Upconversion Emission and Dual-Mode Sensing Characteristics of NaYF4:Yb3+/Er3+ Microcrystals at High and Ultralow Temperatures
Abstract
1. Introduction
2. Experimental Details
2.1. Materials and Equipment
2.2. Sample Preparation
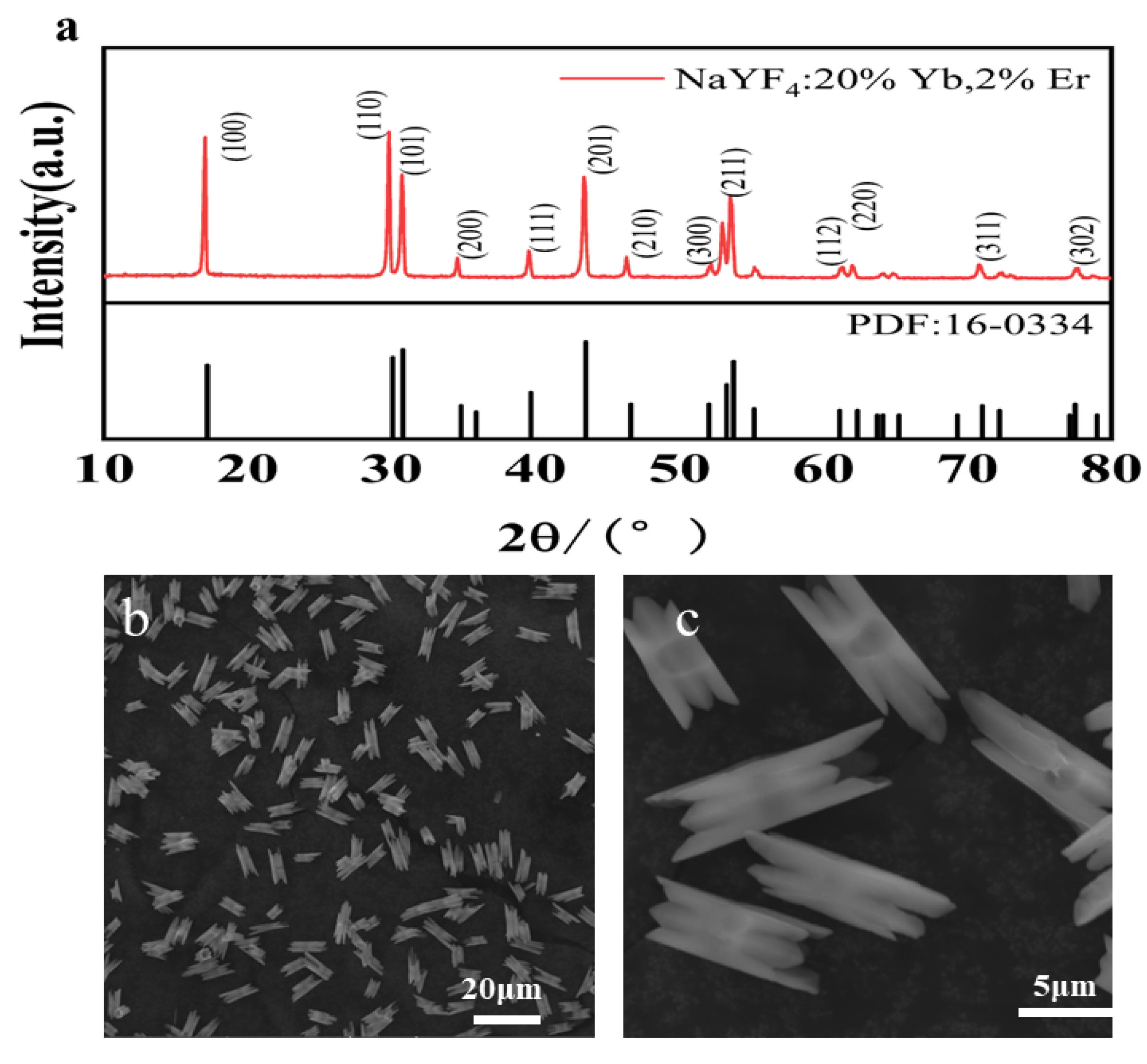
3. Sample Characterizations
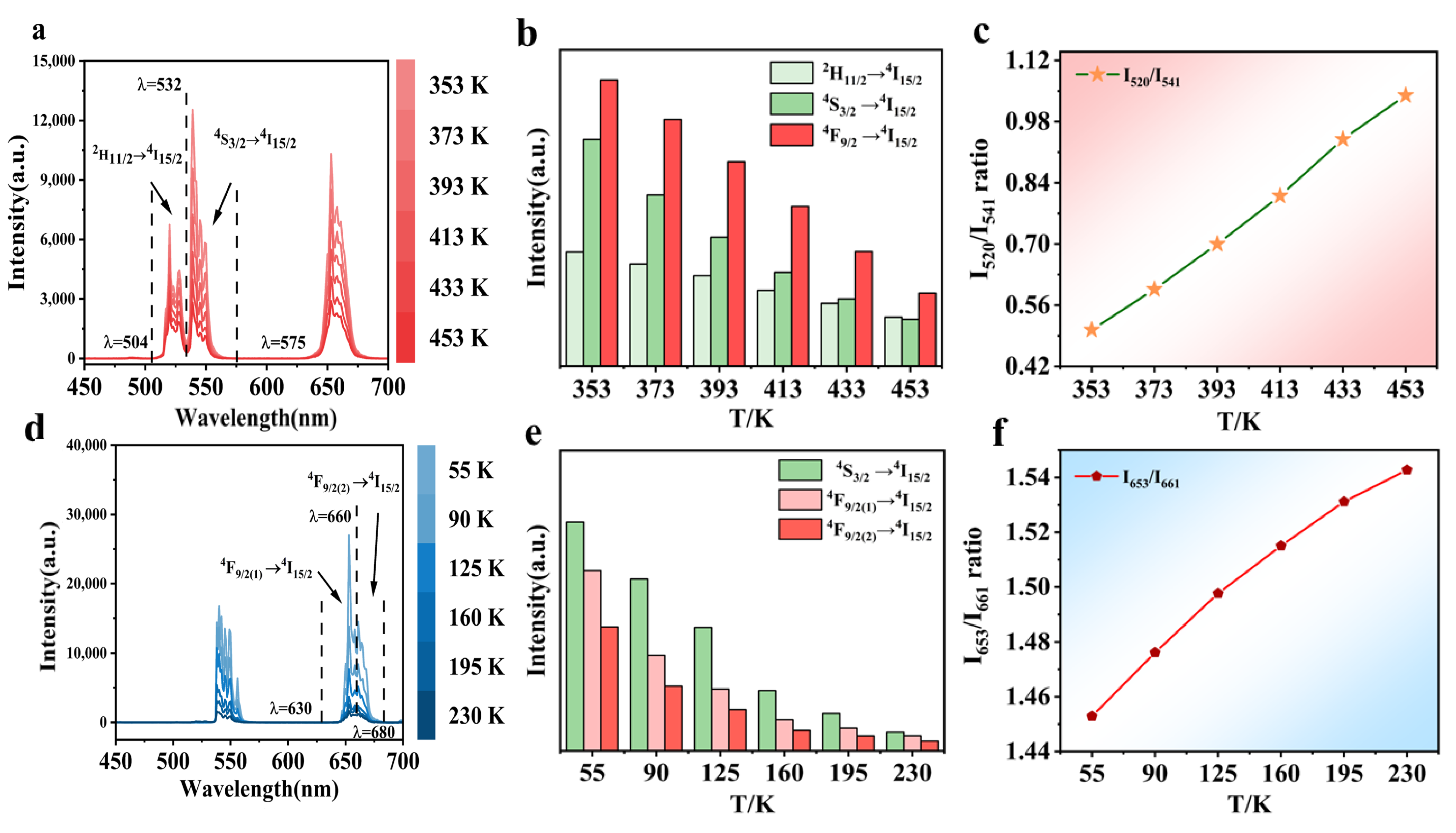
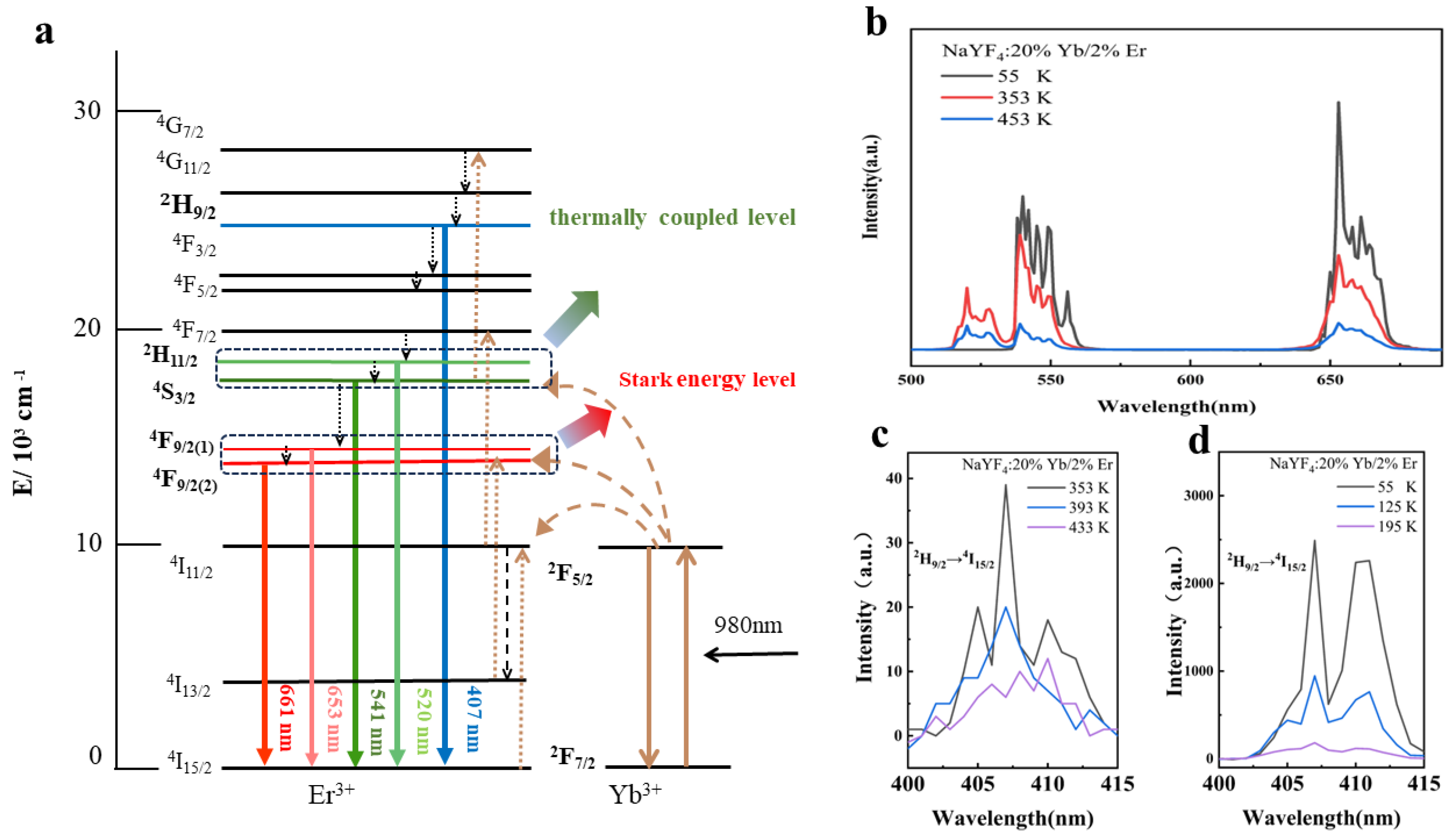
4. Temperature-Dependent Luminescence Characteristics
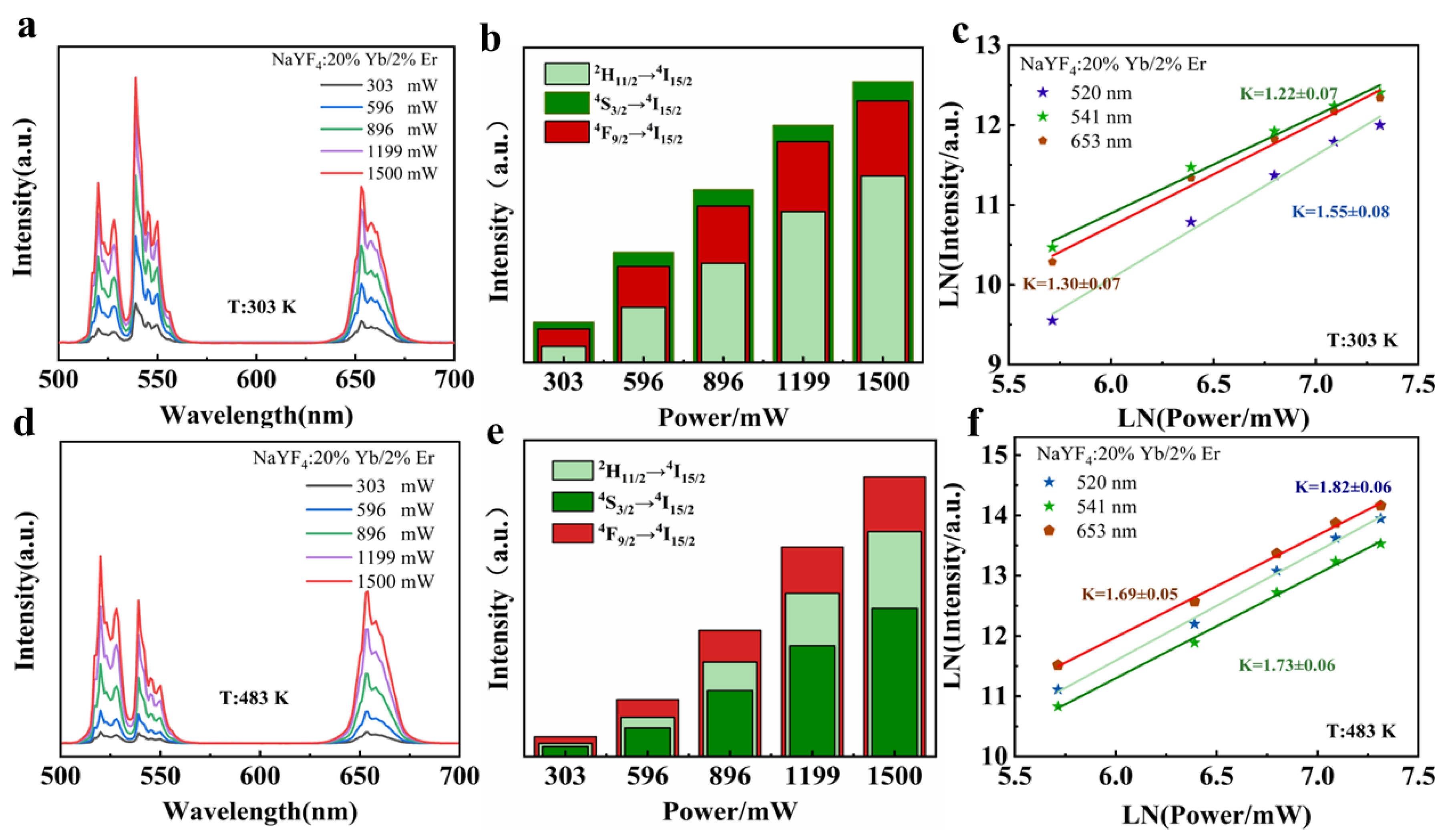
5. Dual-Mode Luminescence Characteristics
5.1. Temperature-Sensing Characteristics in Dual Mode
5.1.1. Dual-Mode Sensing Characteristics at High Temperatures
5.1.2. Dual-Mode Sensing Characteristics at Low-Temperatures
5.1.3. Comparison of the High and Low-Temperature Conditions in Dual Mode
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brites, C.D.; Lima, P.P.; Silva, N.J.; Millan, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef] [PubMed]
- Kilbane, J.D.; Chan, E.M.; Monachon, C.; Borys, N.J.; Levy, E.S.; Pickel, A.D.; Urban, J.J.; Schuck, P.J.; Dames, C. Far-field optical nanothermometry using individual sub-50 nm upconverting nanoparticles. Nanoscale 2016, 8, 11611–11616. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Zhao, X.; Zhang, Z.; Shi, R.; Wu, Y.; Xiang, J.; Guo, C. Local symmetric distortion boosted photon up-conversion and thermometric sensitivity in lanthanum oxide nanospheres. Nanoscale 2018, 10, 9245–9251. