Preparations and Thermal Properties of PDMS-AlN-Al2O3 Composites through the Incorporation of Poly(Catechol-Amine)-Modified Boron Nitride Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
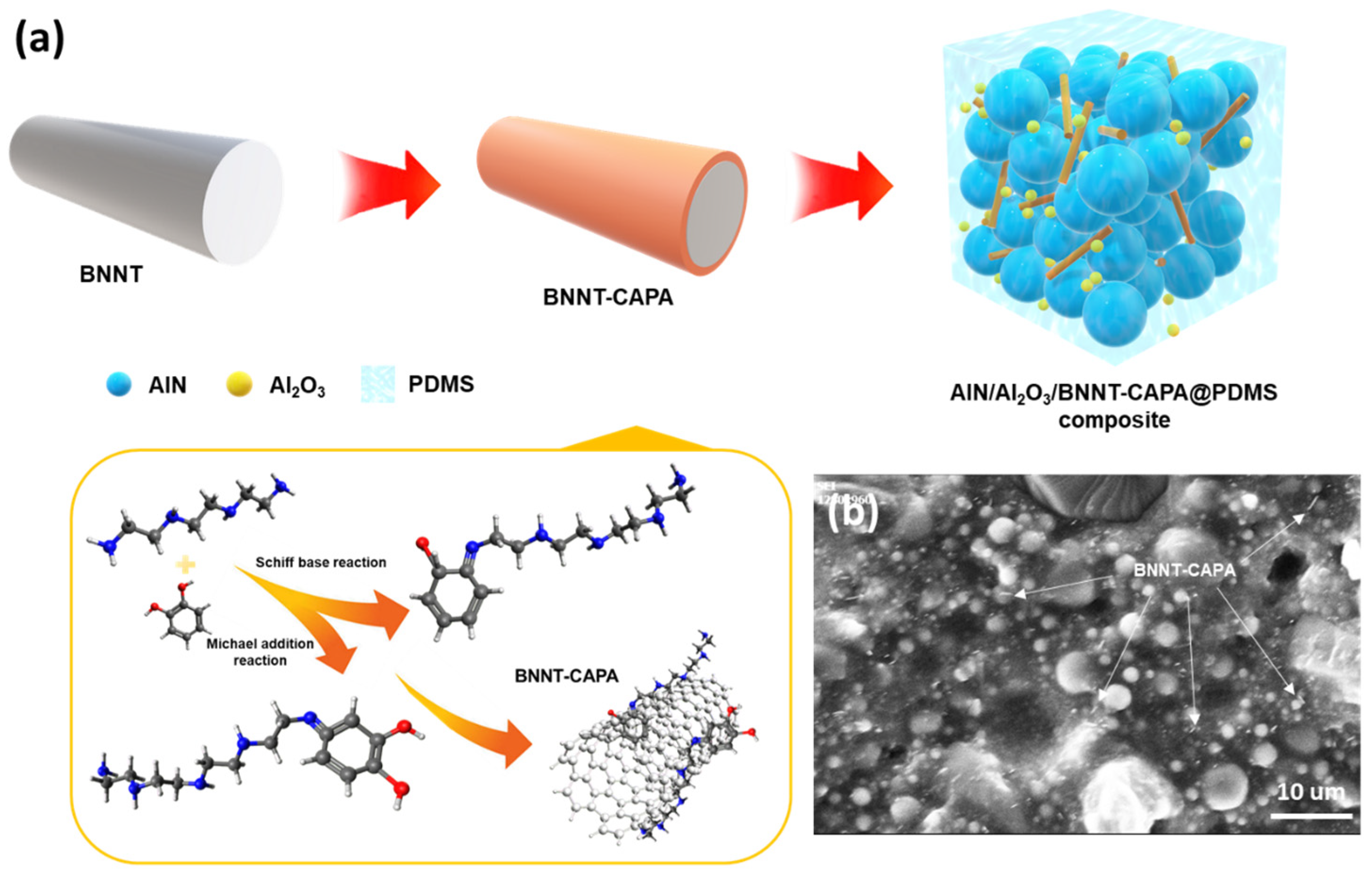
2.2. Preparation of BNNT-CAPA
2.3. Preparation of PDMS/AlN/Al2O3/BNNT-CAPA Composites
2.4. Characterizations
3. Results and Discussion
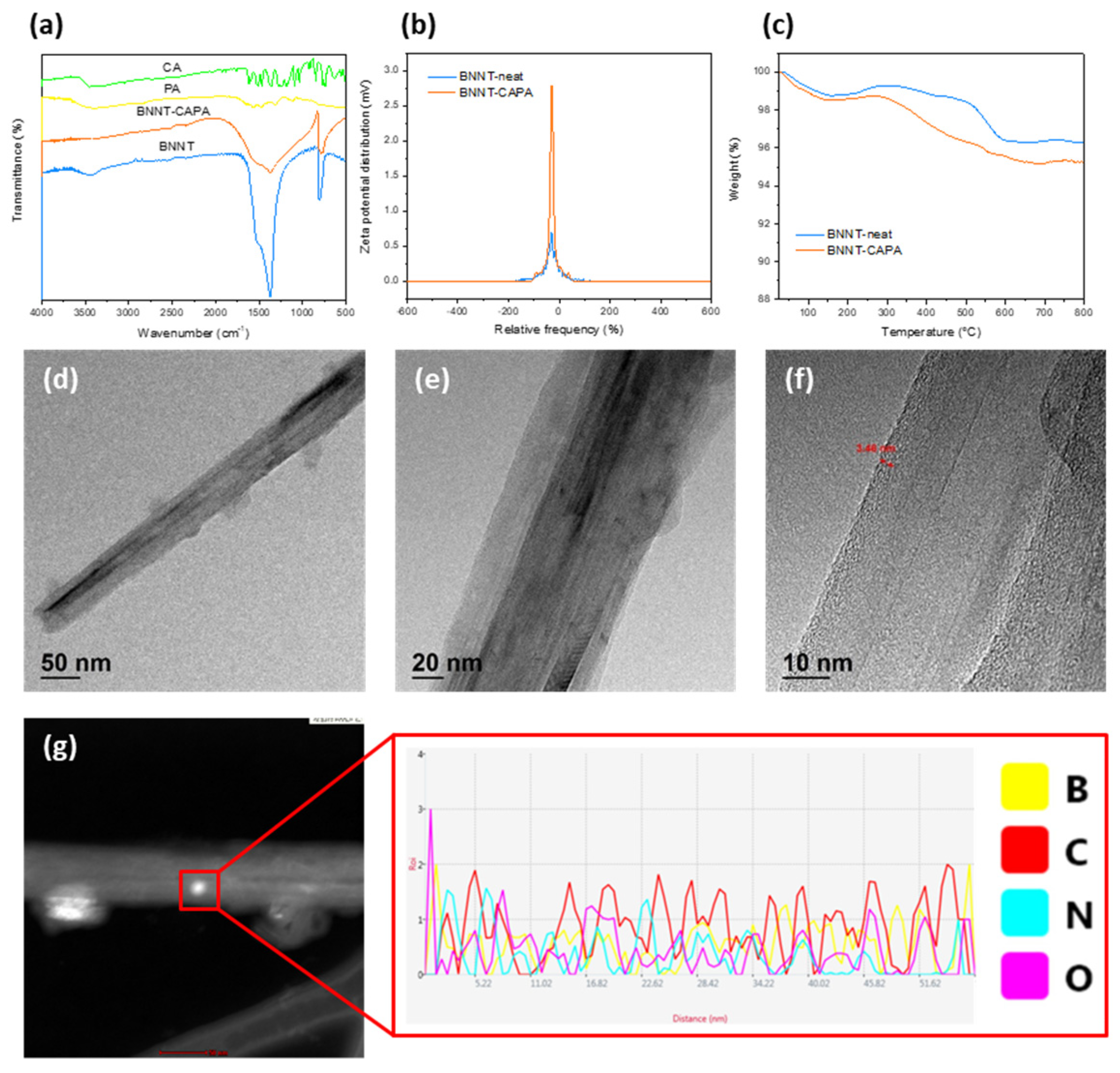
3.1. Chemical and Physical Characterization of BNNT-CAPA
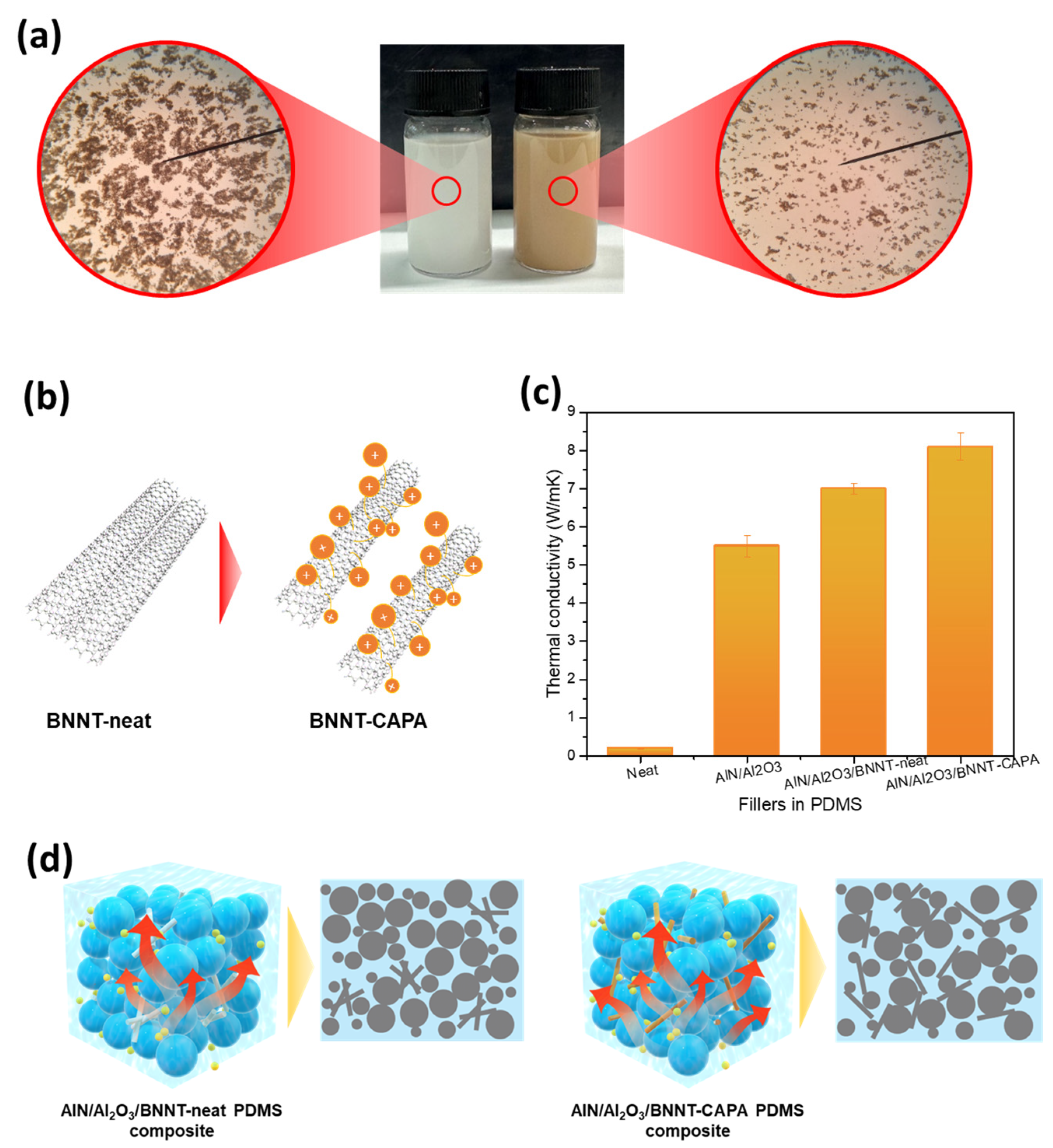
3.2. Modified BNNT Dispersion and Thermal Conductivity Performance
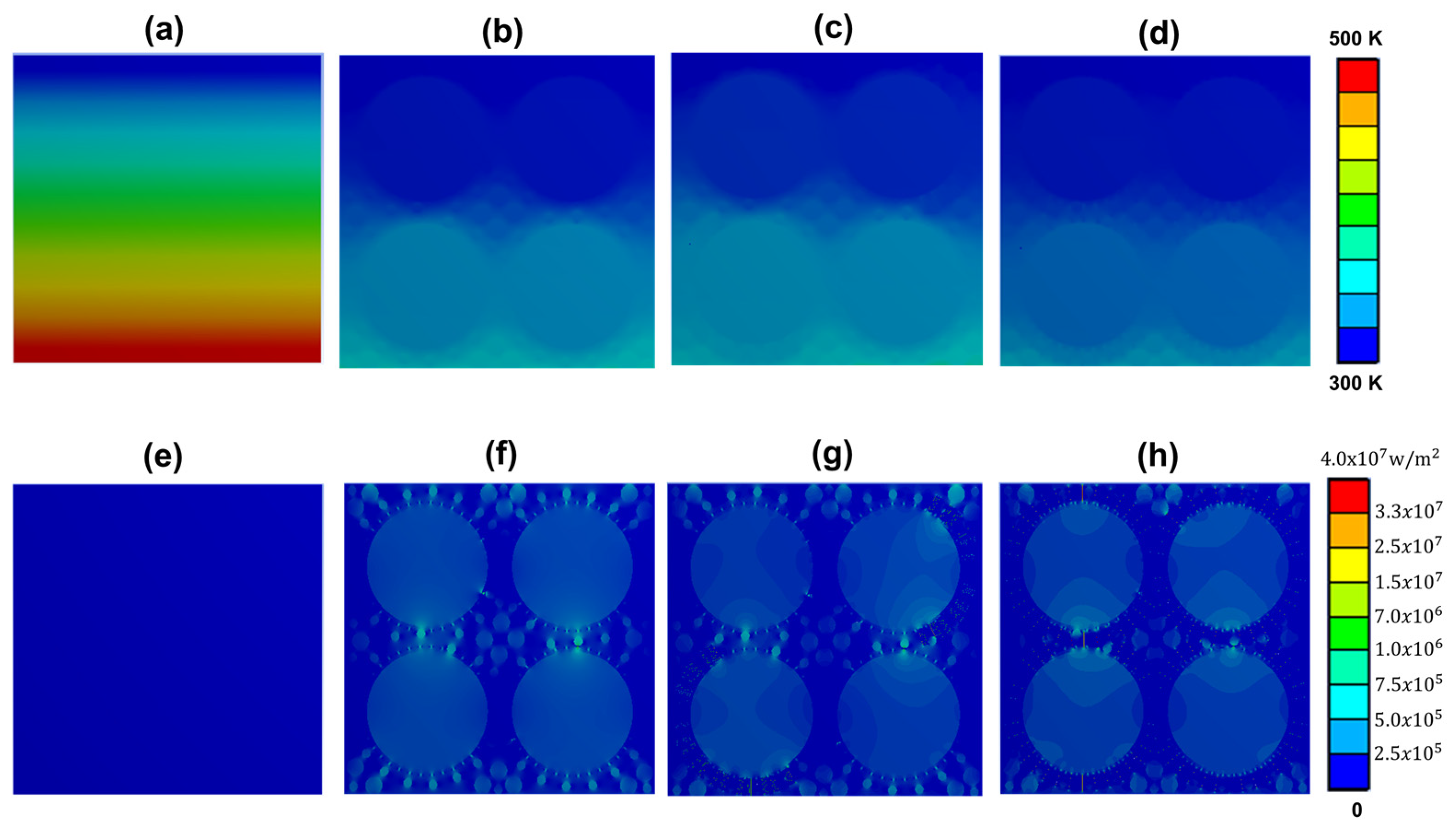
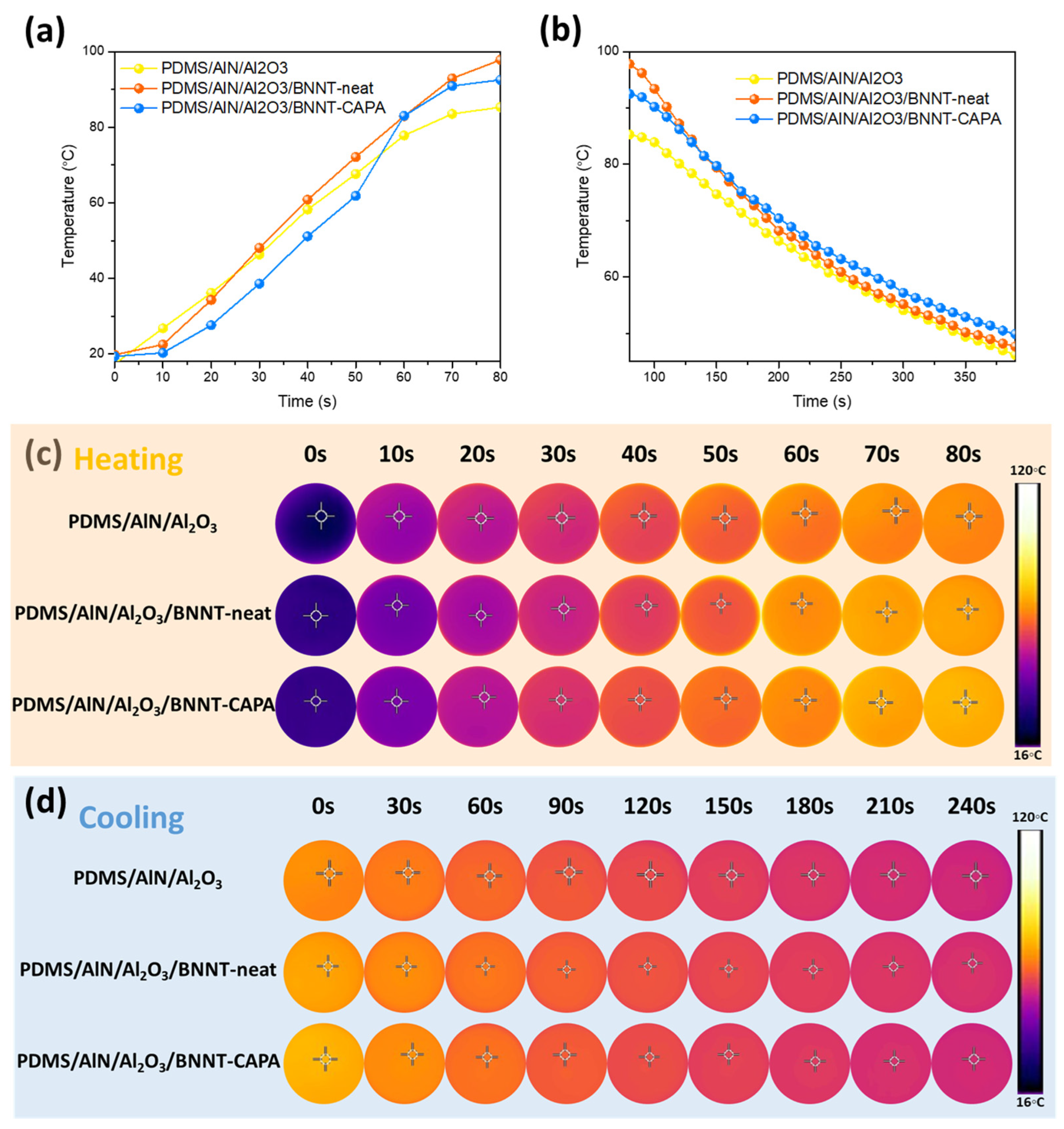
3.3. Practical Thermal Management Demonstration Performance
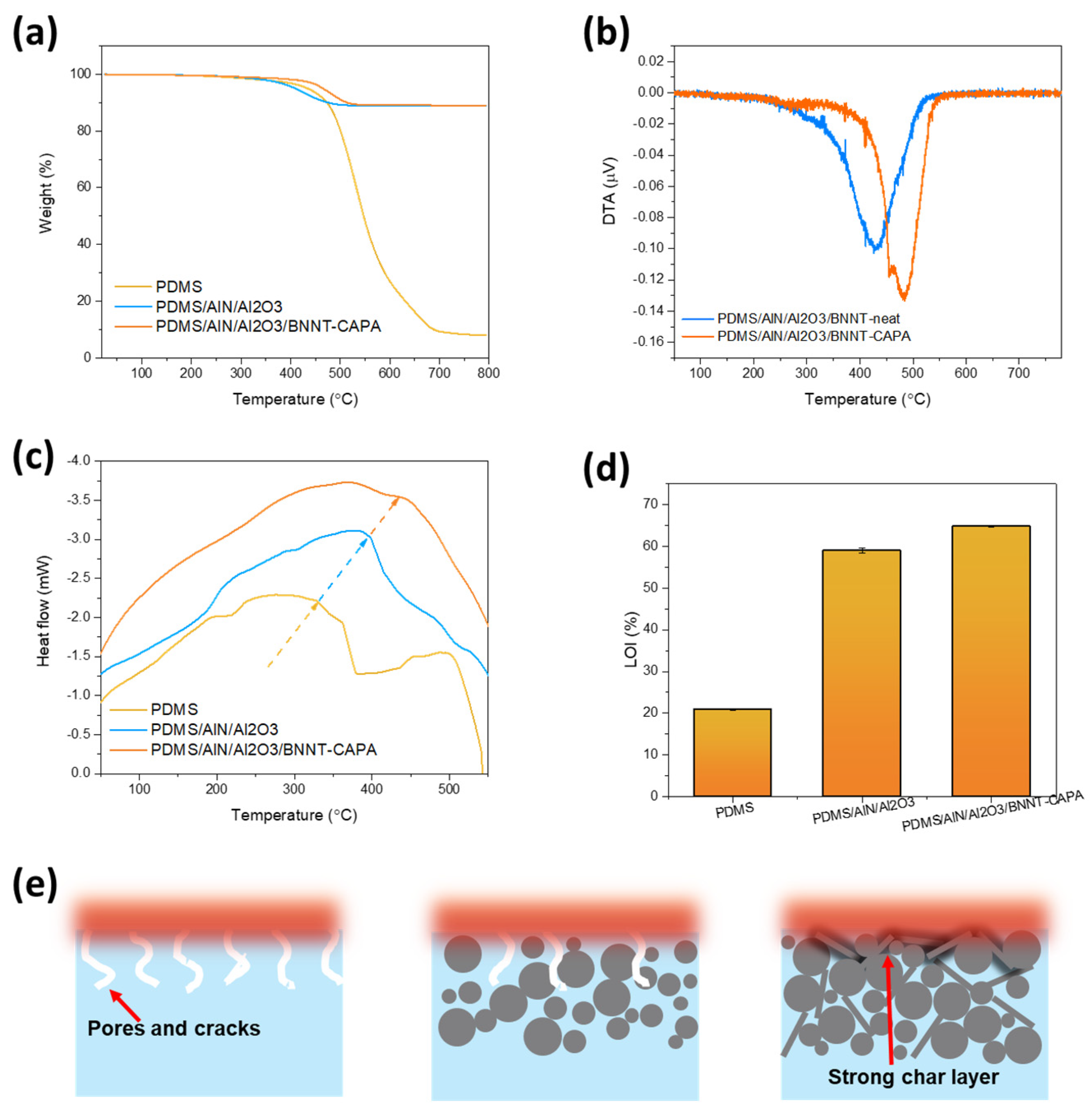
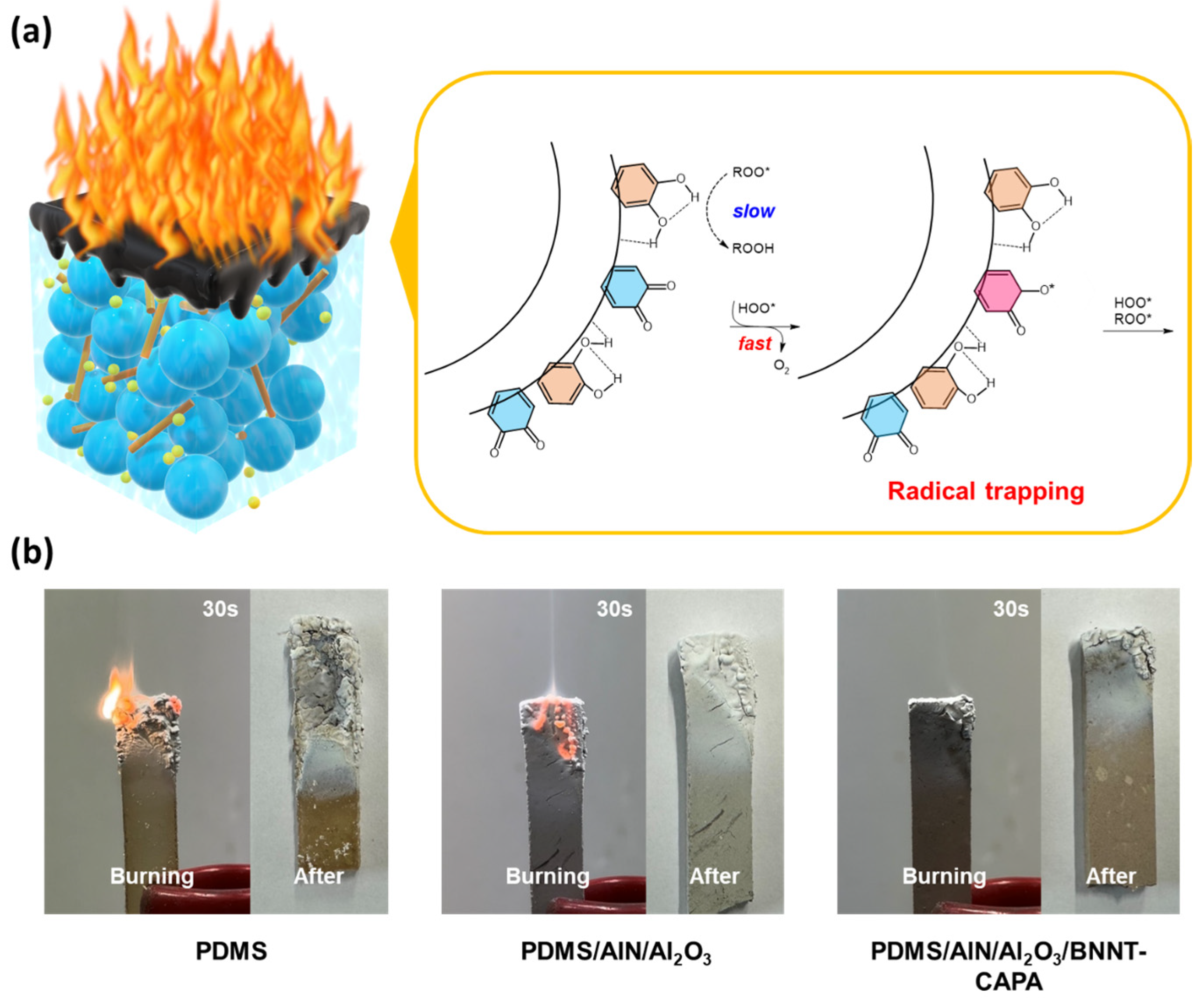
3.4. Fire Repellence Performance Demonstration of PDMS/AlN/Al2O3/BNNT-CAPA Composite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dou, Z.; Zhang, B.; Xu, P.; Fu, Q.; Wu, K. Dry-Contact Thermal Interface Material with the Desired Bond Line Thickness and Ultralow Applied Thermal Resistance. ACS Appl. Mater. Interfaces 2023, 15, 57602–57612. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Lu, Z.; Wang, J.; Ye, N.; Zhang, H.; Zhou, L.; Li, J.; Lu, Y. Construction of Micro-Nano Hybrid Structure Based on Carbon Nanotube Whisker and Alumina for Thermally Conductive yet Electrically Insulating Silicone Rubber Composites. Compos. Sci. Technol. 2024, 249, 110495. [Google Scholar] [CrossRef]
- Han, G.; Zhao, X.; Feng, Y.; Ma, J.; Zhou, K.; Shi, Y.; Liu, C.; Xie, X. Highly Flame-Retardant Epoxy-Based Thermal Conductive Composites with Functionalized Boron Nitride Nanosheets Exfoliated by One-Step Ball Milling. Chem. Eng. J. 2021, 407, 127099. [Google Scholar] [CrossRef]
