A Nanoporous Polymer Modified with Hexafluoroisopropanol to Detect Dimethyl Methylphosphonate
Abstract
:1. Introduction
2. Materials and Methods
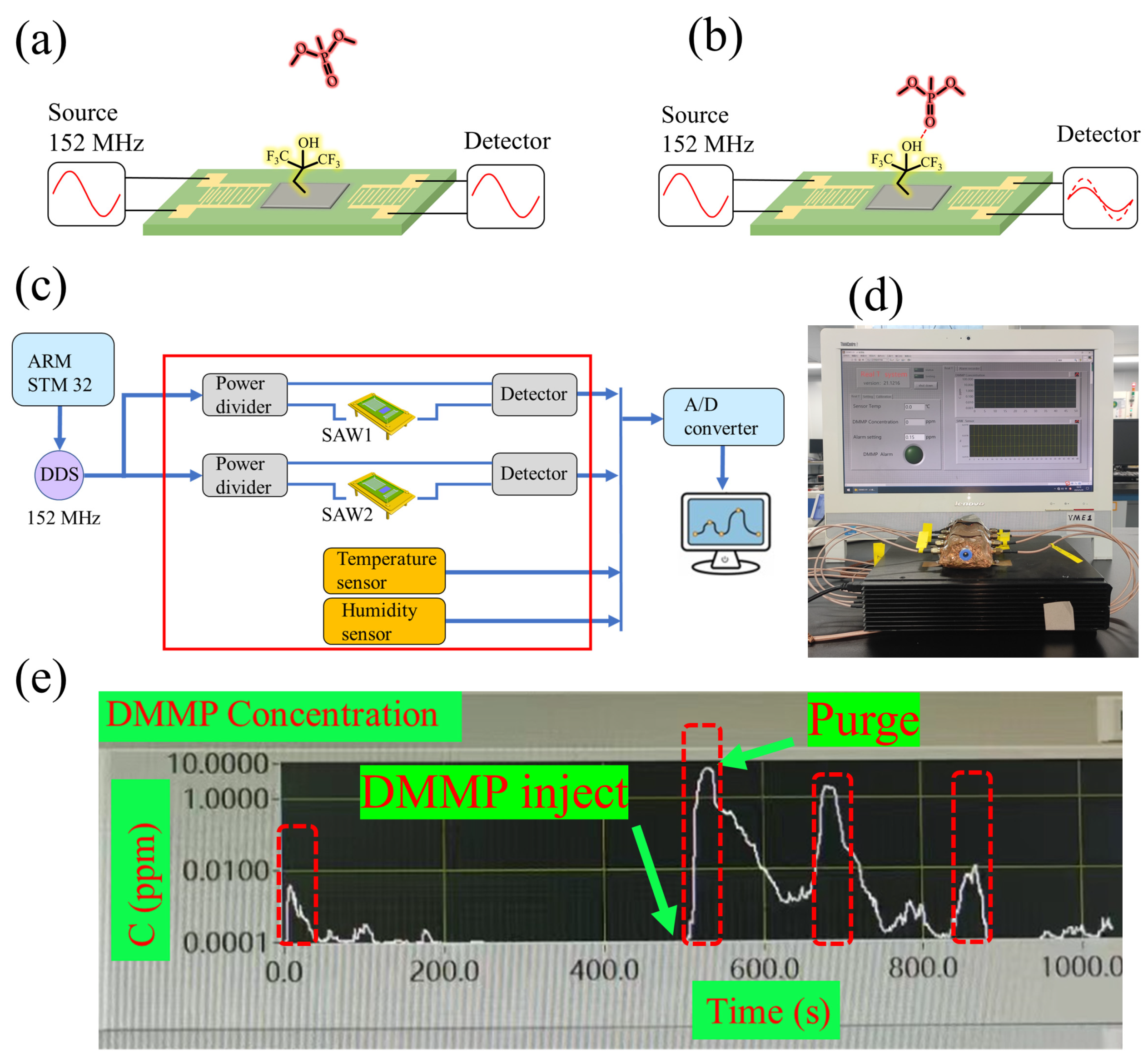
2.1. Design of SAW Device
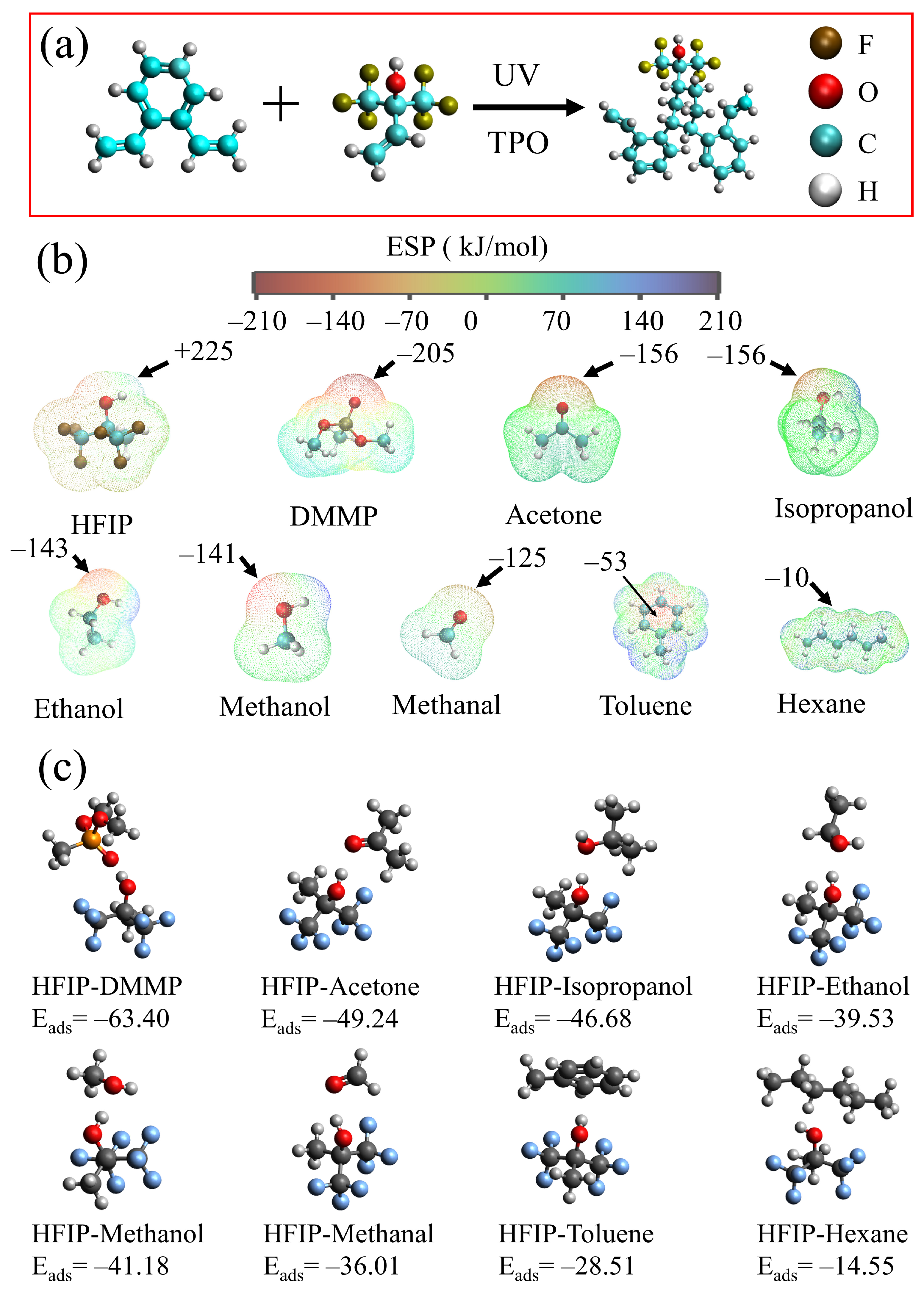
2.2. Preparation of Sensitive Materials
2.3. Material Characterization
2.4. Sensor Measurement
2.5. Calculation of Materials
3. Results
3.1. Material Characterization
3.2. Sensor Performance Measurement and Optimization
4. Discussion
4.1. Sensing Mechanism
4.2. Adsorption Mechanism
4.3. Interaction between HFIPs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costanzi, S.; Machado, J.H.; Mitchell, M. Nerve Agents: What They Are, How They Work, How to Counter Them. ACS Chem. Neurosci. 2018, 9, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-T.; Kuo, J.-C.; Yang, Y.-J. Polymer-dispersed liquid crystal doped with carbon nanotubes for dimethyl methylphosphonate vapor-sensing application. Appl. Phys. Lett. 2013, 102, 191912. [Google Scholar] [CrossRef]
- Pagidi, S.; Pasupuleti, K.S.; Reddeppa, M.; Ahn, S.; Kim, Y.; Kim, J.-H.; Kim, M.-D.; Lee, S.H.; Jeon, M.Y. Resistive type NO2 gas sensing in polymer-dispersed liquid crystals with functionalized-carbon nanotubes dopant at room temperature. Sens. Actuators B Chem. 2022, 370, 132482. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, L.; Zhang, Y.; Xing, Y.; Fei, T.; Liu, S.; Zhang, T. DMMP sensors based on Au-SnO2 hybrids prepared through colloidal assembly approach: Gas sensing performances and mechanism study. Sens. Actuators B Chem. 2022, 369, 132278. [Google Scholar] [CrossRef]
