Propylene Production via Oxidative Dehydrogenation of Propane with Carbon Dioxide over Composite MxOy-TiO2 Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Synthesis and Characterization
2.2. In Situ FTIR Spectroscopy under Reaction Conditions
2.3. Catalytic Performance Experiments
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Adsorption/Desorption Characteristics of CO2 by In Situ DRIFTS
3.3. Temperature-Programmed Desorption of CO2
3.4. Effect of the Nature of MxOy Additive on Catalytic Performance
3.5. Time-On-Stream (TOS) Stability Test
3.6. Oxidative Dehydrogenation of Propane with CO2 Studied by In Situ DRIFTS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New Trends in Olefin Production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Zhang, Y. Mesoporous silica-supported chromium catalyst: Characterization and excellent performance in dehydrogenation of propane to propylene with carbon dioxide. Catal. Commun. 2007, 8, 565–570. [Google Scholar] [CrossRef]
- Tóth, A.; Halasi, G.; Bánsági, T.; Solymosi, F. Reactions of propane with CO2 over Au catalysts. J. Catal. 2016, 337, 57–64. [Google Scholar] [CrossRef]
- Atanga, M.A.; Rezaei, F.; Jawad, A.; Fitch, M.; Rownaghi, A.A. Oxidative dehydrogenation of propane to propylene with carbon dioxide. Appl. Catal. B Environ. 2018, 220, 429–445. [Google Scholar] [CrossRef]
- Michorczyk, P.; Kuśtrowski, P.; Kolak, A.; Zimowska, M. Ordered mesoporous Ga2O3 and Ga2O3–Al2O3 prepared by nanocasting as effective catalysts for propane dehydrogenation in the presence of CO2. Catal. Commun. 2013, 35, 95–100. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Niemczyk, M. Investigation of catalytic activity of CrSBA-1 materials obtained by direct method in the dehydrogenation of propane with CO2. Appl. Catal. A Gen. 2010, 374, 142–149. [Google Scholar] [CrossRef]
- Solymosi, F.; Tolmacsov, P. Decomposition of Propane and Its Reactions with CO2 Over Alumina-Supported Pt Metals. Catal. Lett. 2002, 83, 183–186. [Google Scholar] [CrossRef]
- Igonina, M.; Tedeeva, M.; Kalmykov, K.; Kapustin, G.; Nissenbaum, V.; Mishin, I.; Pribytkov, P.; Dunaev, S.; Kustov, L.; Kustov, A. Properties of CrOx/MCM-41 and Its Catalytic Activity in the Reaction of Propane Dehydrogenation in the Presence of CO2. Catalysts 2023, 13, 906. [Google Scholar] [CrossRef]
- Mukherjee, D.; Park, S.-E.; Reddy, B.M. CO2 as a soft oxidant for oxidative dehydrogenation reaction: An eco benign process for industry. J. CO2 Util. 2016, 16, 301–312. [Google Scholar] [CrossRef]
- Barzegari, F.; Kazemeini, M.; Rezaei, M.; Farhadi, F.; Keshavarz, A.R. Syngas production through CO2 reforming of propane over highly active and stable mesoporous NiO-MgO-SiO2 catalysts: Effect of calcination temperature. Fuel 2022, 322, 124211. [Google Scholar] [CrossRef]
- Alabdullah, M.; Ibrahim, M.; Dhawale, D.; Bau, J.A.; Harale, A.; Katikaneni, S.; Gascon, J. Rhodium Nanoparticle Size Effects on the CO2 Reforming of Methane and Propane. ChemCatChem 2021, 13, 2879–2886. [Google Scholar] [CrossRef]
- Panagiotopoulou, P. Hydrogenation of CO2 over supported noble metal catalysts. Appl. Catal. A Gen. 2017, 542, 63–70. [Google Scholar] [CrossRef]
- Krylov, O.V.; Mamedov, A.K.; Mirzabekova, S.R. The regularities in the interaction of alkanes with CO2 on oxide catalysts. Catal. Today 1995, 24, 371–375. [Google Scholar] [CrossRef]
