.; Xiao, L.G.; Zhang, R.F.; Lv, X.D.; et al. Designing hierarchical nanoporous membranes for highly efficient gas adsorption and storage. Sci. Adv. 2020, 6, eabb0694. [Google Scholar] [CrossRef]
- Sandru, M.; Sandru, E.M.; Ingram, W.F.; Deng, J.; Stenstad, P.M.; Deng, L.Y.; Spontak, R.J. An integrated materials approach to ultrapermeable and ultraselective CO2 polymer membranes. Science 2022, 376, 90–94. [Google Scholar] [CrossRef]
- Murali, R.S.; Ismail, A.F.; Rahman, M.A.; Sridhar, S. Mixed matrix membranes of Pebax-1657 loaded with 4A zeolite for gaseous separations. Sep. Purif. Technol. 2014, 129, 1–8. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Lin, R.J.; Ge, L.; Liu, S.M.; Rudolph, V.; Zhu, Z.H. Mixed-Matrix Membranes with Metal-Organic Framework-Decorated CNT Fillers for Efficient CO2 Separation. ACS Appl. Mater. Interfaces 2015, 7, 14750–14757. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Sánchez-Laínez, J.; Gracia-Guillén, I.; Zornoza, B.; Téllez, C.; Coronas, J. Thin supported MOF based mixed matrix membranes of Pebax® 1657 for biogas upgrade. New J. Chem. 2019, 43, 312–319. [Google Scholar] [CrossRef]
- Zhang, X.H.; Chuah, C.Y.; Dong, P.P.; Cha, Y.H.; Bae, T.H.; Song, M.K. Hierarchically Porous Co-MOF-74 Hollow Nanorods for Enhanced Dynamic CO2 Separation. ACS Appl. Mater. Interfaces 2018, 10, 43316–43322. [Google Scholar] [CrossRef] [PubMed]
- Babaei, H.; McGaughey, A.J.H.; Wilmer, C.E. Transient Mass and Thermal Transport during Methane Adsorption into the Metal Organic Framework HKUST-1. ACS Appl. Mater. Interfaces 2018, 10, 2400–2406. [Google Scholar] [CrossRef]
- Fan, L.L.; Kang, Z.X.; Shen, Y.T.; Wang, S.S.; Zhao, H.R.; Sun, H.Y.; Hu, X.T.; Sun, H.X.; Wang, R.M.; Sun, D.F. Mixed Matrix Membranes Based on Metal-Organic Frameworks with Tunable Pore Size for CO2 Separation. Cryst. Growth Des. 2018, 18, 4365–4371. [Google Scholar] [CrossRef]
- Liu, B.; Li, D.; Yao, J.; Sun, H. Improved CO2 separation performance and interfacial affinity of mixed matrix membrane by incorporating UiO-66-PEI@ [bmim] [Tf2N] particles. Sep. Purif. Technol. 2020, 239, 116519. [Google Scholar] [CrossRef]
- Habib, N.; Shamair, Z.; Tara, N.; Nizami, A.S.; Akhtar, F.H.; Ahmad, N.M.; Gilani, M.A.; Bilad, M.R.; Khan, A.L. Development of highly permeable and selective mixed matrix membranes based on Pebax®1657 and NOTT-300 for CO2 capture. Sep. Purif. Technol. 2020, 234, 116101. [Google Scholar] [CrossRef]
- Jun, H.J.; Yoo, D.K.; Jhung, S.H. Metal-organic framework (MOF-808) functionalized with ethyleneamines: Selective adsorbent to capture CO2 under low pressure. J. CO2 Util. 2022, 58, 101932. [Google Scholar] [CrossRef]
- Dong, H.; Li, L.H.; Feng, Z.D.; Wang, Q.N.; Luan, P.; Li, J.; Li, C. Amine-Functionalized Quasi-MOF for Direct Air Capture of CO2. ACS Mater. Lett. 2023, 5, 2656–2664. [Google Scholar] [CrossRef]
- Zhu, Z.T.; Parker, S.T.; Forse, A.C.; Lee, J.H.; Siegelman, R.L.; Milner, P.J.; Tsai, H.H.; Ye, M.S.; Xiong, S.Y.; Paley, M.V.; et al. Cooperative Carbon Dioxide Capture in Diamine-Appended Magnesium-Olsalazine Frameworks. J. Am. Chem. Soc. 2023, 145, 17151–17163. [Google Scholar] [CrossRef]
