Recent Advances toward Enhanced Photocatalytic Proprieties of BiFeO3-Based Materials
Abstract
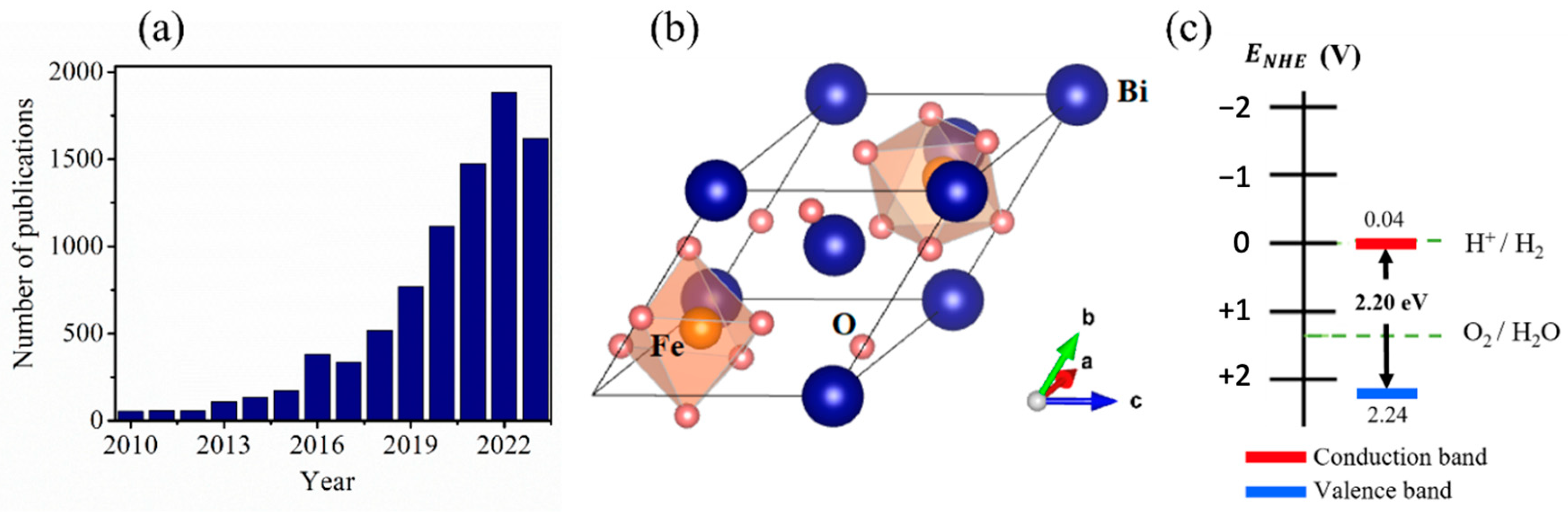
:1. Introduction
2. Bismuth Ferrite
2.1. Size Effect
2.2. Doping and Substitution Effects
2.3. Effect of BFO-Based Heterostructures
3. BFO-Based Materials Photocatalytic Applications
3.1. Degradation of Organic Pollutants
| Photocatalyst | Doping Elements | Band Gap (ev) | Polluant | Degradation Time | Removal Efficiency | Refs. |
|---|---|---|---|---|---|---|
| BiFeO3 | 2.2 eV | Methylene Blue | 240 min | 58% | [122] | |
| 10% Gd-BiFeO3 | Gd | 1.95–1.18 eV | Ciprofloxacin | 240 min | 80% | [121] |
| 10% Gd-BiFeO3 | Gd | 1.95–1.18 eV | Levofloxacin | 240 min | 79% | [121] |
| 10% Gd-BiFeO3 | Gd | 2.38–2.29 eV | Methylene Blue | 180 min | 97% | [122] |
| 10% Gd-BiFeO3 | Gd | 2.03–2.2 eV | Rhodamine B | 240 min | 96% | [124] |
| Bi0.90La0.05Ba0.05FeO3 | La | 2.02–2.11 eV | Methylene Blue | 70 min | 87% | [125] |
| Bi0.8Nd0.2FeO3 | Nd | 1.99 eV | Rhodamine B | 120 min | 59% | [128] |
| Bi0.85Dy0.15FeO3 | Dy | 2.35–2.26 eV | Methylene Blue | 240 min | 92% | [126] |
| Er3%-BFO | Er | 2.12 eV | Tetracycline hydrochloride | 180 min | 75,8 | [127] |
| Bi0.97Sm0.03FeO3 | Sm | 2.14 eV | Methyl orange | 120 min | 86.9% | [129] |
| Bi0.93Ba0.07FeO3 | Ba | 2.11–1.86 eV | Toluene | 50 min | 91% | [130] |
| Bi0.93Ba0.07FeO3 | Ba | 2.11–1.86 eV | Benzene | 50 min | 81% | [130] |
| 10% Mn-doped BFO | Mn | 2.2–1.97 eV | Acid red 85 | 60 min | 100% | [119] |
| BiFe0.925Co0.075O3 | Co | Acid Red 85 | 240 min | 93.79% | [131] | |
| 0.2 wt% Pd-BFO | Pb | 2.10 eV | Malachite green | 95.7% | [132] | |
| Bi0.92La0.08Fe0.95Se0.5O3 | (La, Se) | 1.77 eV | Congo Red | 30 min | 90% | [133] |
| Bi0.92Ce0.08Fe0.92Ni0.08O3 | (Ce, Ni) | 1.9 eV | Methylene Blue | 90 min | 93.29% | [134] |
| Bi0.92Ce0.08Fe0.92Ni0.08O3 | (Ce, Ni) | 1.9 eV | Rhodamine B | 90 min | 96.05% | [134] |
| Bi0.9Ba0.05Fe0.95Ca0.05O3 | (Ba, Ca) | 2.1 eV | Methylene Blue | 90 min | 93% | [125] |
| Bi0.95Nd0.05Fe0.97Ni0.03O3 | (Nd, Ni) | 2.1 eV | Methylene Blue | 90 min | 93% | [135] |
| BiFeO3/BiVO4 | - | 2.7 eV | Tetracycline | 90 min | 95% | [136] |
| BiFeO3/MoS2 | - | 1.8 eV | Rhodamine B | 200 min | 89% | [137] |
| Ag/BiFeO3 | - | 2.2 eV | Methyl orange | 120 min | 96% | [138] |
| BiFeO3/rGO | - | 1.9 eV | Methylene Blue | 300 min | 98% | [139] |
| Cu2O/BiFeO3 | - | 2/2.1 eV | Tetracycline | 120 min | 98% | [140] |
| BiFeO3/Bi2Fe4O9 | - | 2.2/1.9 eV | O-chlorophenol | 240 min | 95% | [141] |
| SnO2/BiFeO3 | - | 3.5/2.07 eV | Rhodamine B | 120 min | 87.2% | [142] |
| BiFeO3/GdFeO3 | - | 2/2.3 eV | Methylene Blue | 540 min | 98% | [143] |
| CuO/BiFeO3 | - | 1.7/2.18 eV | Rhodamine B | 270 min | 50% | [144] |
| BiFeO3/ZnFe2O4 | - | 2.17/2.03 eV | Methylene Blue | 120 min | 97% | [145] |
| BiFeO3/TiO2 | - | 2.2/3.2 eV | Methylene Blue | 180 min | 94.4% | [146] |
| BiFeO3/Fe2O3 | - | 2.25/1.9 eV | Methylene Blue | 60 min | 94% | [147] |
| BiFeO3/CuWO4 | - | 2.1/2.3 eV | Methyl orange | 120 min | 85% | [148] |
| Ag2O/BiFeO3 | - | 1.3/2.1 eV | Rhodamine B | 60 min | 97% | [149] |
| BiFeO3/g-C3N4 | - | 2.13/2.78 eV | Rhodamine B | 60 min | 100% | [150] |
3.2. Solar Water Splitting
4. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM 1.5G | Air Mass 1.5 Global Spectrum |
| CB | Conduction Band |
| CPE | Constant Phase Elements |
| CS | Conventional Sintering |
| DEA | Diethaloamine |
| EIS | Electrochemical Impedance Spectroscopy |
| FTO | Fluorine doped Tin Oxide |
| HER | Hydrogen Evolution Reaction |
| ITO | Indium Tin Oxide |
| LSV | Linear Sweep Voltammetry |
| MB | Methylene Blue |
| MWS | Microwave Sintering |
| NPs | Nanoparticles |
| OER | Oxygen Evolution Reaction |
| PC | Photocatalysis |
| PEC | Photoelectrochemical |
| pH | Potential of Hydrogen |
| PL | Photoluminescence |
| PLD | Pulsed Laser Deposition |
| PMs | Perovskite materials |
| Pr | Remanent Polarization |
| PV | Photovoltaic |
| RHE | Reversible Hydrogen Electrode |
| ROS | Reactive Oxygen Species |
| RT | Room Temperature |
| SCE | Saturated Calomel Electrode |
| SEM | Scanning Electron Microscope |
| SG | Sol–gel |
| STH | Solar-to-Hydrogen |
| TC | Tetracycline hydrochloride |
| UV | Ultra Violet |
| VB | Valence Band |
| WS | Water Splitting |
| εr | Dielectric Constant |
References
- Barbir, F.; Veziro, T.N.; Plass, H.J. Environmental damage due to fossil fuels use. Int. J. Hydrogen Energy 1990, 15, 739–749. [Google Scholar] [CrossRef]
- Hassan, A.; Ilyas, S.Z.; Jalil, A.; Ullah, Z. Monetization of the environmental damage caused by fossil fuels. Environ. Sci. Pollut. Res. 2021, 28, 21204–21211. [Google Scholar] [CrossRef] [PubMed]
- Geerken, T.G.; Timmermans, V.T.; Lassaux, S.L. Hydrogen and its Applications: Review of Life Cycle Assessment Studies and Well-to-Wheel Studies. Hysociety 2005, 1–11. Available online: http://www2.ulg.ac.be/cior-fsa/publicat/erscp_h2.pdf (accessed on 1 December 2023).
