Hexagonal Boron Nitride as Filler for Silica-Based Elastomer Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Elastomeric Materials
2.1.3. Fillers
2.2. Preparation of Functionalized Boron Nitride (hBN-OH)
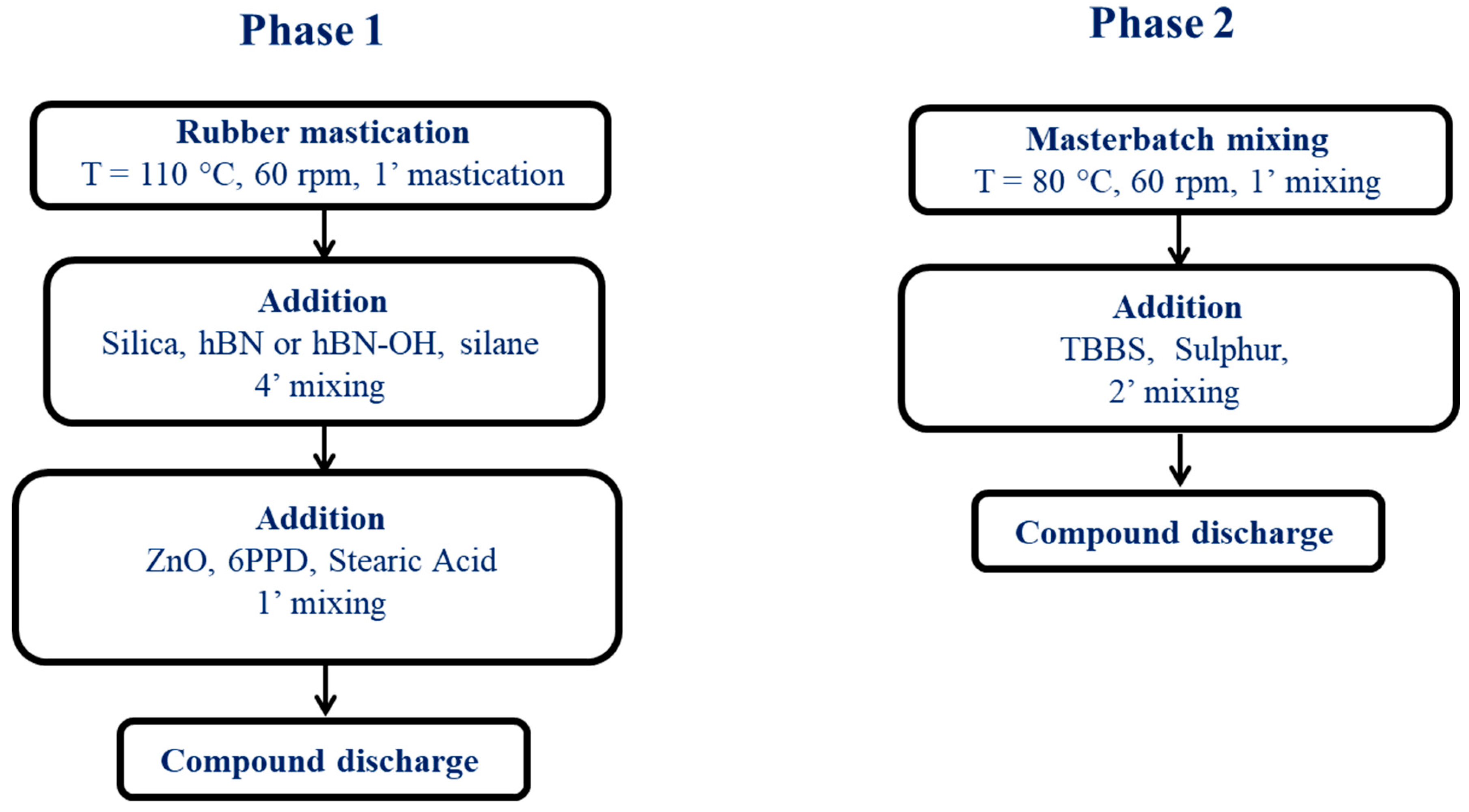
2.3. Preparation of Elastomeric Composites
2.4. Characterization Techniques
2.4.1. Thermogravimetric Analysis
2.4.2. Fourier-Transform Infrared (FT-IR)
2.4.3. Wide-Angle X-ray Diffraction (WAXD)
2.4.4. Crosslinking
2.4.5. Dynamic Mechanical Analysis in the Shear Mode—Strain Sweep Test
2.4.6. Dynamic Mechanical Analysis in the Axial Mode
2.4.7. Tensile Test
2.4.8. High-Pressure Capillary Viscosimeter (HKV)
2.4.9. High-Resolution Transmission Electron Microscopy
2.4.10. Scanning Electron Microscope Coupled with Energy-Dispersive X-ray Spectroscopy (SEM-EDS)
3. Results and Discussion
3.1. Preparation of hBN Samples
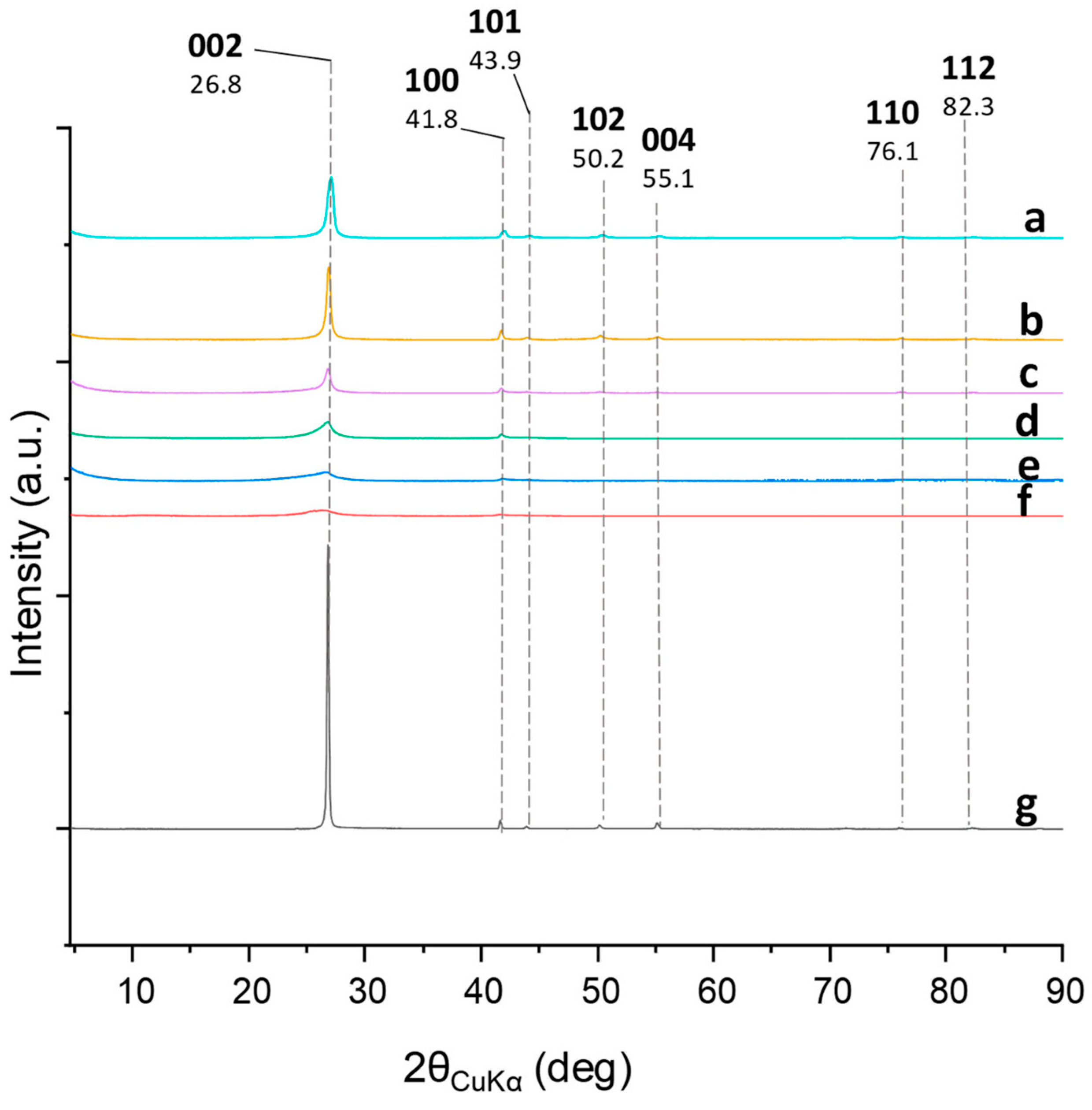
3.2. Characterization of hBN Samples
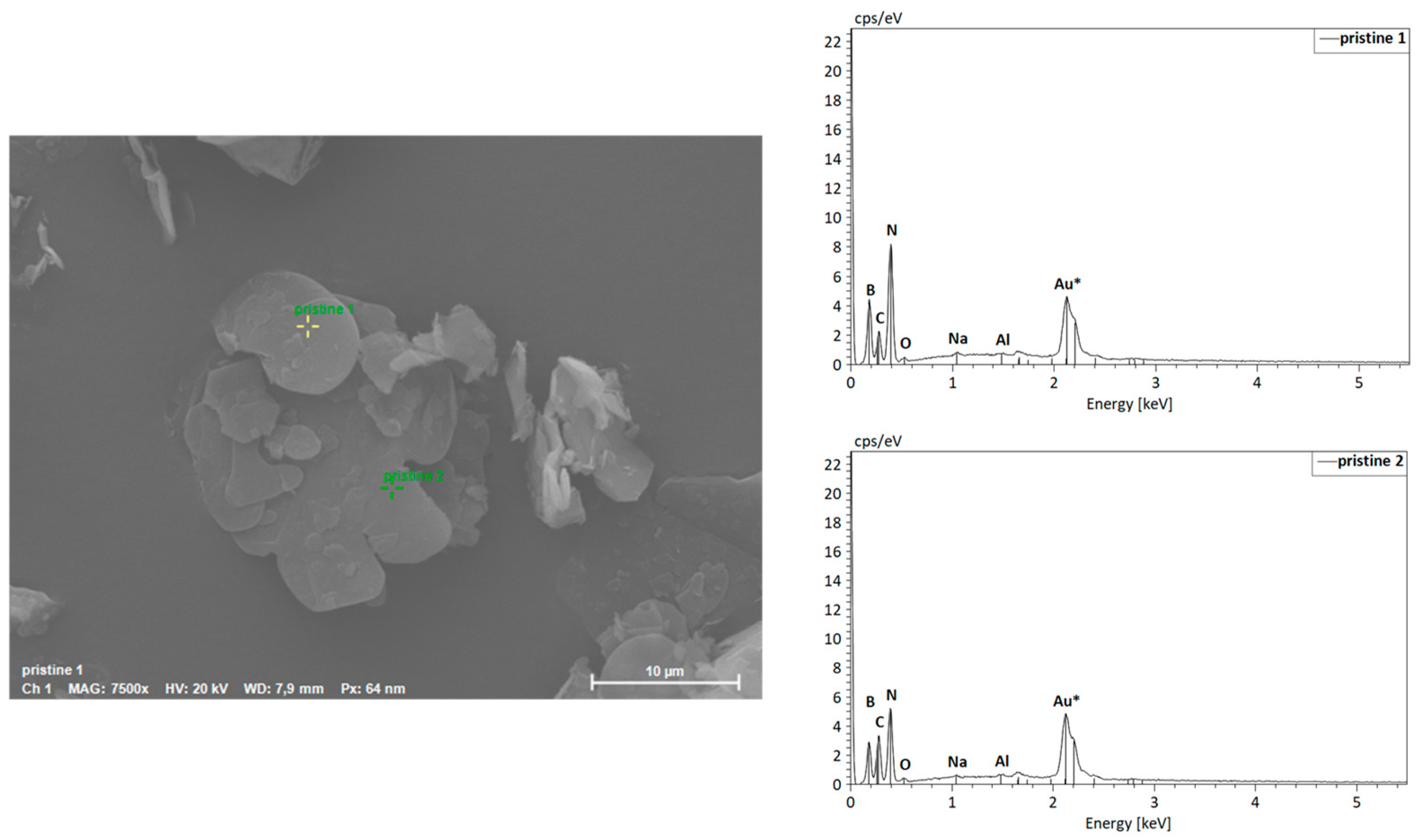
Scanning Electron Microscopy Coupled with Energy-Dispersive X-ray Spectroscopy (SEM-EDS)
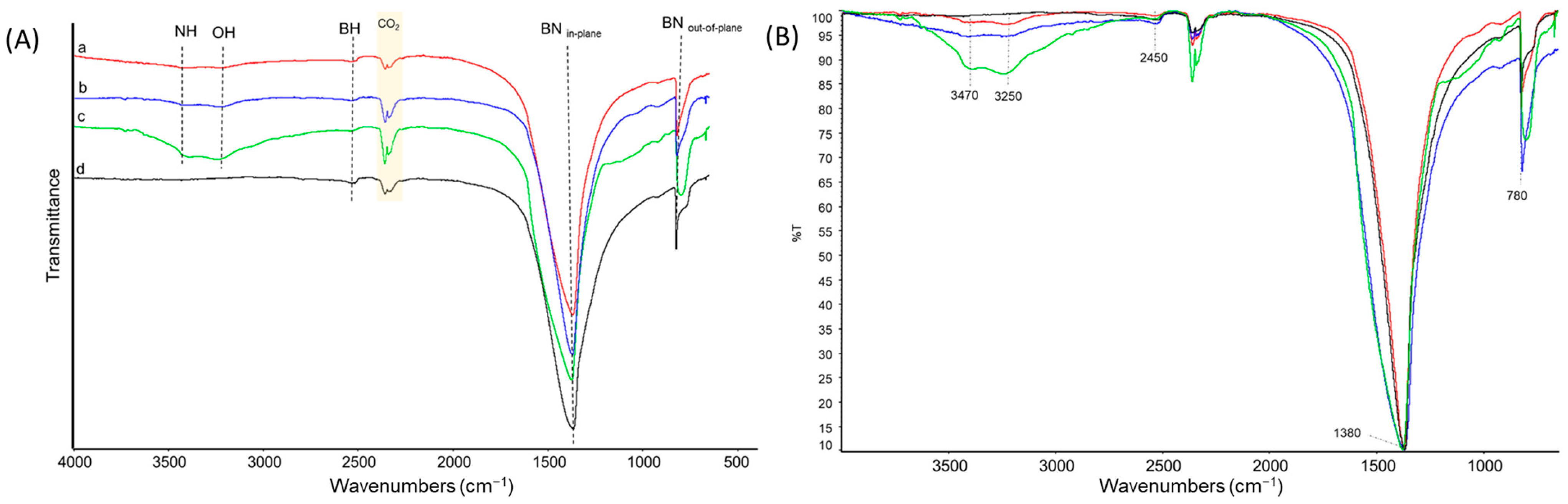
3.3. On the Functionalization of hBN with OH Groups
3.4. Elastomer Composites Containing Silica, hBN-p, and hBN-OH
3.4.1. Sulfur-Based Crosslinking
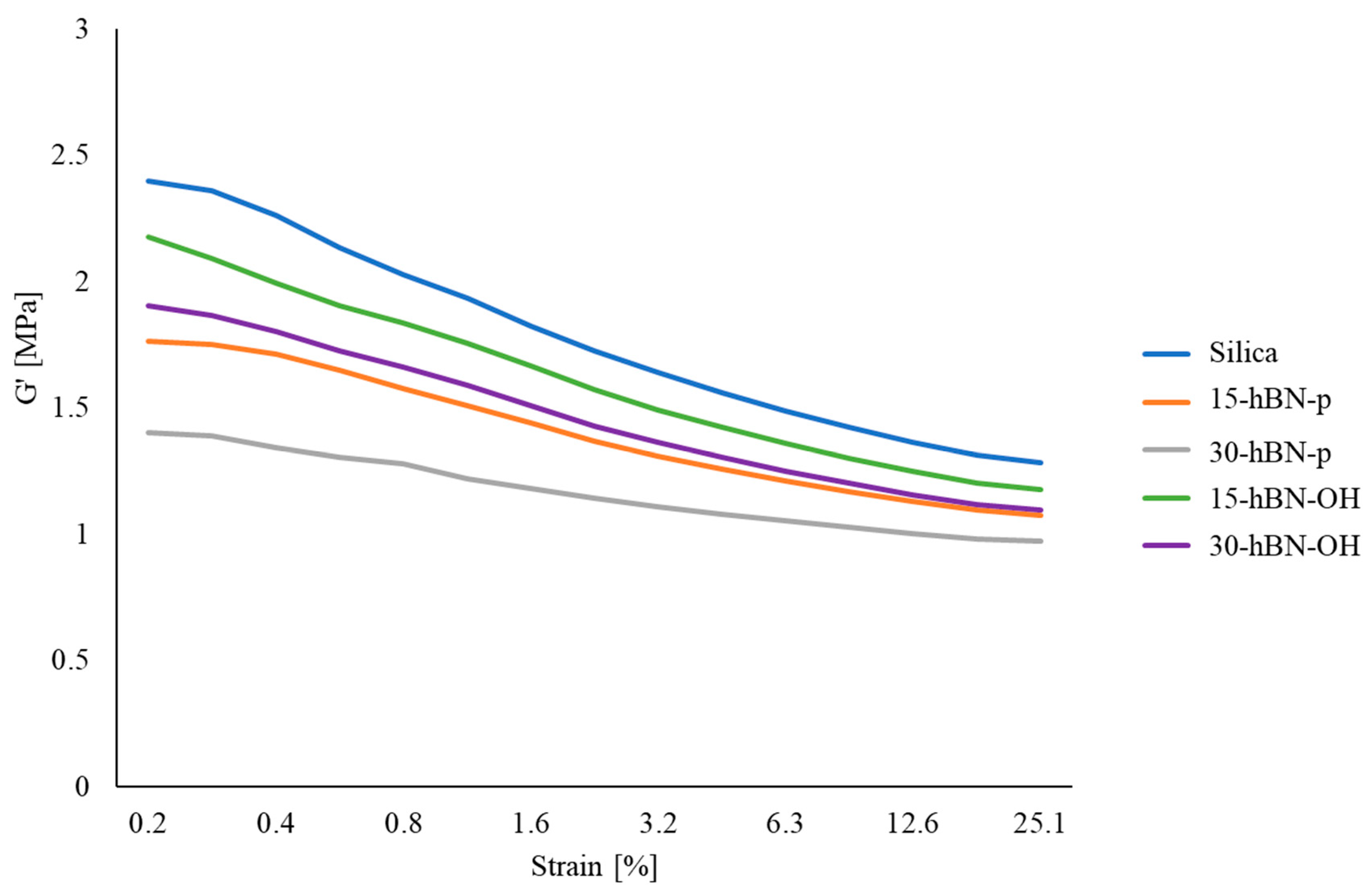
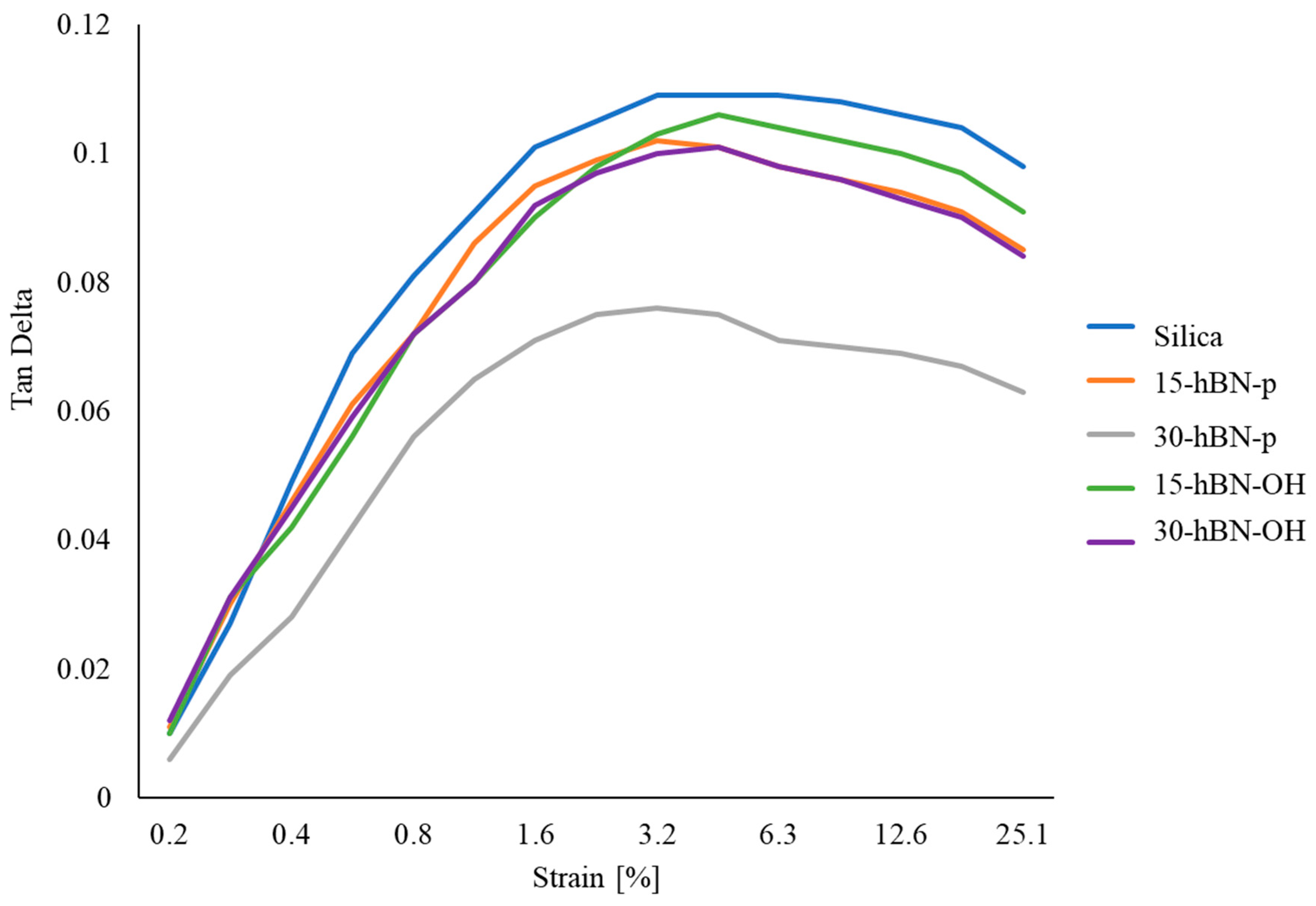
3.4.2. Dynamic Mechanical Properties in the Shear Mode
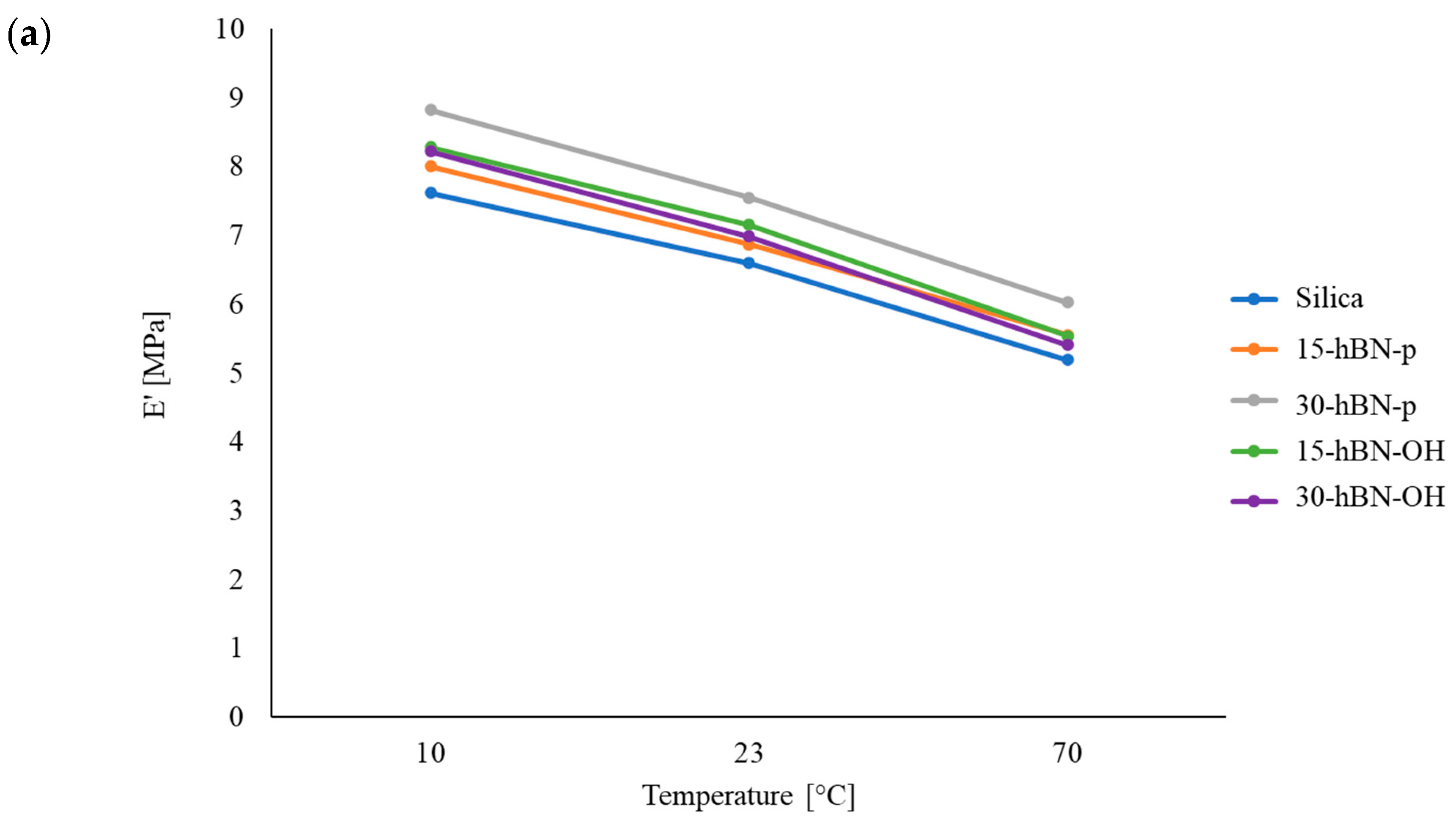
3.4.3. Dynamic Mechanical Properties from Axial Compression Tests
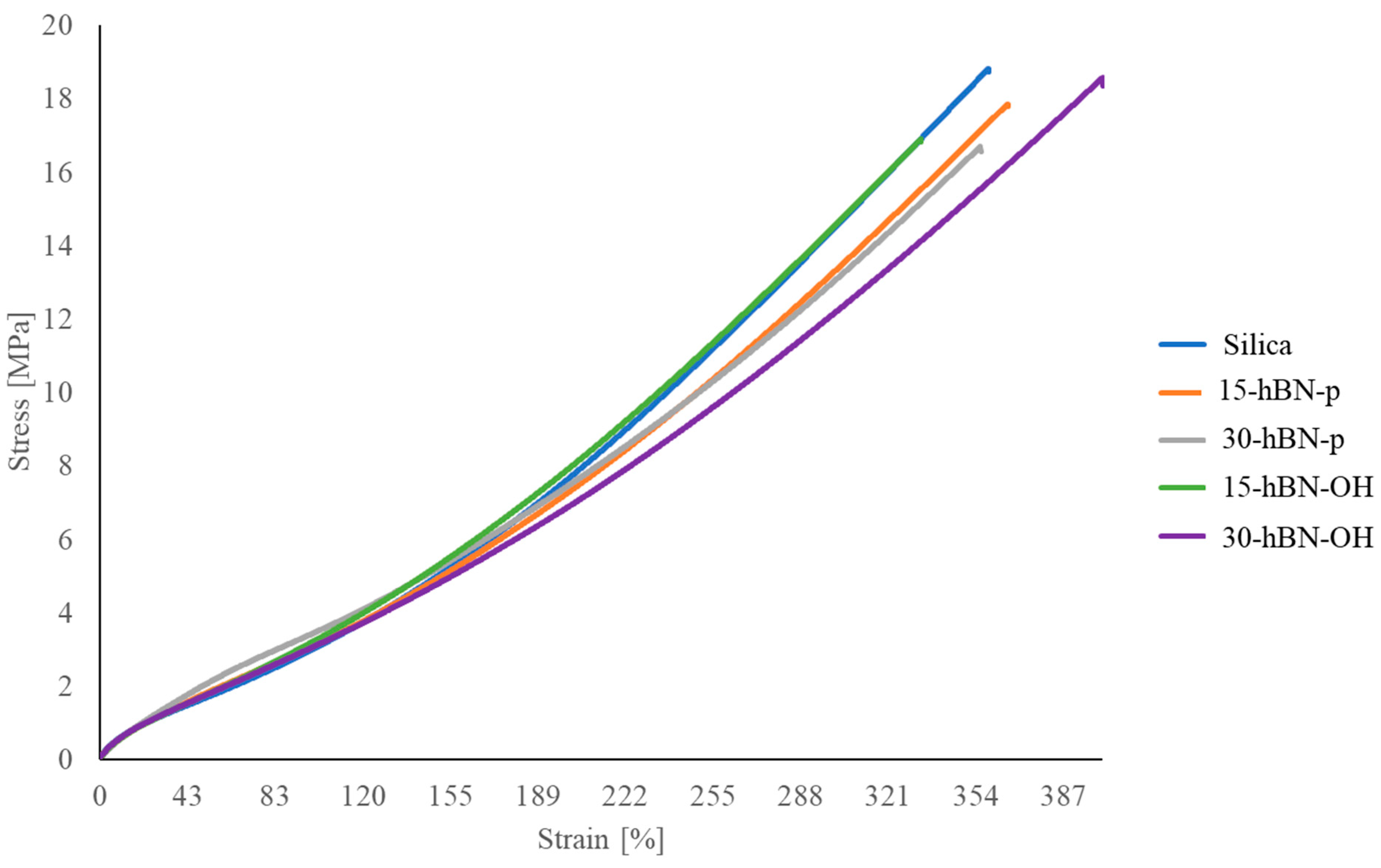
