Research Progress on the Preparation Methods for and Flame Retardant Mechanism of Black Phosphorus and Black Phosphorus Nanosheets
Abstract
:1. Introduction
2. Preparation of Black Phosphorous
2.1. Pressurization
2.1.1. High Temperature and High Pressure Method
2.1.2. Mechanical Ball Milling
2.2. Catalytic Methods
2.2.1. The Mercury Reflux Method and the Bismuth Melting Method
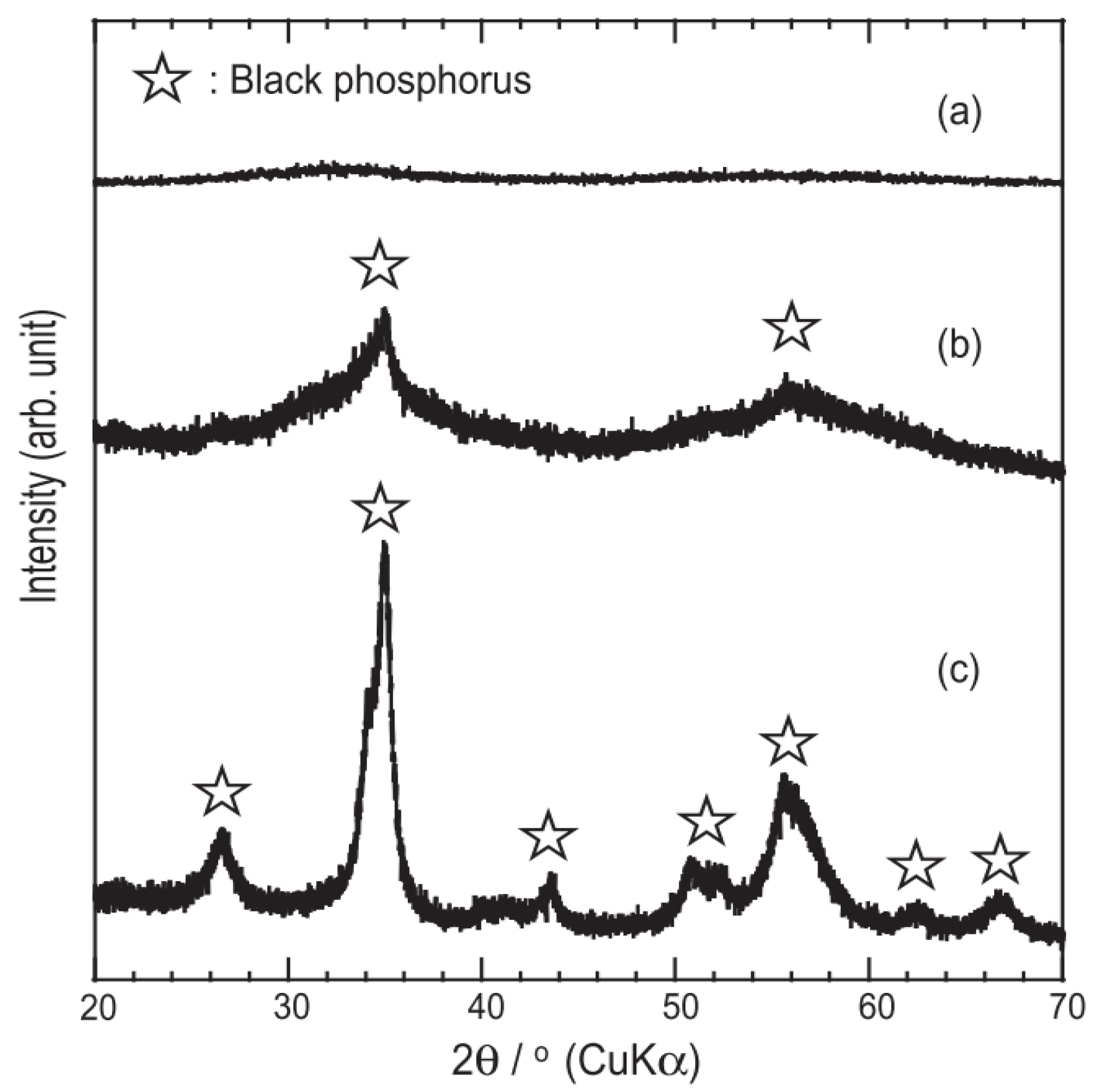
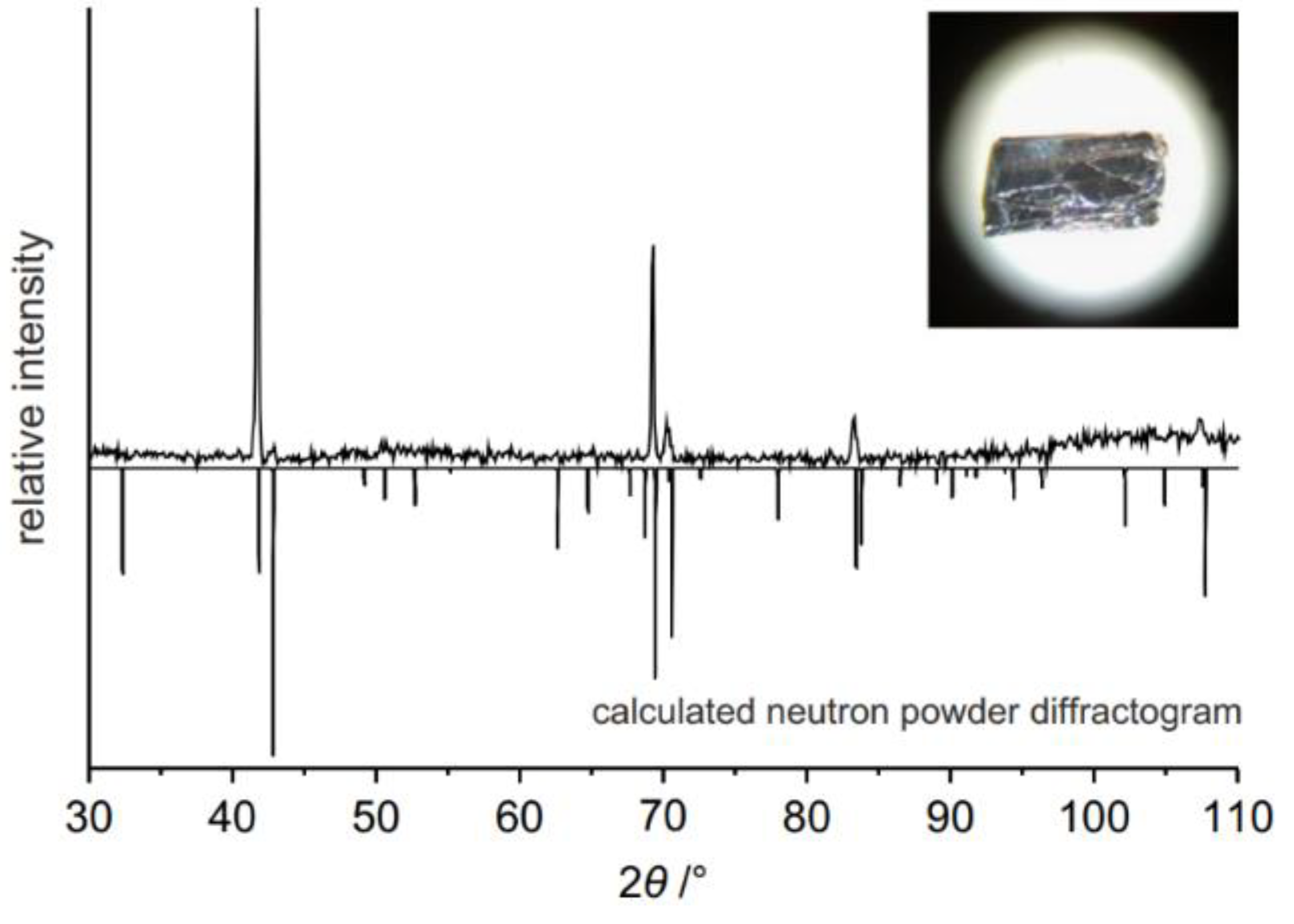
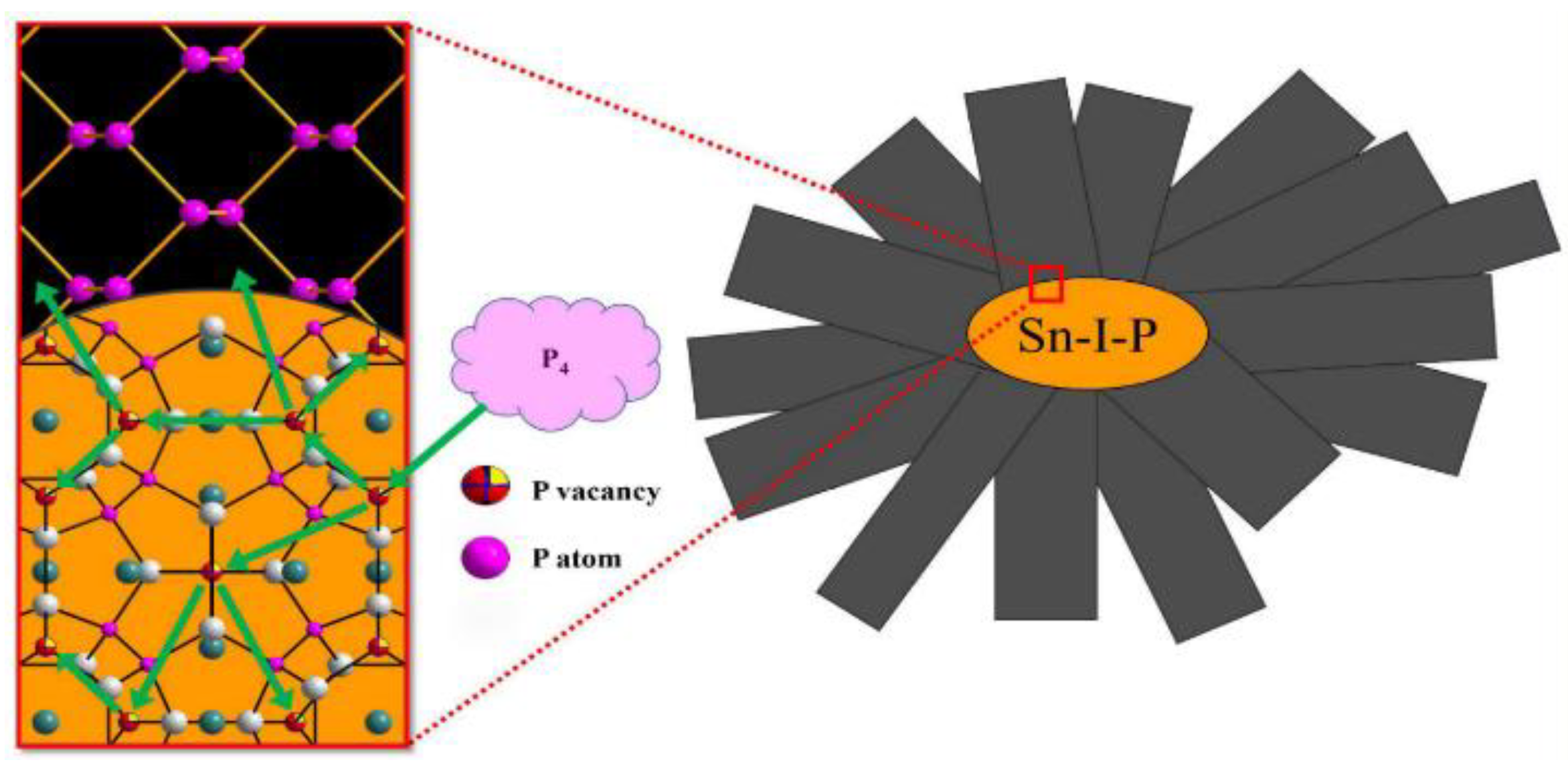
2.2.2. Mineralization
2.3. Comparison of Various Preparation Methods
3. Preparation of Black Phosphorus Nanosheets
3.1. Top–Down Method
3.1.1. Mechanical Stripping Method
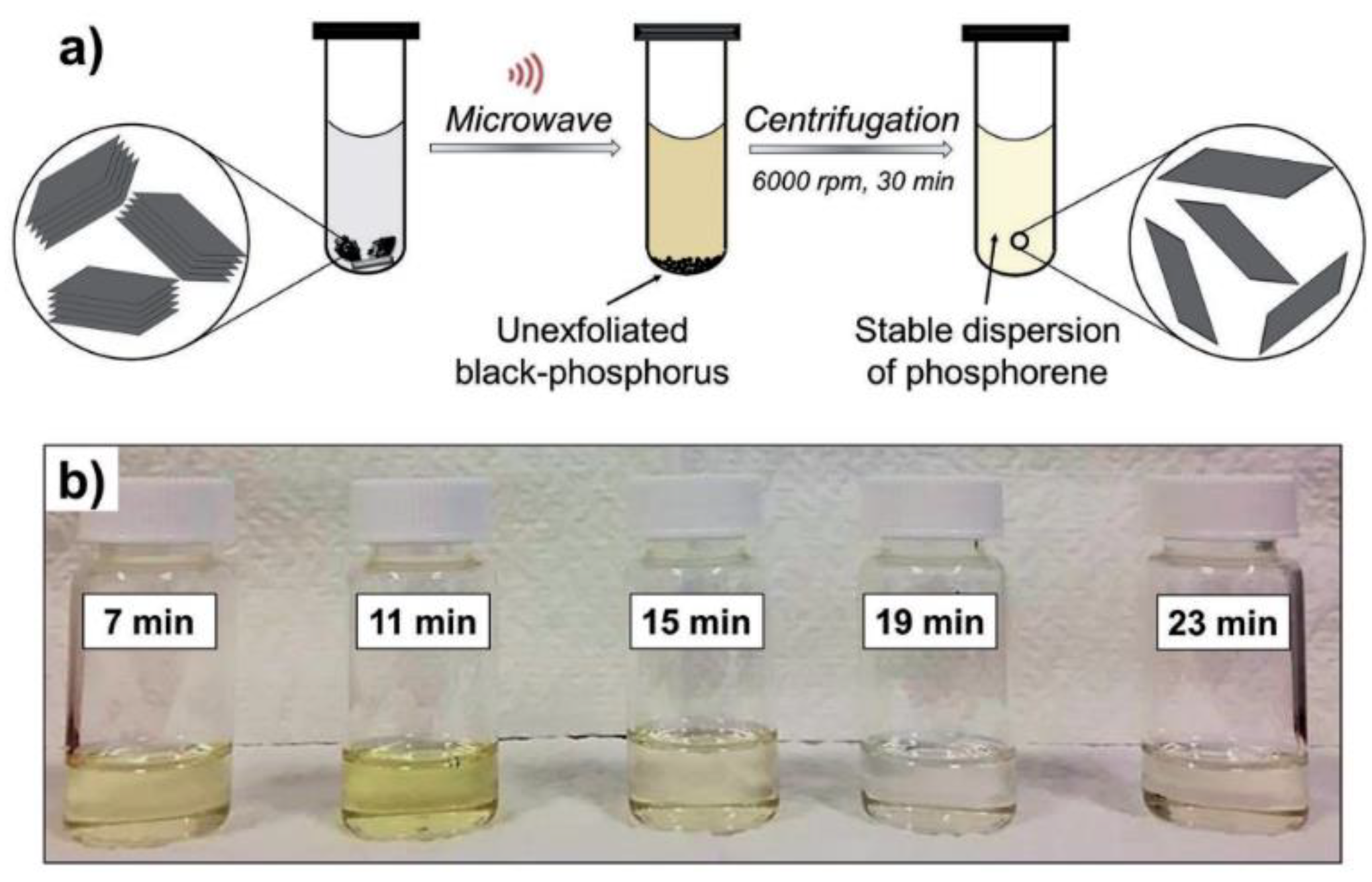
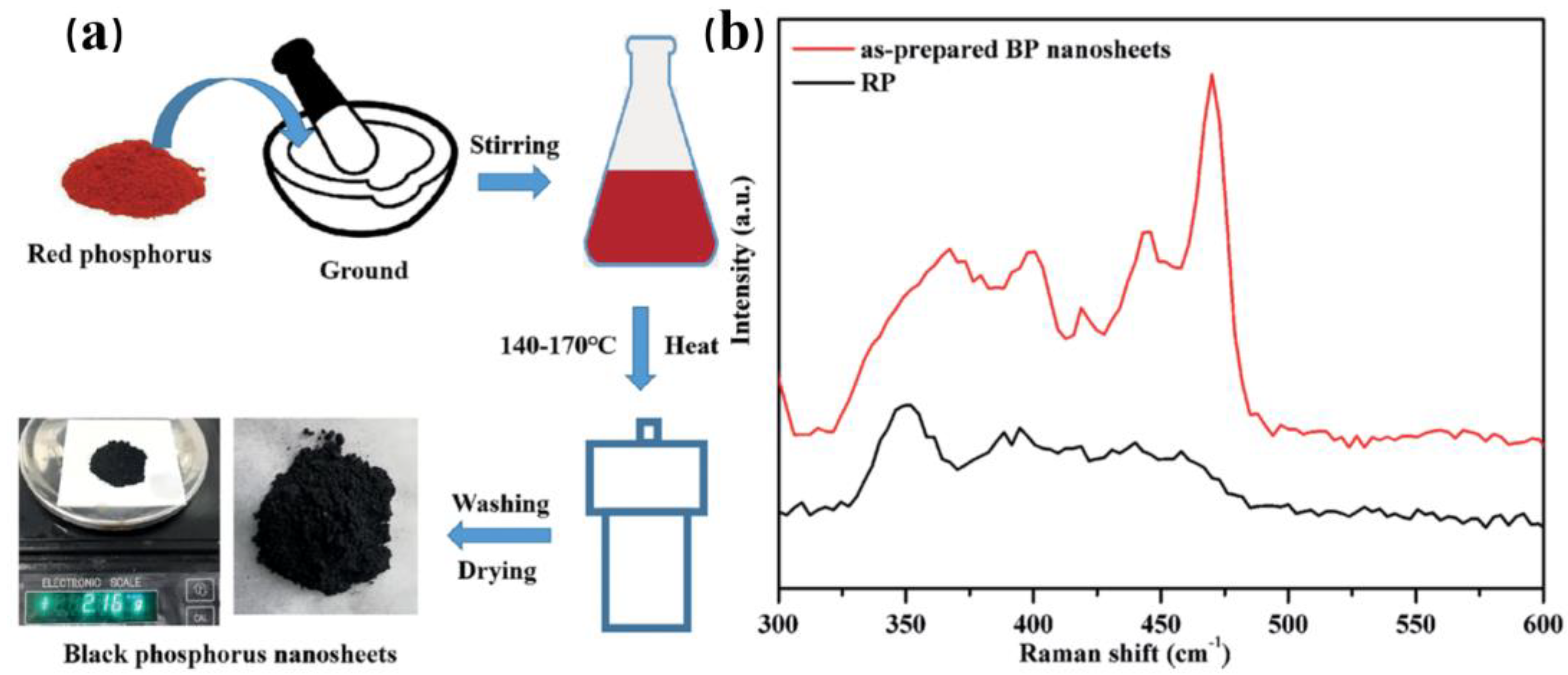
3.1.2. Liquid Phase Exfoliation
3.2. The Bottom–Up Method
3.2.1. Solvothermal Method
3.2.2. Chemical Vapor Deposition
3.2.3. High Pressure Method
3.3. Comparison of Various Preparation Methods
4. Flame Retardant Mechanism and Application
4.1. Flame Retardant Mechanism of Black Phosphazene as Various Flame Retardants
4.2. Black Phosphorus and Black Phosphorus Alkene for Flame Retardant Applications
4.2.1. Polyurethane
4.2.2. Epoxy Resin
4.2.3. Other Materials
5. Summary and Vision for the Future
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Parasana, N.; Shah, M.; Unnarkat, A. Recent advances in developing innovative sorbents for phosphorus removal-perspective and opportunities. Environ. Sci. Pollut. Res. 2022, 29, 38985–39016. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H. Phosphorus Acquisition and Utilization in Plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Niu, X.; Guan, L.; Guan, L.; Qian, H.; Wang, W.; Sha, J.; Wang, Y. Understanding the growth of black phosphorus crystals. CrystEngComm 2016, 18, 7737–7744. [Google Scholar] [CrossRef]
- Antonatos, N.; Bouša, D.; Kovalska, E.; Sedmidubský, D.; Růžička, K.; Vrbka, P.; Veselý, M.; Hejtmánek, J.; Sofer, Z. Large-Scale Production of Nanocrystalline Black Phosphorus Ceramics. ACS Appl. Mater. Interfaces 2020, 12, 7381–7391. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.B.; Pimenta, M.A.; de Matos, C.J.S. Raman spectroscopy in black phosphorus. J. Raman Spectrosc. 2017, 49, 76–90. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, L.; Li, T.; Mei, S.; Wang, C.; Wen, B.; Huang, W.; Li, C.; Zheng, G.; Wang, H.; et al. Two-Dimensional Black Phosphorus Nanomaterials: Emerging Advances in Electrochemical Energy Storage Science. Nano-Micro Lett. 2020, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Zhou, J.; Wang, X.; Wu, L.; Zhong, S.; Li, Y. Recent advances in black-phosphorus-based materials for electrochemical energy storage. Mater. Today 2021, 42, 117–136. [Google Scholar] [CrossRef]
- Lee, G.; Kim, S.; Jung, S.; Jang, S.; Kim, J. Suspended black phosphorus nanosheet gas sensors. Sens. Actuators B Chem. 2017, 250, 569–573. [Google Scholar] [CrossRef]
- Srivastava, A.; Verma, A.; Das, R.; Prajapati, Y.K. A theoretical approach to improve the performance of SPR biosensor using MXene and black phosphorus. Optik 2020, 203, 163430. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Luo, B.; Yu, D. Black-phosphorus-based materials for application in solar cells. Chin. J. Struct. Chem. 2023, 42, 100109. [Google Scholar] [CrossRef]
- Sultana, I.; Rahman, M.M.; Ramireddy, T.; Chen, Y.; Glushenkov, A.M. Correction: High capacity potassium-ion battery anodes based on black phosphorus. J. Mater. Chem. A 2019, 7, 2421. [Google Scholar] [CrossRef]
- Han, Y.; Rong, X.; Wang, M.; Xue, Y.; Dai, H.; Liu, Y. Progress in the preparation, application, and recycling of black phosphorus. Chemosphere 2023, 311, 137161. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Yue, L.; Ren, D. Preparation of High-Intensity Fluorescent Black Phosphorus Nanosheets. Phys. Status Solidi (A)—Appl. Mater. Sci. 2023, 220, 2200806. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Sofer, Z.; Sedmidubský, D.; Luxa, J.; Kherzi, B.; Pumera, M. Metallic impurities in black phosphorus nanoflakes prepared by different synthetic routes. Nanoscale 2018, 10, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Bridgman, P.W. Polymorphism, Principally of the Elements, up to 50,000 kg/cm2. Phys. Rev. 1935, 48, 893–906. [Google Scholar] [CrossRef]
