Thermally Induced Surface Structure and Morphology Evolution in Bimetallic Pt-Au/HOPG Nanoparticles as Probed Using XPS and STM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. X-ray Photoelectron Spectroscopy
2.3. Scanning Tunneling Microscopy
3. Results and Discussion
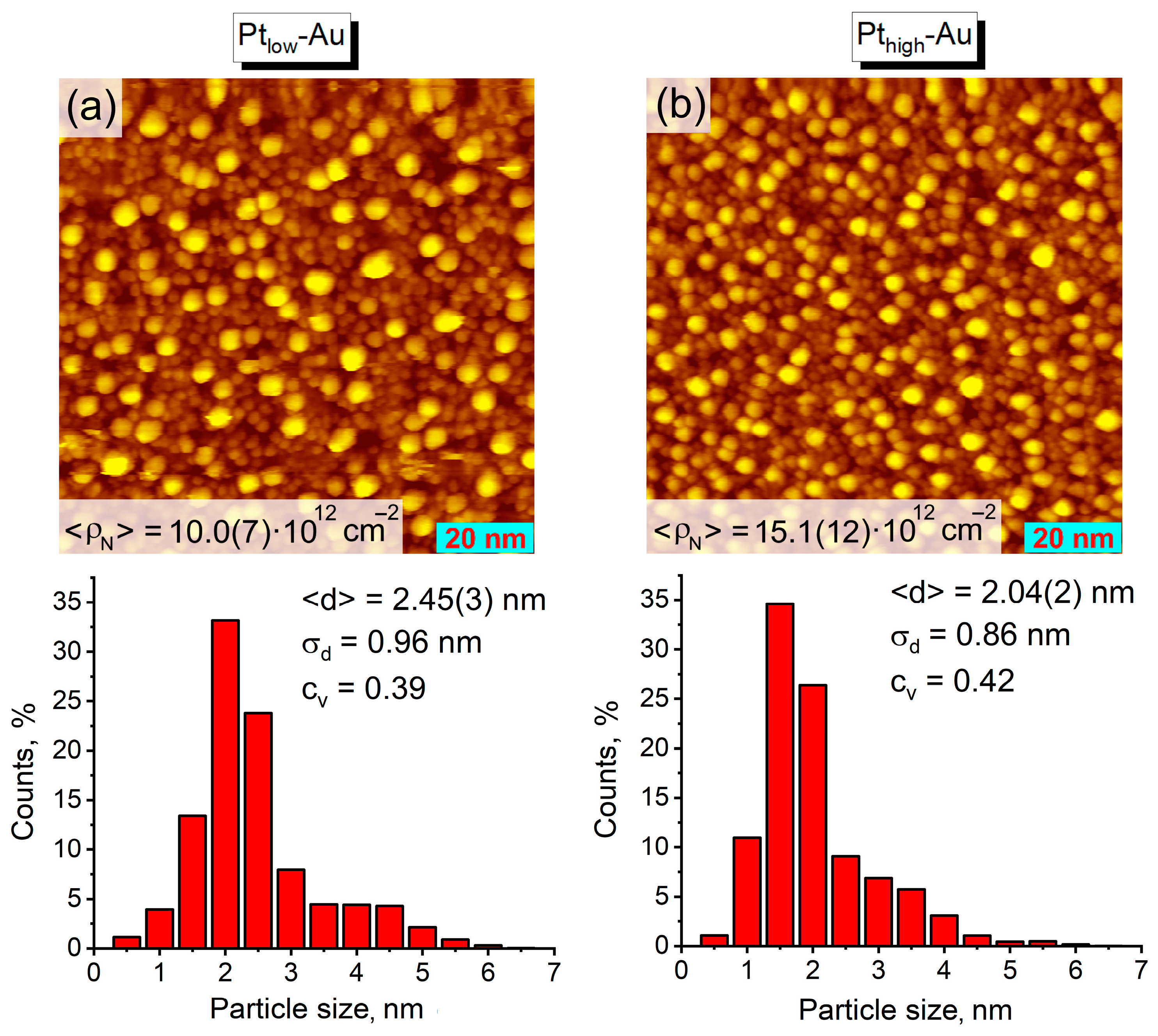
3.1. Thermal Vacuum Deposition of Au and Pt on HOPG
3.2. Theoretical Modeling of the Structure of Bimetallic Pt-Au/HOPG Nanoparticles
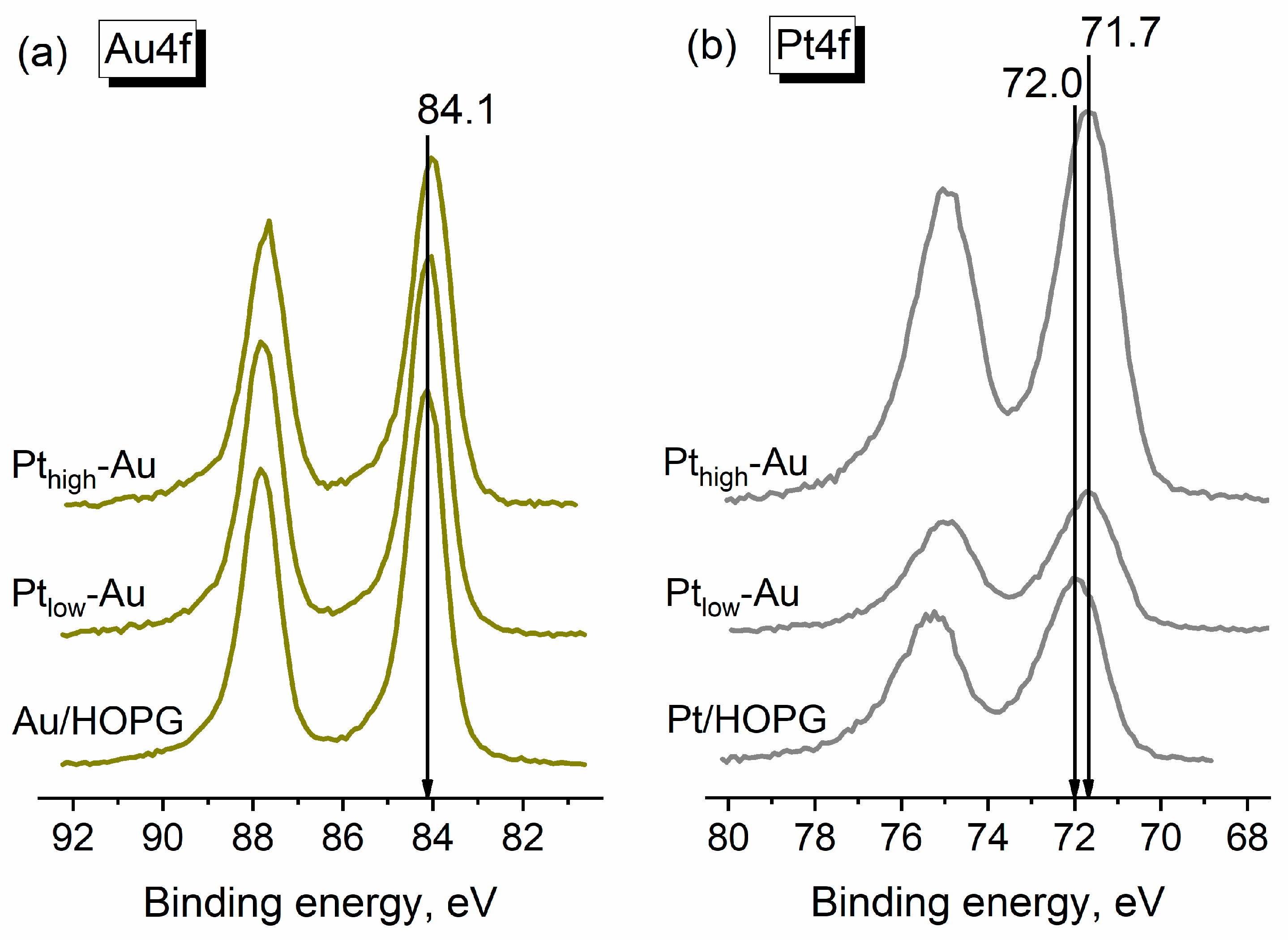
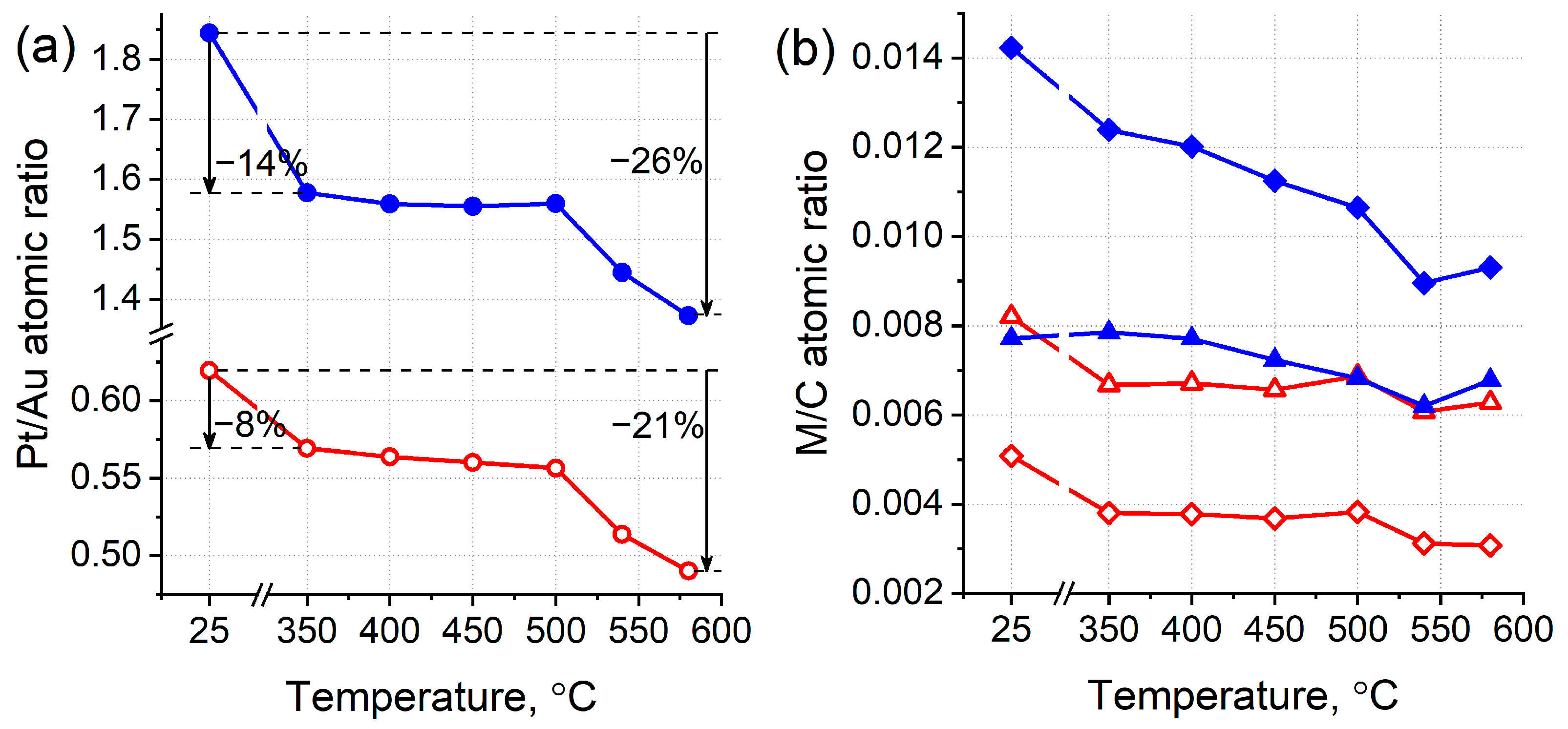
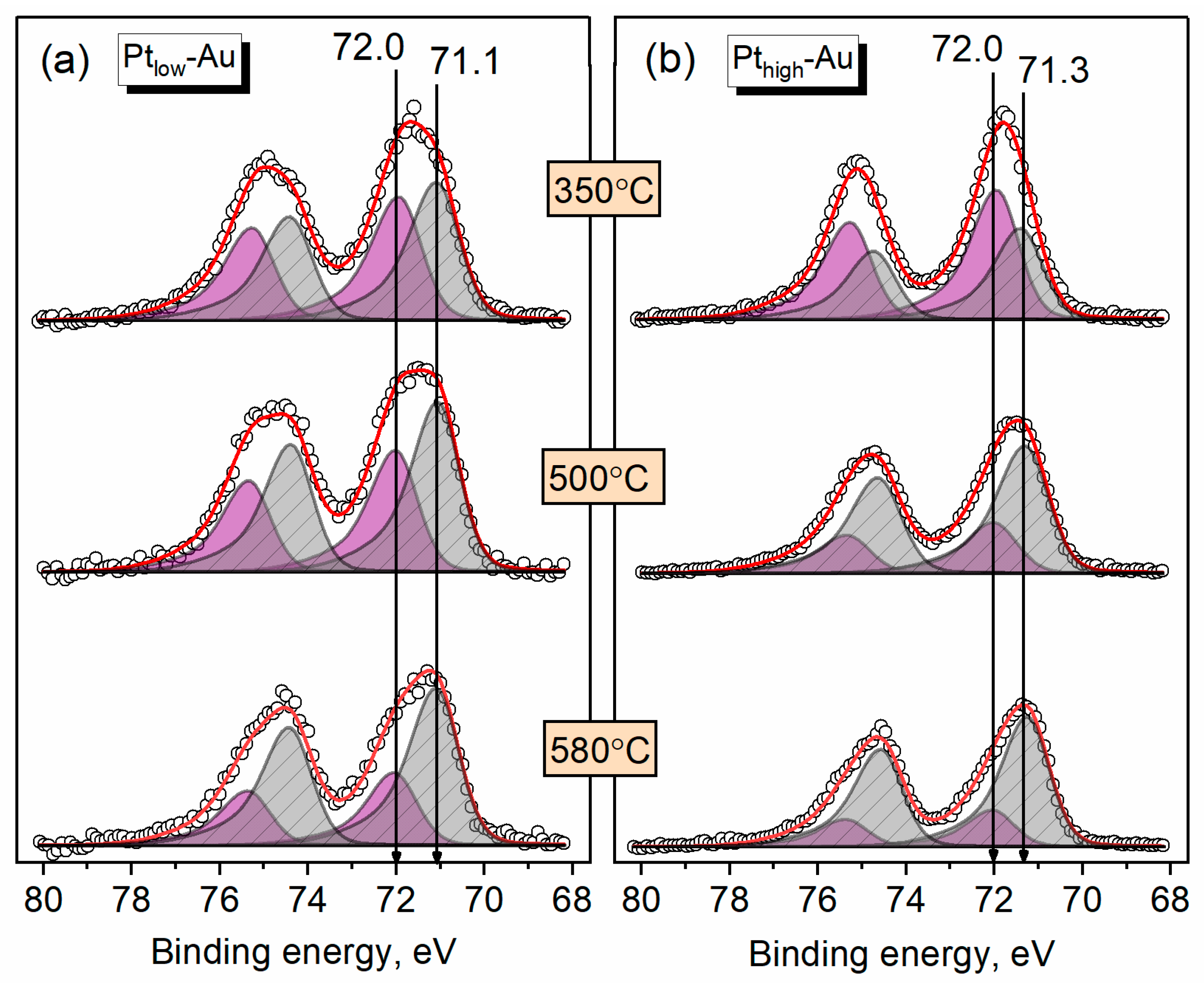
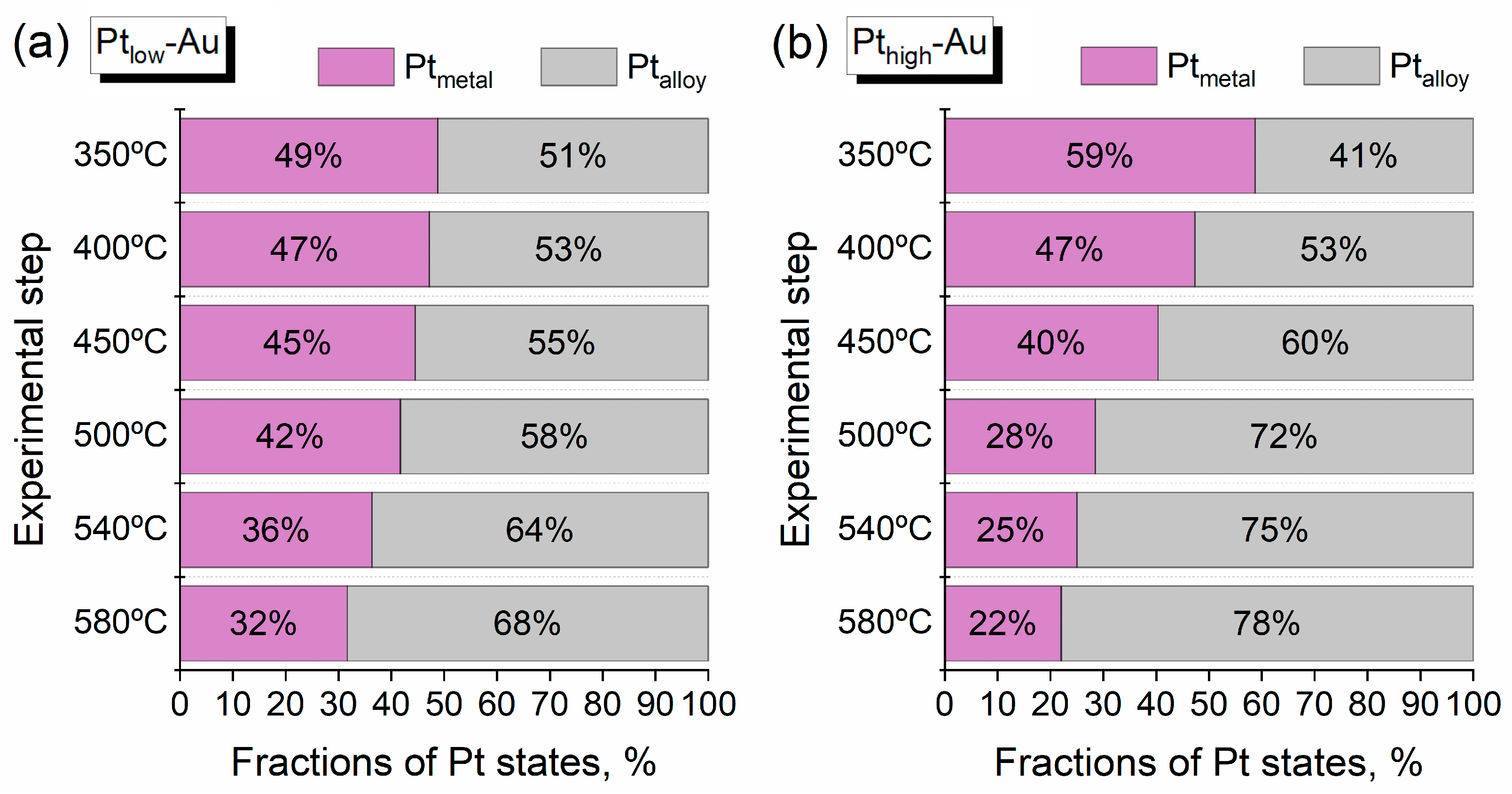
3.3. Thermal Annealing of Bimetallic Pt-Au/HOPG Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
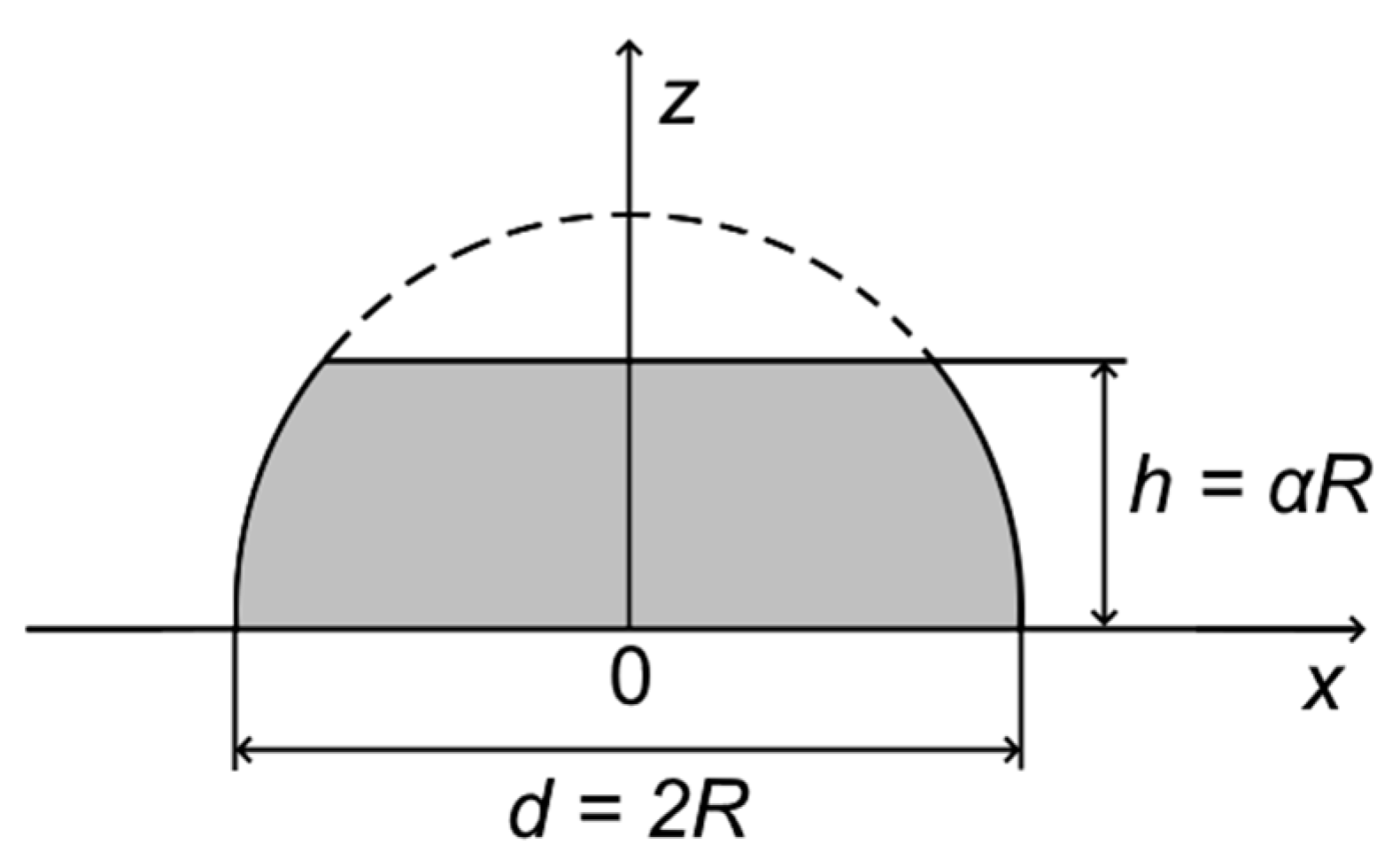
Appendix A
Appendix A.1. Truncated Hemispherical Au/HOPG Nanoparticles: Au4f Intensity Calculation
Appendix A.2. Truncated Hemispherical Ptshell-Aucore/HOPG Nanoparticles: Au4f Intensity Calculation
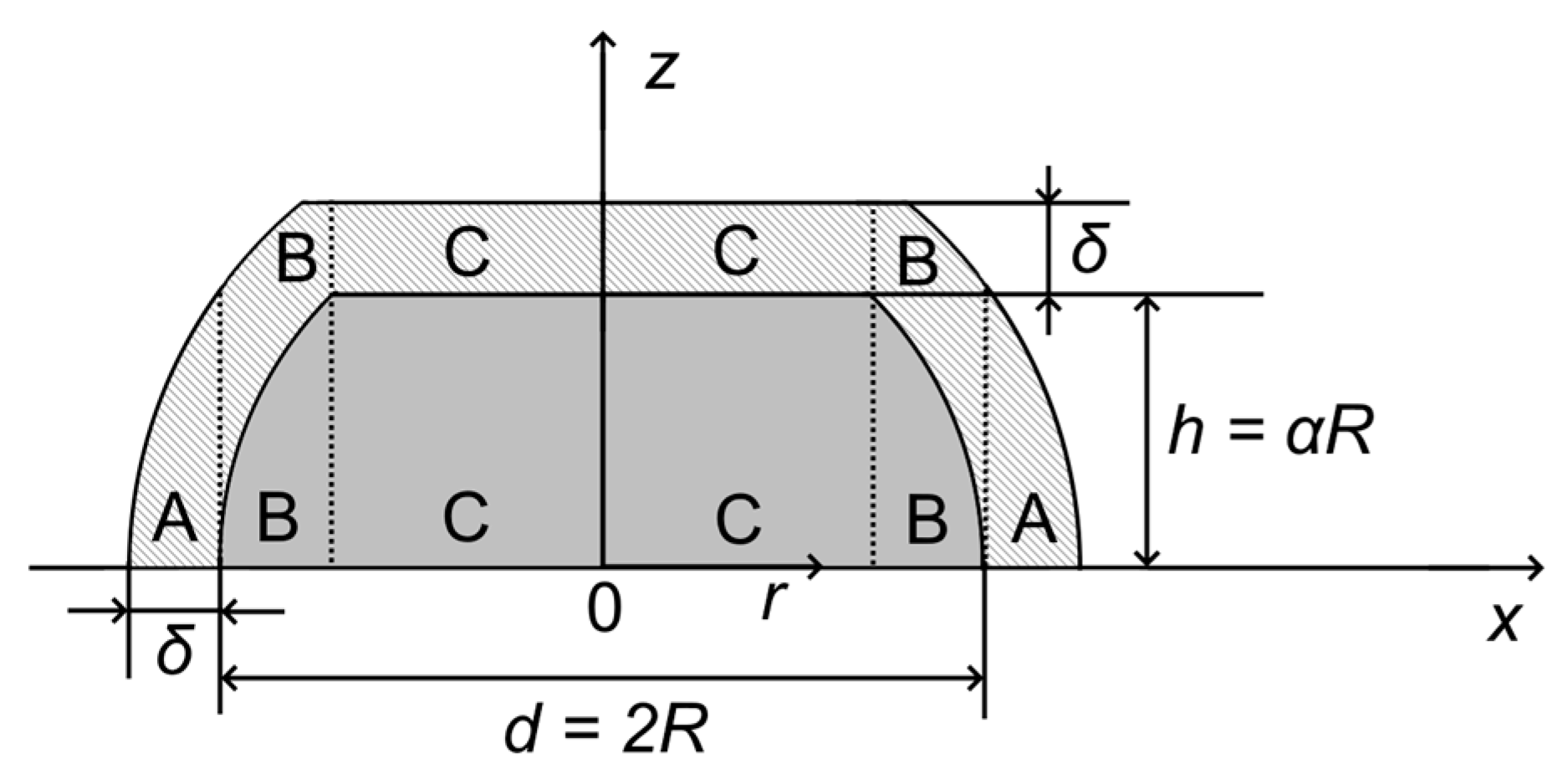
Appendix A.3. Truncated Hemispherical Ptshell-Aucore/HOPG Nanoparticles: Pt4f Intensity Calculation
References
- Zhao, Z.J.; Liu, S.; Zha, S.; Cheng, D.; Studt, F.; Henkelman, G.; Gong, J. Theory-Guided Design of Catalytic Materials Using Scaling Relationships and Reactivity Descriptors. Nat. Rev. Mater. 2019, 4, 792–804. [Google Scholar] [CrossRef]
- Goodman, E.D.; Schwalbe, J.A.; Cargnello, M. Mechanistic Understanding and the Rational Design of Sinter- Resistant Heterogeneous Catalysts. ACS Catal. 2017, 7, 7156–7173. [Google Scholar] [CrossRef]
- Giannakakis, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Single-Atom Alloys as a Reductionist Approach to the Rational Design of Heterogeneous Catalysts. Acc. Chem. Res. 2019, 52, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Zaera, F. Designing Sites in Heterogeneous Catalysis: Are We Reaching Selectivities Competitive with Those of Homogeneous Catalysts? Chem. Rev. 2022, 122, 8594–8757. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Peng, M.; Chen, Y.; Cai, X.; Qin, X.; Wang, N.; Xiao, D.; Jin, L.; Wang, G.; Wen, X.D.; et al. Low-Temperature Acetylene Semi-Hydrogenation over the Pd1-Cu1 Dual-Atom Catalyst. J. Am. Chem. Soc. 2022, 144, 18485–18493. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, E.; Stoltze, P.; Norskov, J.K. Monte Carlo Simulations of Adsorption-Induced Segregation. Surf. Sci. 2002, 505, 200–214. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Role of Strain and Ligand Effects in the Modification of the Electronic and Chemical Properties of Bimetallic Surfaces. Phys. Rev. Lett. 2004, 93, 156801. [Google Scholar] [CrossRef]
- Zafeiratos, S.; Piccinin, S.; Teschner, D. Alloys in Catalysis: Phase Separation and Surface Segregation Phenomena in Response to the Reactive Environment. Catal. Sci. Technol. 2012, 2, 1787–1801. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, S.; Nguyen, L.; Zhang, X. Action of Bimetallic Nanocatalysts under Reaction Conditions and during Catalysis: Evolution of Chemistry from High Vacuum Conditions to Reaction Conditions. Chem. Soc. Rev. 2012, 41, 7980–7993. [Google Scholar] [CrossRef]
- Gao, F.; Goodman, D.W. Pd–Au Bimetallic Catalysts: Understanding Alloy Effects from Planar Models and (Supported) Nanoparticles. Chem. Soc. Rev. 2012, 41, 8009–8020. [Google Scholar] [CrossRef]
- Ellert, O.G.; Tsodikov, M.V.; Nikolaev, S.A.; Novotortsev, V.M. Bimetallic Nanoalloys in Heterogeneous Catalysis of Industrially Important Reactions: Synergistic Effects and Structural Organization of Active Components. Russ. Chem. Rev. 2014, 83, 718–732. [Google Scholar] [CrossRef]
- Rupprechter, G. Sum Frequency Generation and Polarization-Modulation Infrared Reflection Absorption Spectroscopy of Functioning Model Catalysts from Ultrahigh Vacuum to Ambient Pressure. Adv. Catal. 2007, 51, 133–263. [Google Scholar] [CrossRef]
- Yao, Y.; Fu, Q.; Zhang, Z.; Zhang, H.; Ma, T.; Tan, D.; Bao, X. Structure Control of Pt-Sn Bimetallic Catalysts Supported on Highly Oriented Pyrolytic Graphite (HOPG). Appl. Surf. Sci. 2008, 254, 3808–3812. [Google Scholar] [CrossRef]