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mu, X.; Huo, J.; Zhang, B.; Zhang, K. Highly-efficient SERS detection for E. coli using a microfluidic chip with integrated NaYF4:Yb,Er@SiO2@Au under near-infrared laser excitation. Microsyst. Technol. 2020, 27, 3285–3291. [Google Scholar] [CrossRef]
- Yagi, K.; Mori, K.; Odawara, O.; Wada, H. Preparation of spherical upconversion nanoparticles NaYF4:Yb,Er by laser ablation in liquid and optical properties. J. Laser Appl. 2020, 32, 022062. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Leng, Z.; Lin, H.; Zeng, F.; Li, C.; Su, Z. Er, Yb:CeF3 red emission nanoparticles with controllable size and enhanced luminescence properties. J. Mater. Sci. Mater. Electron. 2021, 32, 8213–8225. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, W.; Wang, R.; Shao, J.; Han, Q.; Wang, C.; Zhang, J.; Zhang, T.; Dong, J.; Zheng, H. Influence of SiO2 layer on the plasmon quenched upconversion luminescence emission of core-shell NaYF4:Yb,Er@SiO2@Ag nanocomposites. Mater. Res. Bull. 2016, 83, 515–521. [Google Scholar] [CrossRef]
- Dubey, A.; Soni, A.K.; Kumari, A.; Dey, R.; Rai, V.K. Enhanced green upconversion emission in NaYF4:Er3+/Yb3+/Li+ phosphors for optical thermometry. J. Alloys Compd. 2017, 693, 194–200. [Google Scholar] [CrossRef]
- Ghazyani, N.; Majles Ara, M.H.; Raoufi, M. Nonlinear photoresponse of NaYF4:Yb,Er@NaYF4 nanocrystals under green CW excitation: A comprehensive study. J. Chem. Phys. 2020, 10, 25696–25702. [Google Scholar] [CrossRef]
