Enhanced Redox Cycle of Rod-Shaped MIL-88A/SnFe2O4@MXene Sheets for Fenton-like Degradation of Congo Red: Optimization and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
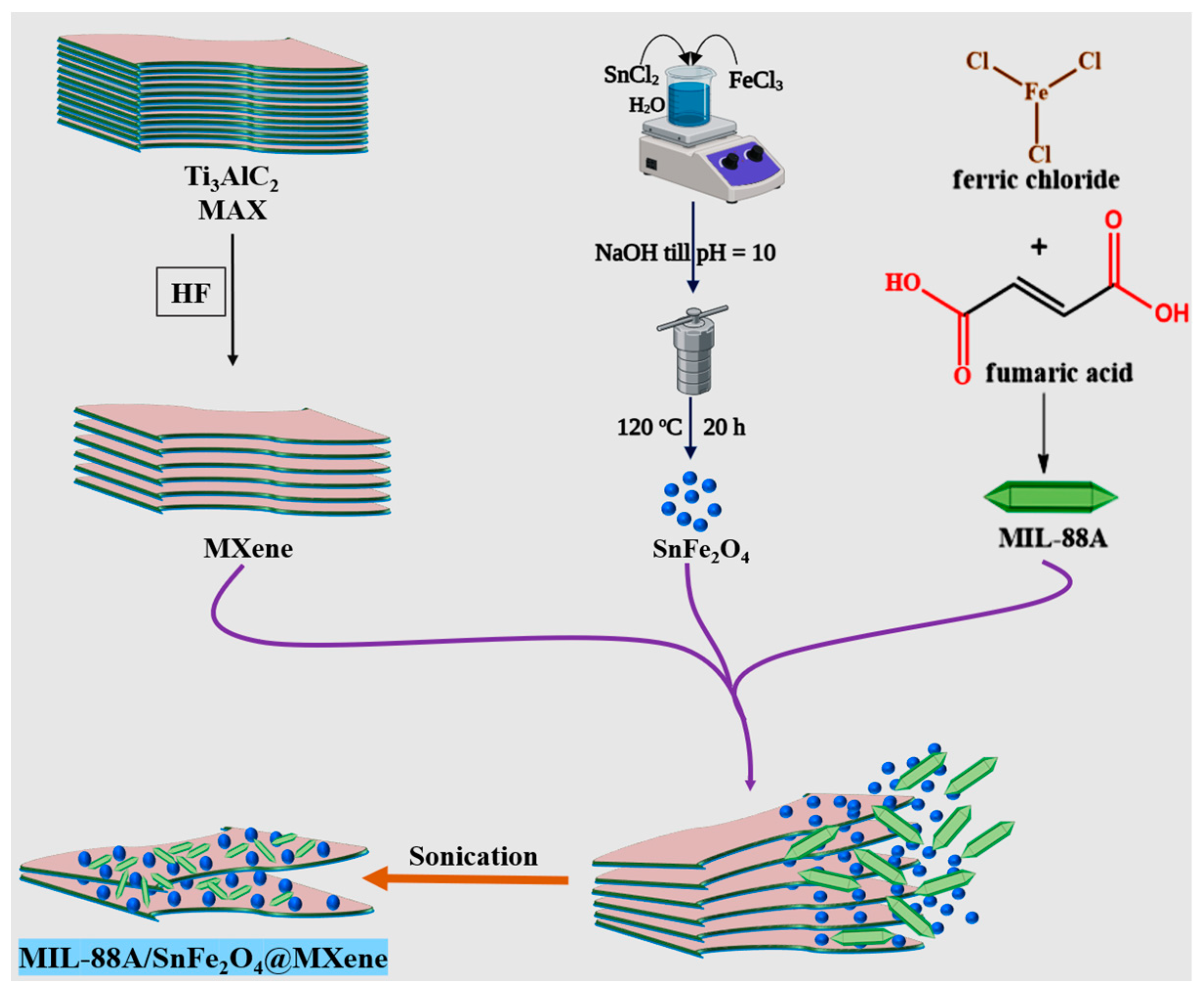
2.2. Synthesis of MIL-88A
2.3. Synthesis of MXene
2.4. Synthesis of SnFe2O4 NPs
2.5. Synthesis of MIL-88A/SnFe2O4@MXene
2.6. Fenton-like Experiments
2.7. Cycling Test
3. Results and Discussions
3.1. Characterization of MIL-88A/SnFe2O4@MXene
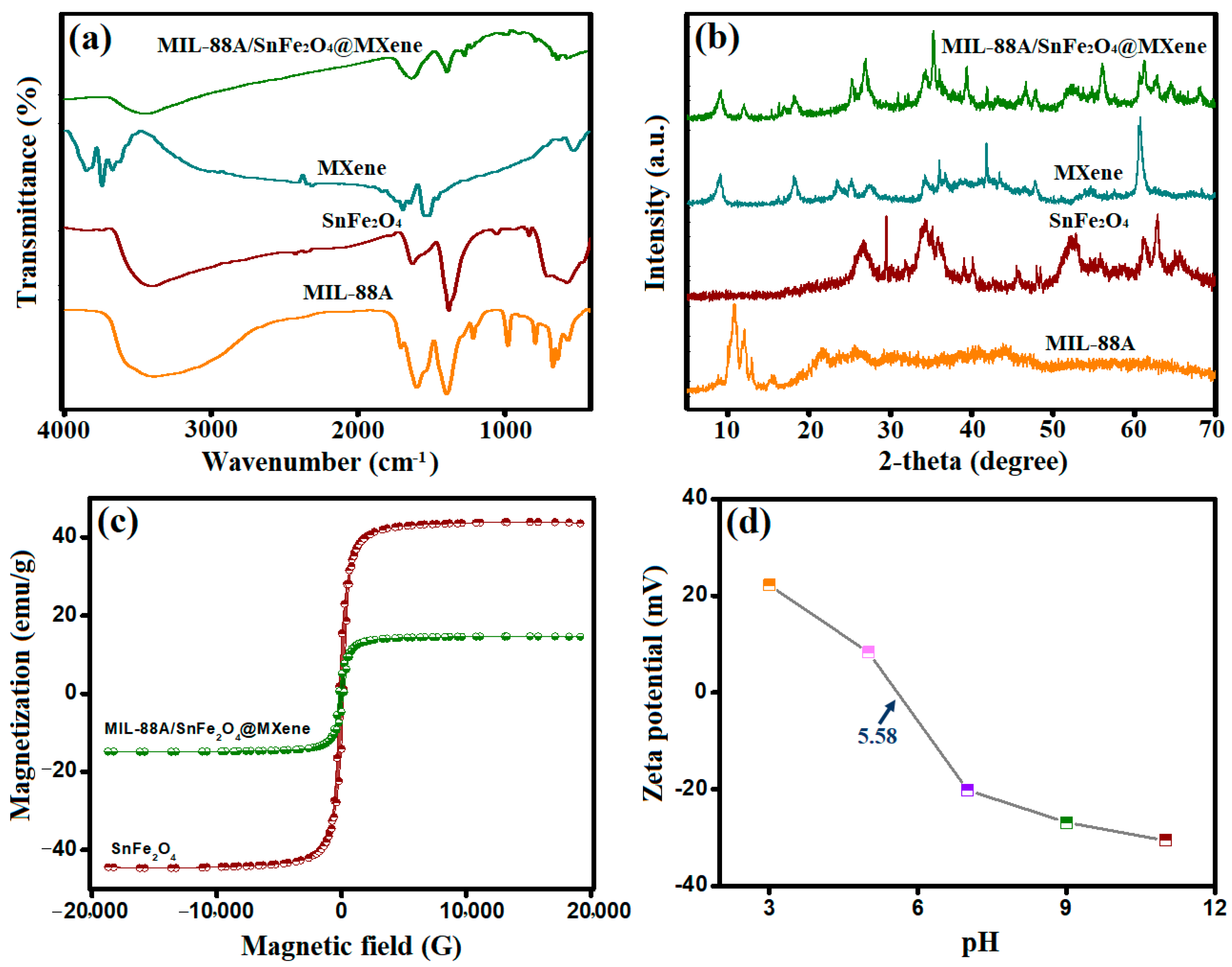