- Demidov, D.V.; Prosvirin, I.P.; Sorokin, A.M.; Bukhtiyarov, V.I. Model Ag/HOPG Catalysts: Preparation and STM/XPS Study. Catal. Sci. Technol. 2011, 1, 1432–1439. [Google Scholar] [CrossRef]
- Panafidin, M.A.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Chetyrin, I.A.; Bukhtiyarov, V.I. Model Bimetallic Pd–Ag/HOPG Catalysts: An XPS and STM Study. Kinet. Catal. 2018, 59, 776–785. [Google Scholar] [CrossRef]
- Kettner, M.; Stumm, C.; Schwarz, M.; Schuschke, C.; Libuda, J. Pd Model Catalysts on Clean and Modified HOPG: Growth, Adsorption Properties, and Stability. Surf. Sci. 2019, 679, 64–73. [Google Scholar] [CrossRef]
- Motin, M.A.; Steiger-Thirsfeld, A.; Stöger-Pollach, M.; Rupprechter, G. Model Catalysis with HOPG-Supported Pd Nanoparticles and Pd Foil: XPS, STM and C2H4 Hydrogenation. Catal. Lett. 2022, 152, 2892–2907. [Google Scholar] [CrossRef]
- Fedorov, A.Y.; Bukhtiyarov, A.V.; Panafidin, M.A.; Prosvirin, I.P.; Zubavichus, Y.V.; Bukhtiyarov, V.I. Alloying Bulk-Immiscible Metals at the Nanoscale: An XPS/STM Study of Bimetallic Ag-Pt/HOPG Nanoparticles. Appl. Surf. Sci. 2023, 636, 157872. [Google Scholar] [CrossRef]
- Khan, N.A.; Uhl, A.; Shaikhutdinov, S.; Freund, H.J. Alumina Supported Model Pd-Ag Catalysts: A Combined STM, XPS, TPD and IRAS Study. Surf. Sci. 2006, 600, 1849–1853. [Google Scholar] [CrossRef]
- Chen, L.; Yelon, A.; Sacher, E. Formation of FePt Alloy Nanoparticles on Highly Oriented Pyrolytic Graphite: A Morphological and in Situ X-ray Photoelectron Spectroscopic Study. J. Phys. Chem. C 2012, 116, 6902–6912. [Google Scholar] [CrossRef]
- Fedorov, A.Y.; Bukhtiyarov, A.V.; Panafidin, M.A.; Prosvirin, I.P.; Chetyrin, I.A.; Smirnova, N.S.; Markov, P.V.; Zubavichus, Y.V.; Stakheev, A.Y.; Bukhtiyarov, V.I. The Effect of CO Treatment on the Surface Structure of Bimetallic Pd-Au/HOPG and Pd-In/HOPG Nanoparticles: A Comparative Study. Nano-Struct. Nano-Objects 2022, 29, 100830. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble Metal-Based Bimetallic Nanoparticles: The Effect of the Structure on the Optical, Catalytic and Photocatalytic Properties. Adv. Colloid. Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef] [PubMed]