3.4.4. Tensile Properties
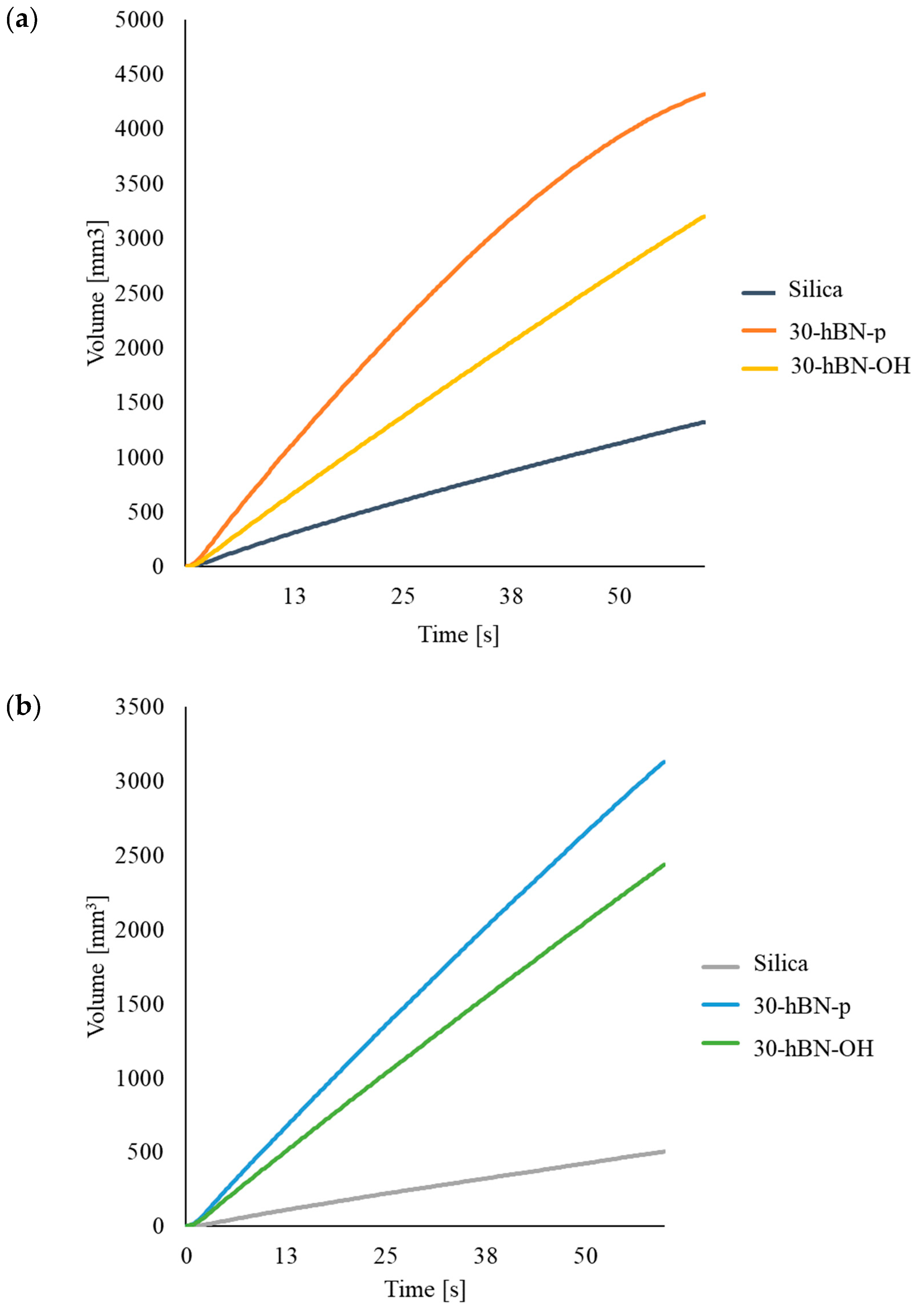
3.4.5. High-Pressure Capillary Viscometer (HKV)
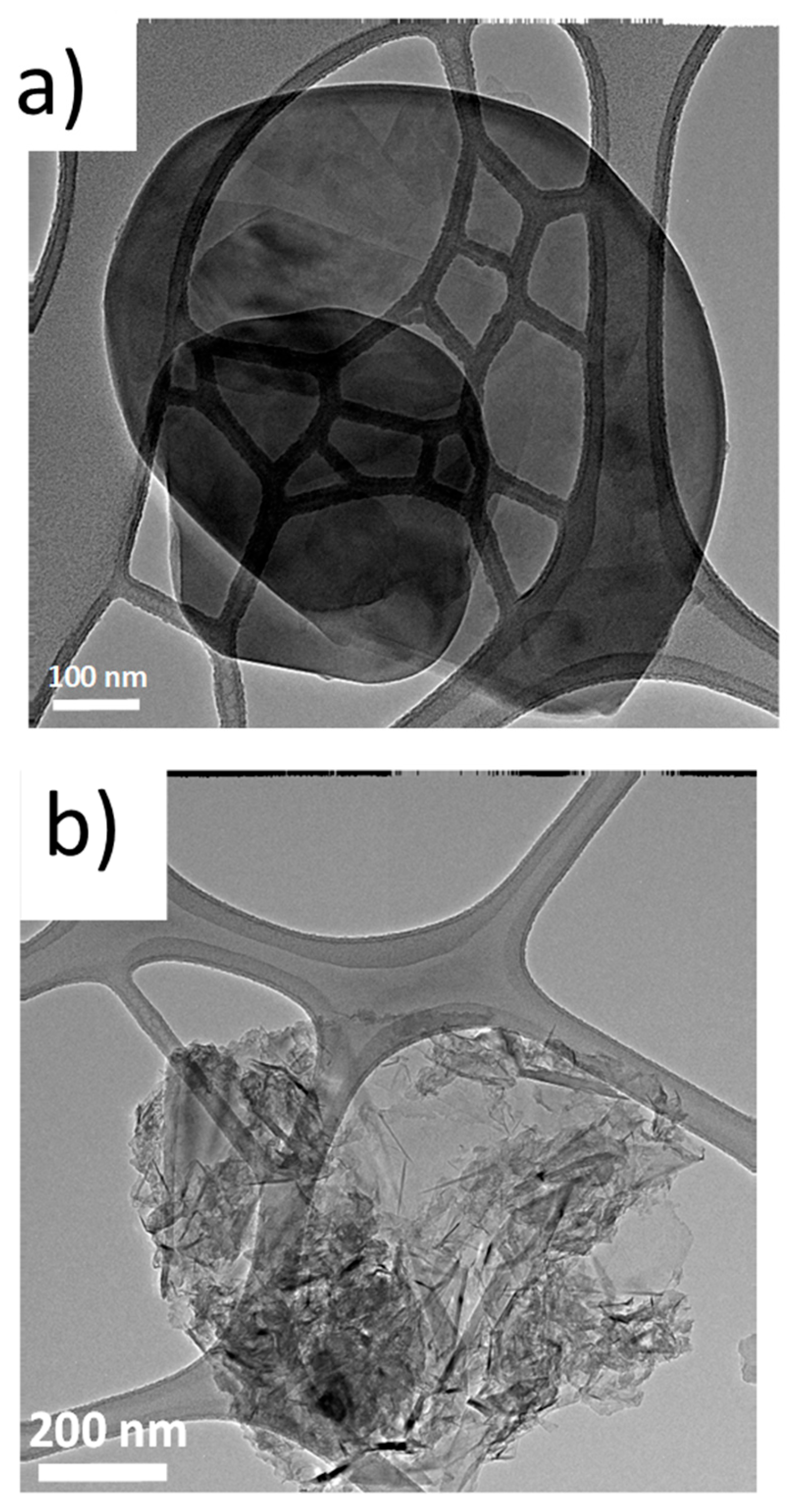
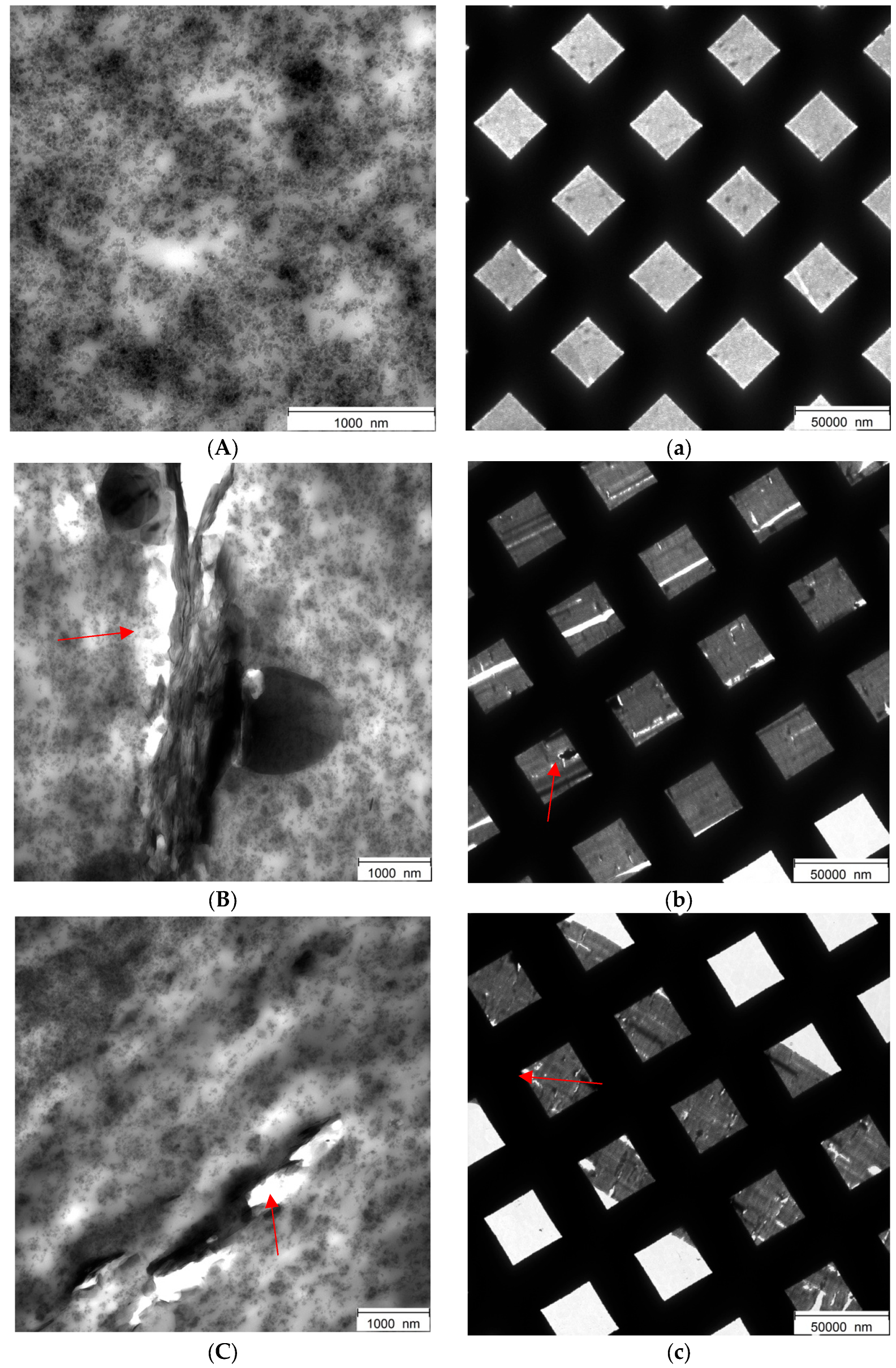
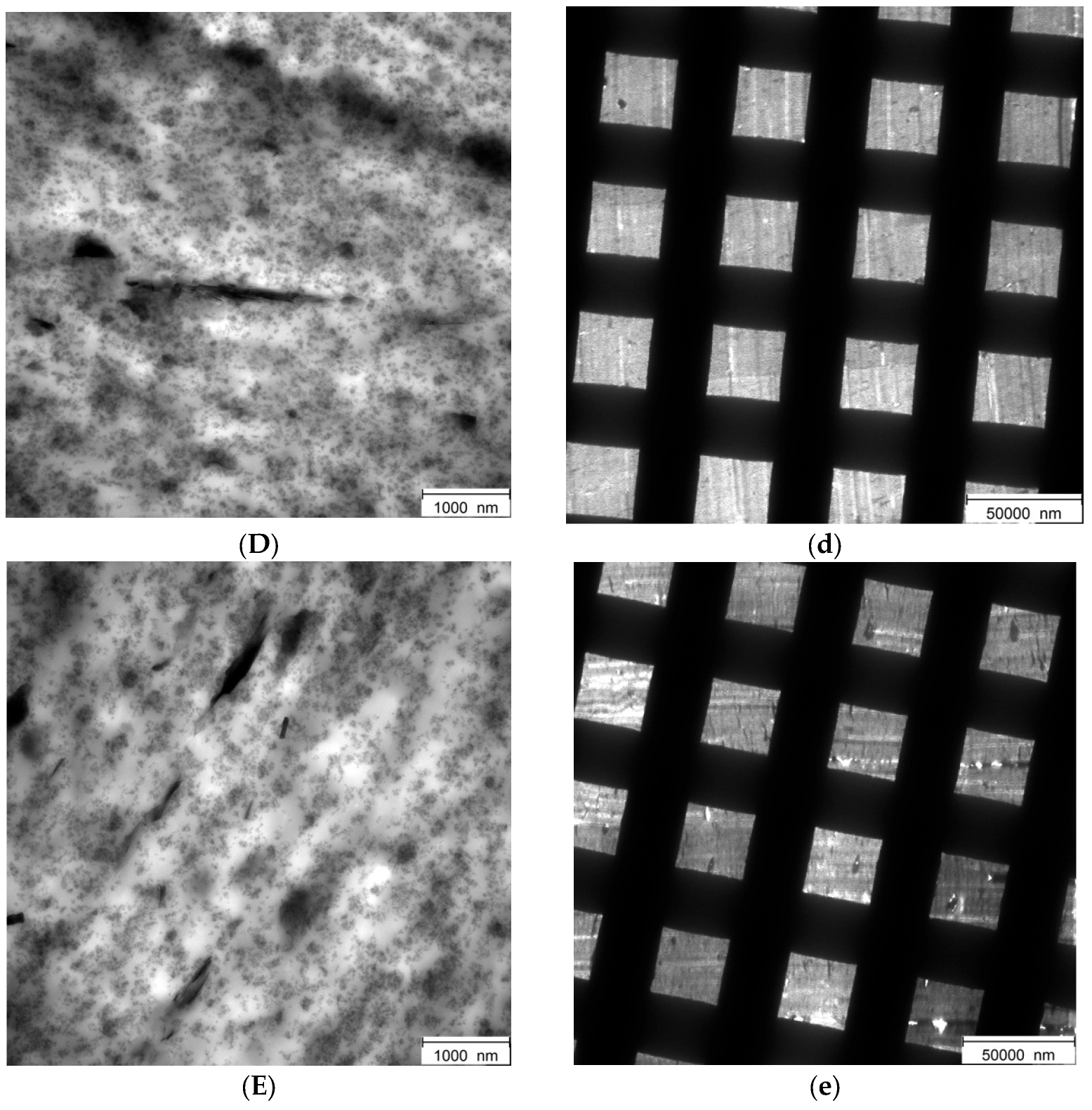
3.4.6. Transmission Electron Microscopy Analyses (TEM) of Rubber Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boehm, H.P.; Clauss, A.; Fischer, G.O.; Hofmann, U. Das adsorptionsverhalten sehr dünner kohlenstoff-folien. Z. Für Anorg. Und Allg. Chem. 1962, 316, 119–127. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; p. 11. [Google Scholar]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-dimensional nanomaterials for biomedical applications: Emerging trends and future prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Wang, R.; Zhang, Y.; Mahmood, A.; Ouyang, Z.; Zhang, H.; Guo, Z. Recent developments in emerging two-dimensional materials and their applications. J. Mater. Chem. C 2019, 8, 387–440. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Qiu, Y.; Yi, X.; Bussche, A.v.D.; Kane, A.; Gao, H.; Koski, K.; Hurt, R. Biological and environmental interactions of emerging two-dimensional nanomaterials. Chem. Soc. Rev. 2016, 45, 1750–1780. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Murali, A.; Lokhande, G.; Deo, K.A.; Brokesh, A.; Gaharwar, A.K. Emerging 2D nanomaterials for biomedical applications. Mater. Today 2021, 50, 276–302. [Google Scholar] [CrossRef]