- Han, G.; Studer, R.M.; Lee, M.J.; Rodriguez, K.M.; Teesdale, J.J.; Smith, Z.P. Post-synthetic modification of MOFs to enhance interfacial compatibility and selectivity of thin-film nanocomposite (TFN) membranes for water purification. J. Membr. Sci. 2023, 666, 121133. [Google Scholar] [CrossRef]
- Kudasheva, A.; Sorribas, S.; Zornoza, B.; Téllez, C.; Coronas, J. Pervaporation of water/ethanol mixtures through polyimide based mixed matrix membranes containing ZIF-8, ordered mesoporous silica and ZIF-8-silica core-shell spheres. J. Chem. Technol. Biotechnol. 2015, 90, 669–677. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Wahab, M.A.; Reddy, K.S.K.; Kakosimos, G.; Abdalla, O.; Favvas, E.P.; Reinalda, D.; Geuzebroek, F.; Abdala, A.; Karanikolos, G.N. Metal Organic Framework—Based Mixed Matrix Membranes for Carbon Dioxide Separation: Recent Advances and Future Directions. Front. Chem. 2020, 8, 25. [Google Scholar] [CrossRef]
- Yang, K.; Dai, Y.; Zheng, W.J.; Ruan, X.H.; Li, H.; He, G.H. ZIFs-modified GO plates for enhanced CO2 separation performance of ethyl cellulose based mixed matrix membranesf. Sep. Purif. Technol. 2019, 214, 87–94. [Google Scholar] [CrossRef]
- Anastasiou, S.; Bhoria, N.; Pokhrel, J.; Reddy, K.S.K.; Srinivasakannan, C.; Wang, K.; Karanikolos, G.N. Metal-organic framework/graphene oxide composite fillers in mixed-matrix membranes for CO2 separation. Mater. Chem. Phys. 2018, 212, 513–522. [Google Scholar] [CrossRef]
- Liu, S.; Sun, L.; Xu, F.; Zhang, J.; Jiao, C.; Li, F.; Li, Z.; Wang, S.; Wang, Z.; Jiang, X.; et al. Nanosized Cu-MOFs induced by graphene oxide and enhanced gas storage capacity. Energy Environ. Sci. 2013, 6, 818–823. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, G.; Ma, W.; Ma, B.; Jin, Z. CdS p-n heterojunction co-boosting with Co3O4 and Ni-MOF-74 for photocatalytic hydrogen evolution. Dalton Trans. 2018, 47, 11176–11189. [Google Scholar] [CrossRef]
- Li, H.Y.; Gong, H.M.; Jin, Z.L. Phosphorus modified Ni-MOF-74/BiVO4 S-scheme heterojunction for enhanced photocatalytic hydrogen evolution. Appl. Catal. B-Environ. 2022, 307, 121166. [Google Scholar] [CrossRef]
- Ding, R.; Dai, Y.; Zheng, W.J.; Li, X.C.; Yan, X.M.; Liu, Y.; Ruan, X.H.; Li, S.J.; Yang, X.C.; Yang, K.; et al. Vesicles-shaped MOF-based mixed matrix membranes with intensified interfacial affinity and CO2 transport freeway. Chem. Eng. J. 2021, 414, 128807. [Google Scholar] [CrossRef]
- Maruthapandian, V.; Kumaraguru, S.; Mohan, S.; Saraswathy, V.; Muralidharan, S. An Insight on the Electrocatalytic Mechanistic Study of Pristine Ni MOF (BTC) in Alkaline Medium for Enhanced OER and UOR. ChemElectroChem 2018, 5, 2795–2807. [Google Scholar] [CrossRef]
- Jang, J.; Ha, J.; Cho, J. Fabrication of Water-Dispersible Polyaniline-Poly(4-styrenesulfonate) Nanoparticles For Inkjet-Printed Chemical-Sensor Applications. Adv. Mater. 2007, 19, 1772–1775. [Google Scholar] [CrossRef]
