Iron Oxide Nanoparticle-Assisted Delamination of Ti3C2Tx MXenes: A New Approach to Produce Magnetic MXene-Based Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MXenes
2.2. Synthesis of Iron Oxide Fe3O4 Nanoparticles
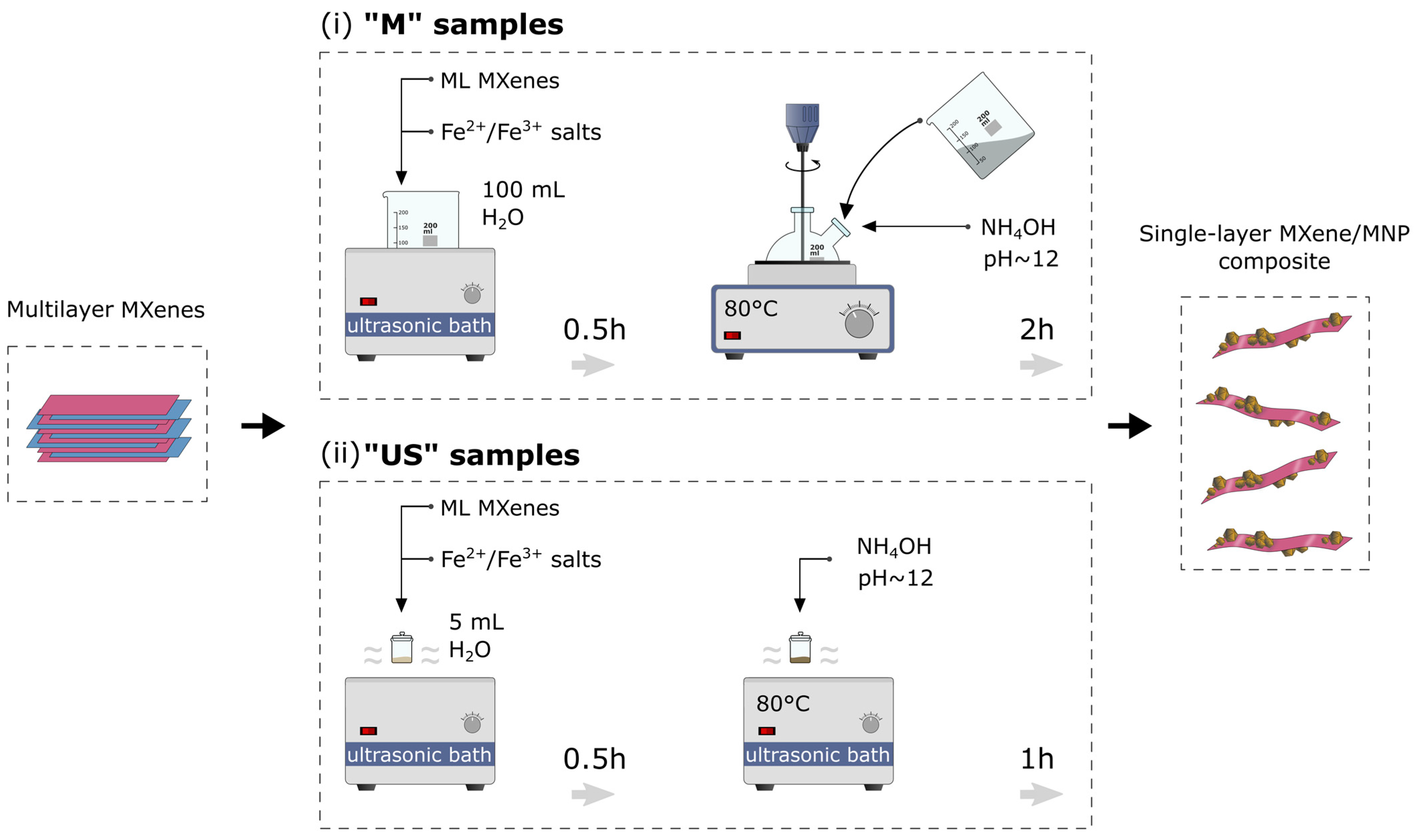
2.3. Synthesis of MXene-MNP Composite Materials
2.3.1. Co-Precipitation (M Samples)
2.3.2. Ultrasound-Assisted Co-Precipitation (US Samples)
2.4. Characterization of the Materials
3. Results and Discussion
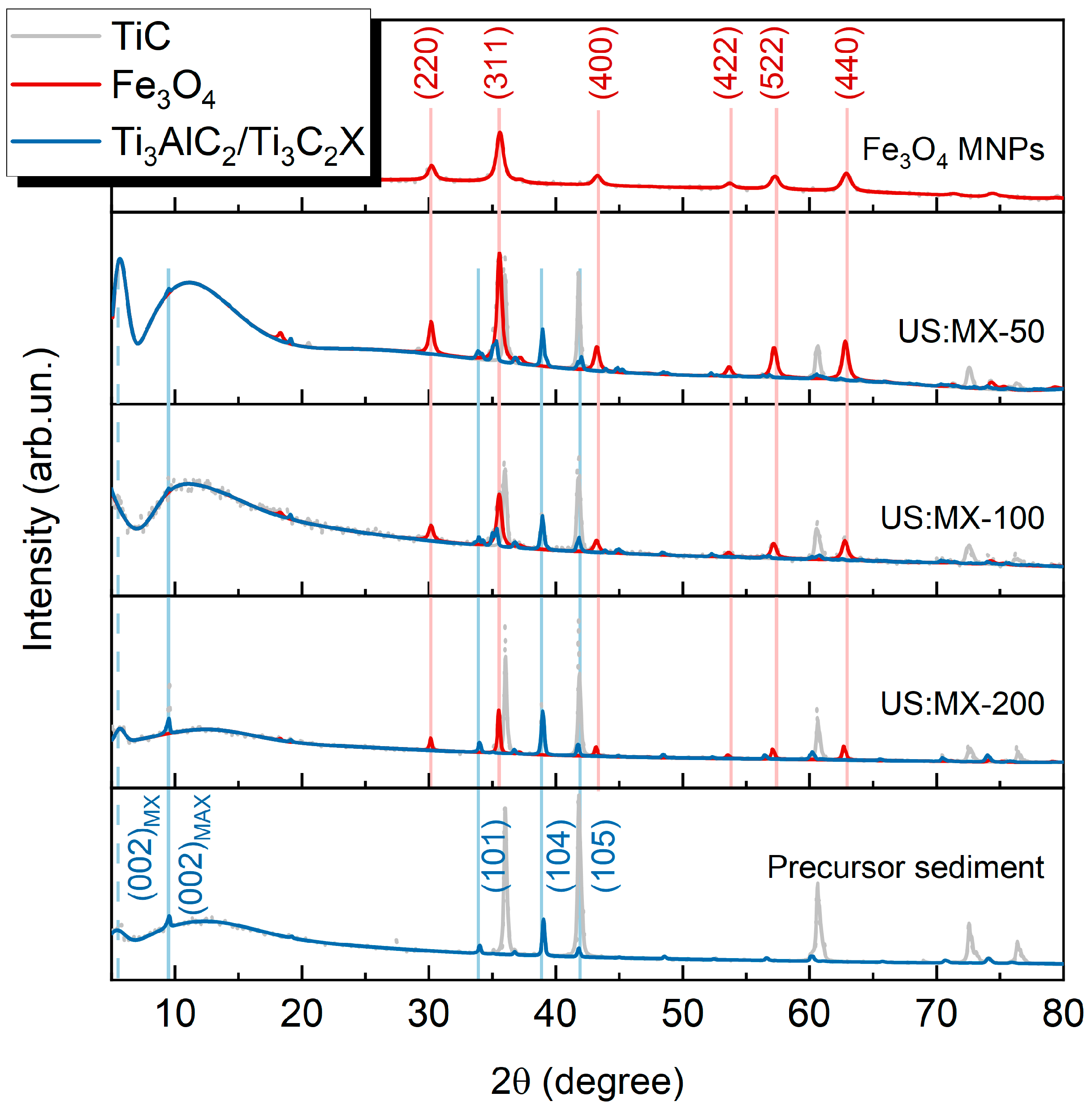
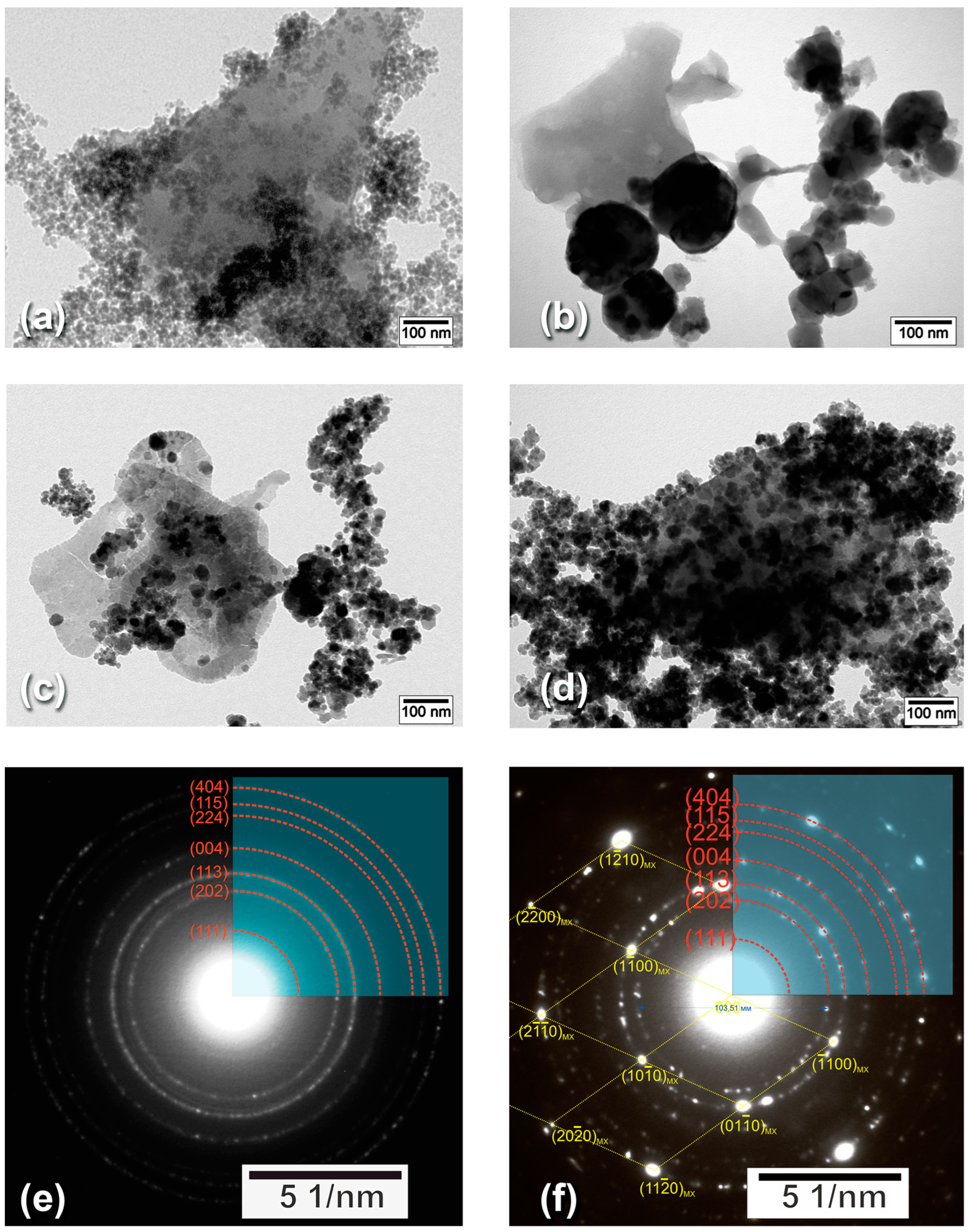
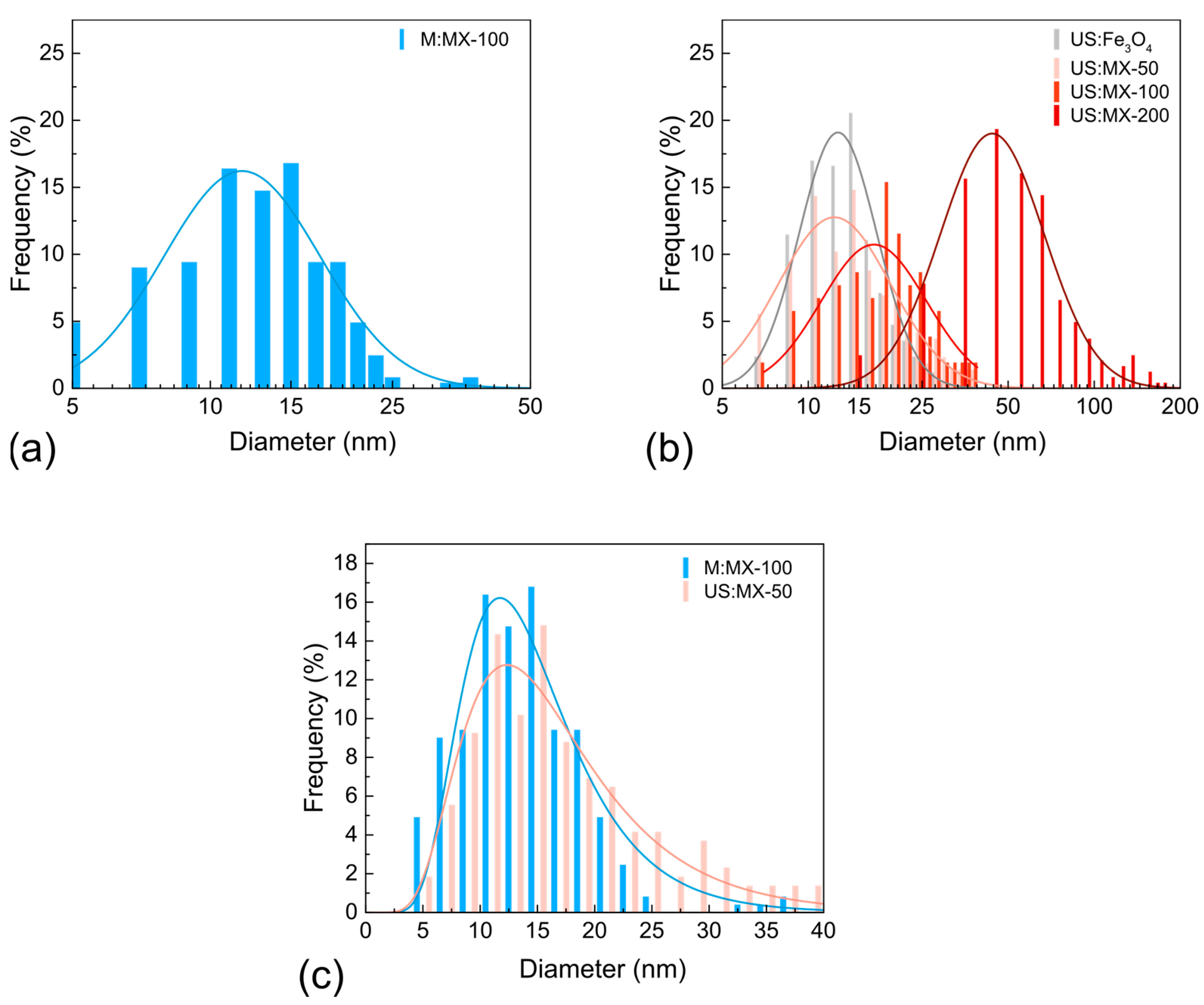
3.1. Characterization of MXene-MNP Composites
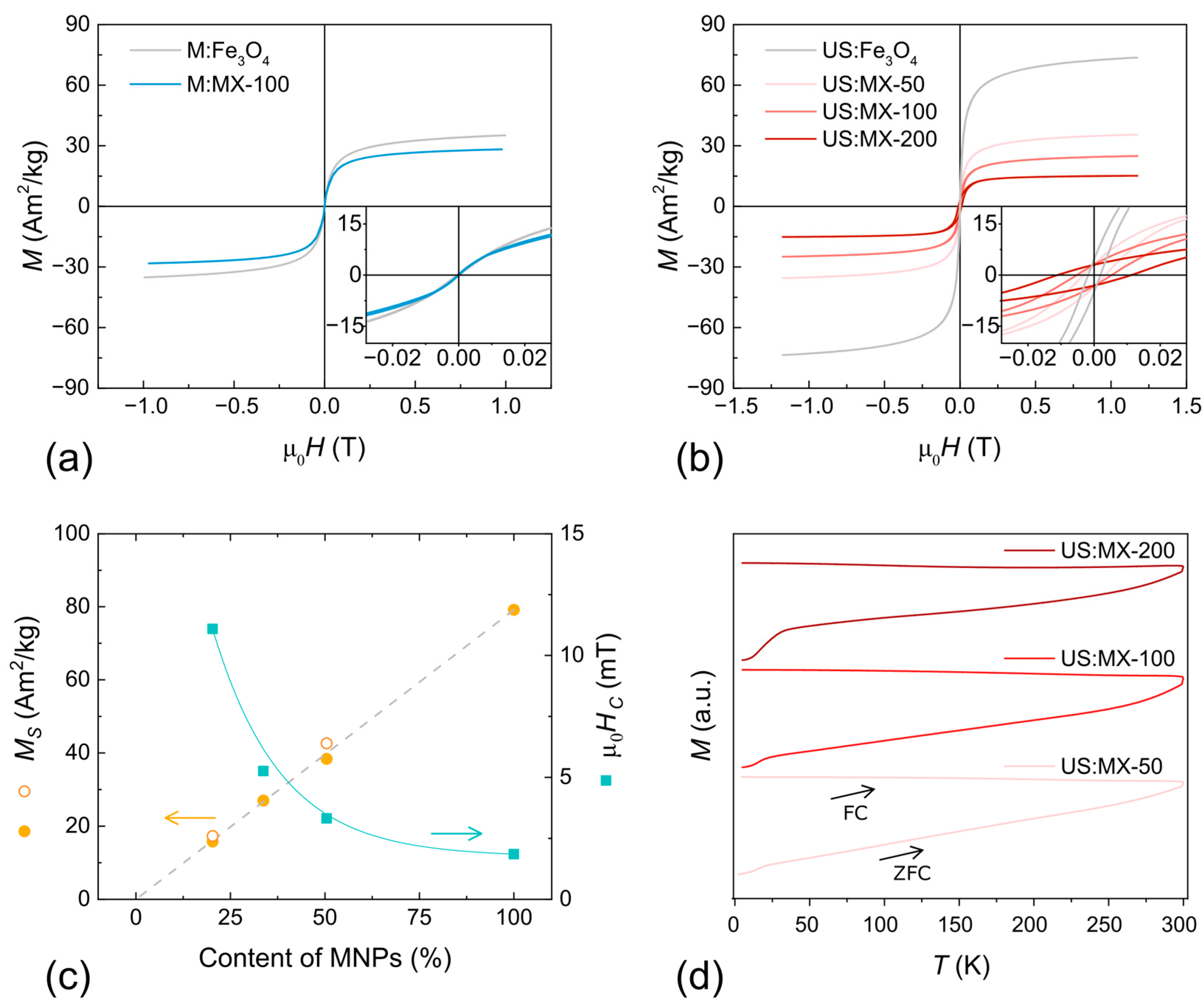
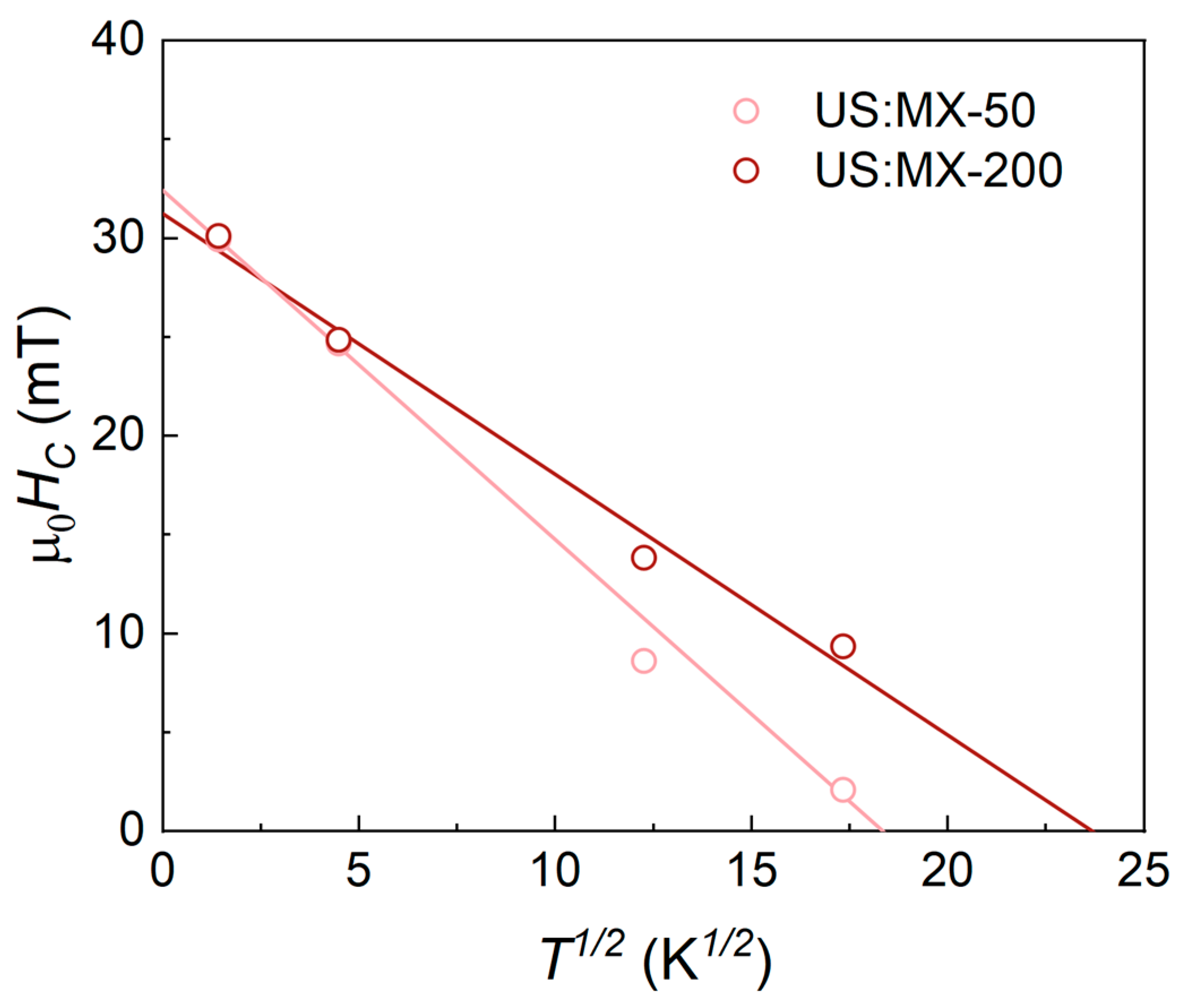
3.2. Magnetic Properties
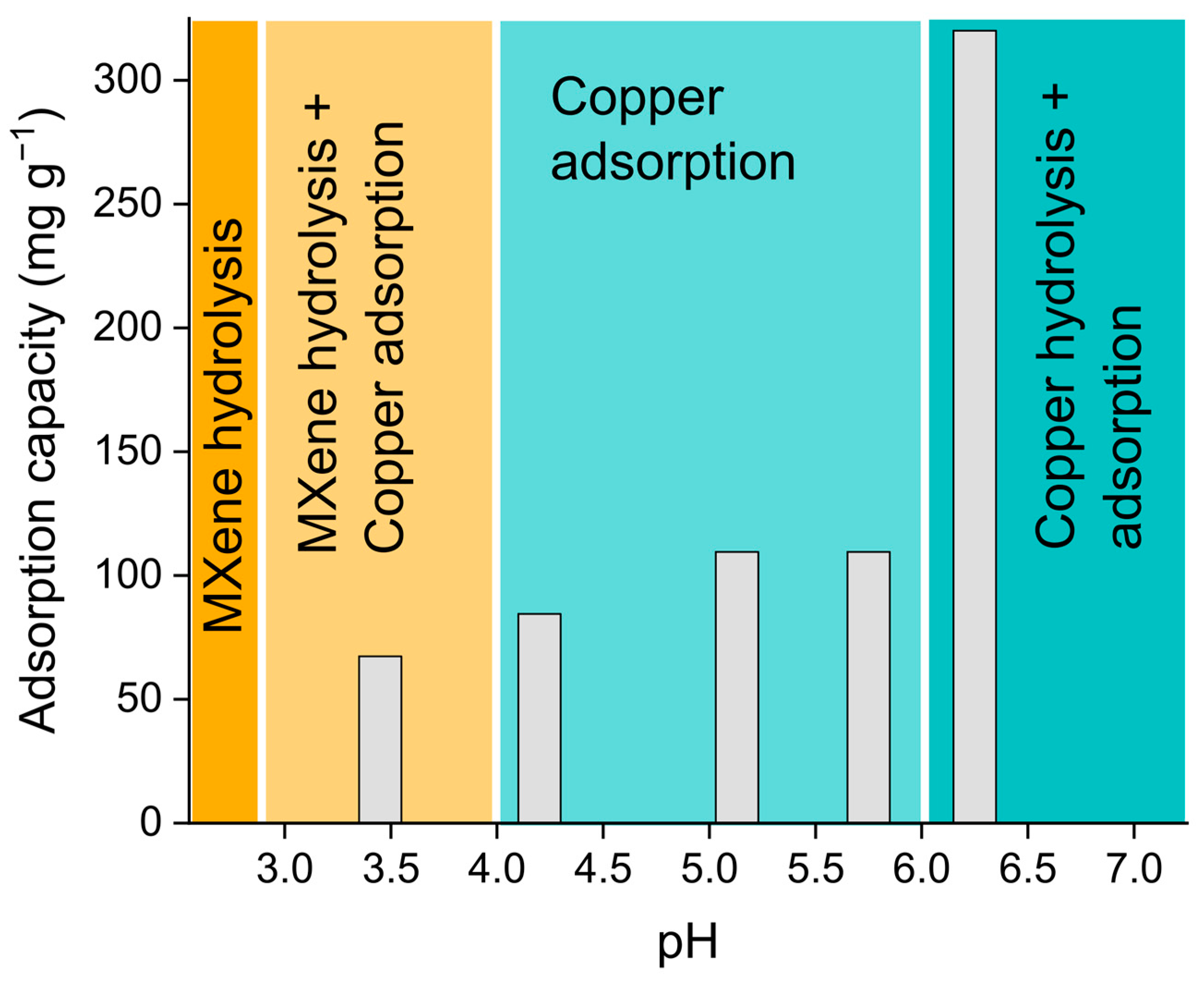
3.3. Adsorptive Properties of MXene-MNP Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248. [Google Scholar]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Mishra, A.; Venkataramanan, N.S.; Singh, A.K.; Yunoki, S. Recent Advances in MXenes: From Fundamentals to Applications. Curr. Opin. Solid State Mater. Sci. 2019, 23, 164. [Google Scholar] [CrossRef]
- Han, M.; Maleski, K.; Shuck, C.E.; Yang, Y.; Glazar, J.T.; Foucher, A.C.; Hantanasirisakul, K.; Sarycheva, A.; Frey, N.C.; May, S.J.; et al. Tailoring electronic and optical properties of MXenes through forming solid solutions. J. Am. Chem. Soc. 2020, 142, 19110–19118. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Ghazaly, A.E.; Rosen, J. i-MXenes for Energy Storage and Catalysis. Adv. Func. Mat. 2020, 30, 2000894. [Google Scholar] [CrossRef]
- Yu, S.; Tang, H.; Zhang, D.; Wang, S.; Qiu, M.; Song, G.; Fu, D.; Hu, B.; Wang, X. MXenes as emerging nanomaterials in water purification and environmental remediation. Sci. Total Environ. 2022, 811, 152280. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive Two-Dimensional Titanium Carbide ‘Clay’ with High Volumetric Capacitance. Nature 2014, 516, 78. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment. Angew. Chem. 2018, 130, 6223. [Google Scholar] [CrossRef]