- Bhati, V.S.; Kumar, M.; Banerjee, R. Gas sensing performance of 2D nanomaterials/metal oxide nanocomposites: A review. J. Mater. Chem. C 2021, 9, 8776–8808. [Google Scholar] [CrossRef]
- Marian, M.; Berman, D.; Rota, A.; Jackson, R.L.; Rosenkranz, A. Layered 2D nanomaterials to tailor friction and wear in machine elements—A review. Adv. Mater. Interfaces 2021, 9, 2101622. [Google Scholar] [CrossRef]
- Chen, W.; Shi, H.; Xin, H.; He, N.R.; Yang, W.; Gao, H. Friction and wear properties of Si3N4-hBN ceramic composites using different synthetic lubricants. Ceram. Int. 2018, 44, 16799–16808. [Google Scholar] [CrossRef]
- Cho, D.-H.; Kim, J.-S.; Kwon, S.-H.; Lee, C.; Lee, Y.-Z. Evaluation of hexagonal boron nitride nano-sheets as a lubricant additive in water. Wear 2013, 302, 981–986. [Google Scholar]
- Corrigan, F.R.; Bundy, F.P. Direct transitions among the allotropic forms of boron nitride at high pressures and temperatures. J. Chem. Phys. 1975, 63, 3812–3820. [Google Scholar] [CrossRef]
- Corso, M.; Auwärter, W.; Muntwiler, M.; Tamai, A.; Greber, T.; Osterwalder, J. Boron nitride nanomesh. Science 2004, 303, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Lago, E.; Santillo, C.; Castillo, A.E.E.D.R.; Hao, S.; Buonocore, G.G.; Chen, Z.; Xia, H.; Lavorgna, M.; Bonaccorso, F. An anisotropic layer-by-layer carbon nanotube/boron nitride/rubber composite and its application in electromagnetic shielding. Nanoscale 2020, 12, 7782–7791. [Google Scholar] [CrossRef] [PubMed]
- Ooi, N.; Rajan, V.; Gottlieb, J.; Catherine, Y.; Adams, J.B. Structural properties of hexagonal boron nitride. Model. Simul. Mater. Sci. Eng. 2006, 14, 515. [Google Scholar] [CrossRef]