- Jacobs Robert, B. Phosphorus at High Temperatures and Pressures. J. Chem. Phys. 1937, 5, 945–953. [Google Scholar] [CrossRef]
- Shirotani, I.J.M.C. Growth of Large Single Crystals of Black Phosphorus at High Pressures and Temperatures, and its Electrical Properties. Jpn. J. Appl. Phys. 1982, 86, 203–211. [Google Scholar] [CrossRef]
- Narita, S.-i.; Akahama, Y.; Tsukiyama, Y.; Muro, K.; Mori, S.; Endo, S.; Taniguchi, M.; Seki, M.; Suga, S.; Mikuni, A.; et al. Electrical and optical properties of black phosphorus single crystals. Physica B+C 1983, 117–118, 422–424. [Google Scholar] [CrossRef]
- Sun, L.-Q.; Li, M.-J.; Sun, K.; Yu, S.-H.; Wang, R.-S.; Xie, H.-M. Electrochemical Activity of Black Phosphorus as an Anode Material for Lithium-Ion Batteries. J. Phys. Chem. C 2012, 116, 14772–14779. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Yang, T.; Tong, Y.; Wang, J.; Luan, J.H.; Jiao, Z.B.; Chen, D.; Yang, Y.; Hu, A.; Liu, C.T.; et al. Heterogeneous precipitation behavior and stacking-fault-mediated deformation in a CoCrNi-based medium-entropy alloy. Acta Mater. 2017, 138, 72–82. [Google Scholar] [CrossRef]
- Günther, P.L.; Gesslle, P.; Rebentisch, W. Untersuchungen zum Diamantproblem. Z. Anorg. Allg. Chem. 1943, 250, 357–372. [Google Scholar] [CrossRef]
- Park, C.-M.; Sohn, H.J. Black Phosphorus and its Composite for Lithium Rechargeable Batteries. Adv. Mater. 2007, 19. [Google Scholar] [CrossRef]
- Nagao, M.; Hayashi, A.; Tatsumisago, M. All-solid-state lithium secondary batteries with high capacity using black phosphorus negative electrode. J. Power Sources 2010, 196, 6902–6905. [Google Scholar] [CrossRef]
- Krebs, H.; Weitz, H.P.; Worms, K.H. Über die Struktur und Eigenschaften der Halbmetalle. VIII. Die katalytische Darstellung des schwarzen Phosphors. Z. Anorg. Allg. Chem. 1955, 280, 119–133. [Google Scholar] [CrossRef]
- Baba, M.; Izumida, F.; Takeda, Y.; Morita, A. Preparation of Black Phosphorus Single Crystals by a Completely Closed Bismuth-Flux Method and Their Crystal Morphology. Jpn. J. Appl. Phys. 1989, 28, 1019. [Google Scholar] [CrossRef]
- Lange, S.; Schmidt, P.; Nilges, T. Au3SnP7@black phosphorus: An easy access to black phosphorus. Inorg. Chem. 2007, 46, 4028–4035. [Google Scholar] [CrossRef] [PubMed]
- Nilges, T.; Kersting, M.; Pfeifer, T. A fast low-pressure transport route to large black phosphorus single crystals. J. Solid State Chem. 2008, 181, 1707–1711. [Google Scholar] [CrossRef]
- Köpf, M.; Eckstein, N.; Pfister, D.; Grotz, C.; Krüger, I.; Greiwe, M.; Hansen, T.; Kohlmann, H.; Nilges, T. Access and in situ growth of phosphorene-precursor black phosphorus. J. Cryst. Growth 2014, 405, 6–10. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Fan, X.; Ni, Y.; Miracle, J.; Theodoropoulou, N.; Sun, J.; Chen, S.; Lv, B.; Yu, Q.J.C.G.; et al. Addition to New Strategy for Black Phosphorus Crystal Growth through Ternary Clathrate. Cryst. Growth Des. 2017, 18, 4206. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, G.; Chen, S.; Guo, Z.; Yu, X.; Zhao, C.; Zhang, H.; Bao, Q.; Wen, S.; Tang, D.; et al. Mechanically exfoliated black phosphorus as a new saturable absorber for both Q-switching and Mode-locking laser operation. Optics Express 2015, 23, 12823–12833. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Gomez, A.; Vicarelli, L.; Prada, E.; Island, J.O.; Narasimha-Acharya, K.L.; Blanter, S.I.; Groenendijk, D.J.; Buscema, M.; Steele, G.A.; Álvarez, J.A.V.; et al. Isolation and characterization of few-layer black phosphorus. 2D Mater. 2014, 1, 025001. [Google Scholar] [CrossRef]
- Lu, W.; Nan, H.; Hong, J.; Chen, Y.; Zhu, C.; Liang, Z.; Ma, X.; Ni, Z.; Jin, C.; Zhang, Z. Plasma-assisted fabrication of monolayer phosphorene and its Raman characterization. Nano Res. 2014, 7, 853–859. [Google Scholar] [CrossRef]