3.1.1. FTIR
3.1.2. XRD
3.1.3. VSM
3.1.4. Zeta Potential
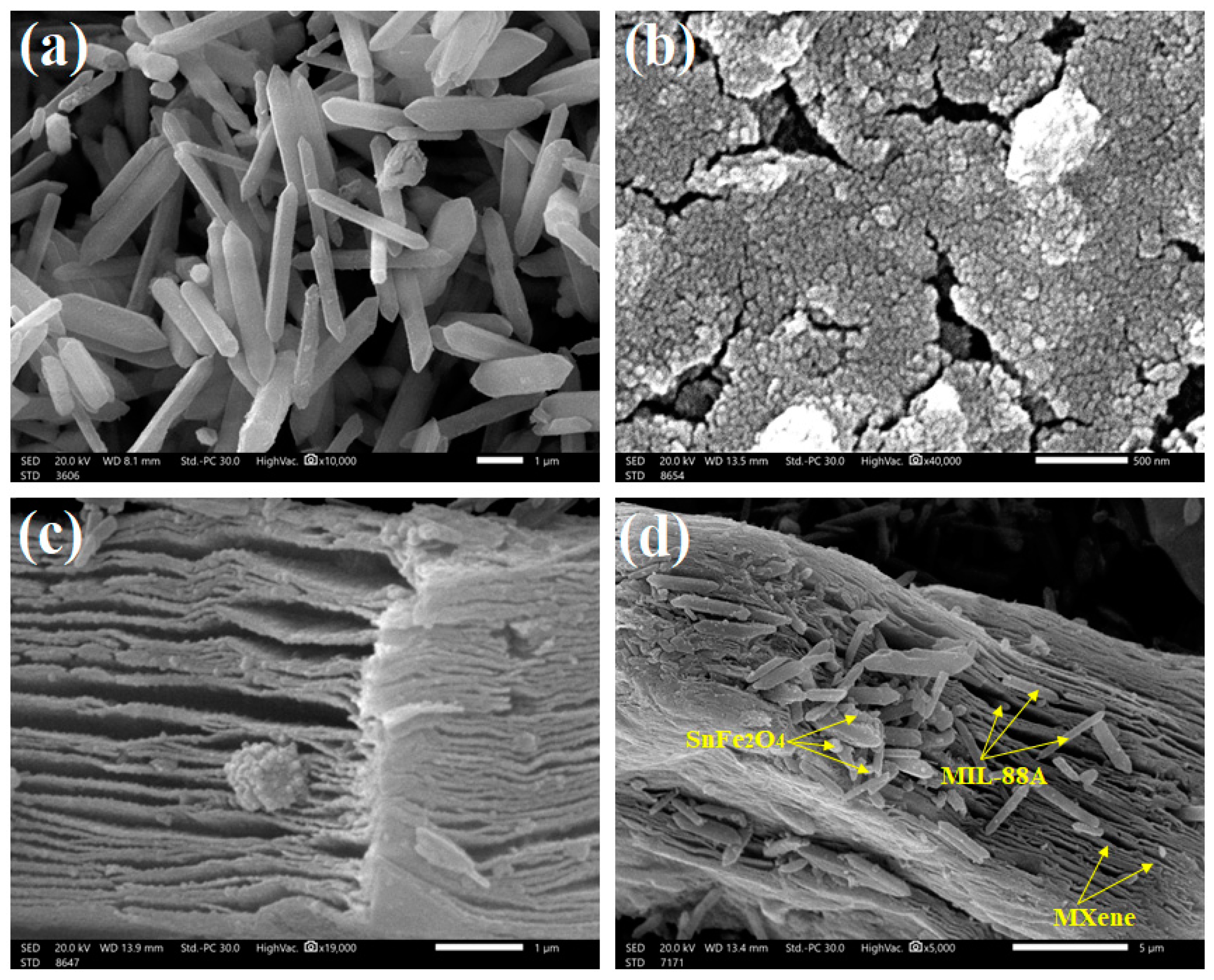
3.1.5. SEM
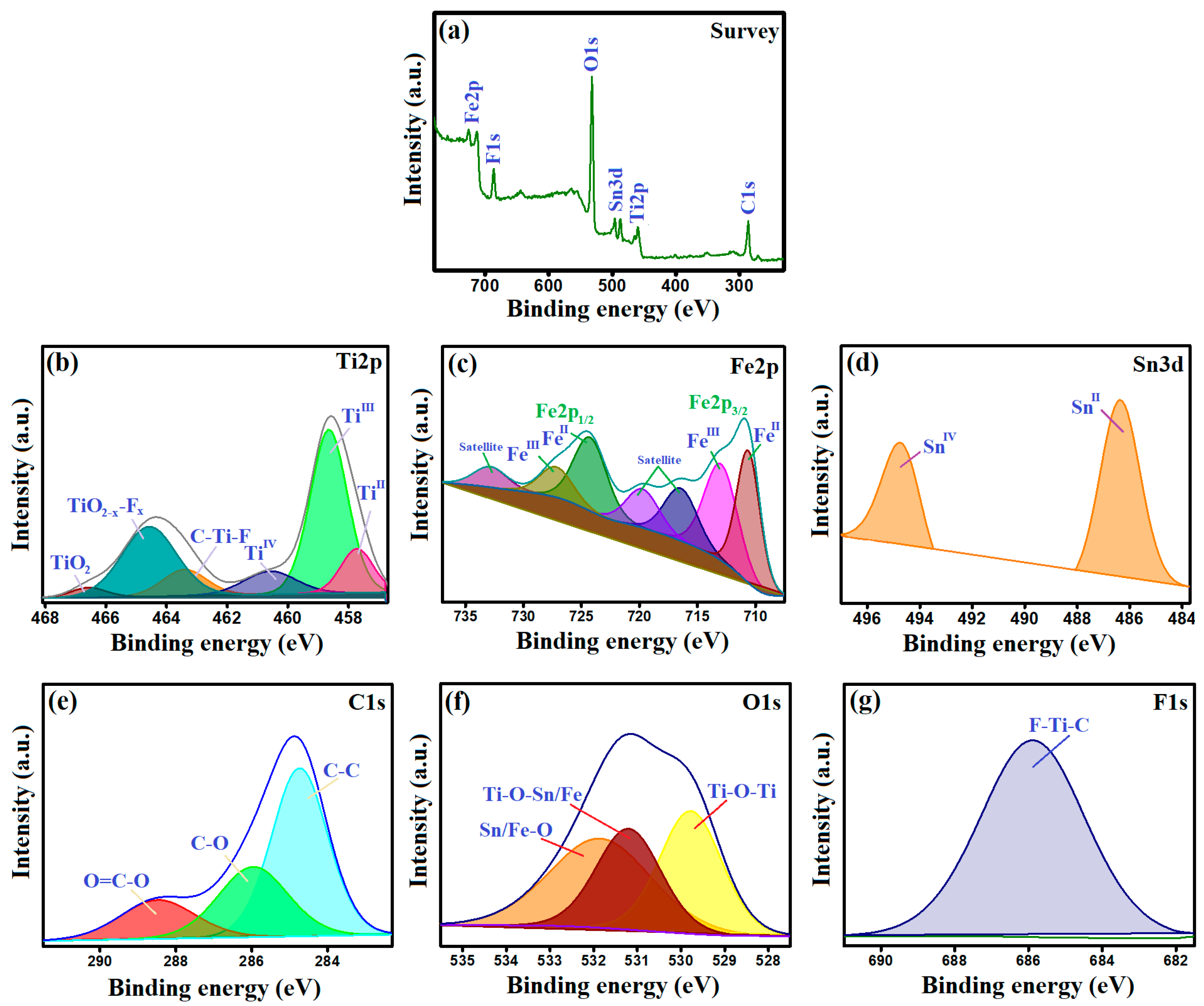
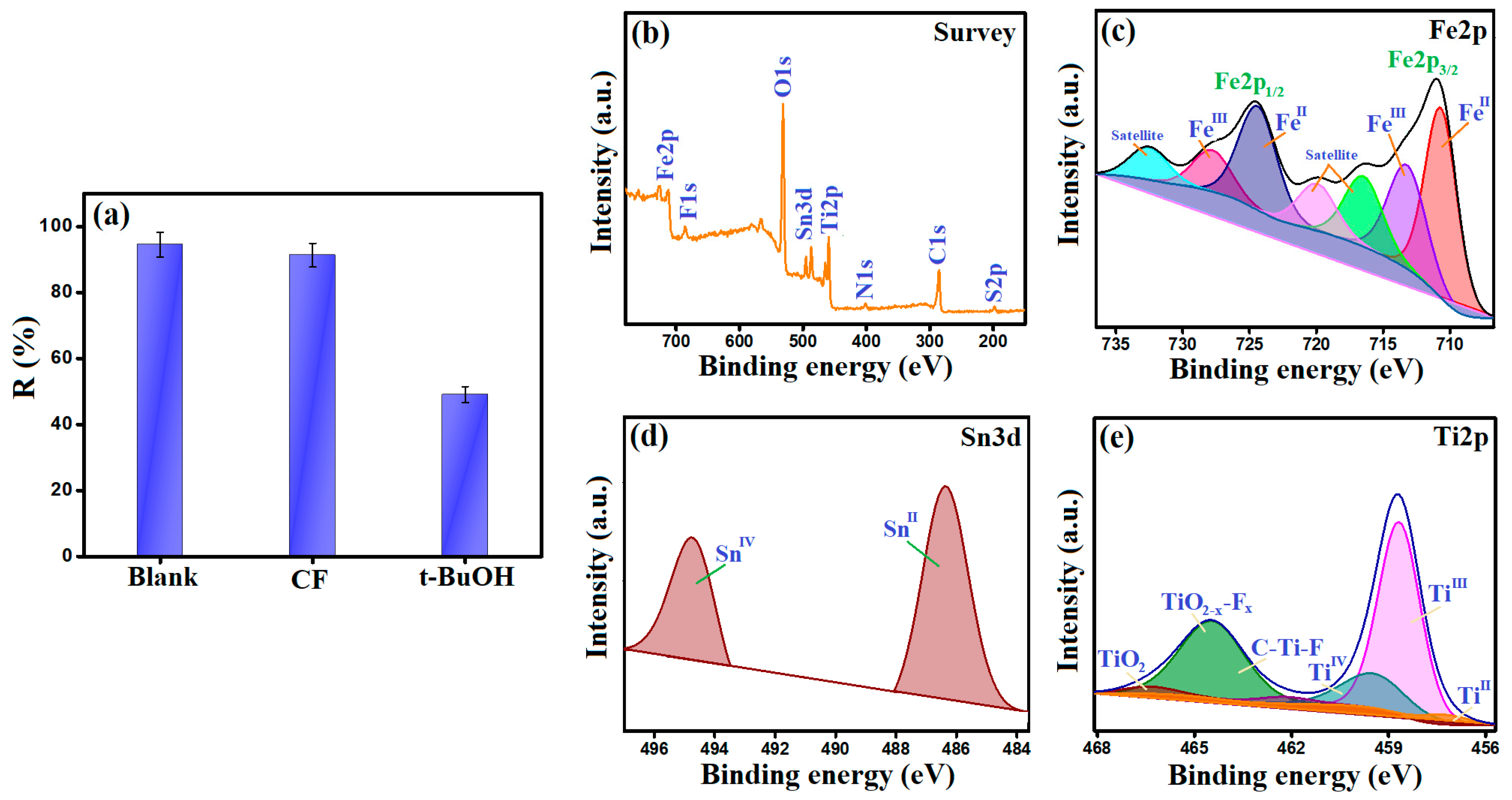
3.1.6. XPS
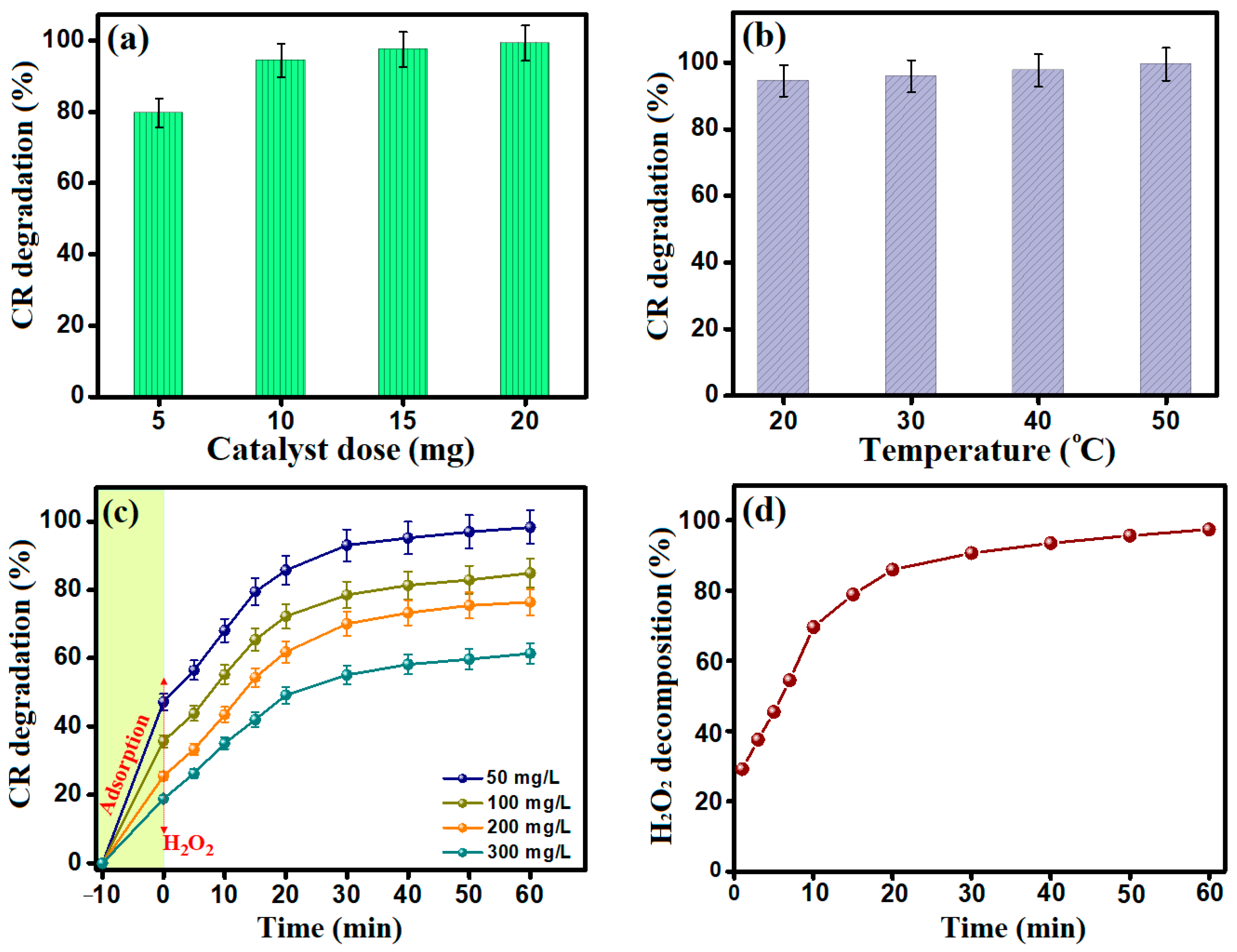
3.2. Optimization of the CR Fenton-like Degradation Process
3.2.1. Comparison Test
3.2.2. The Impact of the Catalytic pH Medium
3.2.3. The Impact of the H2O2 Concentration
3.2.4. The Impact of MIL-88A/SnFe2O4@MXene Dose