- Bao, J.; Jeppson, K.; Edwards, M.; Fu, Y.; Ye, L.; Lu, X.; Liu, J. Synthesis and applications of two-dimensional hexagonal boron nitride in electronics manufacturing. Electron. Mater. Lett. 2016, 12, 1–16. [Google Scholar] [CrossRef]
- Shang, Y.; Yang, G.; Su, F.; Feng, Y.; Ji, Y.; Liu, D.; Yin, R.; Liu, C.; Shen, C. Multilayer polyethylene/hexagonal boron nitride composites showing high neutron shielding efficiency and thermal conductivity. Compos. Commun. 2020, 19, 147–153. [Google Scholar] [CrossRef]
- Fan, Y.; Cho, U.R. Effects of graphite and boron nitride based fillers on mechanical, thermal conductive, and thermo-physical properties in solution styrene–butadiene rubber. Polym. Compos. 2019, 40, E1426–E1433. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, B.; Wang, X.; Hanagata, N.; Li, X.; Liu, D.; Wang, X.; Jiang, X.; Bando, Y.; Golberg, D. Highly water-soluble, porous, and biocompatible boron nitrides for anticancer drug delivery. ACS Nano 2014, 8, 6123–6130. [Google Scholar] [CrossRef]
- Mukheem, A.; Shahabuddin, S.; Akbar, N.; Miskon, A.; Sarih, N.M.; Sudesh, K.; Khan, N.A.; Saidur, R.; Sridewi, N. Boron nitride doped polyhydroxyalkanoate/chitosan nanocomposite for antibacterial and biological applications. Nanomaterials 2019, 9, 645. [Google Scholar] [CrossRef]