- Ranjbar, M.; Taher, M.A.; Sam, A. Mg-MOF-74 nanostructures: Facile synthesis and characterization with aid of 2,6-pyridinedicarboxylic acid ammonium. J. Mater. Sci. Mater. Electron. 2015, 27, 1449–1456. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.; Zhu, Q.; Dong, R.; Yang, R.; Cheng, Y.; He, C. Microwave-Assisted Rapid Synthesis of Well-Shaped MOF-74 (Ni) for CO2 Efficient Capture. Inorg. Chem. 2019, 58, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Cheng, Y.; Chen, C.; Kosari, M.; Jiang, Z.; He, C. Taming structure and modulating carbon dioxide (CO2) adsorption isosteric heat of nickel-based metal organic framework (MOF-74(Ni)) for remarkable CO2 capture. J. Colloid Interface Sci. 2022, 612, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Burress, J.; Bandosz, T.J. The synthesis and characterization of copper-based metal–organic framework/graphite oxide composites. Carbon 2011, 49, 563–572. [Google Scholar] [CrossRef]
- Pazani, F.; Aroujalian, A. Enhanced CO2-selective behavior of Pebax-1657: A comparative study between the influence of graphene-based fillers. Polym. Test. 2020, 81, 106264. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Li, X.; Deng, M. Gas separation properties of poly(amide-6-b-ethylene oxide)/amino modified multi-walled carbon nanotubes mixed matrix membranes. J. Membr. Sci. 2014, 467, 41–47. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, W.; Yang, X.; Ning, M.; Li, X.; Xi, Y.; Yan, X.; Zhang, X.; Dai, Y.; Liu, H.; et al. Pebax-based mixed matrix membranes derived from microporous carbon nanospheres for permeable and selective CO2 separation. Sep. Purif. Technol. 2021, 274, 119015. [Google Scholar] [CrossRef]
- Azizi, N.; Mohammadi, T.; Behbahani, R.M. Synthesis of a new nanocomposite membrane (PEBAX-1074/PEG-400/TiO2) in order to separate CO2 from CH4. J. Nat. Gas Sci. Eng. 2017, 37, 39–51. [Google Scholar] [CrossRef]
- Ghadimi, A.; Amirilargani, M.; Mohammadi, T.; Kasiri, N.; Sadatnia, B. Preparation of alloyed poly(ether block amide)/poly(ethylene glycol diacrylate) membranes for separation of CO2/H2 (syngas application). J. Membr. Sci. 2014, 458, 14–26. [Google Scholar] [CrossRef]
- Azizi, N.; Arzani, M.; Mahdavi, H.R.; Mohammadi, T. Synthesis and characterization of poly(ether-block-amide) copolymers/multi-walled carbon nanotube nanocomposite membranes for CO2/CH4 separation. Korean J. Chem. Eng. 2017, 34, 2459–2470. [Google Scholar] [CrossRef]
- Jiang, H.; Bai, L.; Yang, B.; Zeng, S.; Dong, H.; Zhang, X. The effect of protic ionic liquids incorporation on CO2 separation performance of Pebax-based membranes. Chin. J. Chem. Eng. 2022, 43, 169–176. [Google Scholar] [CrossRef]