- Wautelet, M.; Dauchot, J.P.; Hecq, M. Phase Diagrams of Small Particles of Binary Systems: A Theoretical Approach. Nanotechnology 2000, 11, 6–9. [Google Scholar] [CrossRef]
- Vallée, R.; Wautelet, M.; Dauchot, J.P.; Hecq, M. Size and Segregation Effects on the Phase Diagrams of Nanoparticles of Binary Systems. Nanotechnology 2001, 12, 68–74. [Google Scholar] [CrossRef]
- Krishnan, G.; Verheijen, M.A.; Ten Brink, G.H.; Palasantzas, G.; Kooi, B.J. Tuning Structural Motifs and Alloying of Bulk Immiscible Mo-Cu Bimetallic Nanoparticles by Gas-Phase Synthesis. Nanoscale 2013, 5, 5375–5383. [Google Scholar] [CrossRef]
- Oumellal, Y.; Provost, K.; Ghimbeu, C.M.; De Yuso, A.M.; Zlotea, C. Composition and Size Dependence of Hydrogen Interaction with Carbon Supported Bulk-Immiscible Pd-Rh Nanoalloys. Nanotechnology 2016, 27, 465401. [Google Scholar] [CrossRef]
- Okamoto, H.; Massalski, T.B. Binary Alloy Phase Diagrams; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Li, X.R.; Xu, M.C.; Chen, H.Y.; Xu, J.J. Bimetallic Au@Pt@Au Core-Shell Nanoparticles on Graphene Oxide Nanosheets for High-Performance H2O2 Bi-Directional Sensing. J. Mater. Chem. B 2015, 3, 4355–4362. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, D.; Gutierrez-Wing, C.; Miki-Yoshida, M.; Jose-Yacaman, M. HAADF Study of Au-Pt Core-Shell Bimetallic Nanoparticles. Appl. Phys. A Mater. Sci. Process 2004, 79, 481–487. [Google Scholar] [CrossRef]
- Ataee-Esfahani, H.; Wang, L.; Nemoto, Y.; Yamauchi, Y. Synthesis of Bimetallic Au@Pt Nanoparticles with Au Core and Nanostructured Pt Shell toward Highly Active Electrocatalysts. Chem. Mater. 2010, 22, 6310–6318. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Wang, X.; Ji, H.; Zhao, C.; Wang, Y.; Zhang, Z. Fabrication of Bi-Modal Nanoporous Bimetallic Pt–Au Alloy with Excellent Electrocatalytic Performance towards Formic Acid Oxidation. Green Chem. 2011, 13, 1914–1922. [Google Scholar] [CrossRef]