- Kocoń, M.; Michorczyk, P.; Ogonowski, J. Effect of supports on catalytic activity of chromium oxide-based catalysts in the dehydrogenation of propane with CO2. Catal. Lett. 2005, 101, 53–57. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, B.; Hua, W.; Yue, Y.; Gao, Z. Support effect in dehydrogenation of propane in the presence of CO2 over supported gallium oxide catalysts. J. Catal. 2006, 239, 470–477. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, Y.; Gao, Z. Chromium Oxide Supported on Mesoporous SBA-15 as Propane Dehydrogenation and Oxidative Dehydrogenation Catalysts. Catal. Lett. 2002, 83, 19–25. [Google Scholar] [CrossRef]
- Takahara, I.; Saito, M.; Inaba, M.; Murata, K. Dehydrogenation of propane over a silica-supported vanadium oxide catalyst. Catal. Lett. 2005, 102, 201–205. [Google Scholar] [CrossRef]
- Zheng, B.; Hua, W.; Yue, Y.; Gao, Z. Dehydrogenation of propane to propene over different polymorphs of gallium oxide. J. Catal. 2005, 232, 143–151. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Su, F.-Z.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Fan, K.-N. Dehydrogenation of propane over spinel-type gallia–alumina solid solution catalysts. J. Catal. 2008, 256, 293–300. [Google Scholar] [CrossRef]
- Li, H.; Yue, Y.; Miao, C.; Xie, Z.; Hua, W.; Gao, Z. Dehydrogenation of ethylbenzene and propane over Ga2O3–ZrO2 catalysts in the presence of CO2. Catal. Commun. 2007, 8, 1317–1322. [Google Scholar] [CrossRef]
- Nowicka, E.; Reece, C.; Althahban, S.M.; Mohammed, K.M.H.; Kondrat, S.A.; Morgan, D.J.; He, Q.; Willock, D.J.; Golunski, S.; Kiely, C.J.; et al. Elucidating the Role of CO2 in the Soft Oxidative Dehydrogenation of Propane over Ceria-Based Catalysts. ACS Catal. 2018, 8, 3454–3468. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kajita, C.; Okumura, K.; Ikenaga, N.-O.; Nishitani-Gamo, M.; Ando, T.; Kobayashi, T.; Suzuki, T. Role of Carbon Dioxide in the Dehydrogenation of Ethane over Gallium-Loaded Catalysts. J. Catal. 2001, 203, 87–93. [Google Scholar] [CrossRef]
- Takehira, K.; Ohishi, Y.; Shishido, T.; Kawabata, T.; Takaki, K.; Zhang, Q.; Wang, Y. Behavior of active sites on Cr-MCM-41 catalysts during the dehydrogenation of propane with CO2. J. Catal. 2004, 224, 404–416. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I. Effect of morphological characteristics of TiO2-supported noble metal catalysts on their activity for the water–gas shift reaction. J. Catal. 2004, 225, 327–336. [Google Scholar] [CrossRef]
- Kokka, A.; Ramantani, T.; Yentekakis, I.V.; Panagiotopoulou, P. Catalytic performance and in situ DRIFTS studies of propane and simulated LPG steam reforming reactions on Rh nanoparticles dispersed on composite MxOy-Al2O3 (M: Ti, Y, Zr, La, Ce, Nd, Gd) supports. Appl. Catal. B Environ. 2022, 316, 121668. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, M.-L.; Song, Y.-H.; Liu, Z.-T.; Wang, L.; Liu, Z.-W. Molecular-level investigation on supported CrOx catalyst for oxidative dehydrogenation of propane with carbon dioxide. J. Catal. 2022, 409, 87–97. [Google Scholar] [CrossRef]
- Zhou, W.; Jiang, Y.; Sun, Z.; Zhou, S.; Xing, E.; Hai, Y.; Chen, G.; Zhao, Y. Support Effect of Ga-Based Catalysts in the CO2-Assisted Oxidative Dehydrogenation of Propane. Catalysts 2023, 13, 896. [Google Scholar] [CrossRef]
- Jawad, A.; Ahmed, S. Analysis and process evaluation of metal dopant (Zr, Cr)-promoted Ga-modified ZSM-5 for the oxidative dehydrogenation of propane in the presence and absence of CO2. RSC Adv. 2023, 13, 11081–11095. [Google Scholar] [CrossRef]