- Burdack, A.; Duarte-Herrera, L.; López-Jiménez, G.; Polklas, T.; Vasco-Echeverri, O. Techno-economic calculation of green hydrogen production and export from Colombia. Int. J. Hydrogen Energy 2022, 48, 1685–1700. [Google Scholar] [CrossRef]
- Shahin, M.S.; Orhan, M.F.; Saka, K.; Hamada, A.T.; Uygul, F. Energy assessment of an integrated hydrogen production system. Int. J. Thermofluids 2023, 17, 100262. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Faisal, A.; Hafeez, A.; Javed, F.; Mustafa, M.; Rehman, F. Hydrogen Production through Water Vapors using Optimized Corona-DBD Hybrid Plasma Micro-Reactor. Fuel 2023, 331, 125838. [Google Scholar] [CrossRef]
- Hren, R.; Vujanović, A.; Van Fan, Y.; Klemeš, J.J.; Krajnc, D.; Čuček, L. Hydrogen production, storage and transport for renewable energy and chemicals: An environmental footprint assessment. Renew. Sustain. Energy Rev. 2023, 173, 113113. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An Overview of Hydrogen Production: Current Status, Potential, and Challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Ardo, F.M.; Lim, J.W.; Ramli, A.; Lam, M.K.; Kiatkittipong, W.; Abdelfattah, E.A.; Shahid, M.K.; Usman, A.; Wongsakulphasatch, S.; Sahrin, N.T. A review in redressing challenges to produce sustainable hydrogen from microalgae for aviation industry. Fuel 2022, 330, 125646. [Google Scholar] [CrossRef]
- Aydin, M.I.; Dincer, I. An assessment study on various clean hydrogen production methods. Energy 2022, 245, 123090. [Google Scholar] [CrossRef]
- Mehanovic, D.; Peloquin, J.F.; Dufault, J.F.; Fréchette, L.; Picard, M. Comparative techno-economic study of typically combustion-less hydrogen production alternatives. Int. J. Hydrogen Energy 2022, 48, 7945–7958. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2014, 40, 11094–11111. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Cho, H.H.; Strezov, V.; Evans, T.J. A review on global warming potential, challenges and opportunities of renewable hydrogen production technologies. Sustain. Mater. Technol. 2023, 35, e00567. [Google Scholar] [CrossRef]
- Tahir, M.B.; Riaz, K.N. Fundamentals of Photocatalysis for Energy Conversion. In Nanomaterials and Photocatalysis in Chemistry; Springer: Singapore, 2021; pp. 5–17. [Google Scholar] [CrossRef]
- Zeeshan, H.M.; Sharma, S.; Panahi, M.; Voloshina, E.; Dedkov, Y. Semiconducting eutectic materials for photocatalysis and photoelectrochemistry applications: A perspective. Phys. Chem. Chem. Phys. 2022, 24, 25720–25734. [Google Scholar] [CrossRef] [PubMed]
- Li, R. Latest progress in hydrogen production from solar water splitting via photocatalysis, photoelectrochemical, and photovoltaic-photoelectrochemical solutions. Chin. J. Catal. 2017, 38, 5–12. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, J.; Zhang, M.; Meng, X.; Chen, M.; Feng, Y.; Chen, X.; Ding, Y. Recent advances and perspectives in cobalt-based heterogeneous catalysts for photocatalytic water splitting, CO2 reduction, and N2 fixation. Chin. J. Catal. 2022, 43, 2273–2300. [Google Scholar] [CrossRef]
- Isaacs, M.; Garcia-Navarro, J.; Ong, W.J.; Jiménez-Calvo, P. Is Photocatalysis the Next Technology to Produce Green Hydrogen to Enable the Net Zero Emissions Goal? Glob. Chall. 2022, 7, 2200165. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Wang, G.; Chang, J.; Tang, W.; Xie, W.; Ang, Y.S. 2D materials and heterostructures for photocatalytic water-splitting: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 293002. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L. Progress in designing effective photoelectrodes for solar water splitting. Cuihua Xuebao/Chin. J. Catal. 2018, 39, 369–378. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting—Materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for photoelectrochemical water splitting—Review. Int. J. Hydrogen Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Minggu, L.J.; Daud, W.R.W.; Kassim, M.B. An overview of photocells and photoreactors for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2010, 35, 5233–5244. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Lee, J.S. Recent theoretical progress in the development of photoanode materials for solar water splitting photoelectrochemical cells. J. Mater. Chem. A Mater. 2015, 3, 10632–10659. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Song, J.; Lee, S. Photoelectrochemical Device Designs toward Practical Solar Water Splitting: A Review on the Recent Progress of BiVO4 and BiFeO3 Photoanodes. Appl. Sci. 2018, 8, 1388. [Google Scholar] [CrossRef]
- Wu, H.; Tan, H.L.; Toe, C.Y.; Scott, J.; Wang, L.; Amal, R.; Ng, Y.H. Photocatalytic and Photoelectrochemical Systems: Similarities and Differences. Adv. Mater. 2020, 32, e1904717. [Google Scholar] [CrossRef]
- Guo, Z.; Zhou, J.; Zhu, L.; Sun, Z. MXene: A promising photocatalyst for water splitting. J. Mater. Chem. A Mater. 2016, 4, 11446–11452. [Google Scholar] [CrossRef]
- Sharma, P.; Jang, J.W.; Lee, J.S. Key Strategies to Advance the Photoelectrochemical Water Splitting Performance of α-Fe2O3 Photoanode. ChemCatChem 2019, 11, 157–179. [Google Scholar] [CrossRef]
- Seabold, J.A.; Neale, N.R. All first row transition metal oxide photoanode for water splitting based on Cu3V2O8. Chem. Mater. 2015, 27, 1005–1013. [Google Scholar] [CrossRef]
- Guo, L.J.; Luo, J.W.; He, T.; Wei, S.H.; Li, S.S. Photocorrosion-Limited Maximum Efficiency of Solar Photoelectrochemical Water Splitting. Phys. Rev. Appl. 2018, 10, 064059. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q.; et al. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 2019, 11, 18968–18994. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, G.; Dong, X.; Hu, J. Interface Band Engineering Charge Transfer for 3D MoS2 Photoanode to Boost Photoelectrochemical Water Splitting. ACS Sustain. Chem. Eng. 2017, 5, 3829–3836. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Huang, J.; Li, Q.; Yang, J. Band Structure Tuning of TiO2 for Enhanced Photoelectrochemical Water Splitting. J. Phys. Chem. C 2014, 118, 7451–7457. [Google Scholar] [CrossRef]
- Lv, R.; Wang, T.; Su, F.; Zhang, P.; Li, C.; Gong, J. Facile synthesis of ZnO nanopencil arrays for photoelectrochemical water splitting. Nano Energy 2014, 7, 143–150. [Google Scholar] [CrossRef]