- Yu, B.; Xing, W.; Guo, W.; Qiu, S.; Wang, X.; Lo, S.; Hu, Y. Thermal Exfoliation of Hexagonal Boron Nitride for Effective Enhancements on Thermal Stability, Flame Retardancy and Smoke Suppression of Epoxy Resin Nanocomposites: Via Sol-Gel Process. J. Mater. Chem. A Mater. 2016, 4, 7330–7340. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, J.; Xue, Y.; He, C.; Zhou, X.; Xie, X.; Ye, Y.; Mai, Y.W. Simultaneous Improvement in the Flame Resistance and Thermal Conductivity of Epoxy/Al2O3 Composites by Incorporating Polymeric Flame Retardant-Functionalized Graphene. J. Mater. Chem. A Mater. 2017, 5, 13544–13556. [Google Scholar] [CrossRef]
- Li, Y.-T.; Liu, W.-J.; Shen, F.-X.; Zhang, G.-D.; Gong, L.-X.; Zhao, L.; Song, P.; Gao, J.-F.; Tang, L.-C. Processing, Thermal Conductivity and Flame Retardant Properties of Silicone Rubber Filled with Different Geometries of Thermally Conductive Fillers: A Comparative Study. Compos. B Eng. 2022, 238, 109907. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, Y.; Chen, C.Y.; Moon, K.; Wang, H.; Wong, C.P. The Use of Polyimide-Modified Aluminum Nitride Fillers in AlN@PI/Epoxy Composites with Enhanced Thermal Conductivity for Electronic Encapsulation. Sci. Rep. 2014, 4, 4779. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, J.; Zhang, H.; Wang, W.; Hu, B.; Xia, D.; Lin, K.; Geng, L.; Yang, Y. Multifunctional MOF@COF Nanoparticles Mediated Perovskite Films Management Toward Sustainable Perovskite Solar Cells. Nano-Micro Lett. 2024, 16, 171. [Google Scholar] [CrossRef]
- Tang, L.; Ruan, K.; Liu, X.; Tang, Y.; Zhang, Y.; Gu, J. Flexible and Robust Functionalized Boron Nitride/Poly(p-Phenylene Benzobisoxazole) Nanocomposite Paper with High Thermal Conductivity and Outstanding Electrical Insulation. Nano-Micro Lett. 2024, 16, 38. [Google Scholar] [CrossRef]