- Gashoul Daresibi, F.; Khodadadi, A.A.; Mortazavi, Y. Atomic layer deposition of Ga2O3 on γ-Al2O3 catalysts with higher interactions and improved activity and propylene selectivity in CO2-assisted oxidative dehydrogenation of propane. Appl. Catal. A Gen. 2023, 655, 119117. [Google Scholar] [CrossRef]
- Shimizu, K.-i.; Takamatsu, M.; Nishi, K.; Yoshida, H.; Satsuma, A.; Tanaka, T.; Yoshida, S.; Hattori, T. Alumina-Supported Gallium Oxide Catalysts for NO Selective Reduction: Influence of the Local Structure of Surface Gallium Oxide Species on the Catalytic Activity. J. Phys. Chem. B 1999, 103, 1542–1549. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Schuttlefield, J.; Zeitler, E.; Grassian, V.H. Carbon dioxide adsorption on oxide nanoparticle surfaces. Chem. Eng. J. 2011, 170, 471–481. [Google Scholar] [CrossRef]
- Su, W.; Zhang, J.; Feng, Z.; Chen, T.; Ying, P.; Li, C. Surface Phases of TiO2 Nanoparticles Studied by UV Raman Spectroscopy and FT-IR Spectroscopy. J. Phys. Chem. C 2008, 112, 7710–7716. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, Q.; Kenarsari, S.D.; Hu, X.; Russell, A.G.; Fan, M.; Shen, X. A new mesoporous amine-TiO2 based pre-combustion CO2 capture technology. Appl. Energy 2015, 147, 214–223. [Google Scholar] [CrossRef]
- Nanayakkara, C.E.; Larish, W.A.; Grassian, V.H. Titanium Dioxide Nanoparticle Surface Reactivity with Atmospheric Gases, CO2, SO2, and NO2: Roles of Surface Hydroxyl Groups and Adsorbed Water in the Formation and Stability of Adsorbed Products. J. Phys. Chem. C 2014, 118, 23011–23021. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Li, Y. Spontaneous Dissociation of CO2 to CO on Defective Surface of Cu(I)/TiO2–x Nanoparticles at Room Temperature. J. Phys. Chem. C 2012, 116, 7904–7912. [Google Scholar] [CrossRef]
- Martra, G. Lewis acid and base sites at the surface of microcrystalline TiO2 anatase: Relationships between surface morphology and chemical behaviour. Appl. Catal. A Gen. 2000, 200, 275–285. [Google Scholar] [CrossRef]
- Jin, L.; Shaaban, E.; Bamonte, S.; Cintron, D.; Shuster, S.; Zhang, L.; Li, G.; He, J. Surface Basicity of Metal@TiO2 to Enhance Photocatalytic Efficiency for CO2 Reduction. ACS Appl. Mater. Interfaces 2021, 13, 38595–38603. [Google Scholar] [CrossRef] [PubMed]
- Mino, L.; Spoto, G.; Ferrari, A.M. CO2 Capture by TiO2 Anatase Surfaces: A Combined DFT and FTIR Study. J. Phys. Chem. C 2014, 118, 25016–25026. [Google Scholar] [CrossRef]
- Wang, K.; Cao, M.; Lu, J.; Lu, Y.; Lau, C.H.; Zheng, Y.; Fan, X. Operando DRIFTS-MS investigation on plasmon-thermal coupling mechanism of CO2 hydrogenation on Au/TiO2: The enhanced generation of oxygen vacancies. Appl. Catal. B Environ. 2021, 296, 120341. [Google Scholar] [CrossRef]
- Manríquez, M.E.; López, T.; Gómez, R.; Navarrete, J. Preparation of TiO2–ZrO2 mixed oxides with controlled acid–basic properties. J. Mol. Catal. A Chem. 2004, 220, 229–237. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I. Effects of alkali additives on the physicochemical characteristics and chemisorptive properties of Pt/TiO2 catalysts. J. Catal. 2008, 260, 141–149. [Google Scholar] [CrossRef]
- Androulakis, A.; Yentekakis, I.V.; Panagiotopoulou, P. Dry reforming of methane over supported Rh and Ru catalysts: Effect of the support (Al2O3, TiO2, ZrO2, YSZ) on the activity and reaction pathway. Int. J. Hydrogen Energy 2023, 48, 33886–33902. [Google Scholar] [CrossRef]