- Gu, H.; Wang, J.; Li, Y.; Wang, Z.; Fu, Y. The core-shell-structured NaYF4:Er3+,Yb3+@NaYF4:Eu3+ nanocrystals as dual-mode and multifunctional luminescent mechanism for high-performance dye-sensitized solar cells. Mater. Res. Bull. 2018, 108, 219–225. [Google Scholar] [CrossRef]
- Calabro, R.L.; Karna, P.; Kim, D.Y.; Yang, D.S. Controlled synthesis and characterization of NaYF4:Yb/Er upconverting nanoparticles produced by laser ablation in liquid. J. Chem. Phys. 2020, 153, 064701–064710. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.M.D.; Le, T.T.G.; Nguyen, T.P.N.; Dau, T.a.N.; Nguyen, V.T.; Tran, T.T.V. Investigating the effect of Yb3+ and Er3+ concentration on red/green luminescent ratio in β-NaYF4: Er, Yb nanocrystals using spectroscopic techniques. J. Mol. Struct. 2020, 1210, 128014. [Google Scholar] [CrossRef]
- Cao, T.M.D.; Le, T.T.G.; Turrell, S.; Ferrari, M.; Lam, Q.V.; Tran, T.T.V. Luminescent Ink Based on Upconversion of NaYF4:Er,Yb@MA Nanoparticles: Environmental Friendly Synthesis and Structural and Spectroscopic Assessment. Molecules 2021, 26, 1041. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Wang, T.; Wang, L.; Liu, X.; Zhu, X. Preparation of NaYF4:Yb, Er nanoparticles coated with hydrophilic polystyrene. Mater. Lett. 2019, 247, 159–162. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Han, Q.; Wang, G.; Zhang, M.; Zhang, J.; Gao, W.; Zheng, H. Metal-enhanced upconversion luminescence of NaYF4:Yb/Er with Ag nanoparticles. Mater. Res. Bull. 2017, 88, 182–187. [Google Scholar] [CrossRef]