- Buscema, M.; Groenendijk, D.J.; Blanter, S.I.; Steele, G.A.; van der Zant, H.S.; Castellanos-Gomez, A. Fast and Broadband Photoresponse of Few-Layer Black Phosphorus Field-Effect Transistors. Nano Lett. 2014, 14, 3347–3352. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, T.; Wang, K.; Wang, L.; Zhang, Y.; Gao, S.; Ye, X.; Zhou, Q.; Jiang, S.; Li, X.; et al. Narrow Directed Black Phosphorus Nanoribbons Produced by A Reformative Mechanical Exfoliation Approach. Small 2023, 19, e2207538. [Google Scholar] [CrossRef]
- Cao, Y.; Tian, X.; Gu, J.; Liu, B.; Zhang, B.; Song, S.; Fan, F.; Chen, Y.J.A.C. Covalent Functionalization of Black Phosphorus with Conjugated Polymer for Information Storage. Angew. Chem. 2018, 57, 4543–4548. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liu, L.; Huang, W.; Wu, K. Polyvinylpyrrolidone-assisted solvent exfoliation of black phosphorus nanosheets and electrochemical sensing of p-nitrophenol. Anal. Chim. Acta 2021, 1167, 338594. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Osei Lartey, P.; Guo, K.; Liu, J.; Ren, M.; Zhao, Y.; Yang, Y. Exfoliating two-dimensional materials into few layers via optimized environmentally-friendly ternary solvents. Nanotechnology 2020, 31, 045602. [Google Scholar] [CrossRef]
- Tiouitchi, G.; Ali, M.A.; Benyoussef, A.; Hamedoun, M.; Lachgar, A.; Kara, A.; Ennaoui, A.; Mahmoud, A.; Boschini, F.; Oughaddou, H.; et al. Efficient production of few-layer black phosphorus by liquid-phase exfoliation. R. Soc. Open Sci. 2020, 7, 201210. [Google Scholar] [CrossRef]
- Du, K.; Yang, W.; Deng, S.; Li, X.; Yang, P. High-Quality Black Phosphorus Quantum Dots Fabricated via Microwave-Tailored Technology. Nanomaterials 2020, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Bartus, C.P.; Hegedűs, T.; Kozma, G.; Szenti, I.; Vajtai, R.; Kónya, Z.; Kukovecz, Á. Exfoliation of black phosphorus in isopropanol-water cosolvents. J. Mol. Struct. 2022, 1260, 132862. [Google Scholar] [CrossRef]
- Jia, J.; Jeon, S.; Jeon, J.; Park, J.-H.; Lee, S. Versatile Doping Control of Black Phosphorus and Functional Junction Structures. J. Phys. Chem. C 2019, 123, 10682–10688. [Google Scholar] [CrossRef]
- Kang, J.; Wood, J.D.; Wells, S.A.; Lee, J.H.; Liu, X.; Chen, K.S.; Hersam, M.C. Solvent Exfoliation of Electronic-Grade, Two-Dimensional Black Phosphorus. ACS Nano 2015, 9, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, H.; Lu, S.; Wang, Z.; Tang, S.; Shao, J.; Sun, Z.; Xie, H.; Wang, H.; Yu, X.-F.; et al. From Black Phosphorus to Phosphorene: Basic Solvent Exfoliation, Evolution of Raman Scattering, and Applications to Ultrafast Photonics. Adv. Funct. Mater. 2015, 25, 6996–7002. [Google Scholar] [CrossRef]
- Jia, C.; Zhao, L.; Cui, M.; Yang, F.; Cheng, G.; Yang, G.; Zeng, Z. Surface coordination modification and electrical properties of few-layer black phosphorus exfoliated by the liquid-phase method. J. Alloys Compd. 2019, 799, 99–107. [Google Scholar] [CrossRef]
- Bat-Erdene, M.; Batmunkh, M.; Shearer, C.J.; Tawfik, S.A.; Ford, M.J.; Yu, L.-Y.; Sibley, A.; Slattery, A.D.; Quinton, J.S.; Gibson, C.T.; et al. Efficient and Fast Synthesis of Few-Layer Black Phosphorus via Microwave-Assisted Liquid-Phase Exfoliation. Small Methods 2017, 1, 1700260. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. Greener organic solvents in analytical chemistry. Curr. Opin. Green Sustain. Chem. 2017, 5, 1–4. [Google Scholar] [CrossRef]
- Zhao, W.; Xue, Z.; Wang, J.; Jiang, J.; Zhao, X.; Mu, T. Large-Scale, Highly Efficient, and Green Liquid-Exfoliation of Black Phosphorus in Ionic Liquids. ACS Appl. Mater. Interfaces 2015, 7, 27608–27612. [Google Scholar] [CrossRef]
- Lee, M.; Roy, A.K.; Jo, S.; Choi, Y.; Chae, A.; Kim, B.; Park, S.Y.; In, I. Exfoliation of black phosphorus in ionic liquids. Nanotechnology 2017, 28, 125603. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, G.; Liu, Z.; Ma, X.; Chen, J.; Zhang, Z.; Ma, X.; Li, F.; Cheng, H.M.; Ren, W. Scalable Clean Exfoliation of High-Quality Few-Layer Black Phosphorus for a Flexible Lithium Ion Battery. Adv. Mater. 2016, 28, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, X.; Lian, P.; Chen, R.; Liu, Y.; Mei, Y. Production of Phosphorene from Black Phosphorus via Sonication and Microwave Co-assisted Aqueous Phase Exfoliation. Chem. Lett. 2018, 47, 1478–1481. [Google Scholar] [CrossRef]