- Grabka, M.; Witkiewicz, Z.; Jasek, K.; Piwowarski, K. Acoustic Wave Sensors for Detection of Blister Chemical Warfare Agents and Their Simulants. Sensors 2022, 22, 5607. [Google Scholar] [CrossRef] [PubMed]
- Fehlen, P.; Thomas, G.; Gonzalez-Posada, F.; Guise, J.; Rusconi, F.; Cerutti, L.; Taliercio, T.; Spitzer, D. Gas sensing of organophosphorous compounds with III–V semiconductor plasmonics. Sens. Actuators B Chem. 2023, 376, 132987. [Google Scholar] [CrossRef]
- Dodds, J.N.; Baker, E.S. Ion Mobility Spectrometry: Fundamental Concepts, Instrumentation, Applications, and the Road Ahead. J. Am. Soc. Mass. Spectrom. 2019, 30, 2185–2195. [Google Scholar] [CrossRef]
- McKelvie, K.H.; Thurbide, K.B. Micro-Flame Photometric Detection in Miniature Gas Chromatography on a Titanium Tile. Chromatographia 2019, 82, 935–942. [Google Scholar] [CrossRef]
- Mandal, D.; Banerjee, S. Surface Acoustic Wave (SAW) Sensors: Physics, Materials, and Applications. Sensors 2022, 22, 820. [Google Scholar] [CrossRef]
- Nasri, A.; Pétrissans, M.; Fierro, V.; Celzard, A. Gas sensing based on organic composite materials: Review of sensor types, progresses and challenges. Mater. Sci. Semicond. Process. 2021, 128, 105744. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.; Fu, W.; Lv, H.; Zu, X.; Guo, Y.; Gibson, D.; Fu, Y.-Q. Advances in sensing mechanisms and micro/nanostructured sensing layers for surface acoustic wave-based gas sensors. J. Mater. Chem. A 2023, 11, 9216–9238. [Google Scholar] [CrossRef]