- Feng, Z.; Lin, L.; Wang, Z.; Zheng, Z. NIR optical temperature sensing with efficiently relative sensitivity based on β-NaYF4: Er3+ nanoparticles. J. Lumin. 2019, 221, 117005. [Google Scholar]
- Meng, M.; Zhang, T.; Wang, J.; Cheng, Z.; Yang, J.; Qiao, X.; Wen, J.; Resch-Genger, U.; Ou, J. Fluorescence temperature sensing of NaYF4:Yb3+/Tm3+@NaGdF4:Nd3+/Yb3+ nanoparticles at low and high temperatures. Nanotechnology 2022, 33, 455502. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Xiao, Q.; Lv, L.; Wu, X.; Dong, X.; Fan, Y.; Zhou, N.; Luo, X. Winning color-tunable upconversion luminescence and high-sensitive optical thermometry in K3Gd(PO4)2:Yb3+,Er3+,Tm3+. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 291, 122324. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ren, X.; Yang, K.; Zhao, S.; Huang, L.; Xu, S. Detection of hydrogen concentration based on an all-fiber fluorescence intensity ratio optical thermometer with Er3+/Yb3+ codoped NaBi(WO4)2 phosphors. Optik 2021, 242, 167280. [Google Scholar] [CrossRef]
- Li, H.; Yu, M.; Dai, J.; Zhou, G.; Sun, J. Upconversion Nanoparticle-Based Fluorescent Film for Distributed Temperature Monitoring of Mobile Phones’ Integrated Chips. Nanomaterials 2023, 13, 1704. [Google Scholar] [CrossRef]
- Klier, D.T.; Kumke, M.U. Upconversion Luminescence Properties of NaYF4:Yb:Er Nanoparticles Codoped with Gd3+. J. Phys. Chem. C 2015, 119, 3363–3373. [Google Scholar] [CrossRef]