- Kim, H.-R.; Lee, S.-H.; Lee, K.-H. Scalable production of large single-layered graphenes by microwave exfoliation ‘in deionized water’. Carbon 2018, 134, 431–438. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, D.; Yu, J.; Zhao, Q.; Yuan, X.; Liu, Y.; Guo, S. Two-dimensional transition metal dichalcogenides based composites for microwave absorption applications: A review. J. Phys. Energy 2023, 5, 012001. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, X.; Fu, M.; Deng, S.; Feng, X.; Yang, W.; Yang, P. Synthesis and antioxidation of low-dimensional black phosphorus. Acta Opt. Sin. 2022, 42, 1016001. [Google Scholar]
- Carrasco, D.F.; Paredes, J.I.; Villar-Rodil, S.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D.J.C. An electrochemical route to holey graphene nanosheets for charge storage applications. Carbon 2022, 195, 57–68. [Google Scholar] [CrossRef]
- Zhou, A.; Bai, J.; Hong, W.; Bai, H. Electrochemically reduced graphene oxide: Preparation, composites, and applications. Carbon 2022, 191, 301–332. [Google Scholar] [CrossRef]
- Guo, T.; Wang, L.; Sun, S.; Wang, Y.; Chen, X.; Zhang, K.; Zhang, D.; Xue, Z.; Zhou, X. Layered MoS2@graphene functionalized with nitrogen-doped graphene quantum dots as an enhanced electrochemical hydrogen evolution catalyst. Chin. Chem. Lett. 2019, 30, 1253–1260. [Google Scholar] [CrossRef]
- Erande, M.B.; Pawar, M.S.; Late, D.J. Humidity Sensing and Photodetection Behavior of Electrochemically Exfoliated Atomically Thin-Layered Black Phosphorus Nanosheets. ACS Appl. Mater. Interfaces 2016, 8, 11548–11556. [Google Scholar] [CrossRef]
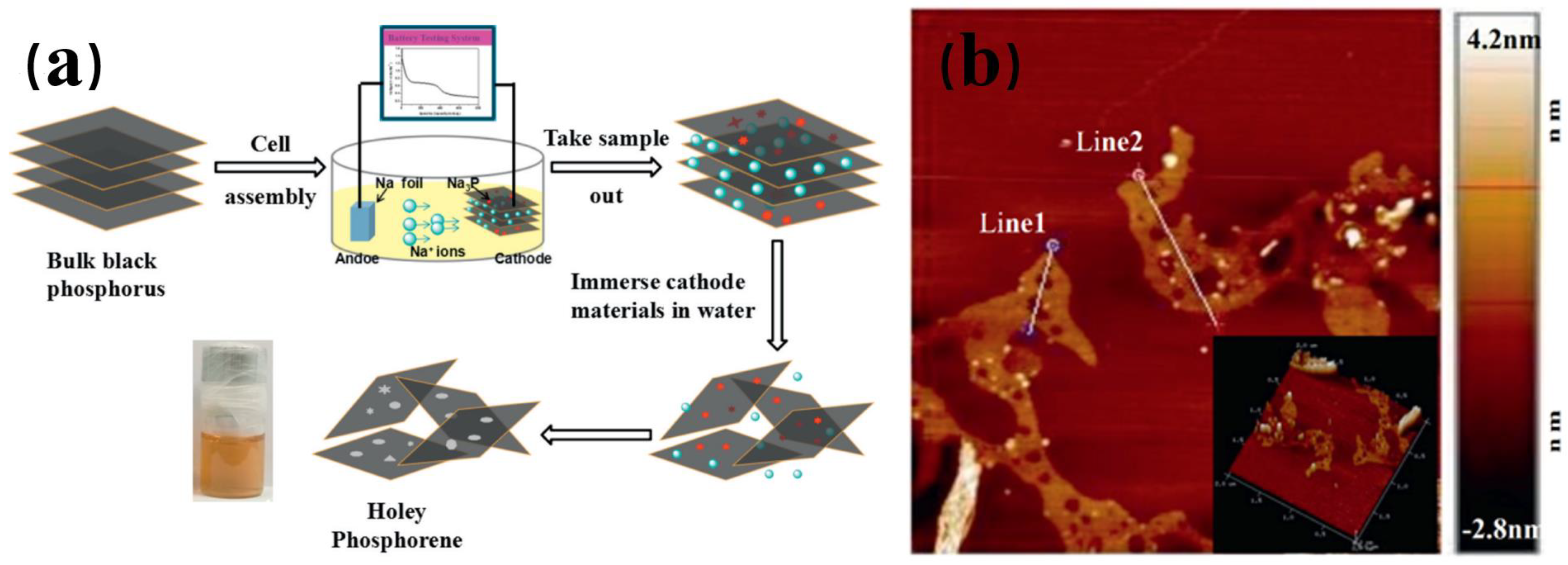
- Jiang, Y.; Hou, R.; Lian, P.; Fu, J.; Lu, Q.; Mei, Y. A facile and mild route for the preparation of holey phosphorene by low-temperature electrochemical exfoliation. Electrochem. Commun. 2021, 128, 107074. [Google Scholar] [CrossRef]
- Rabiei Baboukani, A.; Khakpour, I.; Drozd, V.; Allagui, A.; Wang, C. Single-step exfoliation of black phosphorus and deposition of phosphorene via bipolar electrochemistry for capacitive energy storage application. J. Mater. Chem. A 2019, 7, 25548–25556. [Google Scholar] [CrossRef]
- Liu, H.; Lian, P.; Zhang, Q.; Yang, Y.; Mei, Y. The preparation of holey phosphorene by electrochemical assistance. Electrochem. Commun. 2019, 98, 124–128. [Google Scholar] [CrossRef]
- Mousavi, S.H.; Müller, T.S.; Karos, R.; de Oliveira, P.W. Faster synthesis of CIGS nanoparticles using a modified solvothermal method. J. Alloys Compd. 2016, 659, 178–183. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, T.; Shao, Y.; Wu, Y.; Huang, B.; Hao, X. A Novel Mild Phase-Transition to Prepare Black Phosphorus Nanosheets with Excellent Energy Applications. Small 2017, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Tian, B.; Smith, B.; Scott, M.C.; Hua, R.; Lei, Q.; Tian, Y. Supported black phosphorus nanosheets as hydrogen-evolving photocatalyst achieving 5.4% energy conversion efficiency at 353 K. Nat. Commun. 2018, 9, 1397. [Google Scholar] [CrossRef]
- Tian, B.; Tian, B.; Smith, B.; Scott, M.C.; Lei, Q.; Hua, R.; Tian, Y.; Liu, Y. Facile bottom-up synthesis of partially oxidized black phosphorus nanosheets as metal-free photocatalyst for hydrogen evolution. Proc. Natl. Acad. Sci. USA 2018, 115, 4345–4350. [Google Scholar] [CrossRef] [PubMed]
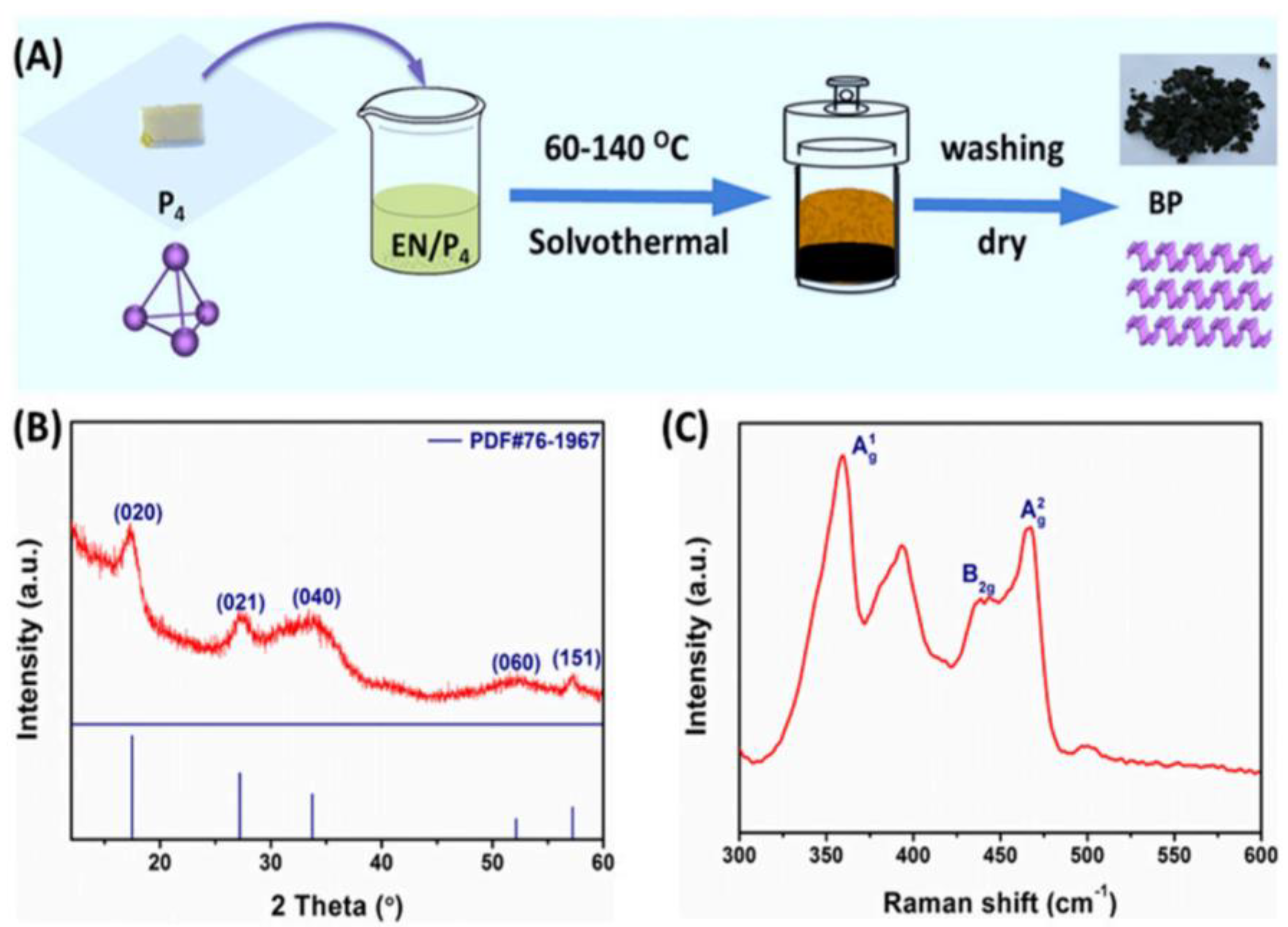
- Zhu, S.; Liang, Q.; Xu, Y.; Fu, H.; Xiao, X.J.E.J.o.I.C. Facile Solvothermal Synthesis of Black Phosphorus Nanosheets from Red Phosphorus for Efficient Photocatalytic Hydrogen Evolution. Eur. J. Inorg. Chem. 2020, 2020, 773–779. [Google Scholar] [CrossRef]
- Li, X.; Colombo, L.; Ruoff, R.S. Synthesis of Graphene Films on Copper Foils by Chemical Vapor Deposition. Adv. Mater. 2016, 28, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wen, S.; Lv, B.; Dou, W.-D. Vertical-Graphene-Assisted Chemical Vapor Deposition for Fast Growth of Macroscaled Graphene Grains. J. Phys. Chem. C 2023, 127, 6991–6997. [Google Scholar] [CrossRef]