- Qiu, S.; Hou, Y.; Xing, W.; Ma, C.; Zhou, X.; Liu, L.; Kan, Y.; Yuen, R.K.K.; Hu, Y. Self-Assembled Supermolecular Aggregate Supported on Boron Nitride Nanoplatelets for Flame Retardant and Friction Application. Chem. Eng. J. 2018, 349, 223–234. [Google Scholar] [CrossRef]
- Niu, H.; Guo, H.; Kang, L.; Ren, L.; Lv, R.; Liu, L.; Bashir, A.; Bai, S. Highly Thermally Conductive and Soft Thermal Interface Materials Based on Vertically Oriented Boron Nitride Film. Compos. B Eng. 2024, 272, 111219. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Zhao, X.; Ye, Y.; Zhou, X.; Liu, H.; Liu, C.; Xie, X. Synergetic Improvement in Thermal Conductivity and Flame Retardancy of Epoxy/Silver Nanowires Composites by Incorporating “Branch-Like” Flame-Retardant Functionalized Graphene. ACS Appl. Mater. Interfaces 2018, 10, 21628–21641. [Google Scholar] [CrossRef]
- Hanif, Z.; Choi, K.-I.; Jung, J.-H.; Pornea, A.G.M.; Park, E.; Cha, J.; Kim, H.-R.; Choi, J.-H.; Kim, J. Dispersion Enhancement of Boron Nitride Nanotubes in a Wide Range of Solvents Using Plant Polyphenol-Based Surface Modification. Ind. Eng. Chem. Res. 2023, 62, 2662–2670. [Google Scholar] [CrossRef]
- He, X.; Ou, D.; Wu, S.; Luo, Y.; Ma, Y.; Sun, J. A Mini Review on Factors Affecting Network in Thermally Enhanced Polymer Composites: Filler Content, Shape, Size, and Tailoring Methods. Adv. Compos. Hybrid. Mater. 2022, 5, 21–38. [Google Scholar] [CrossRef]
- Yanar, N.; Kim, T.Y.-S.; Jung, J.; Dinh, D.K.; Choi, K.; Pornea, A.G.; Yadav, D.; Hanif, Z.; Park, E.; Kim, J. Boron Nitride Nanotube-Aligned Electrospun PVDF Nanofiber-Based Composite Films Applicable to Wearable Piezoelectric Sensors. ACS Appl. Nano Mater. 2024, 247. [Google Scholar] [CrossRef]