- Head, A.R.; Tsyshevsky, R.; Trotochaud, L.; Eichhorn, B.; Kuklja, M.M.; Bluhm, H. Electron Spectroscopy and Computational Studies of Dimethyl Methylphosphonate. J. Phys. Chem. A 2016, 120, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Grate, J.W. Hydrogen-Bond Acidic Polymers for Chemical Vapor Sensing. Chem. Rev. 2008, 108, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wang, Y.; Cheng, Y.; Yang, X.; Yu, K.; Li, J.; Du, X.; Tang, X.; Jiang, Y. Detection of volatile organosulfur compounds by hydrogen-bond acidic polymer: A combined experimental and theoretical study. Mater. Lett. 2019, 237, 282–285. [Google Scholar] [CrossRef]
- Shaik, M.; Rao, V.K.; Ramana, G.V.; Halder, M.; Gutch, P.K.; Pandey, P.; Jain, R. p-Hexafluoroisopropanol phenyl functionalized graphene for QCM based detection of dimethyl methylphosphonate, a simulant of the nerve agent sarin. RSC Adv. 2018, 8, 8240–8245. [Google Scholar] [CrossRef]
- Wu, Q.; Li, X.; Wang, X.; Yuan, Y.; Bu, X.; Wu, H.; Li, X.; Han, C.; Wang, X.; Liu, W. High-performance p-hexafluoroisopropanol phenyl functionalized multi-walled carbon nanotube film on surface acoustic wave device for organophosphorus vapor detection. Nanotechnology 2022, 33, 375501. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Yu, J.; Moharram, D.; Chen, R.; Zhang, H.; Liu, Q.; Zhang, M.; Wang, J. HFIP-functionalized electrospun WO3 hollow nanofibers/rGO as an efficient double layer sensing material for dimethyl methylphosphonate gas under UV-Light irradiation. J. Alloys Compd. 