3.2.5. The Impact of the Temperature of the Catalytic System
3.2.6. The Impact of the Concentration of CR Molecules
3.2.7. Kinetic Study of H2O2 Decomposition
3.3. Kinetic Study
3.3.1. CR Decomposition
3.3.2. H2O2 Decomposition
3.4. Scavenging Test
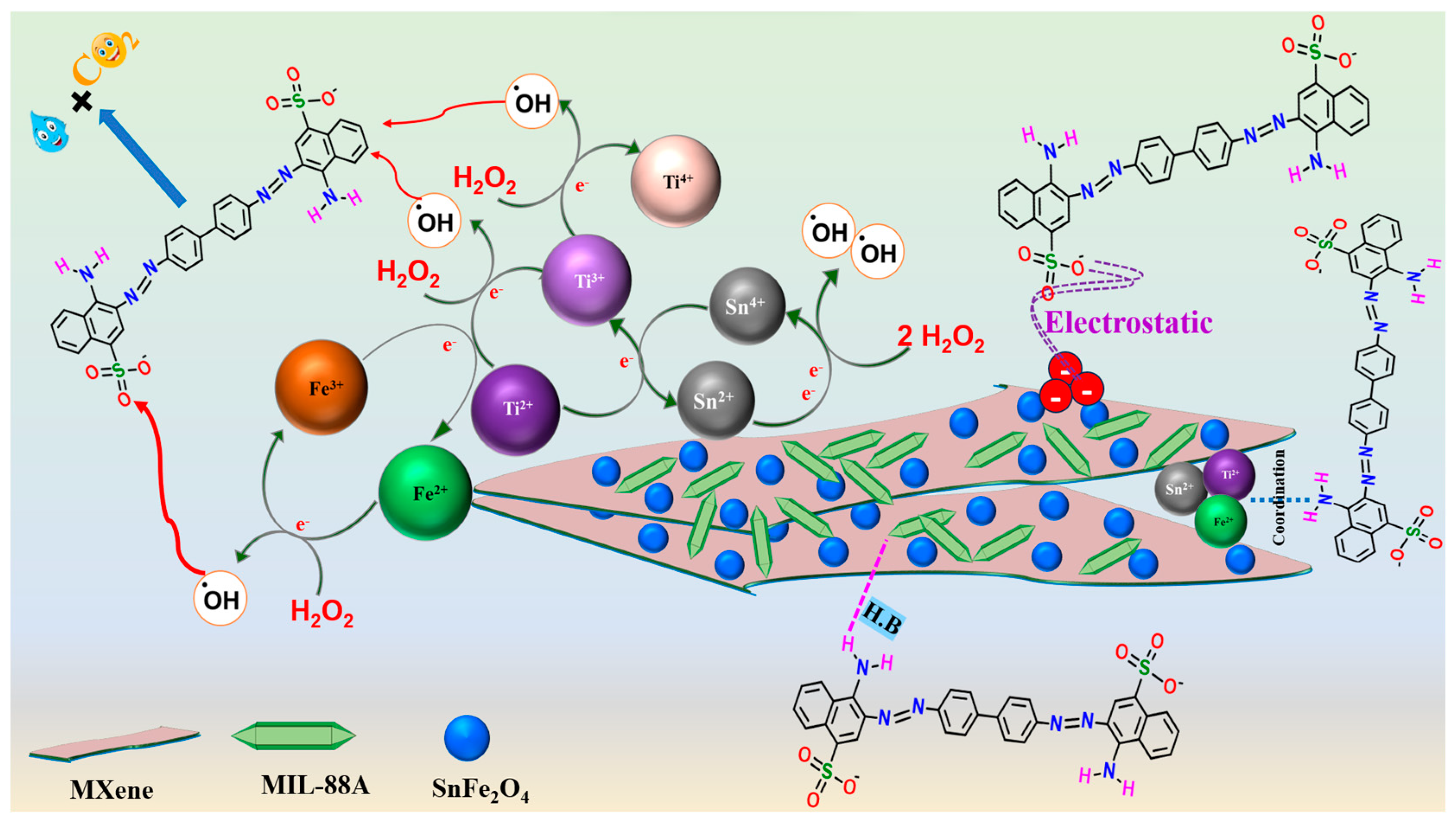
3.5. Degradation Mechanism
- (i)
- FeII species could transfer electrons to activate H2O2 and form •OH radicals, following the Haber–Weiss mechanism, as represented in Equation (10). This proposition was proved by the blue shift of the Fe2p spectrum of the used MIL-88A/SnFe2O4@MXene catalyst (Figure 7c). In addition, the FeIII/FeII ratio of the used catalyst was found to be 0.549, while the ratio of the pure catalyst was 0.264, reflecting the increase in the FeIII amounts after the Fenton-like degradation of CR and contributing of FeII species in the H2O2 activation.
- (ii)
- The SnII ions play a dual role in the Fenton-like degradation of CR molecules since they could provide the needed electrons to activate H2O2 and produce •OH radicals (Equation (11)). The participation of SnII species in the H2O2 activation was confirmed by the detected shifting in the Sn3d peaks and the increase in the SnIV/SnII ratio after the Fenton-like reaction from 0.599 to 0.674 (Figure 7d); additionally, the ability of SnII to recover FeIII to FeII, where the redox potential (Eo) of SnII/SnIV is 0.14 V, and the Eo of FeII/FeIII is 0.77 V (Equation (12)).