- Li, G.; Tan, L.; Zhang, Y.; Wu, B.; Li, L. Highly Efficiently Delaminated Single-Layered MXene Nanosheets with Large Lateral Size. Langmuir 2017, 33, 9000. [Google Scholar] [CrossRef]
- Urbankowski, P.; Anasori, B.; Makaryan, T.; Er, D.; Kota, S.; Walsh, P.L.; Zhao, M.; Shenoy, V.B.; Barsoum, M.W.; Gogotsi, Y. Synthesis of Two-Dimensional Titanium Nitride Ti4N3 (MXene). Nanoscale 2016, 8, 11385. [Google Scholar] [CrossRef] [PubMed]
- VahidMohammadi, A.; Kayali, E.; Orangi, J.; Beidaghi, M. Techniques for MXene Delamination into Single-Layer Flakes. In 2D Metal Carbides and Nitrides (MXenes); Anasori, B., Gogotsi, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Wei, Y.; Zhang, P.; Soomro, R.A.; Zhu, Q.; Xu, B. Advances in the Synthesis of 2D MXenes. Adv. Mater. 2021, 33, 2103148. [Google Scholar] [CrossRef] [PubMed]
- Shuck, C.E.; Sarycheva, A.; Anayee, M.; Levitt, A.; Zhu, Y.; Uzun, S.; Balitskiy, V.; Zahorodna, V.; Gogotsi, O.; Gogotsi, Y. Scalable Synthesis of Ti3C2Tx MXene. Adv. Eng. Mater. 2020, 22, 1901241. [Google Scholar] [CrossRef]
- El Ghazaly, A.; Ahmed, H.; Rezk, A.R.; Halim, J.; Persson, P.O.Å.; Yeo, L.Y.; Rosen, J. One-Step, Salt-Solution-Based Acoustic Synthesis of Ti3C2 MXene. ACS Nano 2021, 15, 4287. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and Delamination of Layered Carbides and Carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef]
- Xuan, J.; Wang, Z.; Chen, Y.; Liang, D.; Cheng, L.; Yang, X.; Liu, Z.; Ma, R.; Sasaki, T.; Geng, F. Organic-Base-Driven Intercalation and Delamination for the Production of Functionalized Titanium Carbide Nanosheets with Superior Photothermal Therapeutic Performance. Angew. Chem. 2016, 128, 14789. [Google Scholar] [CrossRef]
- Yang, Y.; Umrao, S.; Lai, S.; Lee, S. Large-Area Highly Conductive Transparent Two-Dimensional Ti2CTx Film. J. Phys. Chem. Lett. 2017, 8, 859. [Google Scholar] [CrossRef]