- Pokrovski, K.; Jung, K.T.; Bell, A.T. Investigation of CO and CO2 Adsorption on Tetragonal and Monoclinic Zirconia. Langmuir 2001, 17, 4297–4303. [Google Scholar] [CrossRef]
- Dobson, K.D.; McQuillan, A.J. An Infrared Spectroscopic Study of Carbonate Adsorption to Zirconium Dioxide Sol−Gel Films from Aqueous Solutions. Langmuir 1997, 13, 3392–3396. [Google Scholar] [CrossRef]
- Ouyang, F.; Nakayama, A.; Tabada, K.; Suzuki, E. Infrared Study of a Novel Acid−Base Site on ZrO2 by Adsorbed Probe Molecules. I. Pyridine, Carbon Dioxide, and Formic Acid Adsorption. J. Phys. Chem. B 2000, 104, 2012–2018. [Google Scholar] [CrossRef]
- Zecchina, A.; Coluccia, S.; Guglielminotti, E.; Ghiotti, G. Infrared study of surface properties of.alpha.-chromia. III. Adsorption of carbon dioxide. J. Phys. Chem. 1971, 75, 2790–2798. [Google Scholar] [CrossRef]
- Bensalem, A.; Weckhuysen, B.M.; Schoonheydt, R.A. Nature of adsorbed species during the reduction of CrO3/SiO2with CO In situ FTIR spectroscopic study. J. Chem. Soc. Faraday Trans. 1997, 93, 4065–4069. [Google Scholar] [CrossRef]
- Li, M.; Tumuluri, U.; Wu, Z.; Dai, S. Effect of Dopants on the Adsorption of Carbon Dioxide on Ceria Surfaces. ChemSusChem 2015, 8, 3651–3660. [Google Scholar] [CrossRef]
- Wu, Z.; Mann, A.K.P.; Li, M.; Overbury, S.H. Spectroscopic Investigation of Surface-Dependent Acid–Base Property of Ceria Nanoshapes. J. Phys. Chem. C 2015, 119, 7340–7350. [Google Scholar] [CrossRef]
- Binet, C.; Daturi, M.; Lavalley, J.-C. IR study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, T.; Li, Y.; Li, X.; Yin, J.; Wang, C. CO2 photoreduction with H2O vapor on highly dispersed CeO2/TiO2 catalysts: Surface species and their reactivity. J. Catal. 2016, 337, 293–302. [Google Scholar] [CrossRef]
- Agarwal, S.; Zhu, X.; Hensen, E.J.M.; Lefferts, L.; Mojet, B.L. Defect Chemistry of Ceria Nanorods. J. Phys. Chem. C 2014, 118, 4131–4142. [Google Scholar] [CrossRef]
- Pan, Y.-x.; Liu, C.-j.; Mei, D.; Ge, Q. Effects of Hydration and Oxygen Vacancy on CO2 Adsorption and Activation on β-Ga2O3(100). Langmuir 2010, 26, 5551–5558. [Google Scholar] [CrossRef] [PubMed]
- Vimont, A.; Lavalley, J.C.; Sahibed-Dine, A.; Otero Areán, C.; Rodríguez Delgado, M.; Daturi, M. Infrared Spectroscopic Study on the Surface Properties of γ-Gallium Oxide as Compared to Those of γ-Alumina. J. Phys. Chem. B 2005, 109, 9656–9664. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.E.; Baltanás, M.A.; Bonivardi, A.L. Infrared Spectroscopic Study of the Carbon Dioxide Adsorption on the Surface of Ga2O3 Polymorphs. J. Phys. Chem. B 2006, 110, 5498–5507. [Google Scholar] [CrossRef]
- Philipp, R.; Fujimoto, K. FTIR spectroscopic study of carbon dioxide adsorption/desorption on magnesia/calcium oxide catalysts. J. Phys. Chem. 1992, 96, 9035–9038. [Google Scholar] [CrossRef]
- Philipp, R.; Omata, K.; Aoki, A.; Fujimoto, K. On the active site of MgO/CaO mixed oxide for oxidative coupling of methane. J. Catal. 1992, 134, 422–433. [Google Scholar] [CrossRef]
- Zaki, M.I.; Knözinger, H.; Tesche, B.; Mekhemer, G.A.H. Influence of phosphonation and phosphation on surface acid–base and morphological properties of CaO as investigated by in situ FTIR spectroscopy and electron microscopy. J. Colloid Interface Sci. 2006, 303, 9–17. [Google Scholar] [CrossRef]
- Constantinou, D.A.; Fierro, J.L.G.; Efstathiou, A.M. A comparative study of the steam reforming of phenol towards H2 production over natural calcite, dolomite and olivine materials. Appl. Catal. B Environ. 2010, 95, 255–269. [Google Scholar] [CrossRef]