- López De Guereñu, A.; Klier, D.T.; Haubitz, T.; Kumke, M.U. Influence of Gd3+ doping concentration on the properties of Na(Y,Gd)F4:Yb3+, Tm3+ upconverting nanoparticles and their long-term aging behavior. Photochem. Photobiol. Sci. 2022, 21, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, D.; Yue, C. Exceptional low-temperature fluorescence sensing properties in novel KBaY(MoO4)3:Yb3+,Ho3+ materials based on FIR of Ho3+ transitions 5F5(1) → 5I8/5S2 → 5I8. J. Mater. Chem. C 2022, 10, 6603–6610. [Google Scholar] [CrossRef]
- Pollnau, M.; Gamelin, D.R.; Lüthi, S.R.; Güdel, H.U.; Hehlen, M.P. Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys. Rev. B 2000, 61, 3337–3346. [Google Scholar] [CrossRef]
- Mai, H.; Zhang, Y.; Sun, L.; Yan, C. Highly Efficient Multicolor Up-Conversion Emissions and Their Mechanisms of Monodisperse NaYF4:Yb,Er Core and Core/Shell-Structured Nanocrystals. J. Phys. Chem. C 2007, 111, 13721–13729. [Google Scholar] [CrossRef]
- Suyver, J.F.; Grimm, J.; Van Veen, M.K.; Biner, D.; Krämer, K.W.; Güdel, H.U. Upconversion spectroscopy and properties of NaYF4 doped with Er3+, Tm3+, and/or Yb3+. J. Lumin. 2006, 117, 1–12. [Google Scholar] [CrossRef]
- Zhu, Z. Smartphone-based apparatus for measuring upconversion luminescence lifetimes. Anal. Chim. Acta 2019, 1054, 122–127. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S.; Liu, Y.; Hou, J.; Xu, X.; Zhao, X.; Wei, B. Influence of NaYF4 Inert and Active Layer on Upconversion Luminescence. Nanomaterials 2022, 12, 3288–3297. [Google Scholar] [CrossRef]