- Dai, L.; Zhao, Y.; Chi, Q.; Liu, H.; Li, J.; Huang, T. Morphological Control and Evolution of Octahedral and Truncated Trisoctahedral Pt–Au Alloy Nanocrystals under Microwave Irradiation. Nanoscale 2014, 6, 9944–9950. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.B.; Zhao, T.S.; Yang, W.W.; Shen, S.Y. Effect of Surface Composition of Pt-Au Alloy Cathode Catalyst on the Performance of Direct Methanol Fuel Cells. Int. J. Hydrogen Energy 2010, 35, 8699–8706. [Google Scholar] [CrossRef]
- Higareda, A.; Kumar-Krishnan, S.; García-Ruiz, A.F.; Maya-Cornejo, J.; Lopez-Miranda, J.L.; Bahena, D.; Rosas, G.; Pérez, R.; Esparza, R. Synthesis of Au@Pt Core—Shell Nanoparticles as Efficient Electrocatalyst for Methanol Electro-Oxidation. Nanomaterials 2019, 9, 1644. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chen, S. Oxygen Reduction Electrocatalyst of Pt on Au Nanoparticles through Spontaneous Deposition. ACS Appl. Mater. Interfaces 2015, 7, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Lei, J.; Hu, Y.; Bi, W.; Xu, N.; Li, Y.; Chen, X.; Jiang, H.; Li, C. Electron Transfer Effect from Au to Pt in Au-Pt/TiO2 towards Efficient Catalytic Activity in CO Oxidation at Low Temperature. Appl. Surf. Sci. 2020, 521, 146447. [Google Scholar] [CrossRef]
- Xiong, S.; Qi, W.; Huang, B.; Wang, M. Size-, Shape- and Composition-Dependent Alloying Ability of Bimetallic Nanoparticles. ChemPhysChem 2011, 12, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Vega, L.; Aleksandrov, H.A.; Farris, R.; Bruix, A.; Viñes, F.; Neyman, K.M. Chemical Ordering in Pt-Au, Pt-Ag and Pt-Cu Nanoparticles from Density Functional Calculations Using a Topological Approach. Mater. Adv. 2021, 2, 6589–6602. [Google Scholar] [CrossRef]
- Tyson, W.R.; Miller, W.A. Surface Free Energies of Solid Metals: Estimation from Liquid Surface Tension Measurements. Surf. Sci. 1977, 62, 267–276. [Google Scholar] [CrossRef]
- XPSPeak Software. Available online: http://xpspeak.software.informer.com/4.1/ (accessed on 10 August 2023).