- (iii)
- The abundant Ti species (Figure 7e) also contribute to the •OH generation and recover both FeII and SnII species. The TiII and TiIII ions activated H2O2, as elucidated in Equations (13) and (14). The lower Eo value of TiII/TiIII compared to TiIII/TiIV implies a higher affinity of TiII to activate H2O2 than TiIII. Also, the TiIII/TiII ratio drastically increased from 1.3 to 16.87, while the ratio of TiIII/TiIV trivially augmented from 0.274 to 0.277, which reflected the lower contribution of TiIII in the Fenton-like degradation of CR. Such a low Eo value of TiII/TiIII enables it to recover FeII and SnII species and sustains the Fenton-like reaction for a long time (Equations (15) and (16)). These suggestions explained the dramatic decline in the atomic percent of TiII ions and denoted the significance of MXene in the reducibility of iron and tin [38]. Figure 8 exhibits the adsorption and Fenton-like degradation mechanisms of CR by MIL-88A/SnFe2O4@MXene.
3.6. Degradation Pathway of CR Molecules
3.7. Recyclability Test
3.8. Leaching Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- El-Monaem, E.M.A.; Ayoup, M.S.; Omer, A.M.; Hammad, E.N.; Eltaweil, A.S. Sandwich-like construction of a new aminated chitosan Schiff base for efficient removal of Congo red. Appl. Water Sci. 2023, 13, 67. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qiu, Y.; Yang, C.; Zang, J.; Song, Z.; Yang, T.; Li, J.; Fan, Y.; Dang, F.; Wang, W. Efficient Degradation of Congo Red in Water by UV-Vis Driven CoMoO4/PDS Photo-Fenton System. Molecules 2022, 27, 8642. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhou, Y.; Zhang, Z.; Xiao, K. Cobalt–copper oxalate nanofibers mediated Fenton degradation of Congo red in aqueous solutions. J. Ind. Eng. Chem. 2017, 52, 153–161. [Google Scholar] [CrossRef]
- Solano, A.M.S.; Garcia-Segura, S.; Martínez-Huitle, C.A.; Brillas, E. Degradation of acidic aqueous solutions of the diazo dye Congo Red by photo-assisted electrochemical processes based on Fenton’s reaction chemistry. Appl. Catal. B Environ. 2015, 168, 559–571. [Google Scholar] [CrossRef]
- Ismail, G.A.; Sakai, H. Review on effect of different type of dyes on advanced oxidation processes (AOPs) for textile color removal. Chemosphere 2022, 291, 132906. [Google Scholar] [CrossRef]
- Alharbi, R.A.; Alminderej, F.M.; Al-Harby, N.F.; Elmehbad, N.Y.; Mohamed, N.A. Preparation and characterization of a new bis-uracil chitosan-based hydrogel as efficient adsorbent for removal of anionic Congo red dye. Polymers 2023, 15, 1529. [Google Scholar] [CrossRef]
- Modwi, A.; Mustafa, B.; Toghan, A.; Taha, K.K. Scalable fabrication and characterization of Y2O3@g-C3N4 nanocomposite for the enhancement of photocatalytic removal of Congo red dye under visible light. J. Mater. Sci. Mater. Electron. 2023, 34, 298. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Synthesis and characterization of novel uracil-modified chitosan as a promising adsorbent for efficient removal of Congo red dye. Polymers 2022, 14, 271. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Ganiyu, S.O.; Martínez-Huitle, C.A.; Mousset, E.; Olvera-Vargas, H.; Trellu, C.; Zhou, M.; Oturan, M.A. Recent advances in electro-Fenton process and its emerging applications. Crit. Rev. Environ. Sci. Technol. 2023, 53, 887–913. [Google Scholar] [CrossRef]
- Cheng, M.; Song, W.; Ma, W.; Chen, C.; Zhao, J.; Lin, J.; Zhu, H. Catalytic activity of iron species in layered clays for photodegradation of organic dyes under visible irradiation. Appl. Catal. B Environ. 2008, 77, 355–363. [Google Scholar] [CrossRef]
- Liao, J.; Li, H.; Zhang, X.; Xiao, D.; Qiang, N. Facile fabrication of Ti supported CuO film composed of bamboo-leaf-like nanosheets and their high catalytic performance in the oxidative degradation of methylene blue with hydrogen peroxide. Appl. Catal. A Gen. 2015, 491, 94–99. [Google Scholar] [CrossRef]