- Hui, S.; Ji, R.; Zhang, H.; Huang, C.; Xu, F.; Sun, L.; Xia, Y.; Lin, X.; Ma, L.; Peng, H.; et al. N-Octadecane Encapsulated by Assembled BN/GO Aerogels for Highly Improved Thermal Conductivity and Energy Storage Capacity. Nanomaterials 2023, 13, 2317. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Uddin, I.; Watanabe, K.; Taniguchi, T.; Whang, D.; Kim, G.-H. Conversion of Charge Carrier Polarity in MoTe2 Field Effect Transistor via Laser Doping. Nanomaterials 2023, 13, 1700. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, Y.; Xiao, K.; Yang, Y.; Ding, H.; Deng, J.; Sun, W. Direct Synthesis of Vertical Self-Assembly Oriented Hexagonal Boron Nitride on Gallium Nitride and Ultrahigh Photoresponse Ultraviolet Photodetectors. Nanomaterials 2023, 13, 1546. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Du, Y.; Wang, M.; Liu, B.; Liu, J.; Jafri, S.H.M.; Liu, W.; Papadakis, R.; Zheng, X.; Li, H. Advances in the field of two-dimensional crystal-based photodetectors. Nanomaterials 2023, 13, 1379. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shen, Y.; Jia, X.; Xu, M.; Zong, R.; Liu, G.; Liu, B.; Huai, X. Dopamine-Mediated Graphene Bridging Hexagonal Boron Nitride for Large-Scale Composite Films with Enhanced Thermal Conductivity and Electrical Insulation. Nanomaterials 2023, 13, 1210. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ge, X.; Zhang, Z.; Yin, S.; Liang, W.; Ge, J. Silicone-Based Thermally Conductive Gel Fabrication via Hybridization of Low-Melting-Point Alloy–Hexagonal Boron Nitride–Graphene Oxide. Nanomaterials 2023, 13, 490. [Google Scholar] [CrossRef]
- Sun, L.; Zou, Y.; Ma, M.; Li, G.; Wang, X.; Zhang, X.; Zhao, Z. Synthesis of cBN-hBN-SiCw Nanocomposite with Superior Hardness, Strength, and Toughness. Nanomaterials 2022, 13, 37. [Google Scholar] [CrossRef]