- Rao, Z.; Li, Z.; Zhao, X.; Gong, X. Targeted high-precision up-converting thermometer platform over multiple temperature zones with Er3+. Mater. Horiz. 2023, 10, 1816–1824. [Google Scholar] [CrossRef]
- Ding, J.; Liao, H.; Pang, L.; Wang, D.; Ye, S. Improved up-conversion behaviors and temperature sensitivity based on Stark sublevels of Er3+ in β-NaYF4:Yb3+, Er3+ and β-NaYF4:Yb3+, Er3+@NaGdF4. Opt. Mater. 2022, 128, 112304. [Google Scholar] [CrossRef]
- Pandey, A.; Som, S.; Kumar, V.; Kumar, V.; Kumar, K.; Rai, V.K.; Swart, H.C. Enhanced upconversion and temperature sensing study of Er3+–Yb3+ codoped tungsten–tellurite glass. Sens. Actuators B Chem. 2014, 202, 1305–1312. [Google Scholar] [CrossRef]
- Vijaya, N.; Babu, P.; Venkatramu, V.; Jayasankar, C.K.; León-Luis, S.F.; Rodríguez-Mendoza, U.R.; Martín, I.R.; Lavín, V. Optical characterization of Er3+-doped zinc fluorophosphate glasses for optical temperature sensors. Sens. Actuators B Chem. 2013, 186, 156–164. [Google Scholar] [CrossRef]
- Wade, S.A.; Collins, S.F.; Baxter, G.W. Fluorescence intensity ratio technique for optical fiber point temperature sensing. J. Appl. Phys. 2003, 94, 4743–4756. [Google Scholar] [CrossRef]
- Liu, J.; Huang, W.; Xia, Z.; Xu, Y. Facile synthesis of accordion-like Y2O3:Er3+ nanothermometers for ratiometric temperature sensing applications. J. Lumin. 2020, 223, 117207. [Google Scholar] [CrossRef]