- Cui, W.; Kang, X.; Zhang, X.; Cui, X. Gel-like ZnO/Zr-MOF (bpy) nanocomposite for highly efficient adsorption of Rhodamine B dye from aqueous solution. J. Phys. Chem. Solids 2019, 134, 165–175. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Zhang, X.; Huang, Y. Highly efficient Fenton and enzyme-mimetic activities of NH2-MIL-88B (Fe) metal organic framework for methylene blue degradation. Sci. Rep. 2018, 8, 5159. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, S.; Meng, X.; Wang, Z.; Gao, T.; Xiao, S. Facile synthesis of fumarate-type iron-cobalt bimetallic MOFs and its application in photo-Fenton degradation of organic dyes. J. Solid State Chem. 2022, 314, 123431. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, K.; Zhao, L. A Fenton-like method using ZnO doped MIL-88A for degradation of methylene blue dyes. RSC Adv. 2020, 10, 39973–39980. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, D.; Shen, K.; Nie, S.; Liu, M.; Huang, H.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. Biomimetic anchoring of Fe3O4 onto Ti3C2 MXene for highly efficient removal of organic dyes by Fenton reaction. J. Environ. Chem. Eng. 2020, 8, 104369. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, H.; Wang, H.; Show, P.L.; Ivanets, A.; Luo, D.; Wang, C. MXenes as heterogeneous Fenton-like catalysts for removal of organic pollutants: A review. J. Environ. Chem. Eng. 2022, 10, 108954. [Google Scholar]
- Wang, C.; Ye, J.; Liang, L.; Cui, X.; Kong, L.; Li, N.; Cheng, Z.; Peng, W.; Yan, B.; Chen, G. Application of MXene-based materials in Fenton-like systems for organic wastewater treatment: A review. Sci. Total Environ. 2023, 862, 160539. [Google Scholar] [CrossRef]
- Cheng, X.; Zu, L.; Jiang, Y.; Shi, D.; Cai, X.; Ni, Y.; Lin, S.; Qin, Y. A titanium-based photo-Fenton bifunctional catalyst of mp-MXene/TiO2−x nanodots for dramatic enhancement of catalytic efficiency in advanced oxidation processes. Chem. Commun. 2018, 54, 11622–11625. [Google Scholar] [CrossRef]
- Leichtweis, J.; Silvestri, S.; Vieira, Y.; de Lima Burgo, T.A.; Foletto, E.L. A novel tin ferrite/polymer composite use in photo-Fenton reactions. Int. J. Environ. Sci. Technol. 2021, 18, 1537–1548. [Google Scholar] [CrossRef]
- Soufi, A.; Hajjaoui, H.; Elmoubarki, R.; Abdennouri, M.; Qourzal, S.; Barka, N. Heterogeneous Fenton-like degradation of tartrazine using CuFe2O4 nanoparticles synthesized by sol-gel combustion. Appl. Surf. Sci. Adv. 2022, 9, 100251. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, S.; Xing, B.; Liu, Y.; Zhang, C.; Xia, H. Preparation of magnetic adsorbent-photocatalyst composites for dye removal by synergistic effect of adsorption and photocatalysis. J. Clean. Prod. 2022, 348, 131301. [Google Scholar] [CrossRef]
- Liu, N.; Huang, W.; Zhang, X.; Tang, L.; Wang, L.; Wang, Y.; Wu, M. Ultrathin graphene oxide encapsulated in uniform MIL-88A (Fe) for enhanced visible light-driven photodegradation of RhB. Appl. Catal. B Environ. 2018, 221, 119–128. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Chen, Q.; Li, P.; Zhou, A.; Cao, X.; Hu, Q. Preparation, mechanical and anti-friction performance of MXene/polymer composites. Mater. Des. 2016, 92, 682–689. [Google Scholar] [CrossRef]
- Sargazi, S.; Hajinezhad, M.R.; Rahdar, A.; Zafar, M.N.; Awan, A.; Baino, F. Assessment of SnFe2O4 nanoparticles for potential application in theranostics: Synthesis, characterization, in vitro, and in vivo toxicity. Materials 2021, 14, 825. [Google Scholar] [CrossRef] [PubMed]
- Allen-Perry, K.; Straka, W.; Keith, D.; Han, S.; Reynolds, L.; Gautam, B.; Autrey, D.E. Tuning the magnetic properties of two-dimensional MXenes by chemical etching. Materials 2021, 14, 694. [Google Scholar] [CrossRef]