- Mballo, A.; Srivastava, A.; Sundaram, S.; Vuong, P.; Karrakchou, S.; Halfaya, Y.; Gautier, S.; Voss, P.L.; Ahaitouf, A.; Salvestrini, J.P.; et al. Towards P-type conduction in hexagonal boron nitride: Doping study and electrical measurements analysis of hBN/AlGaN heterojunctions. Nanomaterials 2021, 11, 211. [Google Scholar] [CrossRef]
- Roy, S.; Zhang, X.; Puthirath, A.B.; Meiyazhagan, A.; Bhattacharyya, S.; Rahman, M.M.; Ajayan, P.M. Structure, properties and applications of two-dimensional hexagonal boron nitride. Adv. Mater. 2021, 33, 2101589. [Google Scholar] [CrossRef]
- Molaei, M.J.; Younas, M.; Rezakazemi, M. A comprehensive review on recent advances in two-dimensional (2d) hexagonal boron nitride. ACS Appl. Electron. Mater. 2021, 3, 5165–5187. [Google Scholar] [CrossRef]
- Li, M.; Huang, G.; Chen, X.; Yin, J.; Zhang, P.; Yao, Y.; Shen, J.; Wu, Y.; Huang, J. Perspectives on environmental applications of hexagonal boron nitride nanomaterials. Nano Today 2022, 44, 101486. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, R.; Zhang, N.; Chen, L.; Li, X. Flexible composite phase change material with improved hydrophobicity and thermal conductivity characters for thermal management. J. Energy Storage 2023, 72, 108641. [Google Scholar] [CrossRef]
- Transforming Our World: The 2030 Agenda for Sustainable Development|Department of Economic and Social Affairs. Available online: https://un.org/ (accessed on 4 September 2023).