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Standard XPS Spectra of the Elements. In Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., King, R.C., Eds.; Perkin-Elmer: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Scofield, J.H. Hartree-Slater Subshell Photoionization Cross-Sections at 1254 and 1487 eV. J. Electron Spectros. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of Electron Inelastic Mean Free Paths. V. Data for 14 Organic Compounds over the 50–2000 eV Range. Surf. Interface Anal. 1994, 21, 165–176. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A Software for Scanning Probe Microscopy and a Tool for Nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Okunev, A.G.; Mashukov, M.Y.; Nartova, A.V.; Matveev, A.V. Nanoparticle Recognition on Scanning Probe Microscopy Images Using Computer Vision and Deep Learning. Nanomaterials 2020, 10, 1285. [Google Scholar] [CrossRef]
- Okunev, A.G.; Nartova, A.V.; Matveev, A.V. Recognition of Nanoparticles on Scanning Probe Microscopy Images Using Computer Vision and Deep Machine Learning. In Proceedings of the SIBIRCON 2019—International Multi-Conference on Engineering, Computer and Information Sciences, Novosibirsk, Russia, 21–27 October 2019; pp. 0940–0943. [Google Scholar]
- Web Service “ParticlesNN”. Available online: http://particlesnn.nsu.ru/ (accessed on 18 August 2023).
- Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I. XPS/STM Study of Model Bimetallic Pd-Au/HOPG Catalysts. Appl. Surf. Sci. 2016, 367, 214–221. [Google Scholar] [CrossRef]
- Smirnov, M.Y.; Kalinkin, A.V.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I. Using X-ray Photoelectron Spectroscopy to Evaluate Size of Metal Nanoparticles in the Model Au/C Samples. J. Phys. Chem. C 2016, 120, 10419–10426. [Google Scholar] [CrossRef]
- Motin, A.M.; Haunold, T.; Bukhtiyarov, A.V.; Bera, A.; Rameshan, C.; Rupprechter, G. Surface Science Approach to Pt/Carbon Model Catalysts: XPS, STM and Microreactor Studies. Appl. Surf. Sci. 2018, 440, 680–687. [Google Scholar] [CrossRef]
- Lim, D.C.; Lopez-Salido, I.; Dietsche, R.; Bubek, M.; Kim, Y.D. Oxidation of Au Nanoparticles on HOPG Using Atomic Oxygen. Surf. Sci. 2006, 600, 507–513. [Google Scholar] [CrossRef]
- Lopez-Salido, I.; Lim, D.C.; Dietsche, R.; Bertram, N.; Kim, Y.D. Electronic and Geometric Properties of Au Nanoparticles on Highly Ordered Pyrolytic Graphite (HOPG) Studied Using X-ray Photoelectron Spectroscopy (XPS) and Scanning Tunneling Microscopy (STM). J. Phys. Chem. B 2006, 110, 1128–1136. [Google Scholar] [CrossRef]