- Li, S.; Ouyang, D.; Zhang, N.; Zhang, Y.; Murthy, A.; Li, Y.; Liu, S.; Zhai, T. Substrate Engineering for Chemical Vapor Deposition Growth of Large-Scale 2D Transition Metal Dichalcogenides. Adv. Mater. 2023, 35, e2211855. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Li, Y.; Ding, K.; Jiang, D.; Dou, X.; Sun, B. Radiative and Non-Radiative Exciton Recombination Processes in a Chemical Vapor Deposition-Grown MoSe2 Film. J. Phys. Chem. C 2022, 126, 15319–15326. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Liu, J.; Zhang, Y.; Xie, Y. Large-Scale Synthesis h-BN Films on Copper-Nickel Alloy by Atmospheric Pressure Chemical Vapor Deposition. Crystals. 2022, 12, 985. [Google Scholar] [CrossRef]
- Saji, V.S. 2D hexagonal boron nitride (h-BN) nanosheets in protective coatings: A literature review. Heliyon 2023, 9, e19362. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.B.; Hagaman, D.; Ji, H.F. Growth of 2D black phosphorus film from chemical vapor deposition. Nanotechnology 2016, 27, 215602. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Xu, L.; Chen, N.; Zhang, H.; Dai, L.; Wang, S. Facile Synthesis of Black Phosphorus: An Efficient Electrocatalyst for the Oxygen Evolving Reaction. Angew. Chem. Int. Ed. 2016, 55, 13849–13853. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, N.; Myers, J.C.; Seaton, N.C.; Pandey, S.K.; Campbell, S.A. Thin-Film Deposition of Surface Passivated Black Phosphorus. ACS Nano 2019, 13, 7091–7099. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, B.; Wang, X.; Chen, S.; Vaisman, M.; Karato, S.-i.; Pan, G.; Larry Lee, M.; Cha, J.; Wang, H.; et al. Synthesis of thin-film black phosphorus on a flexible substrate. 2D Mater. 2015, 2, 031002. [Google Scholar] [CrossRef]
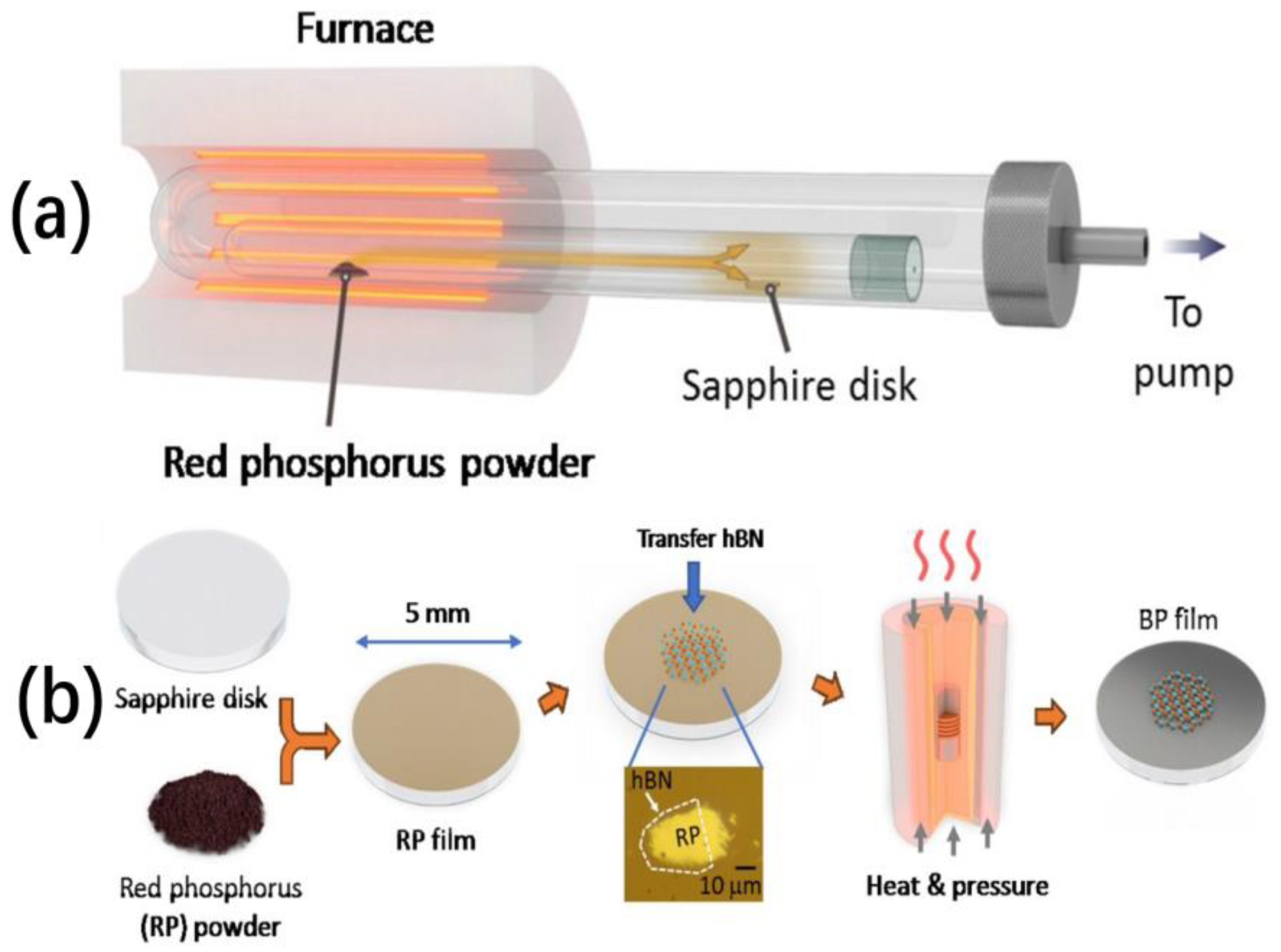
- Li, C.; Wu, Y.Q.; Deng, B.; Xie, Y.; Guo, Q.; Yuan, S.; Chen, X.; Bhuiyan, M.A.; Wu, Z.; Watanabe, K.; et al. Synthesis of Crystalline Black Phosphorus Thin Film on Sapphire. Adv. Mater. 2018, 30, 1703748. [Google Scholar] [CrossRef] [PubMed]
- Bevington, C.; Williams, A.J.; Guider, C.; Baker, N.C.; Meyer, B.; Babich, M.A.; Robinson, S.; Jones, A.; Phillips, K.A. Development of a Flame Retardant and an Organohalogen Flame Retardant Chemical Inventory. Sci. Data 2022, 9, 295. [Google Scholar] [CrossRef]
- Appavoo, D.; Amarnath, N.; Lochab, B. Cardanol and Eugenol Sourced Sustainable Non-halogen Flame Retardants for Enhanced Stability of Renewable Polybenzoxazines. Front. Chem. 2020, 8, 711. [Google Scholar] [CrossRef]
- Cai, T.; Wang, J.; Zhang, C.; Cao, M.; Jiang, S.; Wang, X.; Wang, B.; Hu, W.; Hu, Y. Halogen and halogen-free flame retarded biologically-based polyamide with markedly suppressed smoke and toxic gases releases. Compos. Part B Eng. 2020, 184, 107737. [Google Scholar] [CrossRef]
- Passauer, L. Thermal characterization of ammonium starch phosphate carbamates for potential applications as bio-based flame-retardants. Carbohydr. Polym. 2019, 211, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Luda, M.P.; Costa, L.; Bracco, P.; Levchik, S.V. Relevant factors in scorch generation in fire retarded flexible polyurethane foams: II Reactivity of isocyanate, urea and urethane groups. Polym. Degrad. Stab. 2004, 86, 43–50. [Google Scholar] [CrossRef]
- Deng, C.; Ji, Y.; Zhu, M.; Liang, Y.; Jian, H.; Yan, Z.; Wen, M.; Park, H. Preparation of Organic-Inorganic Phosphorus-Nitrogen-Based Flame Retardants and Their Application to Plywood. Polymers 2023, 15, 3112. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chen, S.; Luo, L.; Yang, Y.; Wang, J.; Chen, Y.; Yang, Y.; Yuan, Z.; Chen, X. Molecules Featuring the Azaheterocycle Moiety toward the Application of Flame-Retardant Polymers. ACS Chem. Health Saf. 2023, 30, 343–361. [Google Scholar] [CrossRef]
- Krumm, E.A.; Patel, V.J.; Tillery, T.S.; Yasrebi, A.; Shen, J.; Guo, G.L.; Marco, S.M.; Buckley, B.T.; Roepke, T.A. Organophosphate Flame-Retardants Alter Adult Mouse Homeostasis and Gene Expression in a Sex-Dependent Manner Potentially Through Interactions With ERα. Toxicol. Sci. 2017, 162, 212–224. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Guan, J.-P.; Tang, R.-C.; Liu, K.-Q. Phytic acid as a bio-based phosphorus flame retardant for poly(lactic acid) nonwoven fabric. J. Clean. Prod. 2016, 124, 114–119. [Google Scholar] [CrossRef]