- Rahman, G.; Joo, O.S. Photoelectrochemical water splitting at nanostructured α-Fe2O3 electrodes. Int. J. Hydrogen Energy 2012, 37, 13989–13997. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Duy, L.T.; Seo, H. Recent Progress in Photoelectrochemical Water Splitting Activity of WO3 Photoanodes. Top. Catal. 2018, 61, 1043–1076. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, H.; Zhu, M.; Li, Y.; Li, W. Interfacial Charge Transport in 1D TiO2 Based Photoelectrodes for Photoelectrochemical Water Splitting. Small 2021, 17, e1903378. [Google Scholar] [CrossRef]
- Muzakkar, M.Z.; Umar, A.A.; Ilham, I.; Saputra, Z.; Zulfikar, L.; Maulidiyah, M.; Wibowo, D.; Ruslan, R.; Nurdin, M. Chalcogenide material as high photoelectrochemical performance Se doped TiO2/Ti electrode: Its application for Rhodamine B degradation. In Journal of Physics: Conference Series; Institute of Physics Publishing: Bristol, UK, 2019. [Google Scholar] [CrossRef]
- Ozawa, K.; Emori, M.; Yamamoto, S.; Yukawa, R.; Yamamoto, S.; Hobara, R.; Fujikawa, K.; Sakama, H.; Matsuda, I. Electron-hole recombination time at TiO2 single-crystal surfaces: Influence of surface band bending. J. Phys. Chem. Lett. 2014, 5, 1953–1957. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, H.; Li, G.; Yan, X.; Yu, Z.; Wang, K.; Wu, Y. Advances in engineering perovskite oxides for photochemical and photoelectrochemical water splitting. In Applied Physics Reviews; American Institute of Physics Inc.: New York, NY, USA, 2021; Volume 8. [Google Scholar] [CrossRef]
- Guerrero, A.; Bisquert, J. Perovskite semiconductors for photoelectrochemical water splitting applications. Curr. Opin. Electrochem. 2017, 2, 144–147. [Google Scholar] [CrossRef]
- Grinberg, I.; West, D.V.; Torres, M.; Gou, G.; Stein, D.M.; Wu, L.; Chen, G.; Gallo, E.M.; Akbashev, A.R.; Davies, P.K.; et al. Perovskite oxides for visible-light-absorbing ferroelectric and photovoltaic materials. Nature 2013, 503, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Young, S.M.; Rappe, A.M. First principles calculation of the shift current photovoltaic effect in ferroelectrics. Phys. Rev. Lett. 2012, 109, 116601. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Salvador, P.A.; Rohrer, G.S. Photocatalysts with internal electric fields. Nanoscale 2014, 6, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Park, N.G. Perovskite Solar Cells: From Materials to Devices. Small 2015, 11, 10–25. [Google Scholar] [CrossRef]
- Sophocleous, M. Global and regional water availability and demand: Prospects for the future. Nat. Resour. Res. 2004, 13, 61–75. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Hashib, M.A. Advances in Heterogeneous Photocatalytic Degradation of Phenols and Dyes in Wastewater: A Review. Water Air Soil Pollut. 2010, 215, 3–29. [Google Scholar] [CrossRef]
- Pandey, A.; Kumar, R.R.; Kalidasan, B.; Laghari, I.A.; Samykano, M.; Kothari, R.; Abusorrah, A.M.; Sharma, K.; Tyagi, V. Utilization of solar energy for wastewater treatment: Challenges and progressive research trends. J. Environ. Manag. 2021, 297, 113300. [Google Scholar] [CrossRef]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Pap. 2022, 77, 677–701. [Google Scholar] [CrossRef]
- Kanhere, P.; Chen, Z. A Review on Visible Light Active Perovskite-Based Photocatalysts. Molecules 2014, 19, 19995–20022. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, J.; Wu, Q.; Zhuang, J.; Guo, H.; Ma, Z.; Ye, Y. Enhanced photovoltaic properties of PbTiO3-based ferroelectric thin films prepared by a sol-gel process. Ceram. Int. 2017, 43, 13063–13068. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.E.; Shvartsman, V.V.; Gobeljic, D.; Gao, Y.; Landers, J.; Wende, H.; Lupascu, D.C. Effect of particle size on ferroelectric and magnetic properties of BiFeO3 nanopowders. Nanotechnology 2013, 24, 355701. [Google Scholar] [CrossRef]
- Qiao, X.; Geng, W.; Sun, Y.; Zheng, D.; Yang, Y.; Meng, J.; He, J.; Bi, K.; Cui, M.; Chou, X. Robust in-plane polarization switching in epitaxial BiFeO3 films. J. Alloys Compd. 2021, 852, 156988. [Google Scholar] [CrossRef]
- Deng, J.; Banerjee, S.; Mohapatra, S.K.; Smith, Y.R.; Misra, M. Bismuth Iron Oxide Nanoparticles as Photocatalyst for Solar Hydrogen Generation from Water. J. Fundam. Renew. Energy Appl. 2011, 1, 1–10. [Google Scholar] [CrossRef]
- Gao, T.; Chen, Z.; Zhu, Y.; Niu, F.; Huang, Q.; Qin, L.; Sun, X.; Huang, Y. Synthesis of BiFeo3 nanoparticles for the visible-light induced photocatalytic property. Mater. Res. Bull. 2014, 59, 6–12. [Google Scholar] [CrossRef]
- Wang, N.; Luo, X.; Han, L.; Zhang, Z.; Zhang, R.; Olin, H.; Yang, Y. Structure, Performance, and Application of BiFeO3 Nanomaterials. Nano-Micro Lett. 2020, 12, 81. [Google Scholar] [CrossRef]
- Qiao, L.; Zhang, S.; Xiao, H.Y.; Singh, D.J.; Zhang, K.H.L.; Liu, Z.J.; Zu, X.T.; Li, S. Orbital controlled band gap engineering of tetragonal BiFeO3 for optoelectronic applications. J. Mater. Chem. C Mater. 2018, 6, 1239–1247. [Google Scholar] [CrossRef]
- Shah, J.H.; Malik, A.S.; Idris, A.M.; Rasheed, S.; Han, H.; Li, C. Intrinsic photocatalytic water oxidation activity of Mn-doped ferroelectric BiFeO3. Chin. J. Catal. 2021, 42, 945–952. [Google Scholar] [CrossRef]
- Yun, Q.; Xing, W.; Chen, J.; Gao, W.; Bai, Y.; Zhao, S. Effect of Ho and Mn co-doping on structural, ferroelectric and ferromagnetic properties of BiFeO3 thin films. Thin Solid Films 2015, 584, 103–107. [Google Scholar] [CrossRef]
- Preethi, A.J.; Ragam, M. Effect of doping in multiferroic BFO: A review. J. Adv. Dielectr. 2021, 11, 2130001. [Google Scholar] [CrossRef]
- Xian, T.; Yang, H.; Dai, J.F.; Wei, Z.Q.; Ma, J.Y.; Feng, W.J. Photocatalytic properties of BiFeO3 nanoparticles with different sizes. Mater. Lett. 2011, 65, 1573–1575. [Google Scholar] [CrossRef]
- Dhawan, A.; Sudhaik, A.; Raizada, P.; Thakur, S.; Ahamad, T.; Thakur, P.; Singh, P.; Hussain, C.M. BiFeO3-based Z scheme photocatalytic systems: Advances, mechanism, and applications. J. Ind. Eng. Chem. 2023, 117, 1–20. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.H.; Zhang, B.P.; Wang, Y.; Nan, C.W. Controlled fabrication of BiFeO3 uniform microcrystals and their magnetic and photocatalytic behaviors. J. Phys. Chem. C 2010, 114, 2903–2908. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.Y.; Yin, K.B.; Dong, S.; Ren, Z.F.; Yuan, F.; Yu, T.; Zou, Z.G.; Liu, J. Visible-Light Photocatalytic Properties of Weak Magnetic BiFeO3 Nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Cheng, X. Spectroscopic study on the valence state of Fe in BiFeO3. J. Solid State Chem. 2022, 311, 123145. [Google Scholar] [CrossRef]