- Dong, B.; Hua, R.N.; Cao, B.S.; Li, Z.P.; He, Y.Y.; Zhang, Z.Y.; Wolfbeis, O.S. Size dependence of the upconverted luminescence of NaYF4:Er,Yb microspheres for use in ratiometric thermometry. Phys. Chem. Chem. Phys. 2014, 16, 20009–20012. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Rai, V.K. An effective way to enhance upconversion emission and temperature sensing via Zn2+ incorporation in Er3+-Yb3+:YMoO 4 nanophosphors. J. Ind. Eng. Chem. 2018, 60, 125–132. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, K.; Wei, X.; Jiang, G.; Duan, C.; Chen, Y.; Yin, M. Upconversion luminescence of NaYF4: Yb3+, Er3+ for temperature sensing. Opt. Commun. 2013, 291, 138–142. [Google Scholar] [CrossRef]
- Mao, Y.; Xian, P.; Jiang, L.; Hu, S.; Tang, J.; Yang, J. Temperature sensing performance based on up-conversion luminescence in hydrothermally synthesized Yb3+/Er3+ co-doped NaScF4 phosphors. Dalton Trans. 2020, 49, 7862–7871. [Google Scholar] [CrossRef]

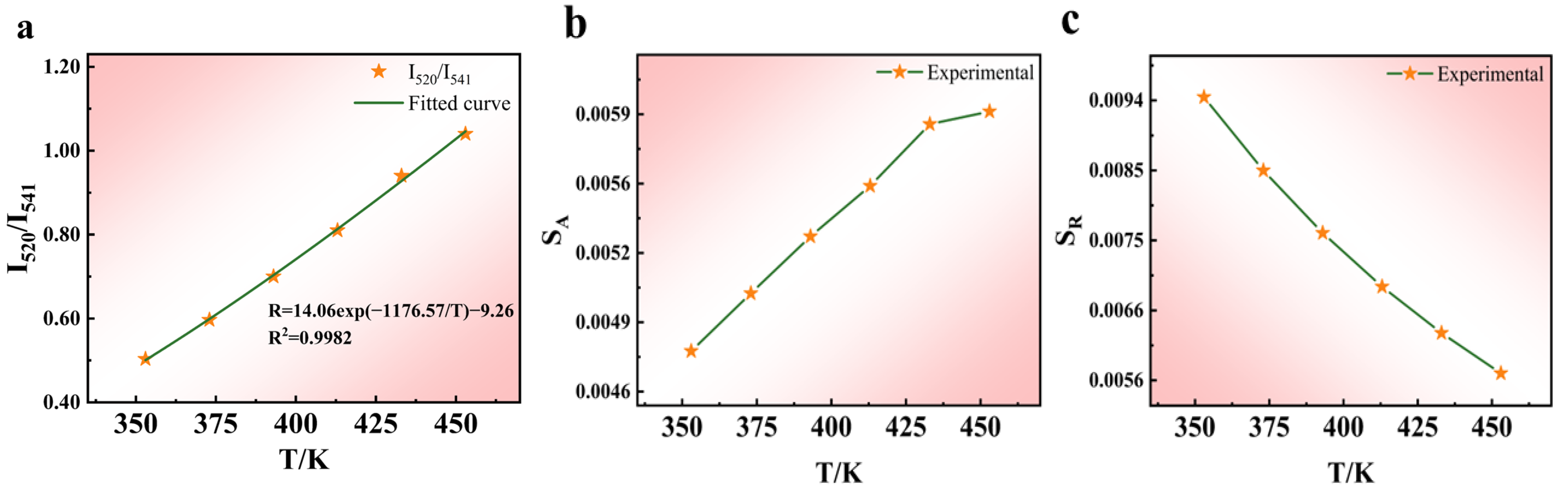
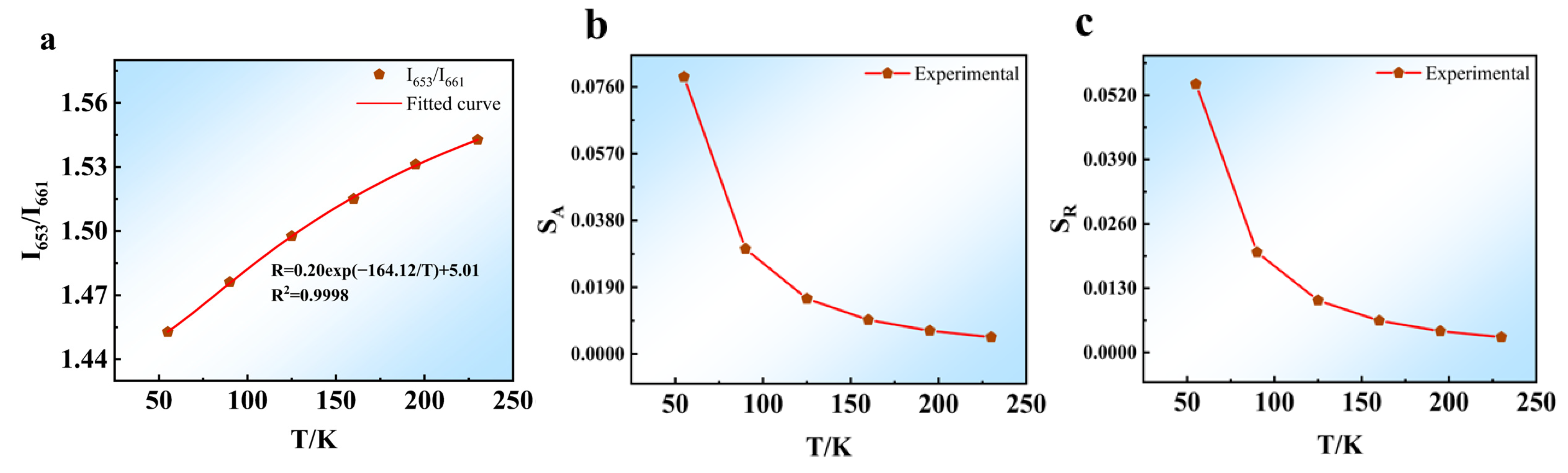
| Phosphors | Transitions | T (K) | SA (K−1) | SR (K−1) | Refs. |
|---|---|---|---|---|---|
| Y2O3:Er3+ | 2H11/2→4I15/2/4S3/2→4I15/2 | 298–393 | 0.0027 | 0.0154 | [34] |
| NaYF4:Yb3+/Er3+ | 2H11/2→4I15/2/4S3/2→4I15/2 | 223–403 | 0.0037 | - | [35] |
| YMoO4:Yb3+/Er3+ | 2H11/2→4I15/2/4S3/2→4I15/2 | 300–523 | 0.0035 | - | [36] |