- Velusamy, K.; Chellam, P.; Kumar, P.S.; Venkatachalam, J.; Periyasamy, S.; Saravanan, R. Functionalization of MXene-Based Nanomaterials for the Treatment of Micropollutants in Aquatic System: A Review. Environ. Pollut. 2022, 301, 119034. [Google Scholar] [CrossRef]
- Carey, M.; Barsoum, M.W. MXene Polymer Nanocomposites: A Review. Mater. Today Adv. 2021, 9, 100120. [Google Scholar] [CrossRef]
- Rethinasabapathy, M.; Bhaskaran, G.; Park, B.; Shin, J.-Y.; Kim, W.-S.; Ryu, J.; Huh, Y.S. Iron Oxide (Fe3O4)-Laden Titanium Carbide (Ti3C2Tx) MXene Stacks for the Efficient Sequestration of Cationic Dyes from Aqueous Solution. Chemosphere 2022, 286, 131679. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhao, X.; Zhou, W.; Wang, C.; Zhang, P. Fe2O3-Decoration and Multilayer Structure Design of Ti3C2 MXene Materials toward Strong and Broadband Absorption of Electromagnetic Waves in the X-Band Region. J. Mater. Sci. Mater. Electron. 2021, 32, 25919. [Google Scholar] [CrossRef]
- Alsafari, I.A.; Munir, S.; Zulfiqar, S.; Saif, M.S.; Warsi, M.F.; Shahid, M. Synthesis, Characterization, Photocatalytic and Antibacterial Properties of Copper Ferrite/MXene (CuFe2O4/Ti3C2) Nanohybrids. Ceram. Int. 2021, 47, 28874. [Google Scholar] [CrossRef]
- Qiu, F.; Wang, Z.; Liu, M.; Wang, Z.; Ding, S. Synthesis, Characterization and Microwave Absorption of MXene/NiFe2O4 Composites. Ceram. Int. 2021, 47, 24713. [Google Scholar] [CrossRef]
- Hu, Y.; Chu, H.; Ma, X.; Li, Y.; Zhao, S.; Li, D. Enhanced Optical Nonlinearities in Ti3C2 MXene Decorated Fe3O4 Nanocomposites for Highly Stable Ultrafast Pulse Generation. Mater. Today Phys. 2021, 21, 100482. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Zhu, Y.; Cao, B.; Zhu, Q.; Zhang, P.; Xu, B.; Wu, F.; Chen, R. Electrostatic Self-assembly of 0D–2D SnO2 Quantum Dots/Ti3C2Tx MXene Hybrids as Anode for Lithium-Ion Batteries. Nano-Micro Lett. 2019, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Mu, D.; Wu, B.; Ma, C.; Bi, J.; Zhang, L.; Yang, H.; Wu, F. Microsphere-Like SiO2/MXene Hybrid Material Enabling High Performance Anode for Lithium Ion Batteries. Small 2020, 16, 1905430. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Qiu, Z.; Wu, X.; Zhou, P.; Zhou, T.; Zhao, J.; Miao, Z.; Zhou, J.; Zhuo, S. Fe3O4@Ti3C2 MXene hybrids with ultrahigh volumetric capacity as an anode material for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 11189–11197. [Google Scholar] [CrossRef]
- Zhang, Z.; Weng, L.; Rao, Q.; Yang, S.; Hu, J.; Cai, J.; Min, Y. Highly-dispersed iron oxide nanoparticles anchored on crumpled nitrogen-doped MXene nanosheets as anode for Li-ion batteries with enhanced cyclic and rate performance. J. Pow. Sour. 2019, 439, 227107. [Google Scholar] [CrossRef]
- Yao, J.; Jiang, W.; Pan, L.; Xiong, J.; Wang, T.; Qiu, T.; Yang, J. Hierarchical structure of in-situ Fe2O3 nanoparticles decorated on crumpled Ti3C2Tx nanosheets with enhanced cycle performance as anode for lithium ion battery, Cer. Int. 2021, 47, 21807–21814. [Google Scholar] [CrossRef]
- Du, Y.; Yu, B.; Wei, L.; Wang, Y.; Zhang, X.; Ye, S. Efficient Removal of Pb(II) by Ti3C2Tx Powder Modified with a Silane Coupling Agent. J. Mater. Sci. 2019, 54, 13283. [Google Scholar] [CrossRef]
- Hu, X.; Chen, C.; Zhang, D.; Xue, Y. Kinetics, Isotherm and Chemical Speciation Analysis of Hg(Ⅱ) Adsorption over Oxygen-Containing MXene Adsorbent. Chemosphere 2021, 278, 130206. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, H.; Yuan, L.; Li, Z.; Zhang, Y.; Gibson, J.K.; Zheng, L.; Chai, Z.; Shi, W. Efficient U(VI) Reduction and Sequestration by Ti2CTx Mxene. Environ. Sci. Technol. 2018, 52, 10748. [Google Scholar] [CrossRef] [PubMed]
- Thirumal, V.; Yuvakkumar, R.; Kumar, P.S.; Keerthana, S.P.; Ravi, G.; Velauthapillai, D.; Saravanakumar, B. Efficient Photocatalytic Degradation of Hazardous Pollutants by Homemade Kitchen Blender Novel Technique via 2D-Material of Few-Layer MXene Nanosheets. Chemosphere 2021, 281, 130984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, M.; Liang, H.; Chen, J.; Xu, L.; Niu, J. Novel Dual-Effective Z-Scheme Heterojunction with g-C3N4, Ti3C2 MXene and Black Phosphorus for Improving Visible Light-Induced Degradation of Ciprofloxacin. Appl. Catal. B Environ. 2021, 291, 120105. [Google Scholar] [CrossRef]