- Hanif, Z.; Khoe, D.D.; Choi, K.-I.; Jung, J.-H.; Pornea, A.G.M.; Yanar, N.; Kwak, C.; Kim, J. Synergistic Effect on Dispersion, Thermal Conductivity and Mechanical Performance of Pyrene Modified Boron Nitride Nanotubes with Al2O3/Epoxy Composites. Compos. Sci. Technol. 2024, 247, 110419. [Google Scholar] [CrossRef]
- Pornea, A.G.M.; Choi, K.-I.; Jung, J.-H.; Hanif, Z.; Kwak, C.; Kim, J. Enhancement of Isotropic Heat Dissipation of Polymer Composites by Using Ternary Filler Systems Consisting of Boron Nitride Nanotubes, h-BN, and Al2O3. ACS Omega 2023, 8, 24454–24466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Feng, Y.; Feng, W. Three-Dimensional Interconnected Networks for Thermally Conductive Polymer Composites: Design, Preparation, Properties, and Mechanisms. Mater. Sci. Eng. R Rep. 2020, 142, 100580. [Google Scholar] [CrossRef]
- Pornea, A.M.; Kim, H. Design and Synthesis of SiO2/TiO2/PDA Functionalized Phase Change Microcapsules for Efficient Solar-Driven Energy Storage. Energy Convers. Manag. 2021, 232, 113801. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Cai, C.; Guo, J.; Fan, H.; Zhu, C.; Dong, H.; Zhao, N.; Xu, J. Mussel Inspired Modification of Polypropylene Separators by Catechol/Polyamine for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 5602–5608. [Google Scholar] [CrossRef]
- Liu, X.; Han, Q.; Yang, D.; Yang, D.; Ni, Y.; Ni, Y.; Yu, L.; Yu, L.; Wei, Q.; Wei, Q.; et al. Thermally Conductive Elastomer Composites with Poly(Catechol-Polyamine)-Modified Boron Nitride. ACS Omega 2020, 5, 14006–14012. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Baschieri, A.; Mollica, F.; Valgimigli, L.; Cedrowski, J.; Litwinienko, G.; Amorati, R. Hydrogen Atom Transfer from HOO to Ortho -Quinones Explains the Antioxidant Activity of Polydopamine. Angew. Chem. Int. Ed. 2021, 60, 15220–15224. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Vasagar, V.; Shanmuganathan, K.; Jones, A.R.; Nazarenko, S.; Ellison, C.J. Bioinspired Catecholic Flame Retardant Nanocoating for Flexible Polyurethane Foams. Chem. Mater. 2015, 27, 6784–6790. [Google Scholar] [CrossRef]
- Si, W.; Lei, W.; Zhang, Y.; Xia, M.; Wang, F.; Hao, Q. Electrodeposition of Graphene Oxide Doped Poly(3,4-Ethylenedioxythiophene) Film and Its Electrochemical Sensing of Catechol and Hydroquinone. Electrochim. Acta 2012, 85, 295–301. [Google Scholar] [CrossRef]
- Güzdemir, Ö.; Kanhere, S.; Bermudez, V.; Ogale, A.A. Boron Nitride-Filled Linear Low-Density Polyethylene for Enhanced Thermal Transport: Continuous Extrusion of Micro-Textured Films. Polymers 2021, 13, 3393. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, T.; Chen, M.; Wu, L. Simultaneous Exfoliation and Functionalization of Large-Sized Boron Nitride Nanosheets for Enhanced Thermal Conductivity of Polymer Composite Film. Chem. Eng. J. 2022, 442, 136237. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, D.; Yang, H.; Qu, H.; Yao, C.; Liu, F.; Yu, P.; Yao, J.; You, F.; Jiang, X. Remarkable Enhancement in Thermal Performance of Polypropylene Carbonate by Using Exfoliated Boron Nitride Nanosheets. Chem. Eng. J. 2022, 450, 138247. [Google Scholar] [CrossRef]
- Gu, J.; Ruan, K. Breaking Through Bottlenecks for Thermally Conductive Polymer Composites: A Perspective for Intrinsic Thermal Conductivity, Interfacial Thermal Resistance and Theoretics. Nano-Micro Lett. 2021, 13, 110. [Google Scholar] [CrossRef]