- Wang, D.; Cui, X.; Xiao, Q.; Hu, Y.; Wang, Z.; Yiu, Y.M.; Sham, T.K. Electronic Behaviour of Au-Pt Alloys and the 4f Binding Energy Shift Anomaly in Au Bimetallics- X-ray Spectroscopy Studies. AIP Adv. 2018, 8, 65210. [Google Scholar] [CrossRef]
- Wolter, S.D.; Brown, B.; Parker, C.B.; Stoner, B.R.; Glass, J.T. The Effect of Gold on Platinum Oxidation in Homogeneous Au-Pt Electrocatalysts. Appl. Surf. Sci. 2010, 257, 1431–1436. [Google Scholar] [CrossRef]
- Plšek, J.; Janda, P.; Bastl, Z. Preparation of Au-Pt Nanostructures on Highly Oriented Pyrolytic Graphite Surfaces by Pulsed Laser Deposition and Their Characterization by XPS and AFM Methods. Collect. Czechoslov. Chem. Commun. 2008, 73, 1299–1313. [Google Scholar] [CrossRef]
- Zhou, W.; Li, M.; Zhang, L.; Chan, S.H. Supported PtAu Catalysts with Different Nano-Structures for Ethanol Electrooxidation. Electrochim. Acta 2014, 123, 233–239. [Google Scholar] [CrossRef]
- Mason, M.G. Electronic Structure of Supported Small Metal Clusters. Phys. Rev. B 1983, 27, 748. [Google Scholar] [CrossRef]
- Yang, D.Q.; Zhang, G.X.; Sacher, E.; José-Yacamán, M.; Elizondo, N. Evidence of the Interaction of Evaporated Pt Nanoparticles with Variously Treated Surfaces of Highly Oriented Pyrolytic Graphite. J. Phys. Chem. B 2006, 110, 8348–8356. [Google Scholar] [CrossRef]
- Smirnov, M.Y.; Kalinkin, A.V.; Bukhtiyarov, V.I. XPS Analysis of the Spacial Distribution of Metals in Au-Ag Bimetallic Particles Considering the Effect of Particle Size Distribution. J. Struct. Chem. 2020, 61, 20–28. [Google Scholar] [CrossRef]
- O’Connor, C.R.; Van Spronsen, M.A.; Karatok, M.; Boscoboinik, J.; Friend, C.M.; Montemore, M.M. Predicting X-ray Photoelectron Peak Shapes: The Effect of Electronic Structure. J. Phys. Chem. C 2021, 125, 10685–10692. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, L. Structures of Platinum Clusters: Planar or Spherical. J. Phys. Chem. A 2004, 108, 8605–8614. [Google Scholar] [CrossRef]
- Sacher, E. Asymmetries in Transition Metal XPS Spectra: Metal Nanoparticle Structure, and Interaction with the Graphene-Structured Substrate Surface. Langmuir 2010, 26, 3807–3814. [Google Scholar] [CrossRef]
- Shevchik, N.J. Local Density of States and Core-Hole Conduction-Electron Interactions in the X-ray Photoemission Spectra of Pt and Ni. Phys. Rev. Lett. 1974, 33, 1336–1339. [Google Scholar] [CrossRef]