- Campos, A.F.C.; de Oliveira, H.A.L.; da Silva, F.N.; da Silva, F.G.; Coppola, P.; Aquino, R.; Mezzi, A.; Depeyrot, J. Core-Shell Bimagnetic Nanoadsorbents for Hexavalent Chromium Removal from Aqueous Solutions. J. Hazard. Mater. 2019, 362, 82. [Google Scholar] [CrossRef]
- Campos, A.F.C.; Michels-Brito, P.H.; da Silva, F.G.; Gomes, R.C.; Gomide, G.; Depeyrot, J. Removal of Direct Yellow 12 from Water Using CTAB-Coated Core-Shell Bimagnetic Nanoadsorbents. J. Environ. Chem. Eng. 2019, 7, 103031. [Google Scholar] [CrossRef]
- Rodovalho, F.L.; Capistrano, G.; Gomes, J.A.; Sodré, F.F.; Chaker, J.A.; Campos, A.F.C.; Bakuzis, A.F.; Sousa, M.H. Elaboration of Magneto-Thermally Recyclable Nanosorbents for Remote Removal of Toluene in Contaminated Water Using Magnetic Hyperthermia. Chem. Eng. J. 2016, 302, 725. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Z.; Sa, B.; Miao, N.; Zhou, J.; Sun, Z. Computational Design of Double Transition Metal MXenes with Intrinsic Magnetic Properties. Nanoscale Horiz. 2022, 7, 276. [Google Scholar] [CrossRef]
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247. [Google Scholar] [CrossRef]
- Salvador, M.; Moyano, A.; Martínez-García, J.C.; Blanco-López, M.C.; Rivas, M. Synthesis of Superparamagnetic Iron Oxide Nanoparticles: SWOT Analysis Towards Their Conjugation to Biomolecules for Molecular Recognition Applications. J. Nanosci. Nanotechnol. 2019, 19, 4839. [Google Scholar] [CrossRef] [PubMed]
- Socoliuc, V.; Peddis, D.; Petrenko, V.I.; Avdeev, M.V.; Susan-Resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vékás, L. Magnetic Nanoparticle Systems for Nanomedicine — A Materials Science Perspective. Magetochemistry 2019, 6, 2. [Google Scholar] [CrossRef]