| NaYF4:Yb3+/Er3+ | 2H11/2→4I15/2/4S3/2→4I15/2 | 50–500 | - | 0.012 | [37] |
| NaScF4:Yb3+/Er3+ | 2H11/2→4I15/2/4S3/2→4I15/2 | 298–573 | 0.0033 | 0.0041 | [38] |
| NaYF4:Yb3+/Er3+ | 2H11/2→4I15/2/4S3/2→4I15/2 | 353–453 | 0.0060 | 0.0094 | This work |
| NaYF4:Yb3+/Er3+ | 4F9/2(1)→4I15/2/4F9/2(2)→4I15/2 | 55–230 | 0.0788 | 0.0543 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wang, Z.; Hou, J.; Zhang, T.; Zhao, X.; Di, S.; Li, Z. Upconversion Emission and Dual-Mode Sensing Characteristics of NaYF4:Yb3+/Er3+ Microcrystals at High and Ultralow Temperatures. Nanomaterials 2024, 14, 871. https://doi.org/10.3390/nano14100871
Xu X, Wang Z, Hou J, Zhang T, Zhao X, Di S, Li Z. Upconversion Emission and Dual-Mode Sensing Characteristics of NaYF4:Yb3+/Er3+ Microcrystals at High and Ultralow Temperatures. Nanomaterials. 2024; 14(10):871. https://doi.org/10.3390/nano14100871
Chicago/Turabian StyleXu, Xinyi, Zhaojin Wang, Jin Hou, Tian Zhang, Xin Zhao, Siyi Di, and Zijie Li. 2024. "Upconversion Emission and Dual-Mode Sensing Characteristics of NaYF4:Yb3+/Er3+ Microcrystals at High and Ultralow Temperatures" Nanomaterials 14, no. 10: 871. https://doi.org/10.3390/nano14100871
APA StyleXu, X., Wang, Z., Hou, J., Zhang, T., Zhao, X., Di, S., & Li, Z. (2024). Upconversion Emission and Dual-Mode Sensing Characteristics of NaYF4:Yb3+/Er3+ Microcrystals at High and Ultralow Temperatures. Nanomaterials, 14(10), 871. https://doi.org/10.3390/nano14100871