- Constantinou, D.A.; Fierro, J.L.G.; Efstathiou, A.M. The phenol steam reforming reaction towards H2 production on natural calcite. Appl. Catal. B Environ. 2009, 90, 347–359. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G.; Jiang, X.; Liu, Y.; Zhu, J.; Ding, F.; Liu, Z.; Guo, X.; Song, C. CO2 Hydrogenation on Unpromoted and M-Promoted Co/TiO2 Catalysts (M = Zr, K, Cs): Effects of Crystal Phase of Supports and Metal–Support Interaction on Tuning Product Distribution. ACS Catal. 2019, 9, 2739–2751. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Zhang, M.; Li, G.; Chen, J.; Jia, H. Suppressive Strong Metal-Support Interactions on Ruthenium/TiO2 Promote Light-Driven Photothermal CO2 Reduction with Methane. Angew. Chem. Int. Ed. 2023, 62, e202300129. [Google Scholar] [CrossRef] [PubMed]
- Al-Shafei, E.; Aljishi, M.; Albahar, M.; Alahmed, A.; Sanhoob, M. Effect of CO2/propane ratio and trimetallic oxide catalysts on maximizing dry reforming of propane. Mol. Catal. 2023, 537, 112945. [Google Scholar] [CrossRef]
- Bermejo-López, A.; Pereda-Ayo, B.; Onrubia-Calvo, J.A.; González-Marcos, J.A.; González-Velasco, J.R. Tuning basicity of dual function materials widens operation temperature window for efficient CO2 adsorption and hydrogenation to CH4. J. CO2 Util. 2022, 58, 101922. [Google Scholar] [CrossRef]
- Leino, E.; Kumar, N.; Mäki-Arvela, P.; Rautio, A.-R.; Dahl, J.; Roine, J.; Mikkola, J.-P. Synthesis and characterization of ceria-supported catalysts for carbon dioxide transformation to diethyl carbonate. Catal. Today 2018, 306, 128–137. [Google Scholar] [CrossRef]
- Makdee, A.; Kidkhunthod, P.; Poo-arporn, Y.; Chanapattharapol, K.C. Enhanced CH4 selectivity for CO2 methanation over Ni-TiO2 by addition of Zr promoter. J. Environ. Chem. Eng. 2022, 10, 107710. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, K.; Choudary, N.V.; Pant, K.K. Effect of support materials on the performance of Ni-based catalysts in tri-reforming of methane. Fuel Process. Technol. 2019, 186, 40–52. [Google Scholar] [CrossRef]
- Nguyen Thanh, D.; Kikhtyanin, O.; Ramos, R.; Kothari, M.; Ulbrich, P.; Munshi, T.; Kubička, D. Nanosized TiO2—A promising catalyst for the aldol condensation of furfural with acetone in biomass upgrading. Catal. Today 2016, 277, 97–107. [Google Scholar] [CrossRef]
- Zhong, C.; Guo, X.; Mao, D.; Wang, S.; Wu, G.; Lu, G. Effects of alkaline-earth oxides on the performance of a CuO–ZrO2 catalyst for methanol synthesis via CO2 hydrogenation. RSC Adv. 2015, 5, 52958–52965. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; He, Z.-H.; Li, L.-Y.; Yang, S.-Y.; He, M.-X.; Sun, Y.-C.; Wang, K.; Chen, J.-G.; Liu, Z.-T. Research progress of CO2 oxidative dehydrogenation of propane to propylene over Cr-free metal catalysts. Rare Met. 2022, 41, 2129–2152. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.; Wang, P.; Wang, X.; Pang, F.; Zhang, Z.; Tan, Y. Dehydrogenation of propane over a hydrothermal-synthesized Ga2O3–Al2O3 catalyst in the presence of carbon dioxide. Catal. Sci. Technol. 2016, 6, 5183–5195. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Kustov, A.L.; Pribytkov, P.V.; Kapustin, G.I.; Leonov, A.V.; Tkachenko, O.P.; Tursunov, O.B.; Evdokimenko, N.D.; Kustov, L.M. Dehydrogenation of propane in the presence of CO2 on GaOx/SiO2 catalyst: Influence of the texture characteristics of the support. Fuel 2022, 313, 122698. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Kustov, A.L.; Stolbov, D.N.; Tedeeva, M.A.; Isaikina, O.Y.; Maslakov, K.I.; Usol’tseva, N.V.; Savilov, S.V. Chromium catalysts supported on carbon nanotubes and graphene nanoflakes for CO2-assisted oxidative dehydrogenation of propane. Appl. Surf. Sci. 2022, 578, 152099. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.-H.; Liu, Z.-T.; Liu, Z.-W. Active and selective nature of supported CrOx for the oxidative dehydrogenation of propane with carbon dioxide. Appl. Catal. B Environ. 2021, 297, 120400. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Liu, Y.-M.; Cao, Y.; He, H.-Y.; Zhuang, J.-H.; Fan, K.-N. Enhanced Activity of Spinel-type Ga2O3–Al2O3 Mixed Oxide for the Dehydrogenation of Propane in the Presence of CO2. Catal. Lett. 2008, 124, 369–375. [Google Scholar] [CrossRef]
- Lavalley, J.C.; Daturi, M.; Montouillout, V.; Clet, G.; Otero Areán, C.; Rodríguez Delgado, M.; Sahibed-Dine, A. Unexpected similarities between the surface chemistry of cubic and hexagonal gallia polymorphs. Phys. Chem. Chem. Phys. 2003, 5, 1301–1305. [Google Scholar] [CrossRef]
- Santhosh Kumar, M.; Hammer, N.; Rønning, M.; Holmen, A.; Chen, D.; Walmsley, J.C.; Øye, G. The nature of active chromium species in Cr-catalysts for dehydrogenation of propane: New insights by a comprehensive spectroscopic study. J. Catal. 2009, 261, 116–128. [Google Scholar] [CrossRef]
- Wang, H.; Tsilomelekis, G. Catalytic performance and stability of Fe-doped CeO2 in propane oxidative dehydrogenation using carbon dioxide as an oxidant. Catal. Sci. Technol. 2020, 10, 4362–4372. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I. Effects of promotion of TiO2 with alkaline earth metals on the chemisorptive properties and water–gas shift activity of supported platinum catalysts. Appl. Catal. B Environ. 2011, 101, 738–746. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Kondarides, D.I. A comparative study of the water-gas shift activity of Pt catalysts supported on single (MOx) and composite (MOx/Al2O3, MOx/TiO2) metal oxide carriers. Catal. Today 2007, 127, 319–329. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V. Infrared spectroscopic identification of species arising from reactive adsorption of carbon oxides on metal oxide surfaces. Mater. Chem. 1982, 7, 89–126. [Google Scholar] [CrossRef]
- Das, D.; Mishra, H.K.; Parida, K.M.; Dalai, A.K. Preparation, physico-chemical characterization and catalytic activity of sulphated ZrO2–TiO2 mixed oxides. J. Mol. Catal. A Chem. 2002, 189, 271–282. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, L.; Long, H.; Li, J.; Tan, W.; Ji, J.; Sun, J.; Tang, C.; Dong, L. Influence of CeO2 loading on structure and catalytic activity for NH3-SCR over TiO2-supported CeO2. J. Rare Earths 2020, 38, 883–890. [Google Scholar] [CrossRef]
- De, S.; Ould-Chikh, S.; Aguilar, A.; Hazemann, J.-L.; Zitolo, A.; Ramirez, A.; Telalovic, S.; Gascon, J. Stable Cr-MFI Catalysts for the Nonoxidative Dehydrogenation of Ethane: Catalytic Performance and Nature of the Active Sites. ACS Catal. 2021, 11, 3988–3995. [Google Scholar] [CrossRef]
- Pratika, R.A.; Wijaya, K.; Trisunaryanti, W. Hydrothermal treatment of SO4/TiO2 and TiO2/CaO as heterogeneous catalysts for the conversion of Jatropha oil into biodiesel. J. Environ. Chem. Eng. 2021, 9, 106547. [Google Scholar] [CrossRef]