- Arifiadi, A.N.; Kim, K.T.; Khairani, I.Y.; Park, C.B.; Kim, K.H.; Kim, S.K. Synthesis and multiferroic properties of high-purity CoFe2O4–BiFeO3 nanocomposites. J. Alloys Compd. 2021, 867, 159008. [Google Scholar] [CrossRef]
- Benyoussef, M.; Saitzek, S.; Rajput, N.S.; Courty, M.; El Marssi, M.; Jouiad, M. Experimental and Theoretical Investigations of Low-Dimensional BiFeO3 System for Photocatalytic Applications. Catalysts 2022, 12, 215. [Google Scholar] [CrossRef]
- Sando, D.; Carrétéro, C.; Grisolia, M.N.; Barthélémy, A.; Nagarajan, V.; Bibes, M. Revisiting the Optical Band Gap in Epitaxial BiFeO3 Thin Films. Adv. Opt. Mater. 2018, 6, 1700836. [Google Scholar] [CrossRef]
- Arazas, A.P.R.; Wu, C.C.; Chang, K.S. Hydrothermal fabrication and analysis of piezotronic-related properties of BiFeO3 nanorods. Ceram. Int. 2018, 44, 14158–14162. [Google Scholar] [CrossRef]
- Subhiksha, V.; Kokilavani, S.; Khan, S.S. Recent advances in degradation of organic pollutant in aqueous solutions using bismuth based photocatalysts: A review. Chemosphere 2022, 290, 133228. [Google Scholar] [CrossRef] [PubMed]
- Benyoussef, M.; Saitzek, S.; Rajput, N.S.; El Marssi, M.; Jouiad, M. Effect of Sr and Ti substitutions on optical and photocatalytic properties of Bi1-xSrxFe1-xTixO3 nanomaterials. Nanoscale Adv. 2023, 5, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhai, T.; Shen, H.; Wang, J.; Min, R.; Ma, K.; Zhang, G. Strategies for enhancing performance of perovskite bismuth ferrite photocatalysts (BiFeO3): A comprehensive review. Chemosphere 2023, 339, 139678. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, H. Ferroelectrics in Photocatalysis. Chem. A Eur. J. 2022, 28, e202103975. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, K.; Li, D.; Li, N.; Xu, J.; Bahnemann, D.W.; Wang, C. Polarization-enhanced photocatalytic activity in non-centrosymmetric materials based photocatalysis: A review. Chem. Eng. J. 2021, 426, 131681. [Google Scholar] [CrossRef]
- Shvartsman, V.V.; Kleemann, W.; Haumont, R.; Kreisel, J. Large bulk polarization and regular domain structure in ceramic BiFeO3. Appl. Phys. Lett. 2007, 90, 172115. [Google Scholar] [CrossRef]
- Lebeugle, D.; Colson, D.; Forget, A.; Viret, M. Very large spontaneous electric polarization in BiFeO3 single crystals at room temperature and its evolution under cycling fields. Appl. Phys. Lett. 2007, 91, 022907. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhou, L.; Zhang, M.F.; Chen, X.Y.; Liu, J.M.; Liu, Z.G. Room-temperature saturated ferroelectric polarization in BiFeO3 ceramics synthesized by rapid liquid phase sintering. Appl. Phys. Lett. 2004, 84, 1731–1733. [Google Scholar] [CrossRef]
- Song, S.H.; Zhu, Q.S.; Weng, L.Q.; Mudinepalli, V.R. A comparative study of dielectric, ferroelectric and magnetic properties of BiFeO3 multiferroic ceramics synthesized by conventional and spark plasma sintering techniques. J. Eur. Ceram. Soc. 2015, 35, 131–138. [Google Scholar] [CrossRef]
- Wahba, M.A.; Yakout, S.M.; Youssef, A.M.; Sharmoukh, W.; Elsayed, A.M.; Khalil, M.S. Chelating Agents Assisted Rapid Synthesis of High Purity BiFeO3: Remarkable Optical, Electrical, and Magnetic Characteristics. J. Supercond. Nov. Magn. 2022, 35, 3689–3704. [Google Scholar] [CrossRef]
- Tahir, M.; Riaz, S.; Ahmad, N.; Khan, U.; Atiq, S.; Iqbal, M.J.; Naseem, S. Anomalous dielectric behavior and correlation of barrier hopping mechanism with ferroelectricity in solvent assisted phase pure bismuth iron oxide nanoparticles. Mater. Res. Bull. 2019, 119, 110543. [Google Scholar] [CrossRef]
- Banoth, P.; Sohan, A.; Kandula, C.; Kollu, P. Structural, dielectric, magnetic, and ferroelectric properties of bismuth ferrite (BiFeO3) synthesized by a solvothermal process using hexamethylenetetramine (HMTA) as precipitating agent. Ceram. Int. 2022, 48, 32817–32826. [Google Scholar] [CrossRef]
- Teague, J.R.; Gerson, R.; James, W.J. Dielectric hysteresis in single crystal BiFeO3. Solid State Commun. 1970, 8, 1073–1074. [Google Scholar] [CrossRef]
- BaoLin, F.; Hao, X.; ZhaoXian, X. Articles Structure and multiferroic properties of Y-doped BiFeO3 ceramics. Chin. Sci. Bull. 2010, 55, 452–456. [Google Scholar] [CrossRef]
- Layek, S.; Verma, H.C. Magnetic and Dielectric Properties of Multiferroic BiFeO3 Nanoparticles Synthesized by a Novel Citrate Combustion Method. Adv. Mater. Lett. 2015, 3, 533–538. [Google Scholar] [CrossRef]
- Du, Y.; Cheng, Z.X.; Shahbazi, M.; Collings, E.W.; Dou, S.X.; Wang, X.L. Enhancement of ferromagnetic and dielectric properties in lanthanum doped BiFeO3 by hydrothermal synthesis. J. Alloys Compd. 2010, 490, 637–641. [Google Scholar] [CrossRef]
- Suresh, P.; Srinath, S. A comprative study of sol-gel and solid-state prepared La3+ doped multiferroic BiFeO3. Adv. Mater. Lett. 2014, 5, 127–130. [Google Scholar] [CrossRef]
- Zhang, G.D.; Dai, J.Q.; Liang, X.L. Enhanced ferroelectric properties in La-doped BiFeO3 films by the sol-gel method. J. Sol-Gel Sci. Technol. 2023, 105, 489–499. [Google Scholar] [CrossRef]
- Sheoran, N.; Kumar, A.; Kumar, V.; Banerjee, A. Structural, Optical, and Multiferroic Properties of Yttrium (Y3+)-Substituted BiFeO3 Nanostructures. J. Supercond. Nov. Magn. 2020, 33, 2017–2029. [Google Scholar] [CrossRef]
- Dhir, G.; Verma, N.K. Correlation of spin, size and structure in sol-gel prepared doped BiFeO3 nanoparticles. J. Mol. Struct. 2020, 1210, 128055. [Google Scholar] [CrossRef]
- Ozdilek, C.; Ozenbas, M. Hydrothermal synthesis of Yb-doped BiFeO3 crystallites and their structural, magnetic and electrical properties. Ceram. Int. 2020, 46, 27800–27808. [Google Scholar] [CrossRef]
- Suresh, S.; Kathirvel, A.; Maheswari, A.U.; Sivakumar, M. Frequency dependent dielectric relaxation of Ba-doped BiFeO3 nanoparticles. Mater. Res. Express 2019, 6, 115057. [Google Scholar] [CrossRef]
- Mazumder, R.; Sen, A. Effect of Pb-doping on dielectric properties of BiFeO3 ceramics. J. Alloys Compd. 2009, 475, 577–580. [Google Scholar] [CrossRef]