- Qiu, S.; Zou, B.; Sheng, H.; Guo, W.; Wang, J.; Zhao, Y.; Wang, W.; Yuen, R.K.K.; Kan, Y.; Hu, Y. Electrochemically Exfoliated Functionalized Black Phosphorene and Its Polyurethane Acrylate Nanocomposites: Synthesis and Applications. ACS Appl. Mater. Interfaces 2019, 11, 13652–13664. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhou, Y.; Zhou, X.; Zhang, T.; Wang, C.; Yuen, R.K.K.; Hu, W.; Hu, Y. Air-Stable Polyphosphazene-Functionalized Few-Layer Black Phosphorene for Flame Retardancy of Epoxy Resins. Small 2019, 15, e1805175. [Google Scholar] [CrossRef]
- Ren, X.; Mei, Y.; Lian, P.; Xie, D.; Yang, Y.; Wang, Y.; Wang, Z. A Novel Application of Phosphorene as a Flame Retardant. Polymers 2018, 10, 227. [Google Scholar] [CrossRef]
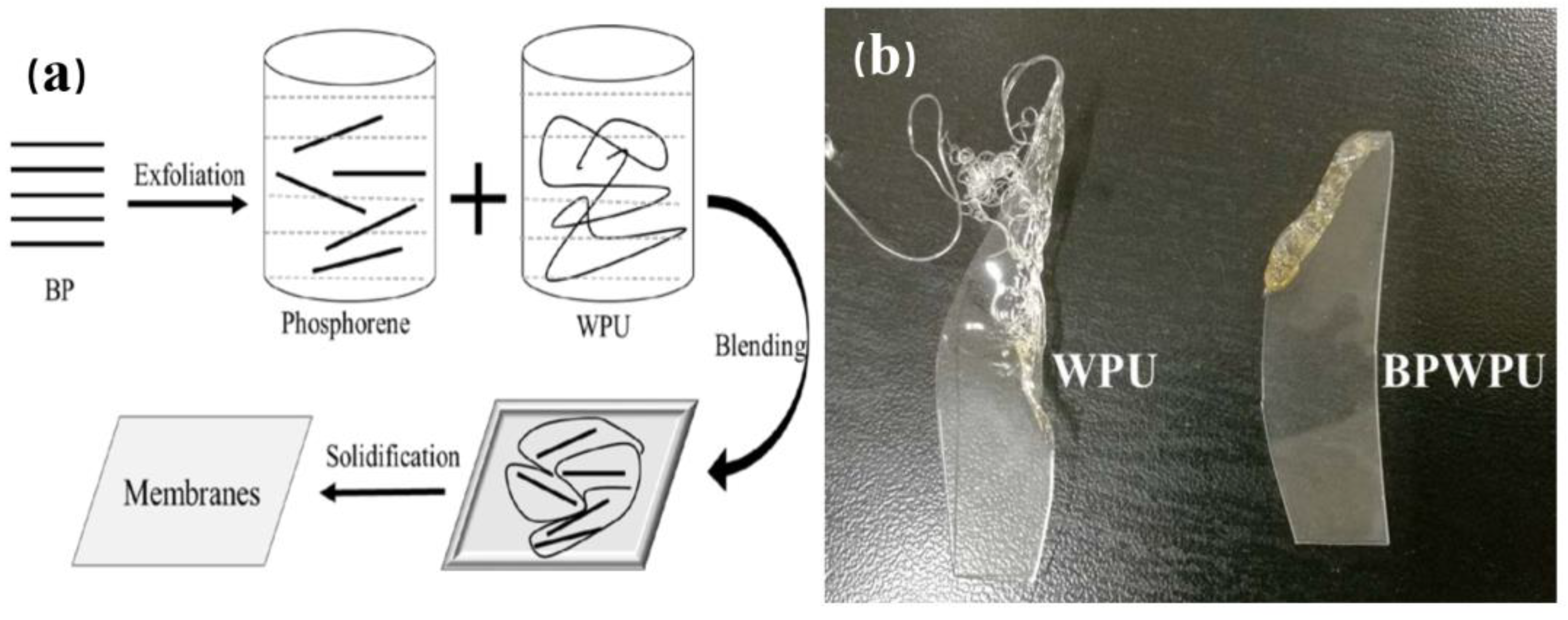
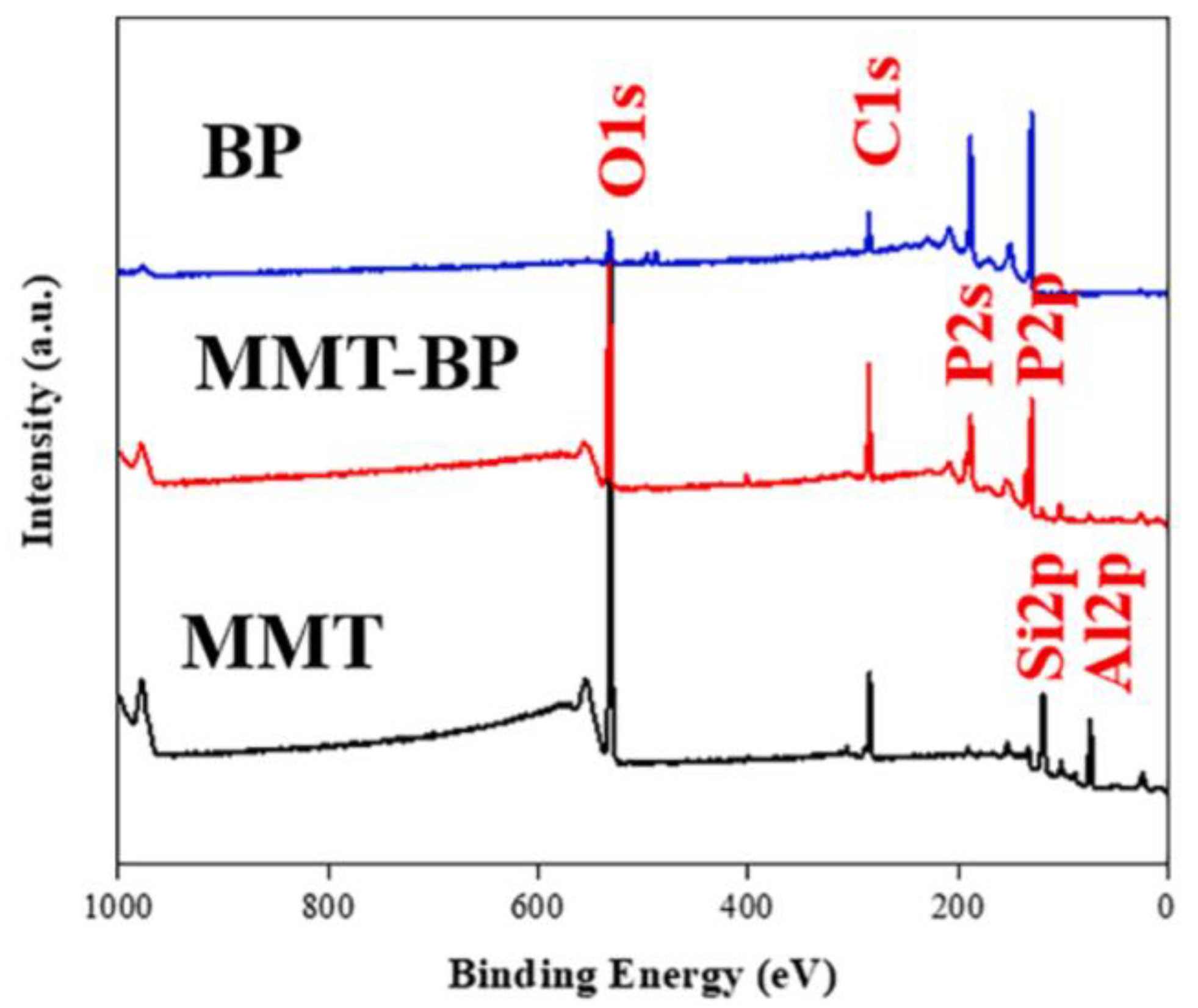
- Yin, S.; Ren, X.; Zheng, R.X.; Li, Y.; Zhao, J.; Xie, D.; Mei, Y.J.C.E.J. Improving fire safety and mechanical properties of waterborne polyurethane by montmorillonite-passivated black phosphorus. Chem. Eng. J. 2023, 464, 142683. [Google Scholar] [CrossRef]
- Ren, X.; Mei, Y.; Lian, P.; Xie, D.; Deng, W.; Wen, Y.; Luo, Y.J.P. Fabrication and Application of Black Phosphorene/Graphene Composite Material as a Flame Retardant. Polymers 2019, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xie, H.; Xie, S.; Hu, A.; Liu, J.; Kang, J.; Hou, J.; Hao, Q.; Liu, H.; Ji, H. A Superior Two-Dimensional Phosphorus Flame Retardant: Few-Layer Black Phosphorus. Molecules 2023, 28, 5062. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Wei, X.; Yu, Q.; Han, Y.; Yu, J.; Wang, Z. High-Performance TPE-S Modified by a Flame-Retardant System Based on Black Phosphorus Nanosheets. ACS Omega 2022, 7, 4224–4233. [Google Scholar] [CrossRef] [PubMed]
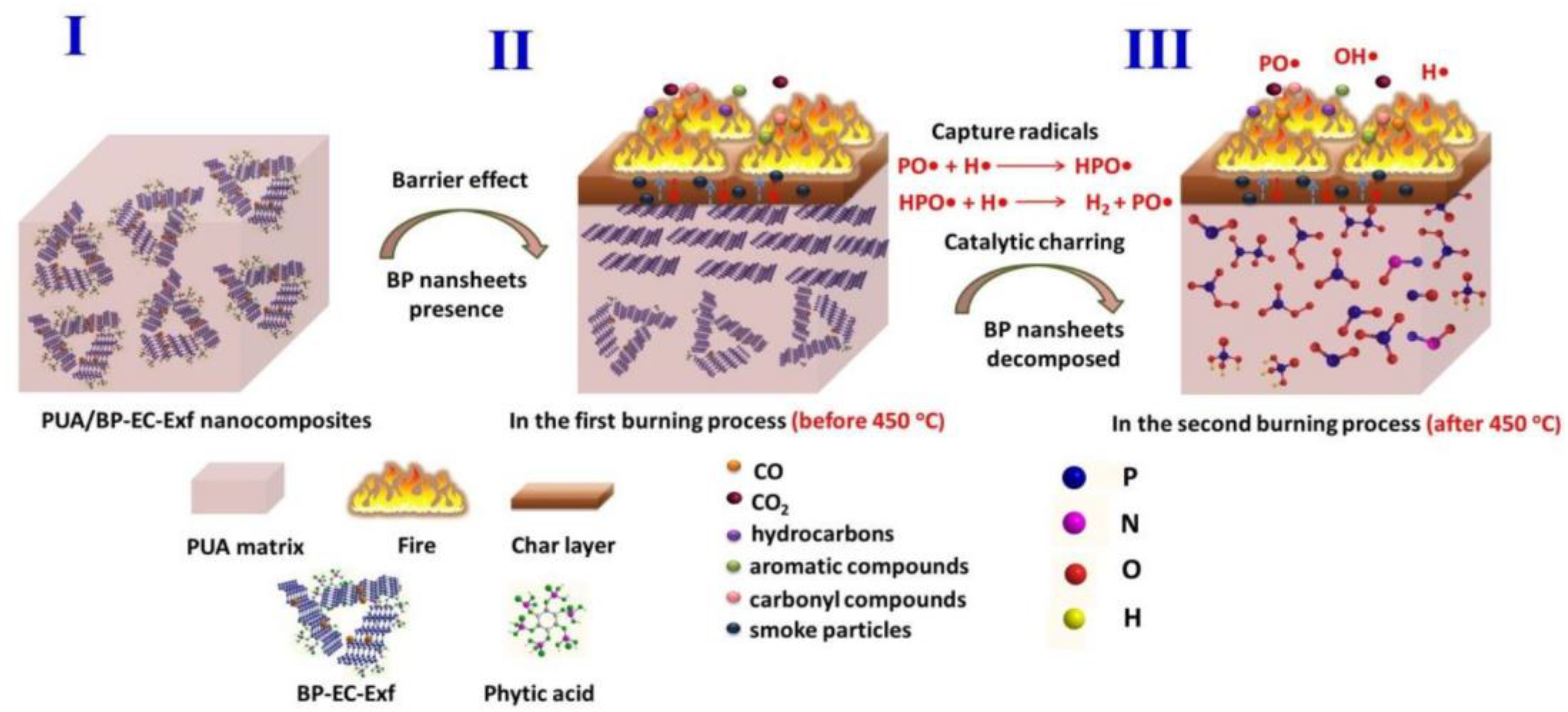
- Yin, S.; Ren, X.; Lian, P.; Zhu, Y.; Mei, Y.J.P. Synergistic Effects of Black Phosphorus/Boron Nitride Nanosheets on Enhancing the Flame-Retardant Properties of Waterborne Polyurethane and Its Flame-Retardant Mechanism. Polymers 2020, 12, 1487. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Cai, T.; He, L.; Chu, F.; Mu, X.; Han, L.; Hu, Y.; Wang, B.; Hu, W. Natural antioxidant functionalization for fabricating ambient-stable black phosphorus nanosheets toward enhancing flame retardancy and toxic gases suppression of polyurethane. J. Hazard. Mater. 2020, 387, 121971. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Mu, X.; Li, Z.; Hu, W.; Hu, Y. Poly(dimethyl siloxane)-grafted black phosphorus nanosheets as filler to enhance moisture-resistance and flame-retardancy of thermoplastic polyurethane. Mater. Chem. Phys. 2022, 286, 126189. [Google Scholar] [CrossRef]
- Yang, W.; Qiu, S.; Zhou, Y.; Wang, J.; Zou, B.; Song, L. Covalent grafting diazotized black phosphorus with ferrocene oligomer towards smoke suppression and toxicity reduction. Chemosphere 2022, 303, 135012. [Google Scholar] [CrossRef] [PubMed]
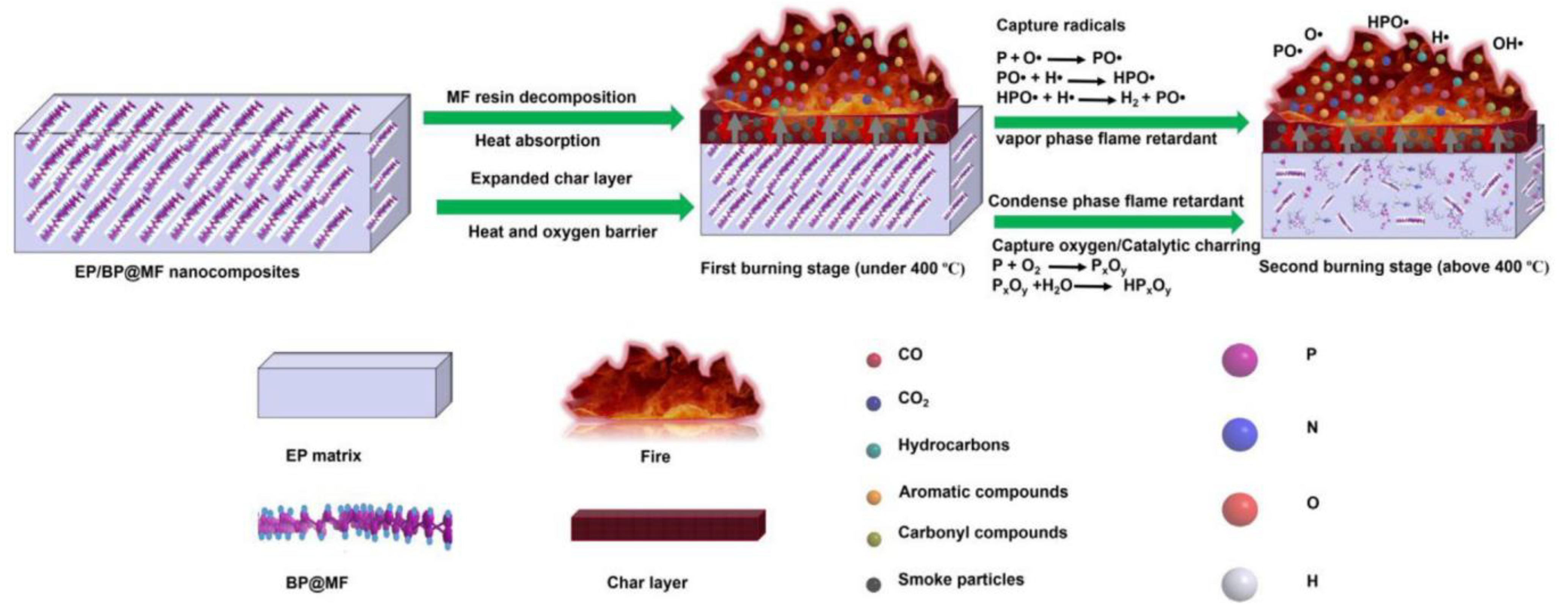
- Qiu, S.; Zou, B.; Zhang, T.; Ren, X.; Yu, B.; Zhou, Y.; Kan, Y.; Hu, Y. Integrated effect of NH2-functionalized/triazine based covalent organic framework black phosphorus on reducing fire hazards of epoxy nanocomposites. Chem. Eng. J. 2020, 401, 126058. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.; Yang, C.; Cheng, K.; Tan, T.; Lv, Y.; Liu, Y. Hybrid Porous Crystalline Materials from Metal Organic Frameworks and Covalent Organic Frameworks. Adv. Sci. 2021, 8, e2101883. [Google Scholar] [CrossRef]
- Li, C.; Cui, X.; Gao, X.; Liu, S.; Sun, Q.; Lian, H.; Zu, L.; Liu, Y.; Wang, X.; Cui, X.J.C.I. Electrochemically prepared black phosphorene micro-powder as flame retardant for epoxy resin. Compos. Interfaces 2020, 28, 693–705. [Google Scholar] [CrossRef]
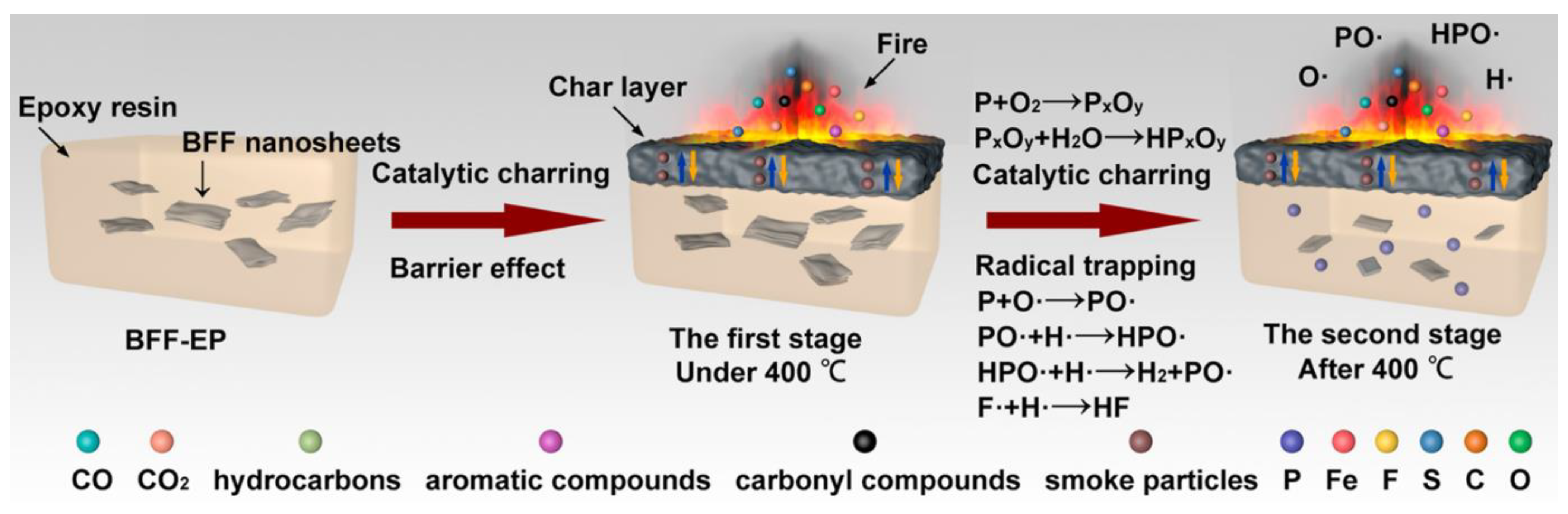
- Ren, X.; Zou, B.; Zhou, Y.; Zhao, Z.; Qiu, S.; Song, L. Construction of few-layered black phosphorus/graphite-like carbon nitride binary hybrid nanostructure for reducing the fire hazards of epoxy resin. J. Coll. Interface Sci. 2021, 586, 692–707. [Google Scholar] [CrossRef] [PubMed]