- World Tire Market Outlook 2020–2025—Global Market Forecast to Reach 2.7 Billion Units by 2025. Available online: https://prnewswire.com/ (accessed on 4 September 2023).
- Hall, D.E.; Moreland, J.C. Fundamentals of rolling resistance. Rubber Chem. Technol. 2001, 74, 525–539. [Google Scholar] [CrossRef]
- Chevalier, Y.; Morawski, J.C. Precipitated Silica with Morphological Properties, Process for Producing It and Its Application, Especially as a Filler. Patent EP 0157703 B1, 29 March 1985. [Google Scholar]
- Legrand, A.P. On the silica edge. In The Surface Properties of Silicas; Legrand, A.P., Ed.; Wiley and Sons: New York, NY, USA, 1998; pp. 1–20. [Google Scholar]
- Locatelli, D.; Bernardi, A.; Rubino, L.R.; Gallo, S.; Vitale, A.; Bongiovanni, R.; Barbera, V.; Galimberti, M. Biosourced Janus Molecules as Silica Coupling Agents in Elastomer Composites for Tires with Lower Environmental Impact. ACS Sustain. Chem. Eng. 2023, 11, 2713–2726. [Google Scholar] [CrossRef]
- Ramier, J.; Chazeau, L.; Gauthier, C.; Guy, L.; Bouchereau, M.N. Influence of silica and its different surface treatments on the vulcanization process of silica filled SBR. Rubber Chem. Technol. 2007, 80, 183–193. [Google Scholar] [CrossRef]
- Guerra, S.; Barbera, V.; Galimberti, M.; Giese, U. Effect of the Functionalization of graphene Nanoplatelets with a Janus Pyrrole Molecule on mechanical and electrical properties of natural Rubber Nanocomposites. KGK Kautsch. Gummi Kunststoffe 2020, 2020, 17–25. [Google Scholar]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for synthesis. Angew. Chem. 2020, 132, 1030–1041. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, Y.; Wang, F.; Yang, Z.; Wang, J. Two dimensional hexagonal boron nitride (2D-hBN): Synthesis, properties and applications. J. Mater. Chem. C 2017, 5, 11992–12022. [Google Scholar] [CrossRef]
- Lin, Y.; Williams, T.V.; Xu, T.-B.; Cao, W.; Elsayed-Ali, H.E.; Connell, J.W. Aqueous dispersions of few-layered and monolayered hexagonal boron nitride nanosheets from sonication-assisted hydrolysis: Critical role of water. J. Phys. Chem. C 2011, 115, 2679–2685. [Google Scholar] [CrossRef]
- Sainsbury, T.; Satti, A.; May, P.; Wang, Z.; McGovern, I.; Gun’ko, Y.K.; Coleman, J. Oxygen radical functionalization of boron nitride nanosheets. J. Am. Chem. Soc. 2012, 134, 18758–18771. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, B.; Park, K.H.; Ryu, H.J.; Jeon, S.; Hong, S.H. Scalable exfoliation process for highly soluble boron nitride nanoplatelets by hydroxide-assisted ball milling. Nano Lett. 2015, 15, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-S.; Ding, H.-L.; Liu, Z.; Cheng, Z.-L. Preparation and tribological properties of hydrothermally exfoliated ultrathin hexagonal boron nitride nanosheets (BNNSs) in mixed NaOH/KOH solution. J. Alloy. Compd. 2019, 784, 807–815. [Google Scholar] [CrossRef]
- Yan, W.; Chen, X.; Lim, J.S.K.; Chen, H.; Gill, V.; Lambourne, A.; Hu, X. Epoxy-assisted ball milling of boron nitride towards thermally conductive impregnable composites. Compos. Part A Appl. Sci. Manuf. 2022, 156, 106868. [Google Scholar] [CrossRef]
- Pakdel, A.; Bando, Y.; Golberg, D. Plasma-assisted interface engineering of boron nitride nanostructure films. ACS Nano 2014, 8, 10631–10639. [Google Scholar] [CrossRef]
- Xiao, F.; Naficy, S.; Casillas, G.; Khan, M.H.; Katkus, T.; Jiang, L.; Liu, H.; Li, H.; Huang, Z. Edge-hydroxylated boron nitride nanosheets as an effective additive to improve the thermal response of hydrogels. Adv. Mater. 2015, 27, 7196–7203. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Kozuch, D.; Robinson, J.A. Large-scale synthesis and functionalization of hexagonal boron nitride nanosheets. Nanoscale 2014, 6, 11671–11675. [Google Scholar] [CrossRef]
- Cui, Z.; Oyer, A.J.; Glover, A.J.; Schniepp, H.C.; Adamson, D.H. Large scale thermal exfoliation and functionalization of boron nitride. Small 2014, 10, 2352–2355. [Google Scholar] [CrossRef]
- Shen, T.; Liu, S.; Yan, W.; Wang, J. Highly efficient preparation of hexagonal boron nitride by direct microwave heating for dye removal. J. Mater. Sci. 2019, 54, 8852–8859. [Google Scholar] [CrossRef]
- ISO9924-1 Standard. Available online: https://www.iso.org/standard/84139.html (accessed on 14 December 2023).
- Standard ISO 37/UNI 6065. Available online: https://www.eurolab.net/it/testler/malzeme-testleri/iso-37-kaucuk-vulkanize-veya-termoplastik-cekme-gerilimi-uzama-ozelliklerinin-tayini/ (accessed on 14 December 2023).