2020, 832, 154999. [Google Scholar] [CrossRef]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and Preparation of Porous Polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef]
- Wang, G.; Sharp, C.; Plonka, A.M.; Wang, Q.; Frenkel, A.I.; Guo, W.; Hill, C.; Smith, C.; Kollar, J.; Troya, D.; et al. Mechanism and Kinetics for Reaction of the Chemical Warfare Agent Simulant, DMMP(g), with Zirconium(IV) MOFs: An Ultrahigh-Vacuum and DFT Study. J. Phys. Chem. C 2017, 121, 11261–11272. [Google Scholar] [CrossRef]
- Farahani, H.S.; Najafi, M.; Behbahani, M.; Naseri, M.T. Magnetic activated carbon as an adsorbent for extraction of DMMP from aqueous samples followed by GC-IMS analysis. Anal. Methods 2023, 15, 4834–4841. [Google Scholar] [CrossRef]
- Villa, C.C.; Galus, S.; Nowacka, M.; Magri, A.; Petriccione, M.; Gutiérrez, T.J. Molecular sieves for food applications: A review. Trends Food Sci. Technol. 2020, 102, 102–122. [Google Scholar] [CrossRef]
- Wu, J.; Xu, F.; Li, S.; Ma, P.; Zhang, X.; Liu, Q.; Fu, R.; Wu, D. Porous Polymers as Multifunctional Material Platforms toward Task-Specific Applications. Adv. Mater. 2019, 31, 1802922. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; El-Mahdy, A.F.M.; Kotp, M.G.; Kuo, S.-W. Advances in porous organic polymers: Syntheses, structures, and diverse applications. Mater. Adv. 2022, 3, 707–733. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Huang, H.; Cao, X.; Chen, X.; Cao, D. Porous organic polymers as a platform for sensing applications. Chem. Soc. Rev. 2022, 51, 2031–2080. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.-M. r2SCAN-3c: A “Swiss army knife” composite electronic-structure method. J. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef] [PubMed]
- Suresh, C.H.; Remya, G.S.; Anjalikrishna, P.K. Molecular electrostatic potential analysis: A powerful tool to interpret and predict chemical reactivity. WIREs Comput. Mol. Sci. 2022, 12, e1601. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. omegaB97M-V: A combinatorially optimized, range-separated hybrid, meta-GGA density functional with VV10 nonlocal correlation. J. Chem. Phys. 2016, 144, 214110. [Google Scholar] [CrossRef]
- Schlumberger, C.; Thommes, M. Characterization of Hierarchically Ordered Porous Materials by Physisorption and Mercury Porosimetry—A Tutorial Review. Adv. Mater. Interfaces 2021, 8, 2002181. [Google Scholar] [CrossRef]
- Wu, Q.; Yuan, Y.; Jiao, M.; Wang, X.; Liu, W.; Bu, X.; Han, C.; Li, X.; Wang, X. Hydrogen-bond acidic organic compound-sensitized ionic gel for dimethyl methylphosphonate detection. Sens. Actuators B Chem. 2023, 391, 134068. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Moharram, D.; Yu, J.; Liu, Q.; Zhu, J.; Li, R.; Wang, J. HFIP-functionalized 3D carbon nanostructure as chemiresistive nerve agents sensors under visible light. Sens. Actuators B Chem. 2022, 358, 131475. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Moharram, D.; Liu, Q.; Yu, J.; Chen, R.; Li, R.; Wang, J. Fabrication of electrospun Co3O4/CuO p-p heterojunctions nanotubes functionalized with HFIP for detecting chemical nerve agent under visible light irradiation. Sens. Actuators B Chem. 2020, 314, 128076. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, Z.; Zhao, L.; Zhang, Y.; Wei, Z.; Xing, C.; Fei, T.; Liu, S.; Zhang, T. A multisite strategy to improve room-temperature DMMP sensing performances on reduced graphene oxide modulated by N-doped carbon nanoparticles and copper ions. Sens. Actuators B Chem. 2023, 393, 134220. [Google Scholar] [CrossRef]
- Jakubik, W.; Wrotniak, J.; Kaźmierczak-Bałata, A.; Stolarczyk, A.; Powroźnik, P. Light-activated SAW sensor structures with photoconductive polymer films for DMMP detection. Sens. Actuators B Chem. 2023, 384, 133597. [Google Scholar] [CrossRef]
- Grabka, M.; Jasek, K.; Pasternak, M. Application of polymethyl[4-(2,3-difluoro-4-hydroxyphenoxy)butyl] siloxane in surface acoustic wave gas sensors for dimethyl methylphosphonate detection. Sens. Actuators B Chem. 2021, 329, 129216. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Aljebawi, K.; Liu, Q.; Chen, R.; Yu, J.; Zhang, M.; Wang, J. 3D hybrid Ni-Multiwall carbon nanotubes/carbon nanofibers for detecting sarin nerve agent at room temperature. J. Alloys Compd. 2019, 780, 680–689. [Google Scholar] [CrossRef]
- You, R.; Liu, J.; Liu, M.; Zhang, Y.; Chen, Z.; He, S. Detecting Small Size Mass Loading Using Transversely Coupled SAW Resonator. Appl. Sci. 2021, 11, 10228. [Google Scholar] [CrossRef]