- Chowdhury, P.; Sikka, K.; De Silva, A.; Seshadri, I. On Thermal Interface Materials With Polydisperse Fillers: Packing Algorithm and Effective Properties. In Proceedings of the ASME 2018 International Technical Conference and Exhibition on Packaging and Integration of Electronic and Photonic Microsystems, San Francisco, CA, USA, 27–30 August 2018. [Google Scholar]
- Wei, Q.; Yang, D.; Yu, L.; Ni, Y.; Zhang, L. Fabrication of Carboxyl Nitrile Butadiene Rubber Composites with High Dielectric Constant and Thermal Conductivity Using Al2O3@PCPA@GO Hybrids. Compos. Sci. Technol. 2020, 199, 108344. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Y.; Bian, R.; Wei, Y.; Jiang, S.; Li, K.; Li, X.; Tian, D.; Zhan, X.; Li, J. Construction of a Boron Nitride Nanosheet Hybrid for Tough, Strong, and Flame-Retardant Phenolic Resins. Chem. Eng. J. 2023, 471, 144463. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Chen, C.; Ye, Y.; Zeng, H.; Qu, H.; Liu, J.; Zhou, X.; Long, S.; Xie, X. Highly Thermally Conductive Flame Retardant Epoxy Nanocomposites with Multifunctional Ionic Liquid Flame Retardant-Functionalized Boron Nitride Nanosheets. J. Mater. Chem. A Mater. 2018, 6, 20500–20512. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Hourlier, D. Heat Treatment of Commercial Polydimethylsiloxane PDMS Precursors: Part I. Towards Conversion of Patternable Soft Gels into Hard Ceramics. Ceram. Int. 2019, 45, 6255–6262. [Google Scholar] [CrossRef]
- Hafiezal, M.R.M.; Khalina, A.; Zurina, Z.A.; Azaman, M.D.M.; Hanafee, Z.M. Thermal and Flammability Characteristics of Blended Jatropha Bio-Epoxy as Matrix in Carbon Fiber–Reinforced Polymer. J. Compos. Sci. 2019, 3, 6. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Deshmukh, A.; Suárez-Villagrán, M.Y.; Das, R.; Pal, V.; Dey, S.; Miller, J.H.; Machado, L.D.; Kumbhakar, P.; Tiwary, C.S. 2D Hexagonal Boron Nitride-Coated Cotton Fabric with Self-Extinguishing Property. ACS Appl. Mater. Interfaces 2020, 12, 45274–45280. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M.; Dogan, S.D.; Savas, L.A.; Ozcelik, G.; Tayfun, U. Flame Retardant Effect of Boron Compounds in Polymeric Materials. Compos. B Eng. 2021, 222, 109088. [Google Scholar] [CrossRef]
- Pornea, A.M.; Kim, H. Synthesis of Hybrid Dual-MOF Encapsulated Phase-Changing Material for Improved Broadband Light Absorption and Photothermal Conversion Enabling Efficient Solar Energy Storage. Sol. Energy Mater. Sol. Cells 2022, 244, 111817. [Google Scholar] [CrossRef]
- Sai, T.; Ran, S.; Guo, Z.; Song, P.; Fang, Z. Recent Advances in Fire-retardant Carbon-based Polymeric Nanocomposites through Fighting Free Radicals. SusMat 2022, 2, 411–434. [Google Scholar] [CrossRef]
- Mai, V.D.; Lee, D.I.; Park, J.H.; Lee, D.S. Rheological Properties and Thermal Conductivity of Epoxy Resins Filled with a Mixture of Alumina and Boron Nitride. Polymers 2019, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Chung, J.Y.; Lee, J.G.; Baek, Y.K.; Shin, P.W. Synergistic Effect of Spherical Al2O3 Particles and BN Nanoplates on the Thermal Transport Properties of Polymer Composites. Compos. Part. A Appl. Sci. Manuf. 2017, 98, 184–191. [Google Scholar] [CrossRef]
- Liu, D.; Ma, C.; Chi, H.; Li, S.; Zhang, P.; Dai, P. Enhancing Thermal Conductivity of Polyimide Composite Film by Electrostatic Self-Assembly and Two-Step Synergism of Al2O3 microspheres and BN Nanosheets. RSC Adv. 2020, 10, 42584–42595. [Google Scholar] [CrossRef]