- Tambe, A.B.; Arbuj, S.S.; Umarji, G.G.; Jawale, N.S.; Rane, S.B.; Kulkarni, S.K.; Kale, B.B. 2D layered MXene/TiO2 nano-heterostructures for photocatalytic H2 generation. Graphene 2d Mater. 2022, 7, 91–106. [Google Scholar] [CrossRef]
- Lu, Y.; Li, D.; Liu, F. Characterizing the chemical structure of Ti3C2Tx MXene by angle-resolved XPS combined with argon ion etching. Materials 2022, 15, 307. [Google Scholar] [CrossRef]
- Jadhav, S.; Pham, H.D.; Padwal, C.; Chougale, M.; Brown, C.; Motta, N.; Ostrikov, K.; Bae, J.; Dubal, D. Enhancing mechanical energy transfer of piezoelectric supercapacitors. Adv. Mater. Technol. 2022, 7, 2100550. [Google Scholar] [CrossRef]
- El-Monaem, E.M.A.; Eltaweil, A.S.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; Omer, A.M. Adsorption of nitrophenol onto a novel Fe3O4-κ-carrageenan/MIL-125 (Ti) composite: Process optimization, isotherms, kinetics, and mechanism. Environ. Sci. Pollut. Res. 2023, 30, 49301–49313. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Wang, H.; Zeng, X.; Han, J.; Zhu, J.; Zhou, M.; Wu, S. High-efficiency photocatalytic activity of type II SnO/Sn3O4 heterostructures via interfacial charge transfer. CrystEngComm 2014, 16, 6841–6847. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Bakr, S.S.; El-Monaem, E.M.A.; El-Subruiti, G.M. Magnetic hierarchical flower-like Fe3O4@ ZIF-67/CuNiMn-LDH catalyst with enhanced redox cycle for Fenton-like degradation of Congo red: Optimization and mechanism. Environ. Sci. Pollut. Res. 2023, 30, 75332–75348. [Google Scholar]
- Youssef, N.A.; Shaban, S.A.; Ibrahim, F.A.; Mahmoud, A.S. Degradation of methyl orange using Fenton catalytic reaction. Egypt. J. Pet. 2016, 25, 317–321. [Google Scholar] [CrossRef]
- Rózsa, G.; Náfrádi, M.; Alapi, T.; Schrantz, K.; Szabó, L.; Wojnárovits, L.; Takács, E.; Tungler, A. Photocatalytic, photolytic and radiolytic elimination of imidacloprid from aqueous solution: Reaction mechanism, efficiency and economic considerations. Appl. Catal. B Environ. 2019, 250, 429–439. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhao, H.; Yao, F.; Cao, J.; Chen, Z.; Ma, F.; Wang, D.; Yang, Q. 2D/2D FeNi-layered double hydroxide/bimetal-MOFs nanosheets for enhanced photo-Fenton degradation of antibiotics: Performance and synergetic degradation mechanism. Chemosphere 2022, 287, 132061. [Google Scholar] [CrossRef]
- Song, H.; Zu, D.; Li, C.; Zhou, R.; Wang, Y.; Zhang, W.; Pan, S.; Cai, Y.; Li, Z.; Shen, Y. Ultrafast activation of peroxymonosulfate by reduction of trace Fe3+ with Ti3C2 MXene under neutral and alkaline conditions: Reducibility and confinement effect. Chem. Eng. J. 2021, 423, 130012. [Google Scholar] [CrossRef]

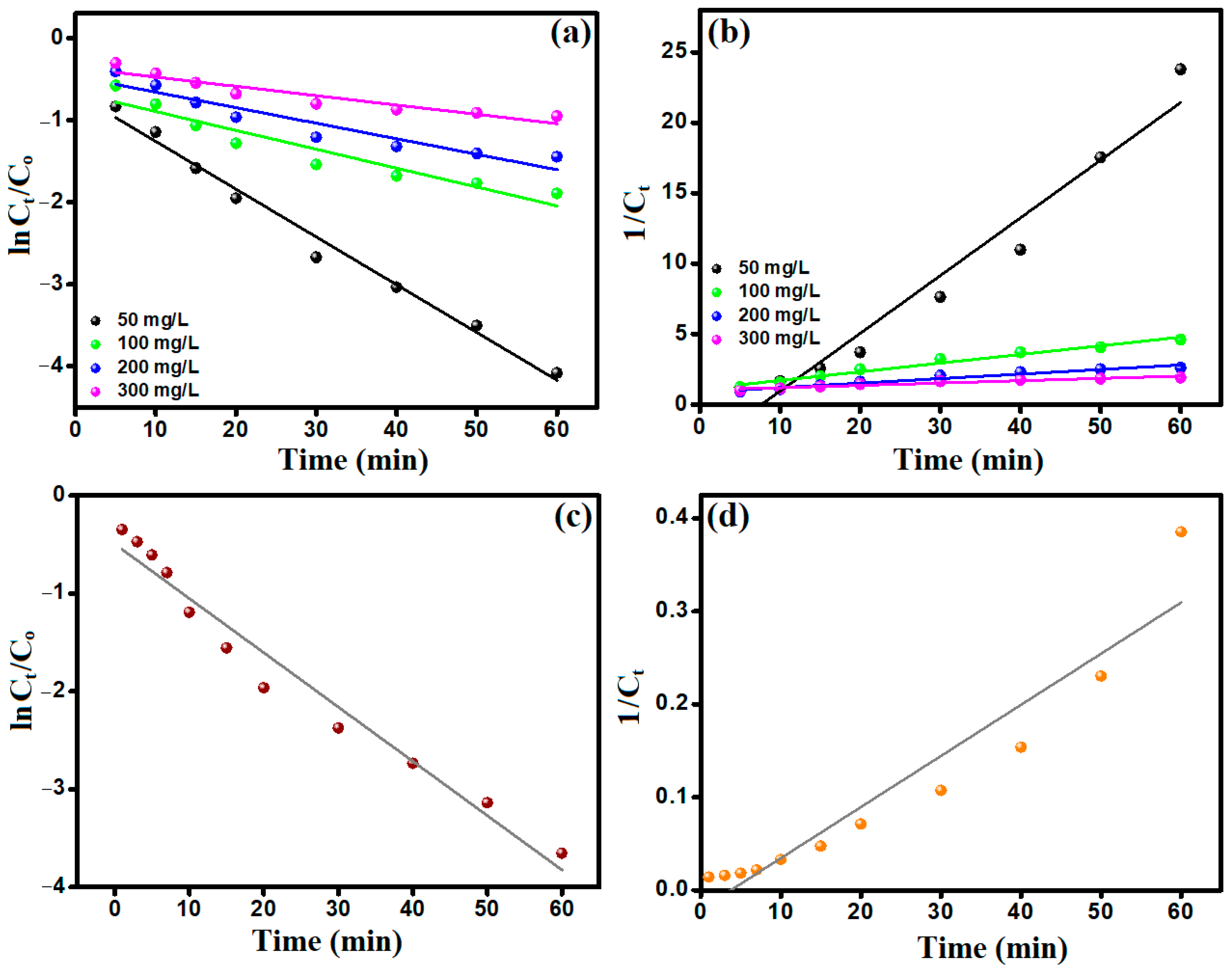


| Kinetic Model | CR Concentrations (mg/L) | |||
|---|---|---|---|---|
| 50 | 100 | 200 | 300 | |
| First-order | ||||
| k1 | 0.0582 | 0.0229 | 0.0189 | 0.0114 |
| R2 | 0.985 | 0.894 | 0.882 | 0.870 |
| Second-order | ||||
| k2 | 0.4098 | 0.0615 | 0.0322 | 0.0166 |
| R2 | 0.949 | 0.974 | 0.946 | 0.918 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Monaem, E.M.; Al Harby, N.; Batouti, M.E.; Eltaweil, A.S. Enhanced Redox Cycle of Rod-Shaped MIL-88A/SnFe2O4@MXene Sheets for Fenton-like Degradation of Congo Red: Optimization and Mechanism. Nanomaterials 2024, 14, 54. https://doi.org/10.3390/nano14010054
Abd El-Monaem EM, Al Harby N, Batouti ME, Eltaweil AS. Enhanced Redox Cycle of Rod-Shaped MIL-88A/SnFe2O4@MXene Sheets for Fenton-like Degradation of Congo Red: Optimization and Mechanism. Nanomaterials. 2024; 14(1):54. https://doi.org/10.3390/nano14010054
Chicago/Turabian StyleAbd El-Monaem, Eman M., Nouf Al Harby, Mervette El Batouti, and Abdelazeem S. Eltaweil. 2024. "Enhanced Redox Cycle of Rod-Shaped MIL-88A/SnFe2O4@MXene Sheets for Fenton-like Degradation of Congo Red: Optimization and Mechanism" Nanomaterials 14, no. 1: 54. https://doi.org/10.3390/nano14010054