- Zheng, Z.; Dazhong, Z.; Zhelong, H. A mass-loading effect LiNbO/sub 3/SAW sensor. In Proceedings of the 6th International Conference on Solid-State and Integrated Circuit Technology, Proceedings (Cat. No.01EX443), Shanghai, China, 22–25 October 2001; Volume 782, pp. 781–784. [Google Scholar]
- van der Lubbe, S.C.C.; Fonseca Guerra, C. The Nature of Hydrogen Bonds: A Delineation of the Role of Different Energy Components on Hydrogen Bond Strengths and Lengths. Chem. An. Asian J. 2019, 14, 2760–2769. [Google Scholar] [CrossRef]
- Wendler, K.; Thar, J.; Zahn, S.; Kirchner, B. Estimating the Hydrogen Bond Energy. J. Phys. Chem. A 2010, 114, 9529–9536. [Google Scholar] [CrossRef]

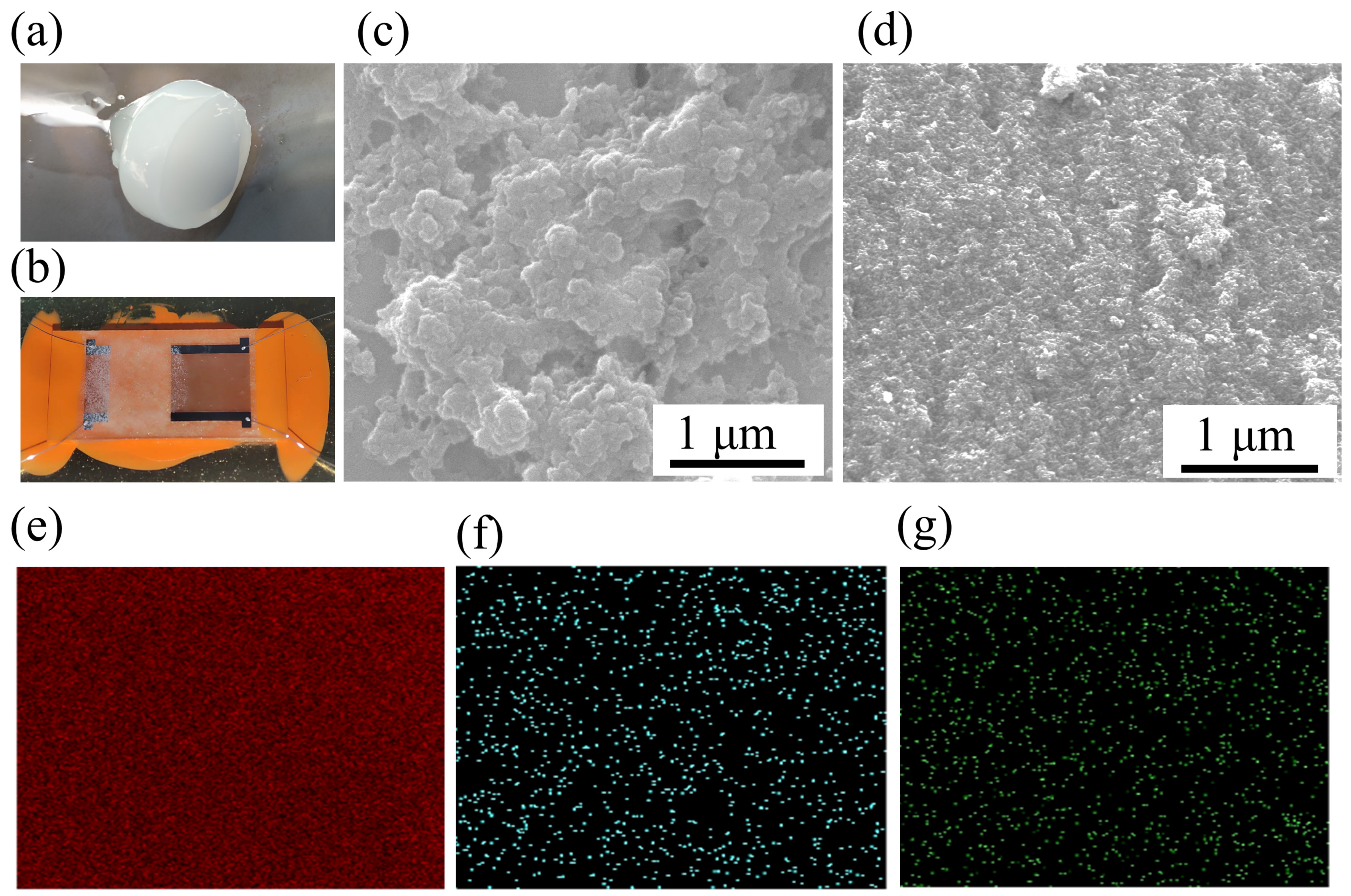
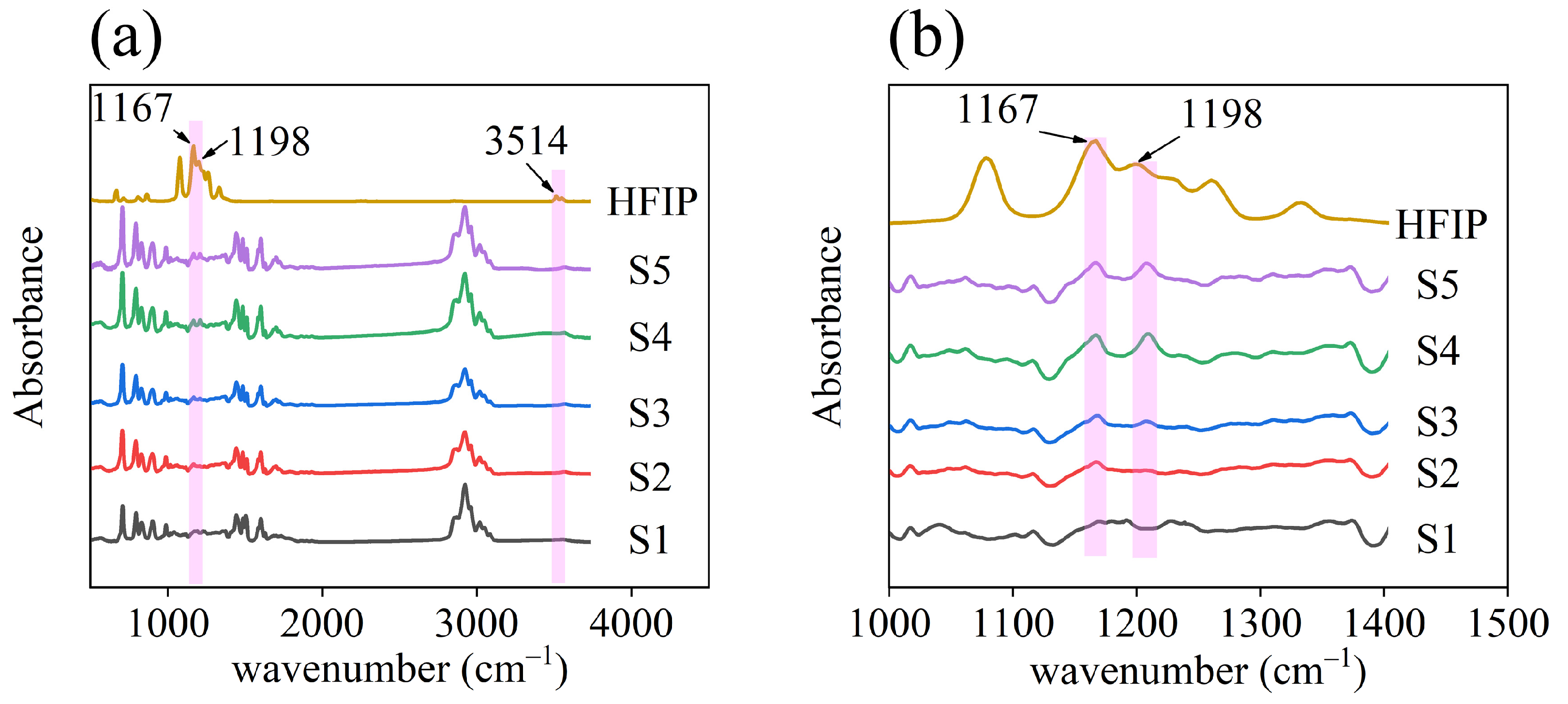



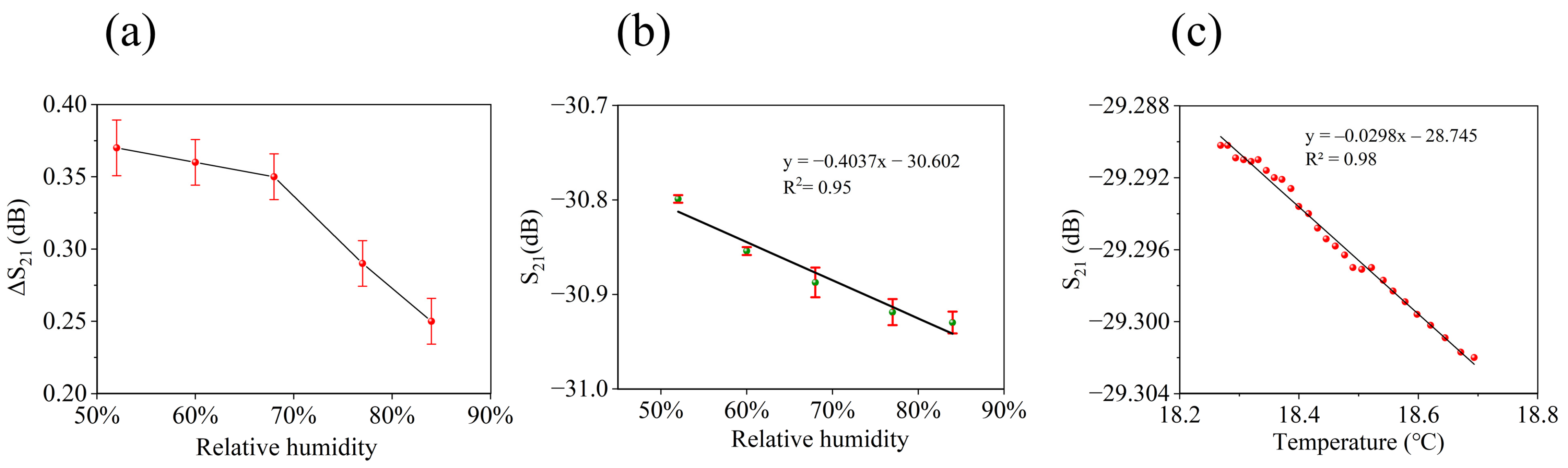
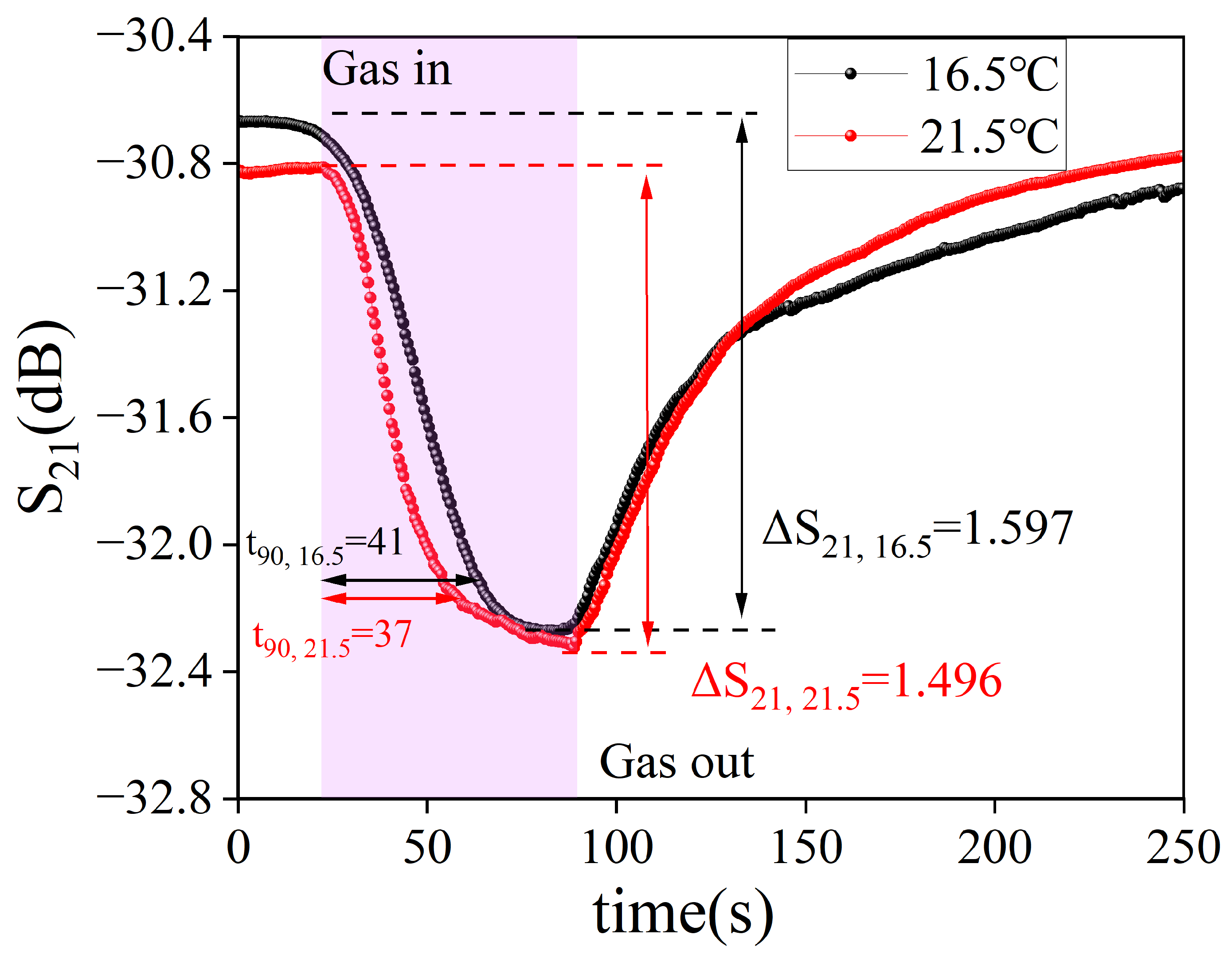


| Sample | BTHB (mL) | DVB (mL) | Xylene (mL) | TPO (g) | HFIP Mass Ratio |
|---|---|---|---|---|---|
| S1 | 0 | 0.565 | 8.5 | 0.0058 | 0% |
| S2 | 0.015 | 7% | |||
| S3 | 0.046 | 19% | |||
| S4 | 0.107 | 36% | |||
| S5 | 0.153 | 44% |
| Materials | Device | Key Process | Response Time/Recovery Time | Detection Concentration (ppm) | Ref. |
|---|---|---|---|---|---|
| HFIP—ionic gel | SAW sensor | Photoinduced polymerization and patterning | 23 s/58 s | 0.2 | [30] |
| HFIP—CNT | SAW sensor | Surface modified HFIP and self-assembly process | 3 s/50 s | 0.1 | [16] |
| HFIP—3D carbon | Resistor | Electrospinning machine and in-situ CVD technique | 2.4 s/4.7 s | 0.1 | [31] |