- Iida, H.; Takayanagi, K.; Nakanishi, T.; Osaka, T. Synthesis of Fe3O4 Nanoparticles with Various Sizes and Magnetic Properties by Controlled Hydrolysis. J. Colloid Interface Sci. 2007, 314, 274. [Google Scholar] [CrossRef] [PubMed]
- Omelyanchik, A.; da Silva, F.G.; Gomide, G.; Kozenkov, I.; Depeyrot, J.; Aquino, R.; Campos, A.F.C.; Fiorani, D.; Peddis, D.; Rodionova, V.; et al. Effect of Citric Acid on the Morpho-Structural and Magnetic Properties of Ultrasmall Iron Oxide Nanoparticles. J. Alloys Compd. 2021, 883, 160779. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Kamzin, A.S.; Valiullin, A.A.; Semenov, V.G.; Vereshchagin, S.N.; Volochaev, M.; Dubrovskiy, A.; Sviridova, T.; Kozenkov, I.; Dolan, E.; et al. Iron Oxide Nanoparticles Synthesized by a Glycine-Modified Coprecipitation Method: Structure and Magnetic Properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129090. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, N.; Uzun, S.; Levitt, A.; Seyedin, S.; Lynch, P.A.; Qin, S.; Han, M.; Yang, W.; Liu, J.; et al. Scalable Manufacturing of Free-Standing, Strong Ti3C2Tx MXene Films with Outstanding Conductivity. Adv. Mater. 2020, 32, 2001093. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhou, Y.; Tan, Y.; Cheng, Z.; Yang, B.; Ma, Y.; Shen, Z.; Jia, J. Exploring Adsorption Behavior and Oxidation Mechanism of Mercury on Monolayer Ti2CO2 (MXenes) from First Principles. Appl. Surf. Sci. 2019, 464, 53. [Google Scholar] [CrossRef]
- Shahzad, A.; Rasool, K.; Miran, W.; Nawaz, M.; Jang, J.; Mahmoud, K.A.; Lee, D.S. Two-Dimensional Ti3C2Tx MXene Nanosheets for Efficient Copper Removal from Water. ACS Sustain. Chem. Eng. 2017, 5, 11481–11488. [Google Scholar]
- Aziz, K.; El Achaby, M.; Mamouni, R.; Saffaj, N.; Aziz, F. A novel hydrogel beads based copper-doped Cerastoderma edule shells@Alginate biocomposite for highly fungicide sorption from aqueous medium. Chemosphere 2022, 311, 136932. [Google Scholar] [CrossRef]