- Pradhan, G.; Sharma, Y.C. A greener and cheaper approach towards synthesis of glycerol carbonate from bio waste glycerol using CaO–TiO2 Nanocatalysts. J. Clean. Prod. 2021, 315, 127860. [Google Scholar] [CrossRef]
- Collado, L.; Reñones, P.; Fermoso, J.; Fresno, F.; Garrido, L.; Pérez-Dieste, V.; Escudero, C.; Hernández-Alonso, M.D.; Coronado, J.M.; Serrano, D.P.; et al. The role of the surface acidic/basic centers and redox sites on TiO2 in the photocatalytic CO2 reduction. Appl. Catal. B Environ. 2022, 303, 120931. [Google Scholar] [CrossRef]
- TA Instruments. Available online: https://www.tainstruments.com/pdf/literature/TA231.pdf (accessed on 12 May 1997).
- Burri, D.R.; Choi, K.M.; Han, S.C.; Burri, A.; Park, S.E. Dehydrogenation of ethylbenzene to styrene with CO2 over TiO2-ZrO2 bifunctional catalyst. Bull. Korean Chem. Soc. 2007, 28, 53–58. [Google Scholar] [CrossRef]
- Burri, A.; Jiang, N.; Yahyaoui, K.; Park, S.-E. Ethylbenzene to styrene over alkali doped TiO2-ZrO2 with CO2 as soft oxidant. Appl. Catal. A Gen. 2015, 495, 192–199. [Google Scholar] [CrossRef]
- Sui, K.; Yang, M.; Zhou, T.; Guan, Z.; Sun, R.; Han, D. Physico-chemical Properties of Nano Cr/Na-ZSM-5 Catalysts for Ethane Dehydrogenation. Integr. Ferroelectr. 2014, 154, 57–63. [Google Scholar] [CrossRef]
- Zhao, D.; Su, T.; Rodríguez-Padrón, D.; Lü, H.; Len, C.; Luque, R.; Yang, Z. Efficient transfer hydrogenation of alkyl levulinates to γ-valerolactone catalyzed by simple Zr–TiO2 metal oxide systems. Mater. Today Chem. 2022, 24, 100745. [Google Scholar] [CrossRef]
- Arena, F.; Giordano, N.; Parmaliana, A. Working Mechanism of Oxide Catalysts in the Partial Oxidation of Methane to Formaldehyde. II. Redox Properties and Reactivity of SiO2, MoO3/SiO2, V2O5/SiO2, TiO2, and V2O5/TiO2 Systems. J. Catal. 1997, 167, 66–76. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, Z.; Shan, W.; Shen, W.; Wang, J. Pd/CeO2–TiO2 catalyst for CO oxidation at low temperature: A TPR study with H2 and CO as reducing agents. J. Catal. 2004, 225, 267–277. [Google Scholar] [CrossRef]
- Song, H.; Zhang, M.; Yu, J.; Wu, W.; Qu, R.; Zheng, C.; Gao, X. The Effect of Cr Addition on Hg0 Oxidation and NO Reduction over V2O5/TiO2 Catalyst. Aerosol Air Qual. Res. 2018, 18, 803–810. [Google Scholar] [CrossRef]
- Karamullaoglu, G.; Dogu, T. Oxidative Dehydrogenation of Ethane over Chromium−Vanadium Mixed Oxide and Chromium Oxide Catalysts. Ind. Eng. Chem. Res. 2007, 46, 7079–7086. [Google Scholar] [CrossRef]
- Phan, T.N.; Kim, H.-S.; Kim, D.-H.; Ko, C.H. Mesoporous Titania as a Support of Gallium-Based Catalysts for Enhanced Ethane Dehydrogenation Performance. Catal. Lett. 2021, 151, 2748–2761. [Google Scholar] [CrossRef]
- Shao, C.-T.; Lang, W.-Z.; Yan, X.; Guo, Y.-J. Catalytic performance of gallium oxide based-catalysts for the propane dehydrogenation reaction: Effects of support and loading amount. RSC Adv. 2017, 7, 4710–4723. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Sooknoi, T.; Resasco, D.E. Catalytic deoxygenation of benzaldehyde over gallium-modified ZSM-5 zeolite. J. Catal. 2009, 268, 68–78. [Google Scholar] [CrossRef]
- Solymosi, F.; Tolmacsov, P.; Zakar, T.S. Dry reforming of propane over supported Re catalyst. J. Catal. 2005, 233, 51–59. [Google Scholar] [CrossRef]
- Boudjemaa, A.; Daniel, C.; Mirodatos, C.; Trari, M.; Auroux, A.; Bouarab, R. In situ DRIFTS studies of high-temperature water-gas shift reaction on chromium-free iron oxide catalysts. Comptes Rendus Chim. 2011, 14, 534–538. [Google Scholar] [CrossRef]