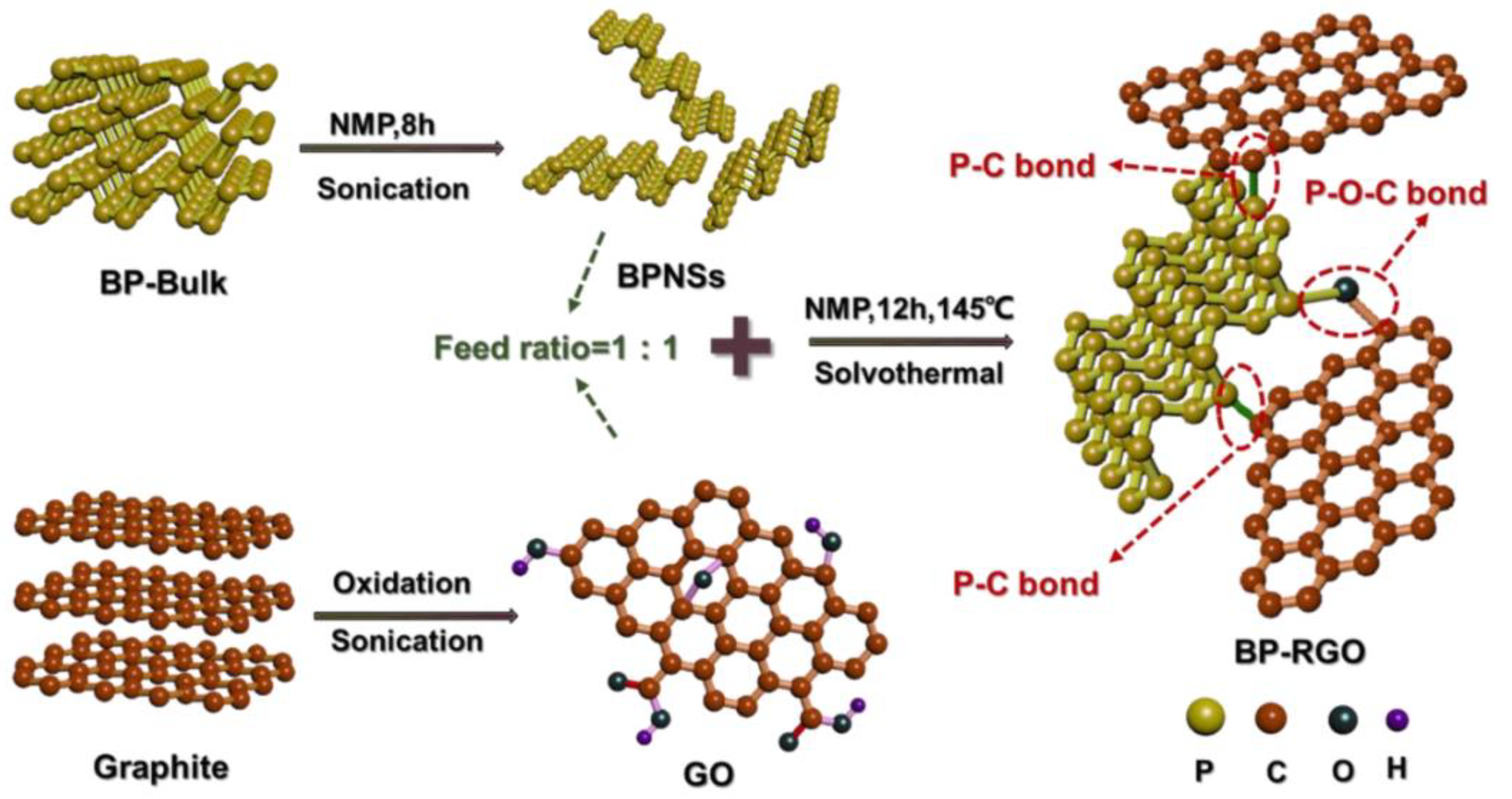
- Zhou, Y.; Chu, F.; Qiu, S.; Guo, W.; Zhang, S.; Xu, Z.; Hu, W.; Hu, Y. Construction of graphite oxide modified black phosphorus through covalent linkage: An efficient strategy for smoke toxicity and fire hazard suppression of epoxy resin. J. Hazard. Mater. 2020, 399, 123015. [Google Scholar] [CrossRef] [PubMed]
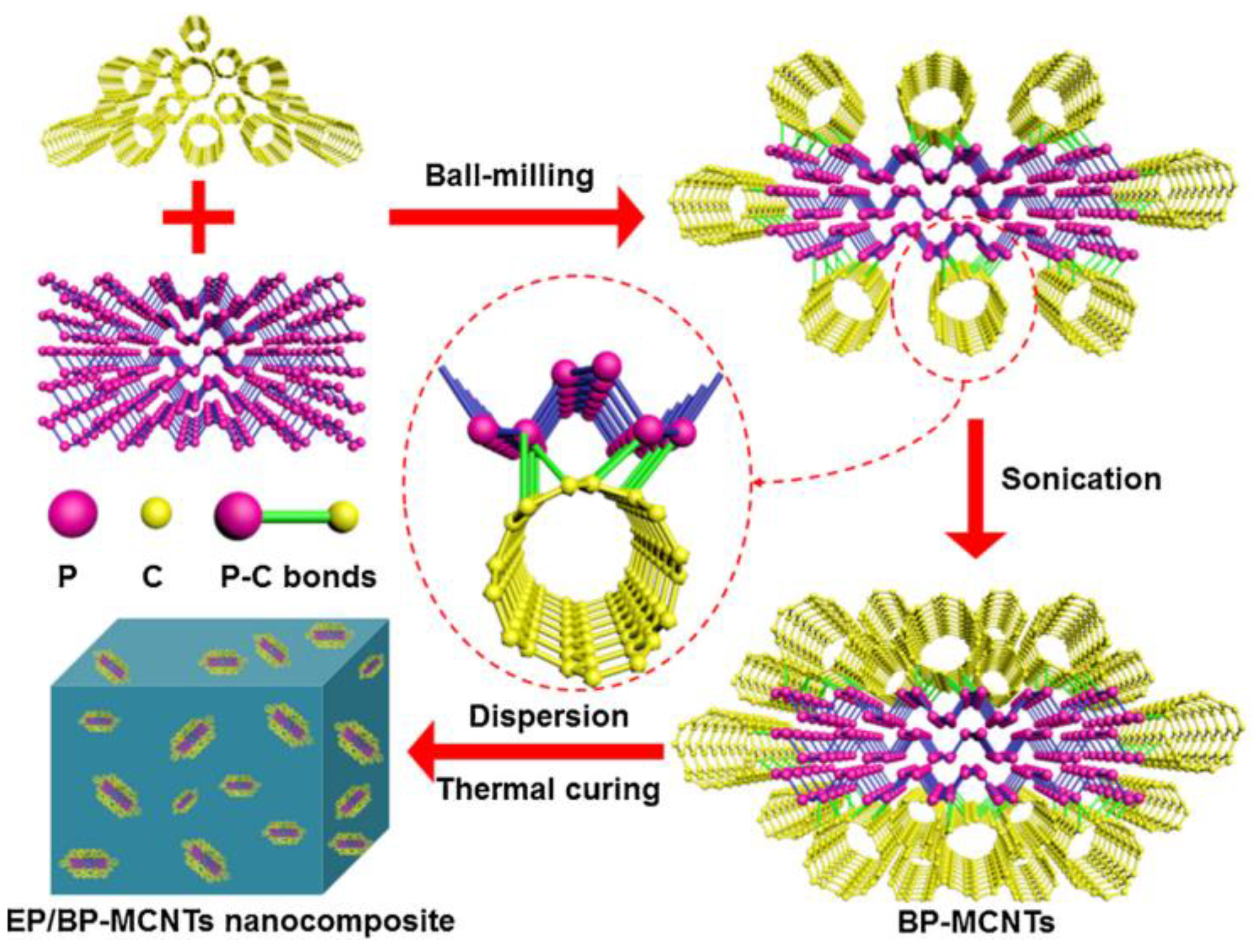
- Zou, B.; Qiu, S.; Ren, X.; Zhou, Y.; Zhou, F.; Xu, Z.; Zhao, Z.; Song, L.; Hu, Y.; Gong, X. Combination of black phosphorus nanosheets and MCNTs via phosphoruscarbon bonds for reducing the flammability of air stable epoxy resin nanocomposites. J. Hazard. Mater. 2020, 383, 121069. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Wu, M.; Liu, X.; Liu, X.; Pan, J.; Liu, S.; Zhao, J.; Xie, W.J.A.A.N.M. Uniformly-Dispersed Black Phosphorene as Flame-Retardant Epoxy Composites via Iterative Dispersion Strategy. ACS Appl. Nano Mater. 2023, 6, 17548–17559. [Google Scholar] [CrossRef]
- Qu, Z.; Wu, K.; Jiao, E.; Chen, W.; Hu, Z.; Xu, C.; Shi, J.; Wang, S.; Tan, Z. Surface functionalization of few-layer black phosphorene and its flame retardancy in epoxy resin. Chem. Eng. J. 2020, 382, 122991. [Google Scholar] [CrossRef]
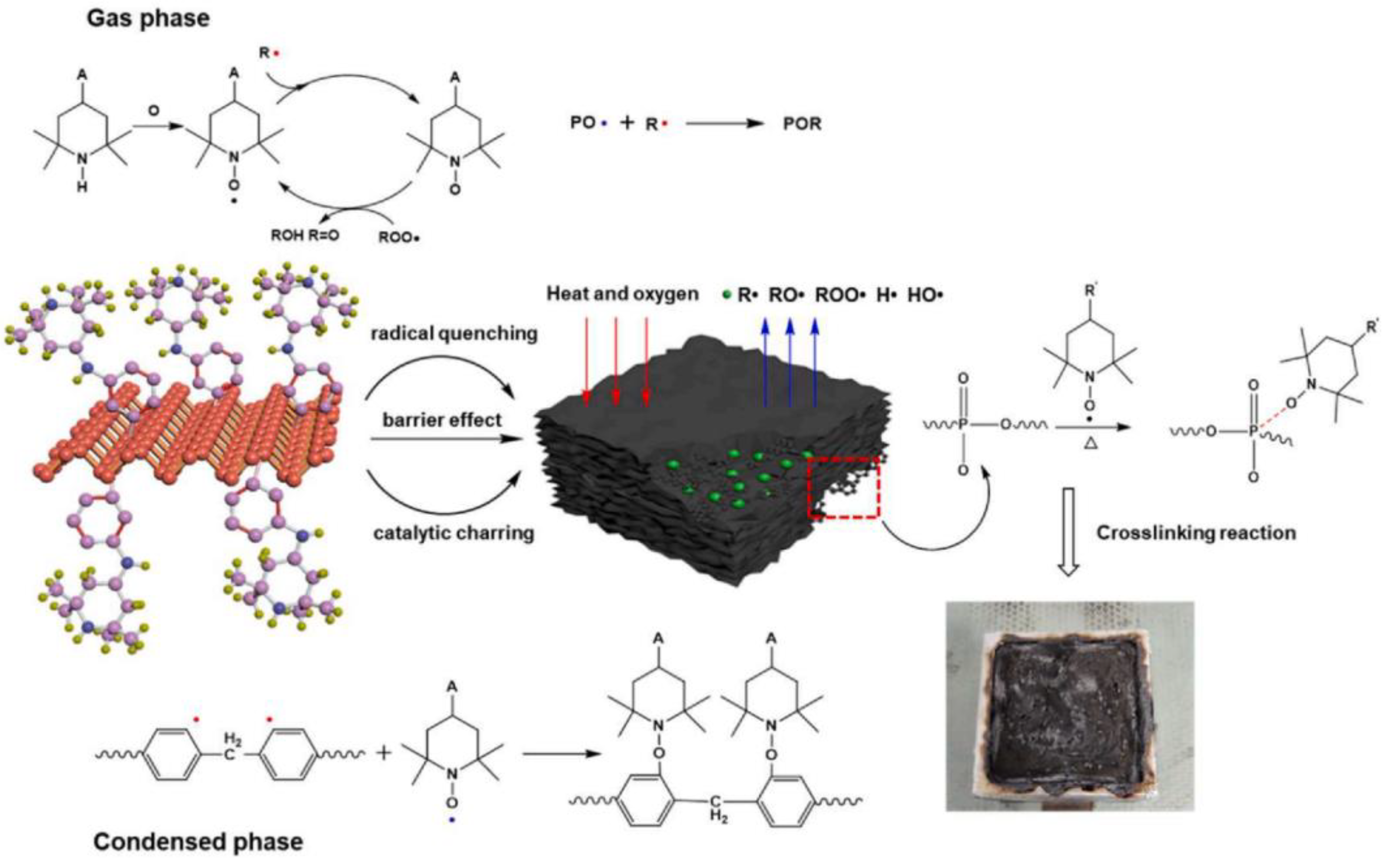
- Qian, Z.; Zou, B.; Xiao, Y.; Qiu, S.; Xu, Z.; Yang, Y.; Jiang, G.; Zhang, Z.; Song, L.; Hu, Y. Targeted modification of black phosphorus by MIL-53(Al) inspired by “Cannikin’s Law” to achieve high thermal stability of flame retardant polycarbonate at ultra-low additions. Compos. Part B Eng. 2022, 238, 109943. [Google Scholar] [CrossRef]
- Qiu, S.; Hou, Y.; Zhou, X.; Zhou, Y.; Wang, J.; Zou, B.; Yang, W.; Song, L.; Hu, Y.J.C.E.J. Light stabilizer and diazo passivation of black phosphorus nanosheets: Covalent functionalization endows air stability and flame retadancy enhancements. Chem. Eng. J. 2021, 425, 131532. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, S.; Chu, F.; Liu, W.; Yang, W.; Hu, W.; Hu, Y. Passivation of black phosphorus by triazine-based silica coating: Hierarchical BP@SiO2-N@Co(OH)2 structure for enhanced fire safety and toughness of unsaturated polyester resins. Compos. Part A Appl. Sci. Manufact. 2023, 164, 107279. [Google Scholar] [CrossRef]
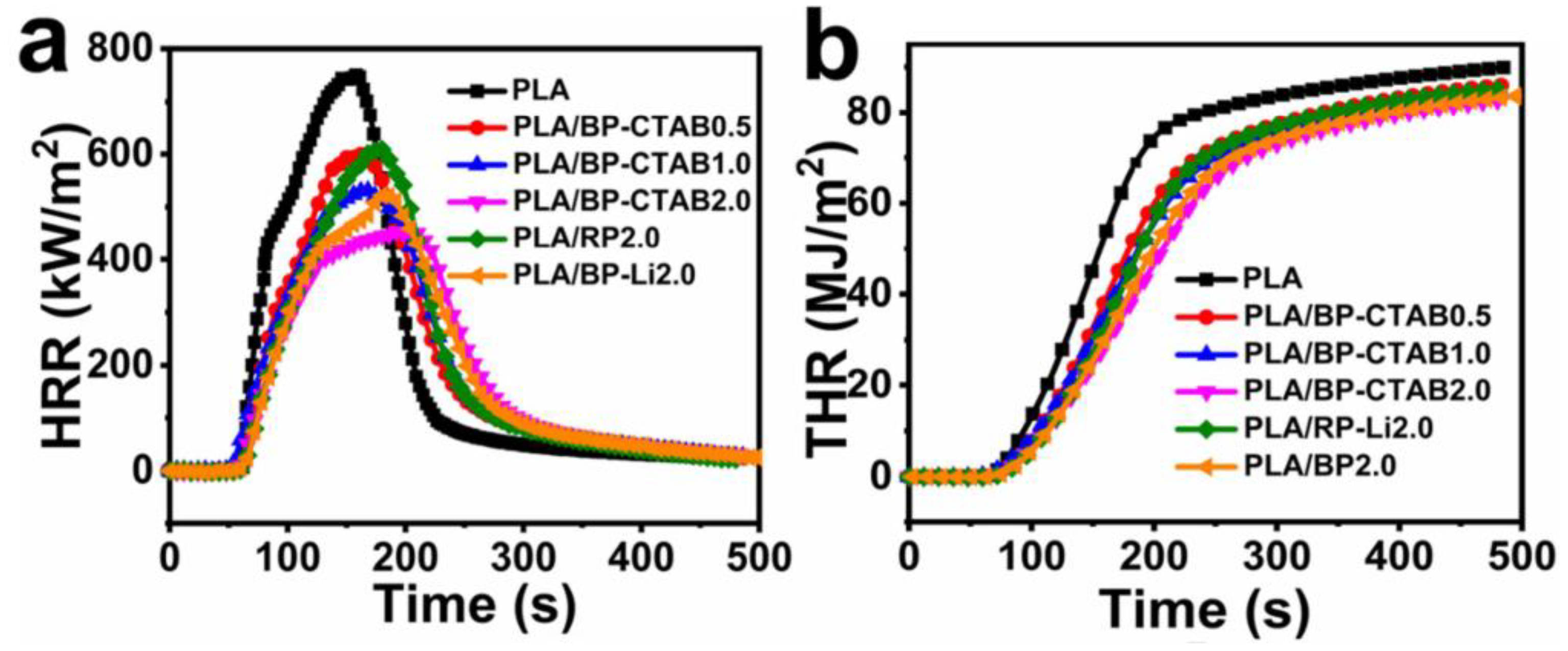
- Zhou, Y.; Huang, J.; Wang, J.; Chu, F.; Xu, Z.; Hu, W.; Hu, Y. Rationally designed functionalized black phosphorus nanosheets as new fire hazard suppression material for polylactic acid. Polym. Degrad. Stab. 2020, 178, 109194. [Google Scholar] [CrossRef]
- Shang, S.; Yuan, B.; Sun, Y.; Chen, G.; Huang, C.; Yu, B.; He, S.; Dai, H.; Chen, X. Facile preparation of layered melamine-phytate flame retardant via supramolecular self-assembly technology. J. Coll. Interface Sci. 2019, 553, 364–371. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Guan, J.-P.; Yang, X.-H.; Tang, R.-C. Improvement of flame retardancy of silk fabric by bio-based phytic acid, nano-TiO2, and polycarboxylic acid. Prog. Org. Coat. 2017, 112, 18–26. [Google Scholar] [CrossRef]
- Liu, W.; Ding, L.; Wang, L.; Zhang, C.; Yang, W.; Liu, D.; Gui, Z.; Hu, W.J.C. A rational design of functionalized black phosphorus cooperates with piperazine pyrophosphate to significantly suppress the fire hazards of polypropylene. Chemosphere 2023, 314, 137686. [Google Scholar] [CrossRef]
- Liu, T.; Lu, Y.; Zhan, R.; Qian, W.; Luo, G. Nanomaterials and nanomaterials-based drug delivery to promote cutaneous wound healing. Adv. Drug Deliv. Rev. 2023, 193, 114670. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, L.; Zhang, L.; Li, C. Simultaneous exfoliation and functionalization of black phosphorus by sucrose-assisted ball milling with NMP intercalating and preparation of flame retardant polyvinyl alcohol film. Polymer 2022, 255, 125036. [Google Scholar] [CrossRef]
- Zou, B.; Qiu, S.; Zhou, Y.; Qian, Z.; Xu, Z.; Wang, J.; Xiao, Y.; Liao, C.; Yang, W.; Han, L.; et al. Photothermal-healing, and record thermal stability and fire safety black phosphorus–boron hybrid nanocomposites: Mechanism of phosphorus fixation effects and charring inspired by cell walls. J. Mater. Chem. A 2022, 10, 14423–14434. [Google Scholar] [CrossRef]