- Liang, D.; Ren, P.; Ren, F.; Jin, Y.; Wang, J.; Feng, C.; Duan, Q. Synergetic Enhancement of Thermal Conductivity by Constructing BN and AlN Hybrid Network in Epoxy Matrix. J. Polym. Res. 2020, 27, 212. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, C.; Ding, J.; Hu, K.; Zheng, K.; Tian, X. Synergetic Enhancement of Thermal Conductivity in the Silica-Coated Boron Nitride (SiO2@BN)/Polymethyl Methacrylate (PMMA) Composites. Colloid. Polym. Sci. 2020, 298, 385–393. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, W.; Li, Y.; Zhao, D.; Yin, H. Hybrid Fillers of Hexagonal and Cubic Boron Nitride in Epoxy Composites for Thermal Management Applications. RSC Adv. 2019, 9, 7388–7399. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pornea, A.G.; Dinh, D.K.; Hanif, Z.; Yanar, N.; Choi, K.-I.; Kwak, M.S.; Kim, J. Preparations and Thermal Properties of PDMS-AlN-Al2O3 Composites through the Incorporation of Poly(Catechol-Amine)-Modified Boron Nitride Nanotubes. Nanomaterials 2024, 14, 847. https://doi.org/10.3390/nano14100847
Pornea AG, Dinh DK, Hanif Z, Yanar N, Choi K-I, Kwak MS, Kim J. Preparations and Thermal Properties of PDMS-AlN-Al2O3 Composites through the Incorporation of Poly(Catechol-Amine)-Modified Boron Nitride Nanotubes. Nanomaterials. 2024; 14(10):847. https://doi.org/10.3390/nano14100847
Chicago/Turabian StylePornea, Arni Gesselle, Duy Khoe Dinh, Zahid Hanif, Numan Yanar, Ki-In Choi, Min Seok Kwak, and Jaewoo Kim. 2024. "Preparations and Thermal Properties of PDMS-AlN-Al2O3 Composites through the Incorporation of Poly(Catechol-Amine)-Modified Boron Nitride Nanotubes" Nanomaterials 14, no. 10: 847. https://doi.org/10.3390/nano14100847
APA StylePornea, A. G., Dinh, D. K., Hanif, Z., Yanar, N., Choi, K.-I., Kwak, M. S., & Kim, J. (2024). Preparations and Thermal Properties of PDMS-AlN-Al2O3 Composites through the Incorporation of Poly(Catechol-Amine)-Modified Boron Nitride Nanotubes. Nanomaterials, 14(10), 847. https://doi.org/10.3390/nano14100847