- Jiao, C.; Li, Z.; Li, X.; Wu, M.; Jiang, H. Improved CO2/N2 separation performance of Pebax composite membrane containing polyethyleneimine functionalized ZIF-8. Sep. Purif. Technol. 2021, 259, 118190. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Y.; Wu, H.; Wu, X.; Yang, H.; Yang, L.; Wang, X.; Wu, Y.; Liu, Y.; Jiang, Z. Amino-functionalized ZIF-7 embedded polymers of intrinsic microporosity membrane with enhanced selectivity for biogas upgrading. J. Membr. Sci. 2020, 602, 117970. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Peng, K.-J.; Liu, Y.-L.; Deng, L. Pebax/PEG Grafted CNT Hybrid Membranes for Enhanced CO2/N2 Separation. Ind. Eng. Chem. Res. 2019, 58, 12226–12234. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on amine and non-amine MIL-53(Al) in Pebax® MH-1657 for CO2 separation. Sep. Purif. Technol. 2018, 200, 177–190. [Google Scholar] [CrossRef]
- Dong, L.; Chen, M.; Li, J.; Shi, D.; Dong, W.; Li, X.; Bai, Y. Metal-organic framework-graphene oxide composites: A facile method to highly improve the CO2 separation performance of mixed matrix membranes. J. Membr. Sci. 2016, 520, 801–811. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Qu, H.; Ma, H.; Chang, R.; Ma, J. A Highly Permeable Mixed Matrix Membrane Containing a Vertically Aligned Metal-Organic Framework for CO2 Separation. ACS Appl. Mater. Interfaces 2021, 13, 50441–50450. [Google Scholar] [CrossRef]
- Sasikumar, B.; Bisht, S.; Arthanareeswaran, G.; Ismail, A.F.; Othman, M.H.D. Performance of polysulfone hollow fiber membranes encompassing ZIF-8, SiO2/ZIF-8, and amine-modified SiO2/ZIF-8 nanofillers for CO2/CH4 and CO2/N2 gas separation. Sep. Purif. Technol. 2021, 264, 118471. [Google Scholar] [CrossRef]
- Song, Z.; Qiu, F.; Zaia, E.W.; Wang, Z.; Kunz, M.; Guo, J.; Brady, M.; Mi, B.; Urban, J.J. Dual-Channel, Molecular-Sieving Core/Shell ZIF@MOF Architectures as Engineered Fillers in Hybrid Membranes for Highly Selective CO2 Separation. Nano Lett. 2017, 17, 6752–6758. [Google Scholar] [CrossRef]

| Sample | Solubility ×108 | Diffusivity ×10−8 | α(CO2/N2) | ||
|---|---|---|---|---|---|
| CO2 | N2 | CO2 | N2 | ||
| Pebax®1657 | 7.45 ± 0.4 | 0.24 ± 0.2 | 9.47 ± 0.9 | 11.47 ± 1.3 | 25.89 ± 1.2 |
| Pebax®1657-MOF-74(Ni) | 9.99 ± 1.1 | 0.12 ± 0.1 | 12.23 ± 2.3 | 12.13 ± 1.4 | 65.58 ± 1.5 |
| Pebax®1657-MOF-74(Ni) @GO-1-10% | 12.89 ± 0.5 | 0.48 ± 0.2 | 6.61 ± 1.6 | 4.18 ± 0.8 | 42.94 ± 1.7 |
| Pebax®1657-MOF-74(Ni) @GO-2-10% | 8.06 ± 0.6 | 0.13 ± 0.1 | 7.98 ± 1.3 | 6.38 ± 1.4 | 76.96 ± 1.3 |
| Pebax®1657-MOF-74(Ni) @GO-3-10% | 7.87 ± 1.2 | 0.15 ± 0.1 | 7.07 ± 2.2 | 1.63 ± 0.4 | 28.76 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Zhang, Q.; Su, J.; Ma, B.; Wan, Y.; Zhong, R.; Zou, R. Graphene-Oxide-Modified Metal–Organic Frameworks Embedded in Mixed-Matrix Membranes for Highly Efficient CO2/N2 Separation. Nanomaterials 2024, 14, 24. https://doi.org/10.3390/nano14010024
Feng L, Zhang Q, Su J, Ma B, Wan Y, Zhong R, Zou R. Graphene-Oxide-Modified Metal–Organic Frameworks Embedded in Mixed-Matrix Membranes for Highly Efficient CO2/N2 Separation. Nanomaterials. 2024; 14(1):24. https://doi.org/10.3390/nano14010024
Chicago/Turabian StyleFeng, Long, Qiuning Zhang, Jianwen Su, Bing Ma, Yinji Wan, Ruiqin Zhong, and Ruqiang Zou. 2024. "Graphene-Oxide-Modified Metal–Organic Frameworks Embedded in Mixed-Matrix Membranes for Highly Efficient CO2/N2 Separation" Nanomaterials 14, no. 1: 24. https://doi.org/10.3390/nano14010024