- Wrzesinska, A.; Khort, A.; Bobowska, I.; Busiakiewicz, A.; Wypych-Puszkarz, A. Influence of the La3+, Eu3+, and Er3+ Doping on Structural, Optical, and Electrical Properties of BiFeO3 Nanoparticles Synthesized by Microwave-Assisted Solution Combustion Method. J. Nanomater. 2019, 2019, 5394325. [Google Scholar] [CrossRef]
- Rani, S.; Sanghi, S.; Agarwal, A.; Kumar, R.; Singh, O. Crystal structure, magnetic and dielectric properties of Er-doped BiFeO3 ceramics. Appl. Phys. A Mater. Sci. Process. 2022, 128, 576. [Google Scholar] [CrossRef]
- Shinjo, Y.; Mori, M.; Fujihara, S.; Hagiwara, M. Ti doping and low-temperature sintering of BiFeO3 nanoparticles synthesized by the solvothermal method. Ceram. Int. 2022, 48, 32723–32729. [Google Scholar] [CrossRef]
- Kathirvel, A.; Krishna, K.N.I.; Ganga, R.; Maheswari, A.U.; Sivakumar, M. Enhanced magnetic, dielectric and photoconductive properties of Zr doped BiFeO3 nanostructures. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 142, 115306. [Google Scholar] [CrossRef]
- Nadeem, M.; Khan, W.; Khan, S.; Husain, S.; Ansari, A. Tailoring dielectric properties and multiferroic behavior of nanocrystalline BiFeO3 via Ni doping. J. Appl. Phys. 2018, 124, 164105. [Google Scholar] [CrossRef]
- Saxena, P.; Kumar, A.; Sharma, P.; Varshney, D. Improved dielectric and ferroelectric properties of dual-site substituted rhombohedral structured BiFeO3 multiferroics. J. Alloys Compd. 2016, 682, 418–423. [Google Scholar] [CrossRef]
- Godara, S.; Kumar, B. Effect of Ba-Nb co-doping on the structural, dielectric, magnetic and ferroelectric properties of BiFeO3 nanoparticles. Ceram. Int. 2015, 41, 6912–6919. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, W.; Cao, W.; Li, W.; Fei, W. Electrical properties of Li and Nb modified BiFeO3 ceramics with reduced leakage current. Ceram. Int. 2021, 47, 4217–4225. [Google Scholar] [CrossRef]
- Sharif, M.K.; Khan, M.A.; Warsi, M.F.; Ramzan, M.; Hussain, A. Structural and ferroelectric properties of hafnium substituted BiFeO3 multiferroics synthesized via auto combustion technique. Ceram. Int. 2018, 44, 20648–20655. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Pradeep, A.; Sathyamoorthy, B.; Chandrasekaran, G. Enhanced multiferroic properties in la and Ce co-doped BiFeO3 nanoparticles. J. Phys. Chem. Solids 2014, 75, 797–802. [Google Scholar] [CrossRef]
- Sen, S.; Mondal, A.; Parida, R.K.; Parida, B.N. Improved optical, dielectric, impedance, and magnetic properties of (BiFeO3)0.6(CaTiO3)0.4 for multifunctional utilities. Inorg. Chem. Commun. 2022, 142, 109664. [Google Scholar] [CrossRef]
- Baloni, M.; Sharma, R.C.; Singh, H.; Singh, M.K.; Kumar, A.; Sati, P.C.; Khan, B.; Thakur, V.N. Effect of Nd doping on structural, dielectric, magnetic and ferroelectric properties of 0.8BiFeO3–0.2PbTiO3 solid solution. J. Alloys Compd. 2022, 905, 164228. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, H.; Liu, F.; Liu, G. Tuning of electric and magnetic properties of BiFeO3-SrTiO3 solid solution ceramics by site-specific doping of Mn. J. Alloys Compd. 2021, 877, 160239. [Google Scholar] [CrossRef]
- Ji, C.; Fan, T.; Chen, G.; Bai, X.; Wang, J.; He, J.; Cai, W.; Gao, R.; Deng, X.; Wang, Z.; et al. Influence of sintering method on microstructure, electrical and magnetic properties of BiFeO3–BaTiO3 solid solution ceramics. Mater. Today Chem. 2021, 20, 100419. [Google Scholar] [CrossRef]
- Zia, L.; Jaffari, G.H.; Khan, N.A.; Rahman, J.U.; Lee, S.; Shah, S.I. Identification and comparison of peculiarities in physical properties of multiferroic morphotrophic phase boundary sintered BiFeO3-xPbTiO3 nano-ceramics. J. Phys. Chem. Solids 2021, 150, 109868. [Google Scholar] [CrossRef]
- Shankar, S.; Maurya, I.; Raj, A.; Singh, S.; Thakur, O.P.; Jayasimhadri, M. Dielectric and tunable ferroelectric properties in BiFeO3–BiCoO3–BaTiO3 ternary compound. Appl. Phys. A Mater. Sci. Process. 2020, 126, 686. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, J.; Shi, R.; Wang, Z.; Zhang, M.; Du, Q.; Qi, X. Structural, dielectric, and multiferroic properties of Ta2O5-modified BiFeO3–BaTiO3–LaFeO3 solid solutions. J. Mater. Sci. Mater. Electron. 2020, 31, 1502–1508. [Google Scholar] [CrossRef]
- Tang, Z.; Zhuang, J.; Bokov, A.A.; Luo, Z.; Kubrin, S.P.; Raevski, I.P.; Ma, M.; Zhang, N.; Zhang, J.; Liu, Z.; et al. Multiscale Domain Structures and Ferroic Properties of Dy-Modified BiFeO3-PbTiO3 Single Crystals. Cryst. Growth Des. 2021, 21, 3082–3092. [Google Scholar] [CrossRef]
- Masso, R.; Tripathy, S.N.; Aponte, F.A.; Pradhan, D.K.; Martinez, R.; Palai, R. Structural and magnetodielectric properties of BiFeO3-GdMnO3 multiferroics. Mater. Res. Express 2021, 8, 016302. [Google Scholar] [CrossRef]
- Kumar, N.; Narayan, B.; Singh, A.K.; Kumar, S. Enhanced magneto-capacitance in Sr2+ modified BiFeO3–PbTiO3 solid solutions. Mater. Chem. Phys. 2020, 252, 123313. [Google Scholar] [CrossRef]
- Praharaj, S.; Singha, A.; Rout, D. Dielectric and piezoelectric properties of lead-free Na0.5Bi0.5TiO3-SrTiO3-BiFeO3 ternary system. J. Alloys Compd. 2021, 867, 159114. [Google Scholar] [CrossRef]
- Ponraj, C.; Vinitha, G.; Daniel, J. Visible light photocatalytic activity of Mn-doped BiFeO3 nanoparticles. Int. J. Green Energy 2020, 17, 71–83. [Google Scholar] [CrossRef]
- Gao, T.; Chen, Z.; Huang, Q.; Niu, F.; Huang, X.; Qin, L.; Huang, Y. A review: Preparation of bismuth ferrite nanoparticles and its applications in visible-light induced photocatalyses. Rev. Adv. Mater. Sci. 2015, 40, 97–109. [Google Scholar]
- Sharmin, F.; Basith, M.A. Highly efficient photocatalytic degradation of hazardous industrial and pharmaceutical pollutants using gadolinium doped BiFeO3 nanoparticles. J. Alloys Compd. 2022, 901, 163604. [Google Scholar] [CrossRef]
- Mohan, S.; Subramanian, B.; Bhaumik, I.; Gupta, P.K.; Jaisankar, S.N. Nanostructured Bi(1−x)Gd(x)FeO3—A multiferroic photocatalyst on its sunlight driven photocatalytic activity. RSC Adv. 2014, 4, 16871–16878. [Google Scholar] [CrossRef]
- Shi, J.; Guo, L. ABO3-based photocatalysts for water splitting. Prog. Nat. Sci. Mater. Int. 2012, 22, 592–615. [Google Scholar] [CrossRef]
- Guo, R.; Fang, L.; Dong, W.; Zheng, F.; Shen, M. Enhanced photocatalytic activity and ferromagnetism in Gd doped BiFeO3 nanoparticles. J. Phys. Chem. C 2010, 114, 21390–21396. [Google Scholar] [CrossRef]
- Vanga, P.R.; Mangalaraja, R.V.; Ashok, M. Structural, magnetic and photocatalytic properties of La and alkaline co-doped BiFeO3 nanoparticles. Mater. Sci. Semicond. Process. 2015, 40, 796–802. [Google Scholar] [CrossRef]