- Sasikala, V.; Vignesh, S.; Sundar, J.K.; El Sayed Massoud, E. Construction of three-dimensional polymeric d-histidine based metal-organic framework (MOF) for selective sorption of CO2 and copper ion sensing applications. Chem. Phys. Lett. 2022, 790. [Google Scholar] [CrossRef]
- Zada, S.; Raza, S.; Khan, S.; Iqbal, A.; Kai, Z.; Ahmad, A.; Ullah, M.; Kakar, M.; Fu, P.; Dong, H.; et al. Microalgal and cyanobacterial strains used for the bio sorption of copper ions from soil and wastewater and their relative study. J. Ind. Eng. Chem. 2022, 105, 463–472. [Google Scholar] [CrossRef]
- Pimneva, L.; Malyshkina, E.; Salnikova, E. Regularities of Sorption of Cations of Zinc and Copper by Natural Sorbent. Procedia Eng. 2016, 165, 853–859. [Google Scholar] [CrossRef]
- Boamah, P.O.; Huang, Y.; Hua, M.; Onumah, J.; Sam-Amoah, L.K.; Boamah, P.O.; Qian, Y.; Zhang, Q. Sorption of copper onto low molecular weight chitosan derivative from aqueous solution. Ecotoxicol. Environ. Saf. 2016, 129, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, L.; Barthen, R.; Stockmann, M.; Gruendig, M.; Franke, K.; Lippmann-Pipke, J. Effect of glutamic acid on copper sorption onto kaolinite-Batch experiments and surface complexation modeling. Chemosphere 2017, 178, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Herschy, R.W. Water quality for drinking: WHO guidelines. Encycl. Earth Sci. Ser. 2012, 876–883. [Google Scholar]
- Peng, C.; Wang, C.-A.; Qi, L.; Huang, Y. A Novel Simple Method to Stably Synthesize Ti3AlC2 Powder with High Purity. Mat. Sci. Forum 2006, 428, 54–58. [Google Scholar] [CrossRef]
- Lu, X.; Zhow, Y. Presureless Sinering and Properties of Ti3AlC2. Int. J. Appl. Cer. Tech. 2013, 7, 744–751. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Sobolev, K.; Shilov, N.; Andreev, N.; Gorshenkov, M.; Rodionova, V. Modification of the Coprecipitation Method for the Synthesis of Iron Oxide Nanoparicles with High Magnetization and Tunable Reaction Yield. Nanobiotechnology Reports in press. 2023.