- Yadav, V.; Kulshrestha, V. Boron nitride: A promising material for proton exchange membranes for energy applications. Nanoscale 2019, 11, 12755–12773. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou, N.; Polychronopoulou, K.; Rebholz, C. Thermal and chemical stability of hexagonal boron nitride (h-BN) nanoplatelets. Vacuum 2015, 112, 42–45. [Google Scholar] [CrossRef]
- Ramier, J.; Gauthier, C.; Chazeau, L.; Stelandre, L.; Guy, L. Payne effect in silica-filled styrene–butadiene rubber: Influence of surface treatment. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 286–298. [Google Scholar] [CrossRef]
- Chazeau, L.; Brown, J.D.; Yanyo, L.C.; Sternstein, S.S. Modulus recovery kinetics and other insights into the Payne effect for filled elastomers. Polym. Compos. 2000, 21, 202–222. [Google Scholar] [CrossRef]
- Hentschke, R. The Payne effect revisited. Express Polym. Lett. 2017, 11, 278–292. [Google Scholar] [CrossRef]
- Çelik, O.N.; Ay, N.; Göncü, Y. Effect of nano hexagonal boron nitride lubricant additives on the friction and wear properties of AISI 4140 steel. Part. Sci. Technol. 2013, 31, 501–506. [Google Scholar] [CrossRef]
- Van Sang, L.; Yano, A.; Osaka, A.I.; Sugimura, N.; Washizu, H. Smoothed particle hydrodynamics and discrete element method coupling for influence of hexagonal boron nitride lubricant particle on friction of elastic coarse-grained micronscale iron. J. Tribol. 2021, 144, 01190. [Google Scholar]
- Samanta, S.; Sahoo, R.R. Covalently linked hexagonal boron nitride-graphene oxide nanocomposites as high-performance oil-dispersible lubricant additives. ACS Appl. Nano Mater. 2020, 3, 10941–10953. [Google Scholar] [CrossRef]
- Agnelli, S.; Pandini, S.; Serafini, A.; Musto, S.; Galimberti, M. Anisotropic nonlinear mechanical behavior in carbon nanotubes/poly (1, 4-cis-isoprene) nanocomposites. Macromolecules 2016, 49, 8686–8696. [Google Scholar] [CrossRef]
- Agnelli, S.; Pandini, S.; Torricelli, F.; Romele, P.; Serafini, A.; Barbera, V.; Galimberti, M. Anisotropic properties of elastomeric nanocomposites based on natural rubber and sp2 carbon allotropes. Express Polym. Lett. 2018, 12, 713–730. [Google Scholar] [CrossRef]
- Bokobza, L.; Chauvin, J.-P. Reinforcement of natural rubber: Use of in situ generated silicas and nanofibres of sepiolite. Polymer 2005, 46, 4144–4151. [Google Scholar] [CrossRef]
- Locatelli, D.; Pavlovic, N.; Barbera, V.; Giannini, L.; Galimberti, M. Sepiolite as reinforcing Filler for Rubber Composites: From the chemical Compatibilization to the commercial Exploitation. KGK Kautsch. Gummi Kunststoffe 2020, 73, 26–35. [Google Scholar]






| Recipes in phr | Silica | 15-hBN-p | 30-hBN-p | 15-hBN-OH | 30-hBN-OH |
|---|---|---|---|---|---|
| [phr] | [phr] | [phr] | [phr] | [phr] | |
| S-SBR 4630 | 70 | 70 | 70 | 70 | 70 |
| NR | 30 | 30 | 30 | 30 | 30 |
| Silica | 50 | 42.5 | 35 | 42.5 | 35 |
| hBN | 0 | 7.5 | 15 | 0 | 0 |
| BN-OH | 0 | 0 | 0 | 7.5 | 15 |
| Experimental Wavenumber (cm−1) | Vibrational Assignment | Relative Abundance per Sample (%) | |||
|---|---|---|---|---|---|
| hBN-Ohas Milled for 2.5 h | hBN-Ohas Milled for 5 h | hBN-Ohas Milled for 10 h | hBN-p | ||
| 3470 | N-H stretching | 2 | 3 | 5 | 0 |
| 3250 | O-H stretching | 2 | 3 | 4 | 0 |
| 2450 | B-H stretching | 1 | 1 | 1 | 1 |
| 1380 | BN in-plane | 100 | 100 | 100 | 100 |
| 780 | BN out-of-plane | 13 | 12 | 9 | 9 |
| Experimental Wavenumber (cm−1) | Vibrational Assignment | Relative Abundance per Sample (%) | |||
|---|---|---|---|---|---|
| hBN-Ohw Milled for 2.5 h | hBN-Ohw Milled for 5 h | hBN-Ohw Milled for 10 h | hBN-p | ||
| 3470 | N-H stretching | 1 | 2 | 6 | 0 |
| 3250 | O-H stretching | 1 | 2 | 6 | 0 |
| 2450 | B-H stretching | 1 | 1 | 1 | 1 |
| 1380 | BN in-plane | 100 | 100 | 100 | 100 |
| 780 | BN out-of-plane | 13 | 12 | 9 | 9 |
| Sample | Milling Time (h) | Temperature Range | |||
|---|---|---|---|---|---|
| T < 150 °C | 150 °C < T < 500 °C | 500 °C < T < 900 °C | T > 900 °C + Residue | ||
| hBN-p | = | 0 | 0 | 1.5 | 98.5 |
| hBN-OHas | 5 | 1.9 | 2.7 | 1.3 | 96.5 |
| hBN-OHw | 5 | 2.5 | 2.52 | 1 | 93.5 |
| hBN-OHas | 10 | 6.8 | 4.5 | 1.6 | 87.2 |
| hBN-OHw | 10 | 6.7 | 6.4 | 0.8 | 86.1 |
| Sample | Milling Time (h) | d002 (nm) | D⊥ (nm) | D// (nm) | D///D⊥ | Layers |
|---|---|---|---|---|---|---|
| hBN-p | 0.31 | 41.53 | 44.56 | 1.07 | 132 | |
| hBN-OHas | 2.5 | 0.31 | 12.82 | 16.80 | 1.31 | 41 |
| hBN-OHw | 2.5 | 0.31 | 18.81 | 26.22 | 1.39 | 60 |
| hBN-OHas | 5 | 0.31 | 11.27 | 19.01 | 1.68 | 36 |
| hBN-OHw | 5 | 0.32 | 15.01 | 21.61 | 2.32 | 40 |
| hBN-OHas | 10 | 0.32 | 2.61 | 3.11 | 1.19 | 8 |
| hBN-OHw | 10 | 0.32 | 2.90 | 3.27 | 1.13 | 9 |
| Silica | 15-hBN-p | 30-hBN-p | 15-hBN-OH | 30-hBN-OH | |
|---|---|---|---|---|---|
| ML [dNm] | 3.8 | 2.5 | 1.9 | 3.4 | 3.1 |
| MH [dNm] | 21.1 | 18.1 | 16.5 | 19.4 | 18.6 |
| MH-ML [dNm] | 17.3 | 15.5 | 14.5 | 16.0 | 15.5 |
| tS1 [min] | 3.1 | 3.4 | 3.4 | 3.2 | 3.4 |
| t90 [min] | 7.8 | 8.5 | 8.2 | 7.5 | 7.7 |
| curing rate [dNM/min] | 3.7 | 3.1 | 3.1 | 3.8 | 3.7 |
| T [°C] | Silica | 15-hBN-p | 30-hBN-p | 15-hBN-OH | 30-hBN-OH | |
|---|---|---|---|---|---|---|
| E′ [MPa] | 10 | 7.6 | 8.0 | 8.8 | 8.3 | 8.2 |
| 23 | 6.6 | 6.9 | 7.5 | 7.1 | 7.0 | |
| 70 | 5.2 | 5.6 | 6.0 | 5.5 | 5.4 | |
| E″ [MPa] | 10 | 3.8 | 3.8 | 4.1 | 4.0 | 4.1 |
| 23 | 2.3 | 2.3 | 2.4 | 2.5 | 2.5 | |
| 70 | 0.6 | 0.6 | 0.5 | 0.6 | 0.6 | |
| Tanδ | 10 | 0.49 | 0.48 | 0.47 | 0.49 | 0.50 |
| 23 | 0.35 | 0.33 | 0.33 | 0.35 | 0.35 | |
| 70 | 0.12 | 0.10 | 0.09 | 0.11 | 0.11 | |
| ΔE′ (E′@10 °C–E′@70 °C) (MPa) | 2.4 | 2.4 | 2.8 | 2.7 | 2.8 |
| Silica | 15-hBN-p | 30-hBN-p | 15-hBN-OH | 30-hBN-OH | |
|---|---|---|---|---|---|
| σ50% [MPa] | 0.03 | 0.05 | 0.03 | 0.013 | |
| σ100% [MPa] | 0.06 | 0.1 | 0.07 | ||
| σ300% [MPa] | 0.13 | 0.36 | 0.34 | ||
| σbreak [MPa] | 1.59 | 1.61 | 2.60 | 1.28 | |
| Ɛbreak [%] | 23.24 | 20.24 | 22.86 | 21.67 | |
| Energy break [MJ/m³] | 3.54 | 3.54 | 3.84 | 3.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magaletti, F.; Prioglio, G.; Giese, U.; Barbera, V.; Galimberti, M. Hexagonal Boron Nitride as Filler for Silica-Based Elastomer Nanocomposites. Nanomaterials 2024, 14, 30. https://doi.org/10.3390/nano14010030
Magaletti F, Prioglio G, Giese U, Barbera V, Galimberti M. Hexagonal Boron Nitride as Filler for Silica-Based Elastomer Nanocomposites. Nanomaterials. 2024; 14(1):30. https://doi.org/10.3390/nano14010030
Chicago/Turabian StyleMagaletti, Federica, Gea Prioglio, Ulrich Giese, Vincenzina Barbera, and Maurizio Galimberti. 2024. "Hexagonal Boron Nitride as Filler for Silica-Based Elastomer Nanocomposites" Nanomaterials 14, no. 1: 30. https://doi.org/10.3390/nano14010030
APA StyleMagaletti, F., Prioglio, G., Giese, U., Barbera, V., & Galimberti, M. (2024). Hexagonal Boron Nitride as Filler for Silica-Based Elastomer Nanocomposites. Nanomaterials, 14(1), 30. https://doi.org/10.3390/nano14010030