- Velasquez Ochoa, J.; Farci, E.; Cavani, F.; Sinisi, F.; Artiglia, L.; Agnoli, S.; Granozzi, G.; Paganini, M.C.; Malfatti, L. CeOx/TiO2 (Rutile) Nanocomposites for the Low-Temperature Dehydrogenation of Ethanol to Acetaldehyde: A Diffuse Reflectance Infrared Fourier Transform Spectroscopy–Mass Spectrometry Study. ACS Appl. Nano Mater. 2019, 2, 3434–3443. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Xu, J.; Li, Y. Integrated CO2 capture and photocatalytic conversion by a hybrid adsorbent/photocatalyst material. Appl. Catal. B Environ. 2015, 179, 489–499. [Google Scholar] [CrossRef]
- Savova, B.; Filkova, D.; Crişan, D.; Crişan, M.; Răileanu, M.; Drăgan, N.; Galtayries, A.; Védrine, J.C. Neodymium doped alkaline-earth oxide catalysts for propane oxidative dehydrogenation. Part I. Catalyst characterisation. Appl. Catal. A Gen. 2009, 359, 47–54. [Google Scholar] [CrossRef]
- Dou, J.; Funderburg, J.; Yang, K.; Liu, J.; Chacko, D.; Zhang, K.; Harvey, A.P.; Haribal, V.P.; Zhou, S.J.; Li, F. CexZr1–xO2-Supported CrOx Catalysts for CO2-Assisted Oxidative Dehydrogenation of Propane—Probing the Active Sites and Strategies for Enhanced Stability. ACS Catal. 2023, 13, 213–223. [Google Scholar] [CrossRef]
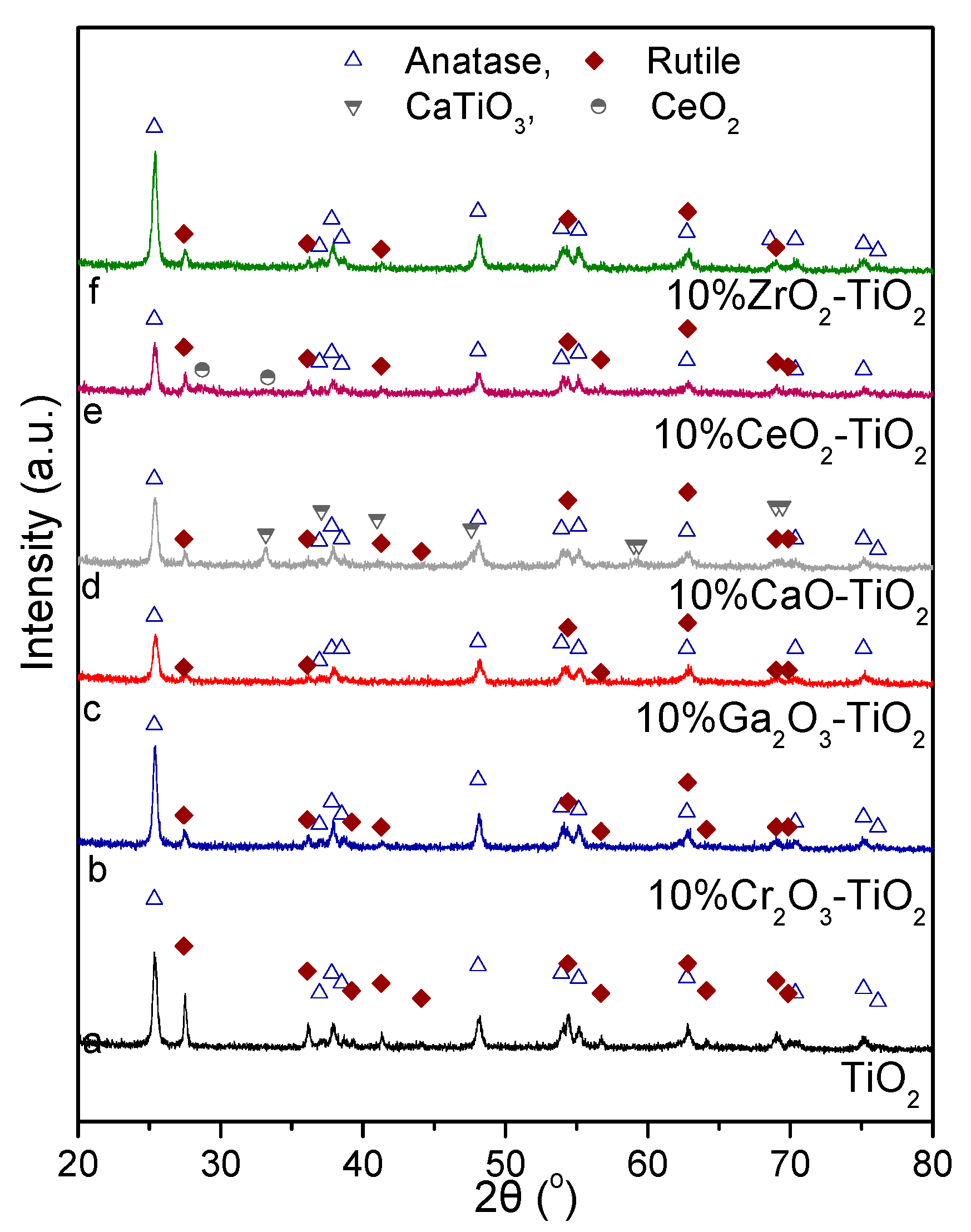
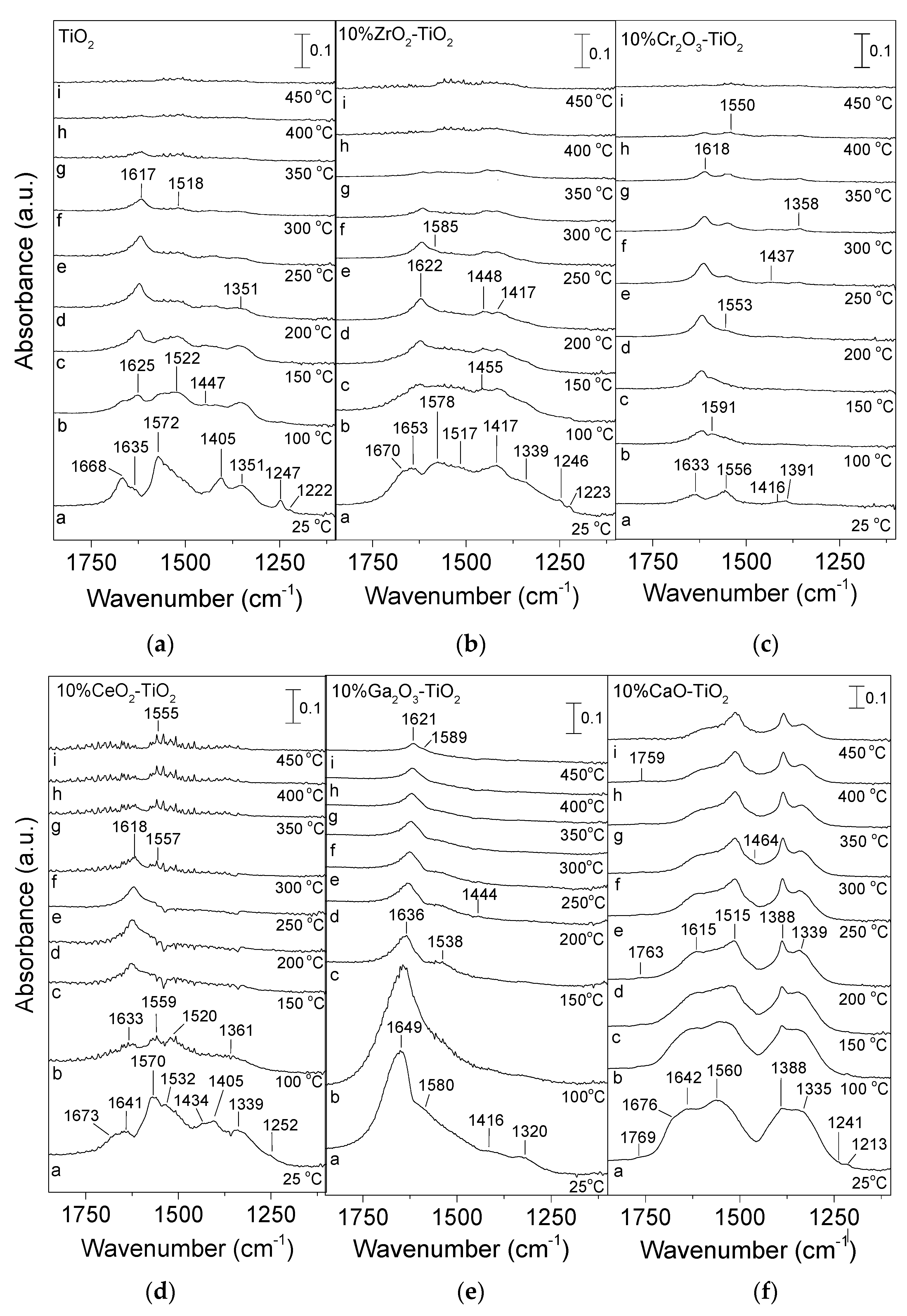
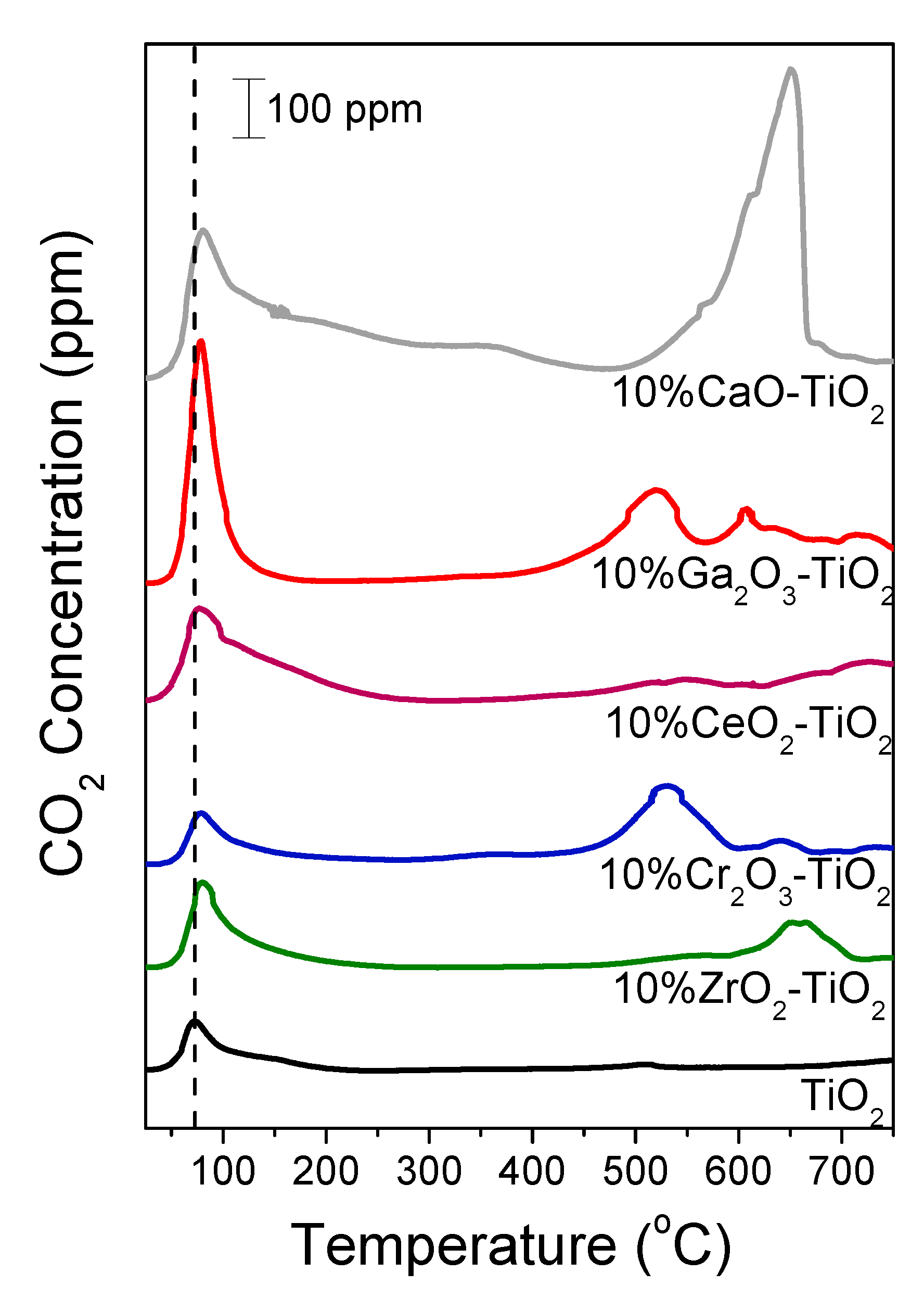

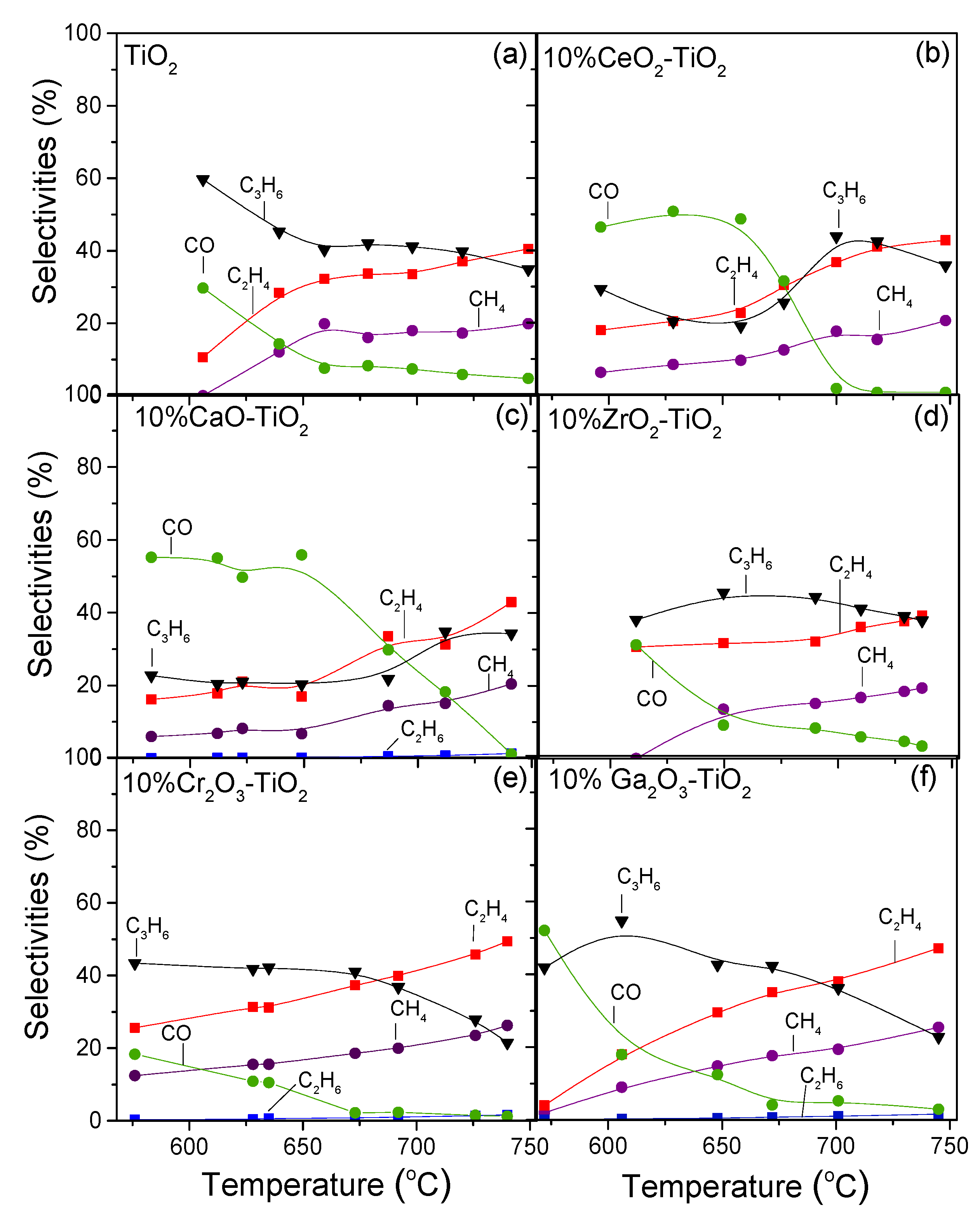
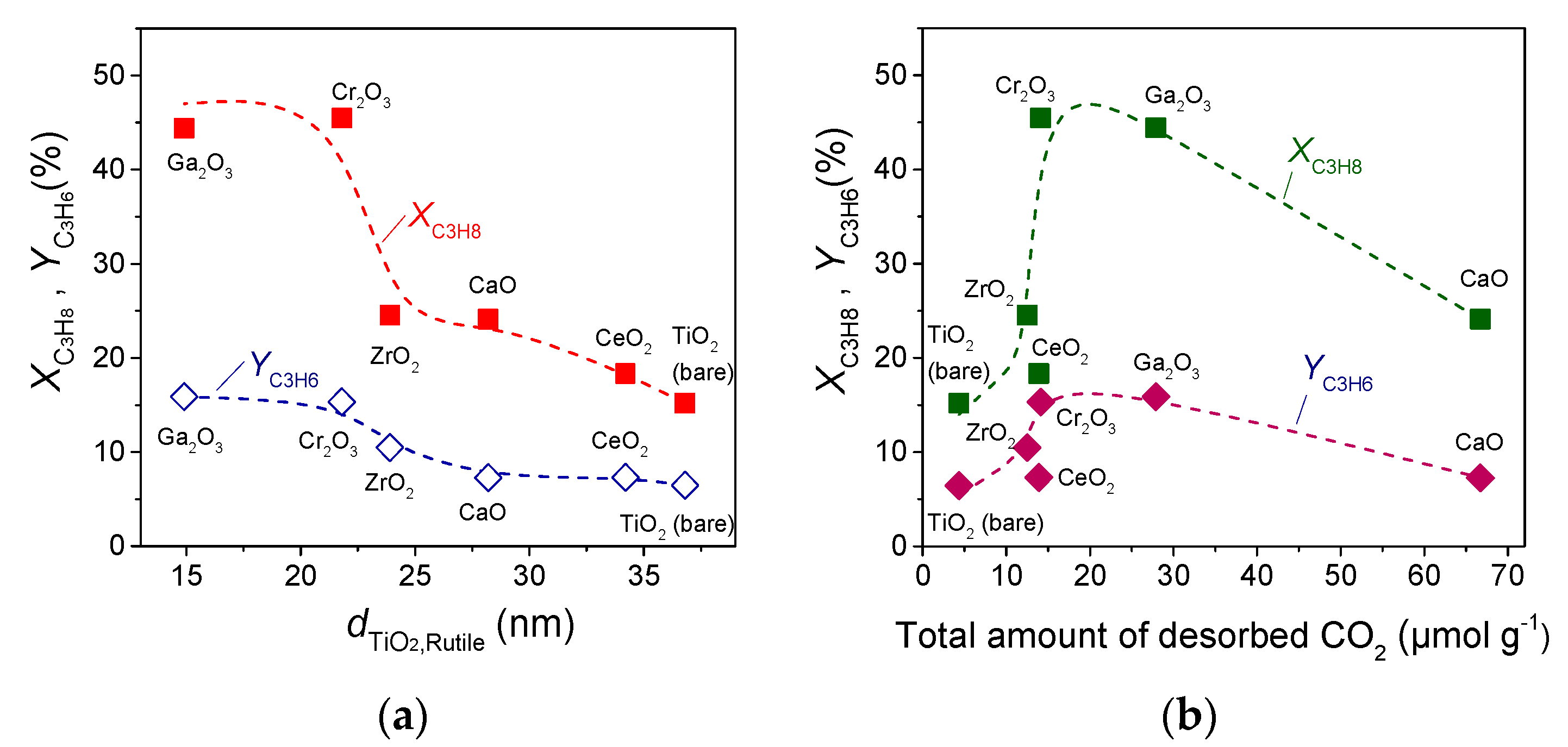
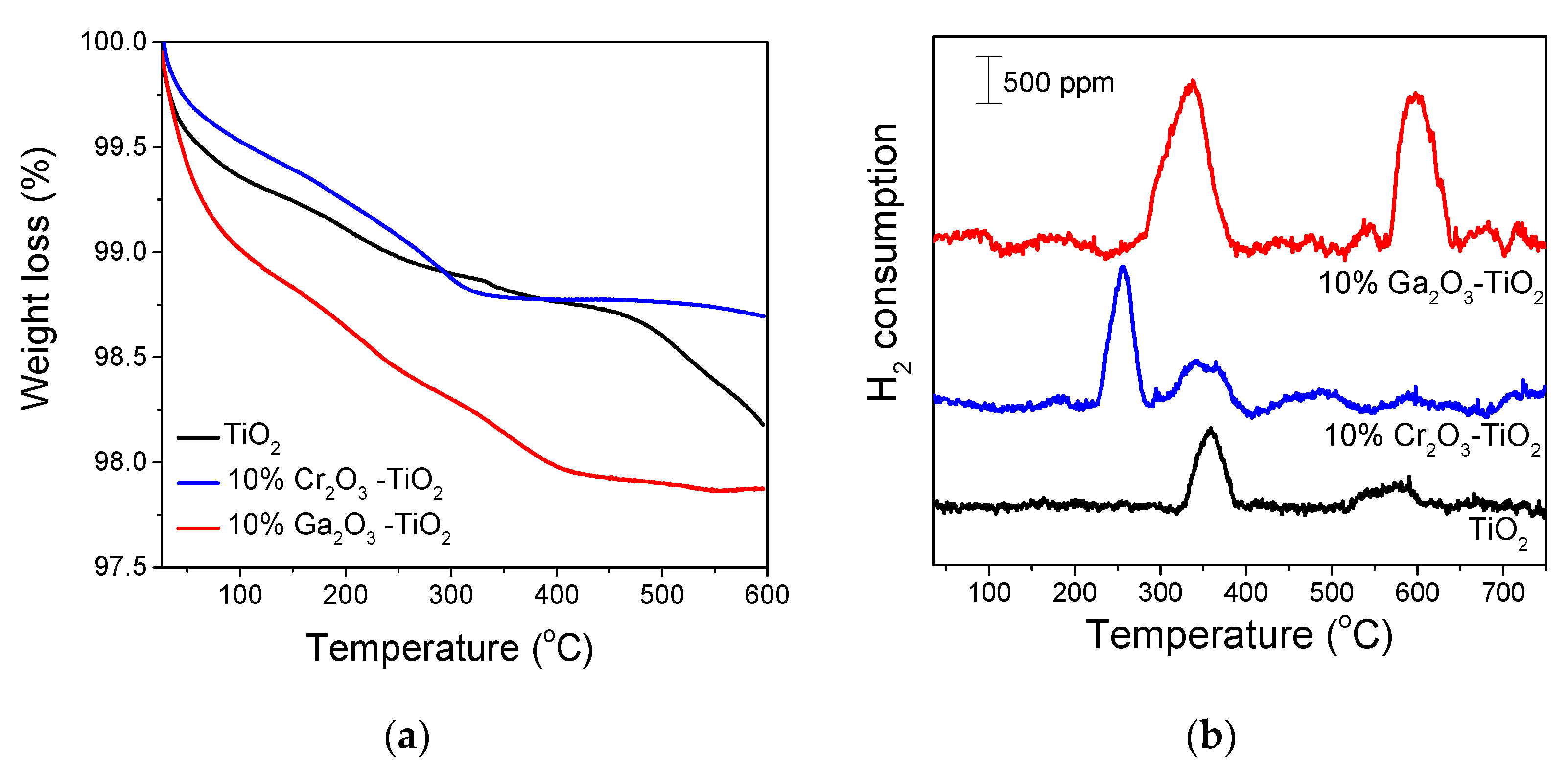

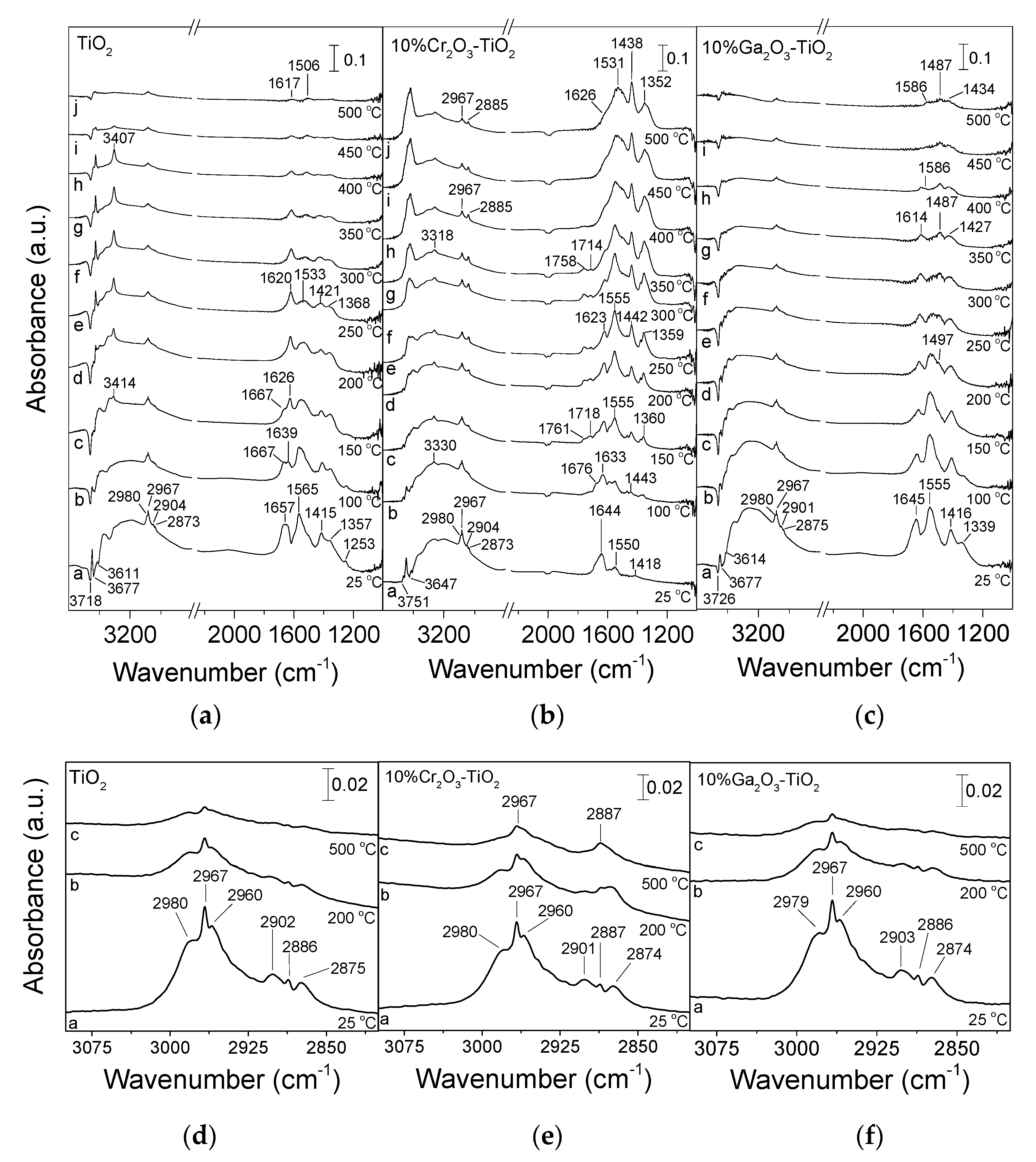
| Catalyst | SSA 1 (m2/g) | Crystallite Size 2 (nm) | Anatase Content 3 (%) | |
|---|---|---|---|---|
| Anatase dTiO2,A | Rutile dTiO2,R | |||
| TiO2 | 36.9 | 22.5 | 36.8 | 59 |
| 10% CeO2-TiO2 | 33.8 | 22.7 | 34.2 | 66 |
| 10% CaO-TiO2 | 33.9 | 19.9 | 28.2 | 79 |
| 10% ZrO2-TiO2 | 41.7 | 19.9 | 23.9 | 83 |
| 10% Cr2O3-TiO2 | 36.4 | 22.7 | 21.8 | 83 |
| 10% Ga2O3-TiO2 | 47.9 | 18.3 | 14.9 | 78 |
| Catalyst | LT Peak (μmol/g) | HT Peak (μmol/g) | Total Amount of Desorbed CO2 (μmol/g) | Total Amount of Desorbed CO2 (μmol/m2) |
|---|---|---|---|---|
| TiO2 | 4.1 | 0.23 | 4.3 | 0.12 |
| 10% ZrO2-TiO2 | 8.0 | 4.5 | 12.5 | 0.30 |
| 10% Cr2O3-TiO2 | 4.8 | 9.3 | 14.1 | 0.39 |
| 10% CeO2-TiO2 | 12.6 | 1.3 | 13.9 | 0.41 |
| 10% Ga2O3-TiO2 | 13.3 | 14.6 | 27.9 | 0.58 |
| 10% CaO-TiO2 | 32.6 | 34.1 | 66.7 | 1.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florou, A.; Bampos, G.; Natsi, P.D.; Kokka, A.; Panagiotopoulou, P. Propylene Production via Oxidative Dehydrogenation of Propane with Carbon Dioxide over Composite MxOy-TiO2 Catalysts. Nanomaterials 2024, 14, 86. https://doi.org/10.3390/nano14010086
Florou A, Bampos G, Natsi PD, Kokka A, Panagiotopoulou P. Propylene Production via Oxidative Dehydrogenation of Propane with Carbon Dioxide over Composite MxOy-TiO2 Catalysts. Nanomaterials. 2024; 14(1):86. https://doi.org/10.3390/nano14010086
Chicago/Turabian StyleFlorou, Alexandra, Georgios Bampos, Panagiota D. Natsi, Aliki Kokka, and Paraskevi Panagiotopoulou. 2024. "Propylene Production via Oxidative Dehydrogenation of Propane with Carbon Dioxide over Composite MxOy-TiO2 Catalysts" Nanomaterials 14, no. 1: 86. https://doi.org/10.3390/nano14010086
APA StyleFlorou, A., Bampos, G., Natsi, P. D., Kokka, A., & Panagiotopoulou, P. (2024). Propylene Production via Oxidative Dehydrogenation of Propane with Carbon Dioxide over Composite MxOy-TiO2 Catalysts. Nanomaterials, 14(1), 86. https://doi.org/10.3390/nano14010086