- Sakar, M.; Balakumar, S.; Saravanan, P.; Bharathkumar, S. Compliments of confinements: Substitution and dimension induced magnetic origin and band-bending mediated photocatalytic enhancements in Bi1−xDyxFeO3 particulate and fiber nanostructures. Nanoscale 2015, 7, 10667–10679. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, L.; Chen, D.; Liang, J.; Qin, L.; Bai, L.; Sun, X.; Huang, Y. Facile synthesis of Er-doped BiFeO3 nanoparticles for enhanced visible light photocatalytic degradation of tetracycline hydrochloride. J. Sol-Gel Sci. Technol. 2019, 90, 535–546. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Wang, X.; Jin, W.; Zhu, C. Ferromagnetism and enhanced photocatalytic activity in Nd doped BiFeO3 nanopowders. J. Mater. Sci. Mater. Electron. 2015, 26, 9929–9940. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, D.; Wang, S.; Zhang, N.; Qin, L.; Huang, Y. Facile synthesis of Sm-doped BiFeO3 nanoparticles for enhanced visible light photocatalytic performance. Mater. Sci. Eng. B 2017, 220, 1–12. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.K. Comparison of benzene and toluene photodegradation under visible light irradiation by Ba-doped BiFeO3 magnetic nanoparticles with fast sonochemical synthesis. Photochem. Photobiol. Sci. 2017, 16, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ponraj, C.; Kumar, P.S.; Sarkar, S.; Krishnamoorthi, C.; Manikandan, N.; Vinitha, G.; Daniel, J. Enhanced visible light photocatalytic activity of magnetic cobalt doped BiFeO3. Surf. Interfaces 2022, 31, 102050. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Lam, S.M.; Sin, J.C.; Zeng, H.; Mohamed, A.R. Magnetically recoverable Pd-loaded BiFeO3 microcomposite with enhanced visible light photocatalytic performance for pollutant, bacterial and fungal elimination. Sep. Purif. Technol. 2020, 236, 116195. [Google Scholar] [CrossRef]
- Umar, M.; Mahmood, N.; Awan, S.U.; Fatima, S.; Mahmood, A.; Rizwan, S. Rationally designed La and Se co-doped bismuth ferrites with controlled bandgap for visible light photocatalysis. RSC Adv. 2019, 9, 17148–17156. [Google Scholar] [CrossRef]
- Kebede, M.T.; Devi, S.; Tripathi, B.; Chauhan, S.; Dillu, V. Structural transition and enhanced magnetic, optical and photocatalytic properties of novel Ce–Ni co-doped BiFeO3 nanoparticles. Mater. Sci. Semicond. Process. 2022, 152, 107086. [Google Scholar] [CrossRef]
- Vanga, P.R.; Mangalaraja, R.V.; Ashok, M. Effect of (Nd, Ni) co-doped on the multiferroic and photocatalytic properties of BiFeO3. Mater. Res. Bull. 2015, 72, 299–305. [Google Scholar] [CrossRef]
- Soltani, T.; Tayyebi, A.; Lee, B.K. BiFeO3/BiVO4 p−n heterojunction for efficient and stable photocatalytic and photoelectrochemical water splitting under visible-light irradiation. Catal. Today 2020, 340, 188–196. [Google Scholar] [CrossRef]
- Bargozideh, S.; Tasviri, M.; Kianifar, M. Construction of novel magnetic BiFeO3/MoS2 composite for enhanced visible-light photocatalytic performance towards purification of dye pollutants. Int. J. Environ. Anal. Chem. 2020, 102, 6390–6404. [Google Scholar] [CrossRef]
- Xu, J.; Qin, T.; Chen, W.; Lv, J.; Zeng, X.; Sun, J.; Li, Y.-Y.; Zhou, J. Synergizing piezoelectric and plasmonic modulation of Ag/BiFeO3 fibrous heterostructure toward boosted photoelectrochemical energy conversion. Nano Energy 2021, 89, 106317. [Google Scholar] [CrossRef]
- Ghorbani, M.; Sheibani, S.; Abdizadeh, H.; Golobostanfard, M.R. Modified BiFeO3/rGO nanocomposite by controlled synthesis to enhance adsorption and visible-light photocatalytic activity. J. Mater. Res. Technol. 2023, 22, 1250–1267. [Google Scholar] [CrossRef]
- Wang, X.; He, X.-S.; Li, C.-Y.; Liu, S.-L.; Lu, W.; Xiang, Z.; Wang, Y. Sonocatalytic removal of tetracycline in the presence of S-scheme Cu2O/BiFeO3 heterojunction: Operating parameters, mechanisms, degradation pathways and toxicological evaluation. J. Water Process. Eng. 2023, 51, 103345. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Sun, J.; Wu, X.; Liang, H.; Qu, Y.; Jing, L. BiFeO3/Bi2Fe4O9 S-scheme heterojunction hollow nanospheres for high-efficiency photocatalytic o-chlorophenol degradation. Appl. Catal. B Environ. 2022, 319, 121893. [Google Scholar] [CrossRef]
- Marwat, M.A.; Ullah, H.; Usman, M.; Ehsan, M.A.; Zhang, H.; Khan, M.F.; Ali, S.; Yousaf, M. Significantly improved photocatalytic activity of the SnO2/BiFeO3 heterojunction for pollutant degradation and mechanism. Ceram. Int. 2022, 48, 14789–14798. [Google Scholar] [CrossRef]
- Subramanian, Y.; Ramasamy, V.; Karthikeyan, R.; Srinivasan, G.R.; Arulmozhi, D.; Gubendiran, R.K.; Sriramalu, M. Investigations on the enhanced dye degradation activity of heterogeneous BiFeO3–GdFeO3 nanocomposite photocatalyst. Heliyon 2019, 5, e01831. [Google Scholar] [CrossRef]
- Niu, F.; Chen, D.; Qin, L.; Zhang, N.; Wang, J.; Chen, Z.; Huang, Y. Facile Synthesis of Highly Efficient p–n Heterojunction CuO/BiFeO3 Composite Photocatalysts with Enhanced Visible-Light Photocatalytic Activity. ChemCatChem 2015, 7, 3279–3289. [Google Scholar] [CrossRef]
- Ghasemi, A.; Hasheminiasari, M.; Masoudpanah, S.M.; Safizade, B. Enhanced Photocatalytic Activity of Two-Pot-Synthesized BiFeO3–ZnFe2O4 Heterojunction Nanocomposite. J. Electron. Mater. 2018, 47, 2225–2229. [Google Scholar] [CrossRef]
- Liao, X.; Li, T.-T.; Ren, H.-T.; Mao, Z.; Zhang, X.; Lin, J.-H.; Lou, C.-W. Enhanced photocatalytic performance through the ferroelectric synergistic effect of p-n heterojunction BiFeO3/TiO2 under visible-light irradiation. Ceram. Int. 2021, 47, 10786–10795. [Google Scholar] [CrossRef]
- Banoth, P.; Narsaiah, B.P.; De Los Santos Valladares, L.; Kargin, J.; Kollu, P. Single-phase BiFeO3 and BiFeO3–Fe2O3 nanocomposite photocatalysts for photodegradation of organic dye pollutants. Nanoscale Adv. 2023, 5, 2646–2656. [Google Scholar] [CrossRef]
- Ramezanalizadeh, H.; Manteghi, F. Design and development of a novel BiFeO3/CuWO4 heterojunction with enhanced photocatalytic performance for the degradation of organic dyes. J. Photochem. Photobiol. A Chem. 2017, 338, 60–71. [Google Scholar] [CrossRef]