- Dehsari, H.S.; Ksenofontov, V.; Möller, A.; Jakob, G.; Asadi, K. Determining Magnetite/Maghemite Composition and Core-Shell Nanostructure from Magnetization Curve for Iron Oxide Nanoparticles. J. Phys. Chem. C 2018, 122, 28292. [Google Scholar] [CrossRef]
- Slimani, S.; Concas, G.; Congiu, F.; Barucca, G.; Yaacoub, N.; Talone, A.; Smari, M.; Dhahri, E.; Peddis, D.; Muscas, G. Hybrid Spinel Iron Oxide Nanoarchitecture Combining Crystalline and Amorphous Parent Material. J. Phys. Chem. C 2021, 125, 10611. [Google Scholar] [CrossRef]
- Li, Y.; Shao, H.; Lin, Z.; Lu, J.; Liu, L.; Duployer, B.; Persson, P.O.Å.; Eklund, P.; Hultman, L.; Li, M.; et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 2020, 19, 894. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, K.; Zhou, K.; Gu, J. Controlled Distributed Ti3C2Tx Hollow Microspheres on Thermally Conductive Polyimide Composite Films for Excellent Electromagnetic Interference Shielding. Adv. Mater. 2023, 35, 2211642. [Google Scholar] [CrossRef] [PubMed]
- Batlle, X.; Pérez, N.; Guardia, P.; Iglesias, O.; Labarta, A.; Bartolomé, F.; Serna, C. Magnetic nanoparticles with bulklike properties (invited). J. Appl. Phys. 2011, 109, 07B524. [Google Scholar] [CrossRef]
- Knobel, M.; Nunes, W.C.; Socolovsky, L.M.; De Biasi, E.; Vargas, J.M.; Denardin, J.C. Superparamagnetism and Other Magnetic Features in Granular Materials: A Review on Ideal and Real Systems. J. Nanosci. Nanotechnol. 2008, 8, 2836–2857. [Google Scholar] [CrossRef]
- Kneller, E.F.; Luborsky, F.E. Particle Size Dependence of Coercivity and Remanence of Single-Domain Particles. J. Appl. Phys. 1963, 34, 656–658. [Google Scholar] [CrossRef]
- Elumalai, S.; Yoshimura, M.; Ogawa, M. Simultaneous Delamination and Rutile Formation on the Surface of Ti3C2Tx MXene for Copper Adsorption. Chem. Asian J. 2020, 15, 1044–1051. [Google Scholar] [CrossRef]
| Sample | Agitation Type | Volume of Water, mL | Fe2+, mmol | Fe3+, mmol | Temperature, °C |
|---|---|---|---|---|---|
| M:Fe3O4 | mechanical | 100 | 1 | 2 | 80 |
| M:MX-100 | mechanical | 100 | 1 | 2 | 80 |
| US:Fe3O4 | ultrasonic | 5 | 0.25 | 0.5 | 80 |
| US:MX-50 | ultrasonic | 5 | 0.25 | 0.5 | 80 |
| US:MX-100 | ultrasonic | 5 | 0.25 | 0.5 | 80 |
| US:MX-200 | ultrasonic | 5 | 0.25 | 0.5 | 80 |
| Sample | dTEM [nm] | σ | DXRD [nm] | a [Å] | Fe3O4 [%] | TiC [%] | Ti3C2Tx [%] |
|---|---|---|---|---|---|---|---|
| M:Fe3O4 | — | — | 7.5 ± 0.8 | 8.385 ± 0.007 | 100 | — | — |
| M:MX-100 | 14 | 0.39 | — | — | — | — | — |
| US:Fe3O4 | 14 | 0.31 | 12 ± 1 | 8.375 ± 0.008 | 100 | — | — |
| US:MX-50 | 15 | 0.45 | 14 ± 1 | 8.377 ± 0.004 | 50 ± 5 | 32 ± 5 | 18 ± 3 |
| US:MX-100 | 20 | 0.42 | 15 ± 3 | 8.351 ± 0.006 | 33 ± 4 | 45 ± 5 | 22 ± 3 |
| US:MX-200 | 52 | 0.42 | 27 ± 5 | 8.378 ± 0.003 | 22 ± 3 | 50 ± 5 | 28 ± 4 |
| Sample | dh [nm] | FWHM | SSABET [m2/g] |
|---|---|---|---|
| US:MX-50 | 209 | 25 | 34.2 |
| US:MX-100 | 286 | 28 | 18.7 |
| US:MX-200 | 447 | 162 | 7.9 |
| Sample | MS [A m2 kg−1] | MR/MS | µ0HC [mT] |
|---|---|---|---|
| M:Fe3O4 | 39 ± 3 | — | — |
| M:MX-100 | 30 ± 3 | — | — |
| US:Fe3O4 | 79 ± 4 | 0.06 | 1.9 ± 0.1 |
| US:MX-50 | 38 ± 3 | 0.08 | 3.3 ± 0.1 |
| US:MX-100 | 27 ± 3 | 0.12 | 5.3 ± 0.1 |
| US:MX-200 | 16 ± 2 | 0.19 | 11.1 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolev, K.; Omelyanchik, A.; Shilov, N.; Gorshenkov, M.; Andreev, N.; Comite, A.; Slimani, S.; Peddis, D.; Ovchenkov, Y.; Vasiliev, A.; et al. Iron Oxide Nanoparticle-Assisted Delamination of Ti3C2Tx MXenes: A New Approach to Produce Magnetic MXene-Based Composites. Nanomaterials 2024, 14, 97. https://doi.org/10.3390/nano14010097
Sobolev K, Omelyanchik A, Shilov N, Gorshenkov M, Andreev N, Comite A, Slimani S, Peddis D, Ovchenkov Y, Vasiliev A, et al. Iron Oxide Nanoparticle-Assisted Delamination of Ti3C2Tx MXenes: A New Approach to Produce Magnetic MXene-Based Composites. Nanomaterials. 2024; 14(1):97. https://doi.org/10.3390/nano14010097
Chicago/Turabian StyleSobolev, Kirill, Alexander Omelyanchik, Nikolai Shilov, Mikhail Gorshenkov, Nikolai Andreev, Antonio Comite, Sawssen Slimani, Davide Peddis, Yevgeniy Ovchenkov, Alexander Vasiliev, and et al. 2024. "Iron Oxide Nanoparticle-Assisted Delamination of Ti3C2Tx MXenes: A New Approach to Produce Magnetic MXene-Based Composites" Nanomaterials 14, no. 1: 97. https://doi.org/10.3390/nano14010097
APA StyleSobolev, K., Omelyanchik, A., Shilov, N., Gorshenkov, M., Andreev, N., Comite, A., Slimani, S., Peddis, D., Ovchenkov, Y., Vasiliev, A., Magomedov, K. E., & Rodionova, V. (2024). Iron Oxide Nanoparticle-Assisted Delamination of Ti3C2Tx MXenes: A New Approach to Produce Magnetic MXene-Based Composites. Nanomaterials, 14(1), 97. https://doi.org/10.3390/nano14010097