| HFIP—Co3O4/CuO | Resistor | Electrospinning machine and surface modified HFIP | 7.3 s/5.2 s | 0.5 | [32] |
| HFIP—WO3/rGO | Resistor | Electrospinning machine and surface modified HFIP | 23 s/25 s | 0.1 | [17] |
| rGO—GNC | Resistor | Hydrothermal method and drop-casting | 105 s/150 s | 10 | [33] |
| Poly-3-hexylthiophene | SAW sensor | Spray-coating technology and light activation of the sensitive material | 100 s/240 s | 0.133 | [34] |
| PMFOS | SAW sensor | Chemical synthesis of PMFOS and thermal evaporation coating | 100 s/480 s | 0.013 | [35] |
| 3D hybrid Ni-CNT | Resistor | Electrospinning machine and and drop-casting | 11 s/12 s | 0.1 | [36] |
| HFIP porous polymers | SAW sensor | Photoinduced polymerization and drop-casting | 29.8 s/43.8 s | 0.8 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, X.; Wu, Q.; Yuan, Y.; Liu, W.; Han, C.; Wang, X. A Nanoporous Polymer Modified with Hexafluoroisopropanol to Detect Dimethyl Methylphosphonate. Nanomaterials 2024, 14, 89. https://doi.org/10.3390/nano14010089
Wang X, Li X, Wu Q, Yuan Y, Liu W, Han C, Wang X. A Nanoporous Polymer Modified with Hexafluoroisopropanol to Detect Dimethyl Methylphosphonate. Nanomaterials. 2024; 14(1):89. https://doi.org/10.3390/nano14010089
Chicago/Turabian StyleWang, Xuming, Xin Li, Qiang Wu, Yubin Yuan, Weihua Liu, Chuanyu Han, and Xiaoli Wang. 2024. "A Nanoporous Polymer Modified with Hexafluoroisopropanol to Detect Dimethyl Methylphosphonate" Nanomaterials 14, no. 1: 89. https://doi.org/10.3390/nano14010089