- Tran, V.T.; Chen, D.H. Ag2O@BiFeO3 heterostructure composite coupling built-in electric field with piezopotential for enhanced photocatalytic pollutant degradation and photoelectrochemical water splitting. Appl. Surf. Sci. 2023, 625, 157175. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Z.; Cao, G.; Wu, Y.; Song, J.; Li, Y.; Zhang, L.; Mu, J.; Chou, X. Facilitated Photocatalytic Degradation of Rhodamine B over One-Step Synthesized Honeycomb-Like BiFeO3/g-C3N4 Catalyst. Nanomaterials 2022, 12, 3970. [Google Scholar] [CrossRef]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Qi, J.; Liu, H.; Feng, M.; Xu, H.; Liu, H.; Wang, C.; Wang, A.; Lü, W. Enhanced hydrogen evolution reaction in Sr doped BiFeO3 by achieving the coexistence of ferroelectricity and ferromagnetism at room temperature. J. Energy Chem. 2020, 53, 93–98. [Google Scholar] [CrossRef]
- Man, S.; Leng, X.; Bai, J.; Kan, S.; Cui, Y.; Wang, J.; Xu, L. Enhancement of photoelectrochemical performance of BiFeO3 by Sm3+ doping. Ceram. Int. 2023, 49, 10255–10264. [Google Scholar] [CrossRef]
- Vishwakarma, A.K.; Tripathi, P.; Srivastava, A.; Sinha, A.S.K.; Srivastava, O.N. Band gap engineering of Gd and Co doped BiFeO3 and their application in hydrogen production through photoelectrochemical route. Int. J. Hydrogen Energy 2017, 42, 22677–22686. [Google Scholar] [CrossRef]
- Khoomortezaei, S.; Abdizadeh, H.; Golobostanfard, M.R. Ferro-photocatalytic Enhancement of Photoelectrochemical Water Splitting Using the WO3/BiFeO3 Heterojunction. Energy Fuels 2021, 35, 9623–9634. [Google Scholar] [CrossRef]
- Wu, X.; Li, H.; Wang, X.; Jiang, L.; Xi, J.; Du, G.; Ji, Z. Ferroelectric enhanced photoelectrochemical water splitting in BiFeO3/TiO2 composite photoanode. J. Alloys Compd. 2019, 783, 643–651. [Google Scholar] [CrossRef]
- Zhang, T.; Shen, Y.; Qiu, Y.; Liu, Y.; Xiong, R.; Shi, J.; Wei, J. Facial Synthesis and Photoreaction Mechanism of BiFeO3/Bi2Fe4O9 Heterojunction Nanofibers. ACS Sustain. Chem. Eng. 2017, 5, 4630–4636. [Google Scholar] [CrossRef]
- Yan, X.; Pu, R.; Xie, R.; Zhang, B.; Shi, Y.; Liu, W.; Ma, G.; Yang, N. Design and fabrication of Bi2O3/BiFeO3 heterojunction film with improved photoelectrochemical performance. Appl. Surf. Sci. 2021, 552, 149442. [Google Scholar] [CrossRef]
- Bera, S.; Ghosh, S.; Shyamal, S.; Bhattacharya, C.; Basu, R.N. Photocatalytic hydrogen generation using gold decorated BiFeO3 heterostructures as an efficient catalyst under visible light irradiation. Sol. Energy Mater. Sol. Cells 2019, 194, 195–206. [Google Scholar] [CrossRef]
- Kolivand, A.; Sharifnia, S. Enhanced photocatalytic hydrogen evolution from water splitting by Z-scheme CdS/BiFeO3 heterojunction without using sacrificial agent. Int. J. Energy Res. 2021, 45, 2739–2752. [Google Scholar] [CrossRef]
- Khoomortezaei, S.; Abdizadeh, H.; Golobostanfard, M.R. Triple Layer Heterojunction WO3/BiVO4/BiFeO3 Porous Photoanode for Efficient Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2019, 2, 6428–6439. [Google Scholar] [CrossRef]
- Zhu, J.; He, Y.; Yang, Y.; Liu, Y.; Chen, M.; Cao, D. BiFeO3/Cu2O Heterojunction for Efficient Photoelectrochemical Water Splitting Under Visible-Light Irradiation. Catal. Lett. 2021, 151, 382–389. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Gong, J. Mechanistic Understanding of the Plasmonic Enhancement for Solar Water Splitting. Adv. Mater. 2015, 27, 5328–5342. [Google Scholar] [CrossRef]
- Ghosh, S.; Mallik, A.K.; Basu, R.N. Enhanced photocatalytic activity and photoresponse of poly(3,4-ethylenedioxythiophene) nanofibers decorated with gold nanoparticle under visible light. Sol. Energy 2018, 159, 548–560. [Google Scholar] [CrossRef]
- Tiburcio, J.; Sacari, E.; Chacaltana, J.; Medina, J.; Gamarra, F.; Polo, C.; Mamani, E.; Quispe, A. Influence of Cr Doping on Structural, Optical, and Photovoltaic Properties of BiFeO3 Synthesized by Sol-Gel Method. Energies 2023, 16, 786. [Google Scholar] [CrossRef]
- Anjum, N.; Lamia, S.N.E.; Arafat, M.Y.; Mahboob, M.; Basith, M.A. Photocatalytic properties of Ti-doped BiFeO3 bulk and nanoparticles for solar hydrogen fuel generation. In AIP Conference Proceedings; American Institute of Physics Inc.: Melville, NY, USA, 2018. [Google Scholar] [CrossRef]
- Haydous, F.; Scarisoreanu, N.D.; Birjega, R.; Ion, V.; Lippert, T.; Dumitrescu, N.; Moldovan, A.; Andrei, A.; Teodorescu, V.S.; Ghica, C.; et al. Rolling dopant and strain in Y-doped BiFeO3 epitaxial thin films for photoelectrochemical water splitting. Sci. Rep. 2018, 8, 15826. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Lu, M.; Qin, G.; Wu, X.; Yu, T.; Zhang, L.; Li, K.; Cheng, X.; Lan, Y. Piezo-Photocatalytic Synergy in BiFeO3@COF Z-Scheme Heterostructures for High-Efficiency Overall Water Splitting. Angew. Chem. Int. Ed. 2022, 61, e202210700. [Google Scholar] [CrossRef] [PubMed]
- Sepahvand, H.; Sharifnia, S. Photocatalytic overall water splitting by Z-scheme g-C3N4/BiFeO3 heterojunction. Int. J. Hydrogen Energy 2019, 44, 23658–23668. [Google Scholar] [CrossRef]
- Arif, N.; Ma, Y.; Iqbal, M.A.; Zafar, M.N.; Liang, H.; Zhang, Q.; Zeng, Y.-J. Enhanced charge separation in dual Z-scheme Au decorated LaFeO3-g-C3N4-BiFeO3 system for efficient H2 production. Fuel 2023, 336, 126832. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Li, L.; Yan, W.; Wang, H.; Mao, W.; Cui, Y.; Li, Y.; Zhu, X. Synergizing the internal electric field and ferroelectric polarization of the BiFeO3/ZnIn2S4 Z-scheme heterojunction for photocatalytic overall water splitting. J. Mater. Chem. A 2023, 11, 434–446. [Google Scholar] [CrossRef]






| Material | Route | Precursors | Size (nm) | ԑr RT; 102 Hz | Pr (µC·cm−2) | Ref. |
|---|---|---|---|---|---|---|
| BiFeO3 bulk | Solid-state | Bi2O3, Fe2O3 | - | - | 40 | [80] |
| BiFeO3 single cristal | Spontaneous crystallization | Bi2O3, Fe2O3 | - | - | 75 | [81] |
| BiFeO3 | Solid-state | Bi2O3, Fe2O3 | 103 | - | 8.9 | [82] |
| BiFeO3 | Solid-state | Bi2O3, Fe2O3 | 2 × 102 | 25 | 7.5 | [83] |
| BiFeO3 | Combustion | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, Organic fuel | 40 | 1118 | - | [84] |
| BiFeO3 | Sol-gel | Bi(NO3)3, 5H2O/Fe(NO3)3, 9H2O | 12 | 84.5 | 8.2 | [85] |
| BiFeO3 | Solvothermal | BiCl3, FeCl3·6H2O, HMTA | - | 4000 | 6.7 | [86] |
| BiFeO3 single cristal | Solid-state | Bi2O3, Fe2O3 | 106 | - | 6.1 | [87] |
| BiFeO3 | Solid-state | Bi2O3, Fe2O3 | 2 × 103 | 121 | 0.3 | [88] |
| BiFeO3 | Combustion | Bi(NO3)3, 5H2O/Fe(NO3)3, 9H2O | 47 | 91 | - | [89] |