- Smirnov, M.Y.; Kalinkin, A.V.; Vovk, E.I.; Simonov, P.A.; Gerasimov, E.Y.; Sorokin, A.M.; Bukhtiyarov, V.I. Comparative XPS Study of Interaction of Model and Real Pt/C Catalysts with NO2. Appl. Surf. Sci. 2018, 428, 972–976. [Google Scholar] [CrossRef]
- Isaifan, R.J.; Ntais, S.; Baranova, E.A. Particle Size Effect on Catalytic Activity of Carbon-Supported Pt Nanoparticles for Complete Ethylene Oxidation. Appl. Catal. A Gen. 2013, 464–465, 87–94. [Google Scholar] [CrossRef]
- Şen, F.; Gökaǧaç, G. Different Sized Platinum Nanoparticles Supported on Carbon: An XPS Study on These Methanol Oxidation Catalysts. J. Phys. Chem. C 2007, 111, 5715–5720. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, D.; Sacher, E. X-ray Photoelectron Spectroscopic Analysis of Pt Nanoparticles on Highly Oriented Pyrolytic Graphite, Using Symmetric Component Line Shapes. J. Phys. Chem. C 2007, 111, 565–570. [Google Scholar] [CrossRef]
- Smirnov, M.Y.; Kalinkin, A.V.; Bukhtiyarov, V.I. Using XPS data for determining spatial distribution of metals in bimetallic particles supported on a flat surface. J. Struct. Chem. 2018, 59, 1849–1857. [Google Scholar] [CrossRef]

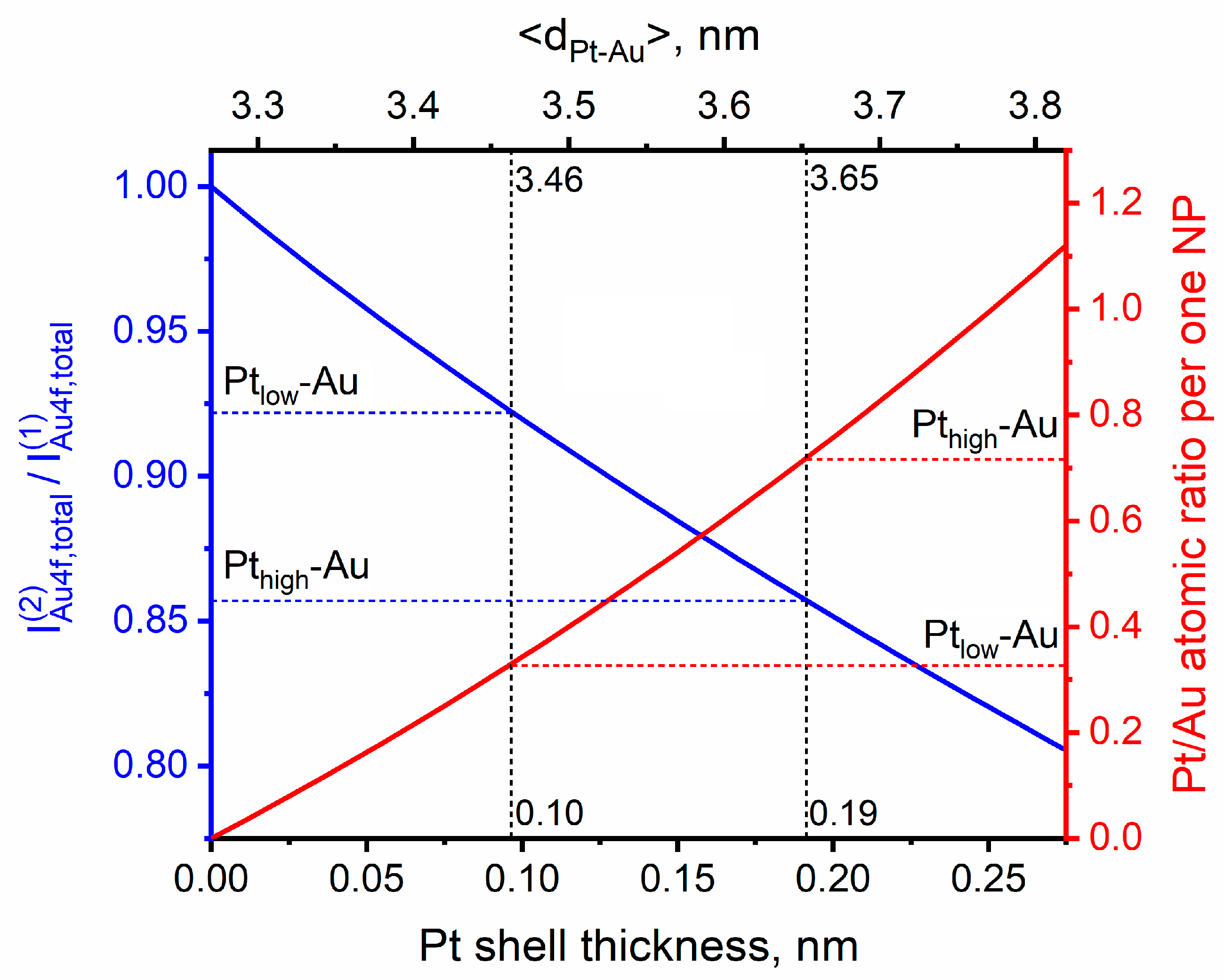
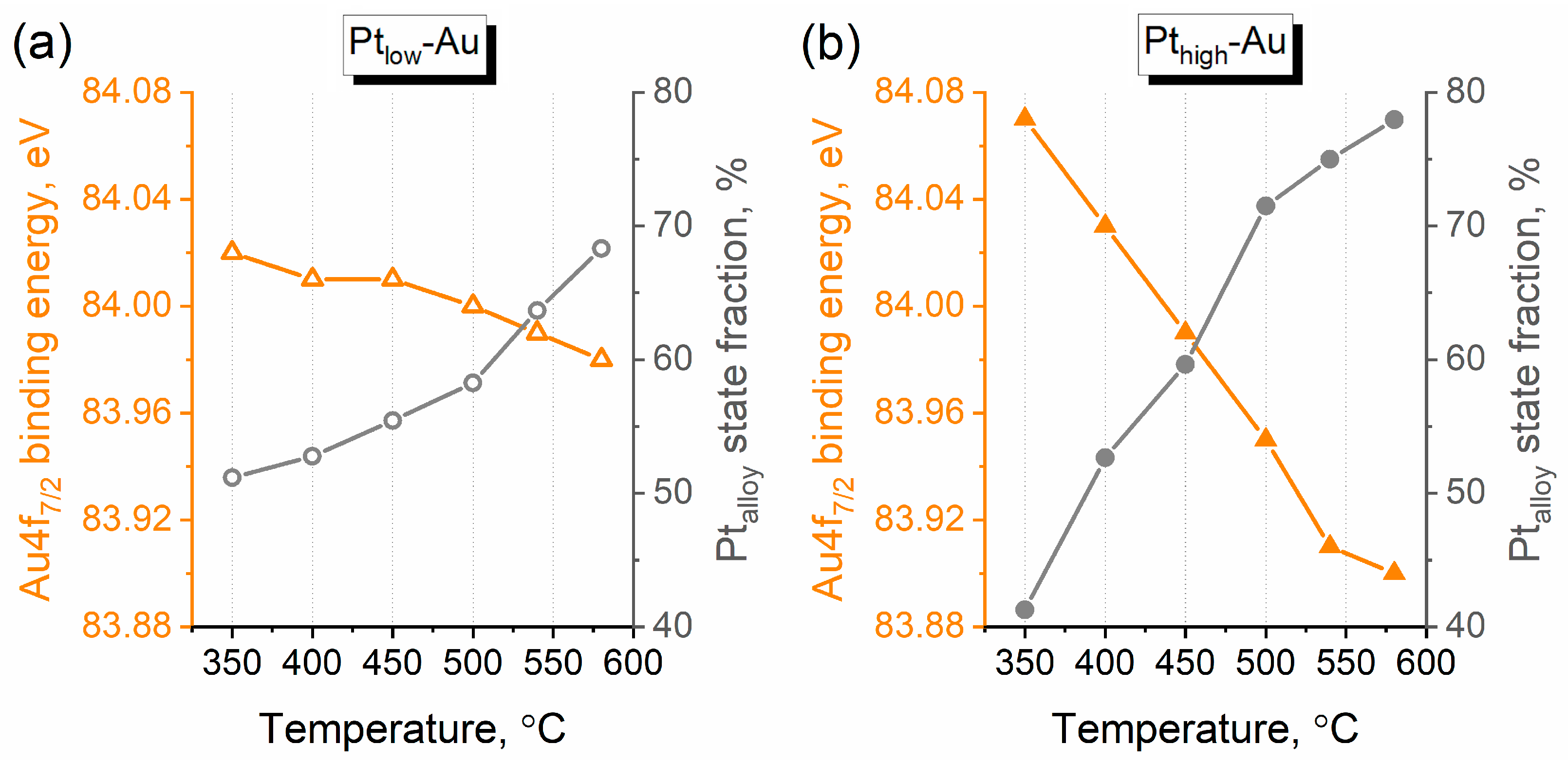
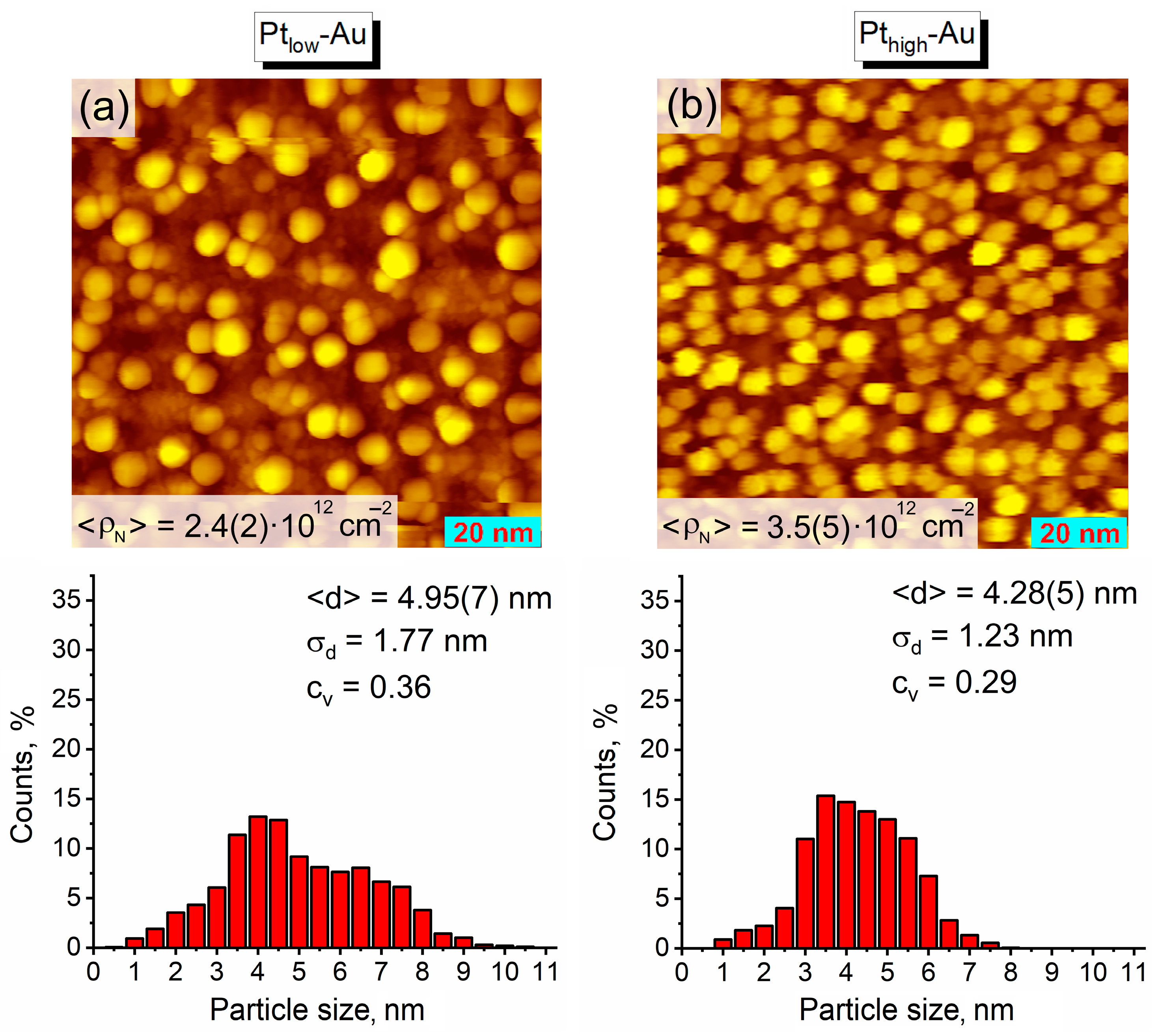
| Sample | Au/C | Pt/C | Pt/Au | |
|---|---|---|---|---|
| Ptlow-Au | 0.008 | 0.005 | 0.62 | 0.92 |
| Pthigh-Au | 0.008 | 0.014 | 1.8 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorov, A.Y.; Bukhtiyarov, A.V.; Panafidin, M.A.; Prosvirin, I.P.; Zubavichus, Y.V.; Bukhtiyarov, V.I. Thermally Induced Surface Structure and Morphology Evolution in Bimetallic Pt-Au/HOPG Nanoparticles as Probed Using XPS and STM. Nanomaterials 2024, 14, 57. https://doi.org/10.3390/nano14010057
Fedorov AY, Bukhtiyarov AV, Panafidin MA, Prosvirin IP, Zubavichus YV, Bukhtiyarov VI. Thermally Induced Surface Structure and Morphology Evolution in Bimetallic Pt-Au/HOPG Nanoparticles as Probed Using XPS and STM. Nanomaterials. 2024; 14(1):57. https://doi.org/10.3390/nano14010057
Chicago/Turabian StyleFedorov, Alexey Yu., Andrey V. Bukhtiyarov, Maxim A. Panafidin, Igor P. Prosvirin, Yan V. Zubavichus, and Valerii I. Bukhtiyarov. 2024. "Thermally Induced Surface Structure and Morphology Evolution in Bimetallic Pt-Au/HOPG Nanoparticles as Probed Using XPS and STM" Nanomaterials 14, no. 1: 57. https://doi.org/10.3390/nano14010057