- McDowell, M.T.; Xiong, H.; Nazemi, M.; Peng, J.; Lutkenhaus, J.L.; Wang, R.; Djire, A.; Sankaran, A.; Leem, J.; Gogotsi, Y. Nanomaterials in the future of energy research. Cell Rep. Phys. Sci. 2023, 4, 101605. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Gao, S.; Yang, X.; Fan, R.; Zhi, M.; Fu, M. Recent advances in the flame retardancy role of graphene and its derivatives in epoxy resin materials. Compos. Part A Appl. Sci. Manufact. 2021, 149, 106539. [Google Scholar] [CrossRef]
- Ge, Q.; Chu, J.; Cao, W.; Yi, F.; Ran, Z.; Jin, Z.; Mao, B.; Li, Z.; Novoselov, K.S.J.A.F.M. Graphene-Based Textiles for Thermal Management and Flame Retardancy. Adv. Funct. Mater. 2022, 32, 2205934. [Google Scholar] [CrossRef]














| Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| High temperature and high pressure method | High temperature and high pressure make white phosphorus and red phosphorus phase-change | Short time and easy to reproduce | Small product size, high cost, high equipment requirements |
| Mechanical ball milling | The high-speed impact of the ball milling medium on the red phosphorus causes it to undergo a phase change | The inert gas prevents black phosphorus from being oxidized to some extent | Poor crystal shape and time-consuming |
| Mercury reflux method, bismuth melting method | Mercury and bismuth are used to reduce the activation energy of the reaction | The reaction conditions are mild | Small product size, time-consuming, more polluting, and costly |
| Mineralization | The mineralizer reacts with red phosphorus in a series of temperature changes | Good crystallinity, high reproducibility, green environmental protection, low cost | Difficult-to-achieve scale |
| Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Mechanical stripping method | Use external force to peel, such as tape, etc. | Simple operation | Time-consuming, low yield, easily oxidized |
| Liquid phase exfoliation | In the mixed solution with external field force, such as ultrasonic wave, microwave, electric field, etc. | The black phosphene prepared has many forms, such as BPNSs, BPQDs, porous black phosphene and so on | Higher organic solvent contamination, low yield, easily oxidized |
| Solvothermal method | Black phosphorus nanosheets were catalyzed by temperature, pressure and solvent in a closed system | Simple operation and low cost | Poor crystallinity, thicker black phosphorus nanosheets |
| Chemical vapor deposition | Nano black phosphorus was prepared by high temperature gas–solid and phase catalysis | Simple operation, low cost and not time-consuming | It is not yet mature and has impurities |
| High pressure method | Red phosphorus film is directly converted into black phosphorus film by applying high pressure | The black phosphorus film has a large size and thin thickness | High energy consumption and harsh reaction conditions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Lai, D.; Yang, J.; Liu, L.; Wu, H.; Wang, J.; Liu, Y. Research Progress on the Preparation Methods for and Flame Retardant Mechanism of Black Phosphorus and Black Phosphorus Nanosheets. Nanomaterials 2024, 14, 892. https://doi.org/10.3390/nano14100892
Cao W, Lai D, Yang J, Liu L, Wu H, Wang J, Liu Y. Research Progress on the Preparation Methods for and Flame Retardant Mechanism of Black Phosphorus and Black Phosphorus Nanosheets. Nanomaterials. 2024; 14(10):892. https://doi.org/10.3390/nano14100892
Chicago/Turabian StyleCao, Wuyan, Dengwang Lai, Jun Yang, Li Liu, Hao Wu, Jin Wang, and Yuejun Liu. 2024. "Research Progress on the Preparation Methods for and Flame Retardant Mechanism of Black Phosphorus and Black Phosphorus Nanosheets" Nanomaterials 14, no. 10: 892. https://doi.org/10.3390/nano14100892
APA StyleCao, W., Lai, D., Yang, J., Liu, L., Wu, H., Wang, J., & Liu, Y. (2024). Research Progress on the Preparation Methods for and Flame Retardant Mechanism of Black Phosphorus and Black Phosphorus Nanosheets. Nanomaterials, 14(10), 892. https://doi.org/10.3390/nano14100892