| Material | Route | Precursors | Size (nm) | ԑr RT; 102 Hz | Pr (µC·cm−2) | Ref. |
|---|---|---|---|---|---|---|
| Bi0.8La0.2FeO3 | Hydrothermal | Bi(NO3)3, Fe(NO3)3, La(NO3)3, KOH | 105 | 225 | - | [90] |
| Bi0.95La0.05FeO3 | Sol–gel | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, La(NO3)3⋅6H2O | 4 × 102 | 50,000 | - | [91] |
| Bi0.98La0.02FeO3 | Sol–gel | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, La(NO3)3⋅6H2O | 80 | 161.8 | 140 | [92] |
| Bi0.8Y0.2FeO3 | Sol–gel & combustion | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, Y(NO3)3⋅6H2O | 41 | 500 | 16 | [93] |
| Bi0.85Gd0.15FeO3 | Sol–gel | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, Gd(NO3)3.3H2O | 16 | 2193 | 7 | [94] |
| Bi0.97Yb0.03FeO3& Bi0.9Yb0.1FeO3 | Hydrothermal | Bi(NO3)3, Fe(NO3)3, Yb(NO3)3, KOH | 29 | 150 | 0.4 | [95] |
| Bi0.985Ba0.015FeO3 | Hydrothermal | Bi(NO3)3, Fe(NO3)3, Ba(NO₃)₂, KOH | 57 | 125 | - | [96] |
| Bi0.97Pb0.03FeO3 | Precipitation | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, Pb(NO3)3·5H2O, | - | 2500 | 0.8 | [97] |
| Bi0.9Eu0.1FeO3 | Microwave-assisted | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, Eu(NO3)3⋅6H2O | 18 | 150 | - | [98] |
| BiFe0.95Ti0.05O3 | Solvothermal | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, TiO2 | 7 × 102 | 1000 | - | [100] |
| BiFe0.85 Hf(3/4)0.15O3 | Combustion | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, HfCl4 | 30 | - | 0.2 | [106] |
| BiFe0.975Zr0.025O3 | Hydrothermal | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, ZrOCl2.8H2O | 46 | 366 | - | [101] |
| BiFe0.99Ni0.01O3 | Sol–gel | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, Ni(NO3)3⋅6H2O | - | 2000 | 2.6 | [102] |
| Bi0.99La0.1Fe0.95Ni0.05O3 | Solid-state | Bi2O3, Fe2O3, La2O3, & NiO | - | 2083 | 0.2 | [103] |
| Bi0.99Ba0.1Fe0.99Nb0.1O3 | Sol–gel & combustion | Bi(NO3)3, Fe(NO3)3, Ba(NO₃)₂, C6H4NNbO12 | 27 | 115 | 3.2 | [104] |
| Bi0.9La0.075Ce0.025FeO3 | Combustion | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, La(NO3)3⋅6H2O, Ce(NO3)3⋅6H2O | 25 | 105 | 3.2 | [107] |
| Bi0.85Er0.15FeO3 | Solid-state | Bi2O3, Fe2O3, Er2O3, | - | 500 | 0.1 | [99] |
| BiFe0.99(Li0⋅5Nb0.5)0.01O3 | Ceramic sintering | Bi2O3, Fe2O3, Li2O, Nb2O5 | - | 1050 | 0.2 | [105] |
| Material | Route | Precursors | Size (nm) | ԑr RT; 102 Hz | Pr (µC·cm−2) | Ref. |
|---|---|---|---|---|---|---|
| (BiFeO3)0.6(CaTiO3)0.4 | Solid-state | CaCO3, Bi2O3, TiO2, Fe2O3 | - | 1075 | 0.1 | [108] |
| (Bi0.95Nd0.05FeO3)0.8(PbTiO3)0.2 | Solid-state | Bi2O3, Nd2O3, Fe2O3, PbO, TiO2 | 2 × 102 | 1625 | 0.8 | [109] |
| Mn-doped-(BiFeO3)0.5(SrTiO3)0.5 | Solid-state | Bi2O3, Fe2O3, SrO, TiO2, MnO2 | - | 720 | 6 | [110] |
| (BiFeO3)0.65(BaTiO3)0.35 | Solid-state | BaCO3, TiO2, Bi2O3, Fe2O3 | 3 × 102 | 4300 | 3.7 | [111] |
| (BiFeO3)0.66(PbTiO3)0.34 | Sol–gel | Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, Pb(CH3COO)2·3H2O, Ti[OCH(CH3)2]4 | - | 587 | 95 | [112] |
| [(Bi0.9Dy0.1)FeO3]0.5–(PbTiO3)0.5 | Combustion | PbO, Bi2O3, Fe2O3, Dy2O3, TiO2 | - | 103 | 9 | [115] |
| (BiFeO3)0.8–(GdMnO3)0.2 | Combustion | Fe(NO3)3·9H2O, Bi(NO3)3.5H2O, Gd2O3, (CH3COO)2 Mn·4H2O | 25 | 688 | 0.4 | [116] |
| (BiFeO3)0.7-PbTiO3)0.3 | Solid-state | Bi2O3, Fe2O3, PbO, TiO2, SrCO3 | 103 | 295 | 7 | [117] |
| (Na0.5Bi0.5TiO3)0.775–(SrTiO3)0.2–BiFeO3)0.025 | Solid-state | Na2CO3, Bi2O3, SrCO3, Fe2O3, TiO2 | - | - | 24 | [118] |
| 0.655BiFeO3–0.025BiCoO3–0.32BaTiO3 | Solid-state | Bi2O3, Fe2O3, Co3O4, La2O3, BaCO3, TiO2 | 60 | 2000 | 5 | [113] |
| 0.675BiFeO3–0.3BaTiO3–0.025LaFeO3–1.25Ta2O5 | Solid-state | Bi2O3, Fe2O3, La2O3, TiO2, Ta2O5, BaCO3 | - | 1149 | 2 | [114] |
| Material | Application | Band Gap (ev) | HER/ Efficiency | Photocurrent Density | Refs. |
|---|---|---|---|---|---|
| BiFeO3 | Solar WS | 2.2 | − | 40 μA⋅cm−2 @ 0.6 V | [72] |
| Bi0.95Sm0.05FeO3 | Photoelectrocatalytic | 2.2 | − | 0.11 mA⋅cm−2 | [153] |
| BiFe0.9Cr0.1O3 | Solar cells | 1.9 | − | 0.3 mA⋅cm−2 | [165] |
| BiFe0.9Ti0.1O3 | Solar WS | 2.3 | − | − | [166] |
| Bi0.85Sr0.15FeO3 | Solar WS | − | − | 0.5 mA⋅cm−2 @ 1.4 V | [152] |
| Bi0.97Y0.03FeO3 | Solar WS | − | − | 0.7 mA⋅cm−2 @ 1.4V | [167] |
| Bi0.875SrxFe0.875Ti0.125O3 | Solar WS | 2.5 | 191 μmol·h−1·g−1/ − | 0.2 μA⋅cm−2 | [76] |
| Bi0.75Gd0.25Fe1−yCoyO3 | Solar WS | 1.8 | 74.6 μmol·h−1·cm−2/ − | 2 mA⋅cm−2 @ 1 V | [154] |
| WO3/BiFeO3 | Solar WS | 3/2.2 | − | 35.2 mA⋅cm−2 @ 2 V | [155] |
| BiFeO3/BiVO4 | Solar WS | 2.7 | − | 0.2 mA⋅cm−2 @ 1 V | [136] |
| Bi2O3/BiFeO3 | Solar WS | 2.8/2.7 | − | −84 μA⋅cm−2 @ −0.7 V | [158] |
| BiFeO3/Cu2O | Solar WS | 2.6 | − | −0.5 mA⋅cm−2 @−0.7 V | [162] |
| BiFeO3/TiO2 | Solar WS | 2.1/3.2 | − | 28.8 mA⋅cm−2 @ 1.5 V | [156] |
| BiFeO3/Bi2Fe4O9 | Solar WS | 2.2/1.9 | 800 μmol·g−1 for 8h/ − | 1.8 μA⋅cm−2 | [157] |
| Au/BiFeO3 | Solar WS | 2.1 | 2.1 mmol·h−1 for 2h/ − | 2.1 μA⋅cm−2 @ 0.6 V | [159] |
| BiFeO3@COF Z-Scheme | Solar WS | − | 1416.4 μmol·h−1·g−1/ − | 3.8 μA⋅cm−2 @ 0.6 V | [168] |
| WO3/BiVO4/BiFeO3 | Solar WS | 3/2.4/2.1 | − | 47 mA⋅cm−2 @ 2.5 V | [161] |
| CdS/BiFeO3 | Solar WS | 2.4/2.1 | 600 μmol·h−1·g−1/ − | 1.2 mA⋅cm−2 | [160] |
| g-C3N4/BiFeO3 Z-scheme | Solar WS | 2.8/2.3 | 23.31 μmol·h−1·g−1/ − | − | [169] |
| Z-scheme Au-LaFeO3-g-C3N4-BiFeO3 | Solar WS | 2/2.7/2 | 698.4 μmol·h−1·g−1/ − | 1.2 μA⋅cm−2 | [170] |
| BiFeO3/ZnIn2S4 Z-scheme | Solar WS | 2/2.3 | 87.3 μmol·h−1·g−1/ − | 0.5 μA⋅cm−2 | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassereddine, Y.; Benyoussef, M.; Asbani, B.; El Marssi, M.; Jouiad, M. Recent Advances toward Enhanced Photocatalytic Proprieties of BiFeO3-Based Materials. Nanomaterials 2024, 14, 51. https://doi.org/10.3390/nano14010051
Nassereddine Y, Benyoussef M, Asbani B, El Marssi M, Jouiad M. Recent Advances toward Enhanced Photocatalytic Proprieties of BiFeO3-Based Materials. Nanomaterials. 2024; 14(1):51. https://doi.org/10.3390/nano14010051
Chicago/Turabian StyleNassereddine, Yassine, Manal Benyoussef, Bouchra Asbani, Mimoun El Marssi, and Mustapha Jouiad. 2024. "Recent Advances toward Enhanced Photocatalytic Proprieties of BiFeO3-Based Materials" Nanomaterials 14, no. 1: 51. https://doi.org/10.3390/nano14010051
APA StyleNassereddine, Y., Benyoussef, M., Asbani, B., El Marssi, M., & Jouiad, M. (2024). Recent Advances toward Enhanced Photocatalytic Proprieties of BiFeO3-Based Materials. Nanomaterials, 14(1), 51. https://doi.org/10.3390/nano14010051









