Carbon Nanofiber Membranes Loaded with MXene@g-C3N4: Preparation and Photocatalytic Property
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Experimental Materials
2.2. Preparation of g-C3N4
2.3. Preparation of Ti3C2 MXenes
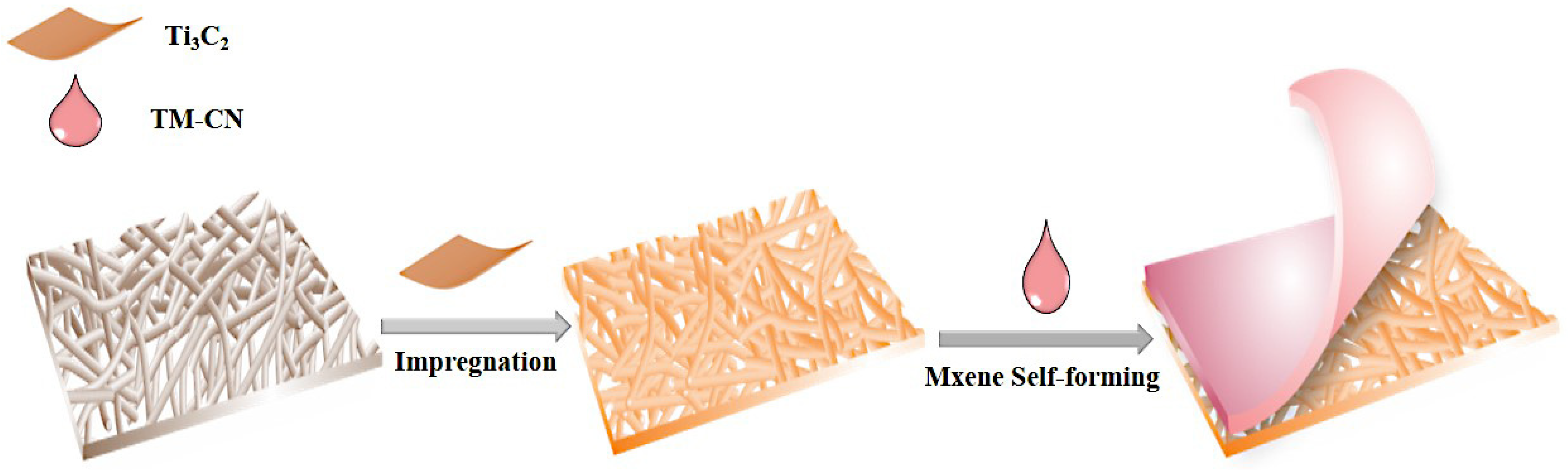
2.4. Preparation of TM-CN
2.5. Preparation of TM-CN-Loaded Carbon Fibers
2.6. Characterization
2.7. Photocatalytic Property Characterizations of C-TM-CN
3. Results and Discussion
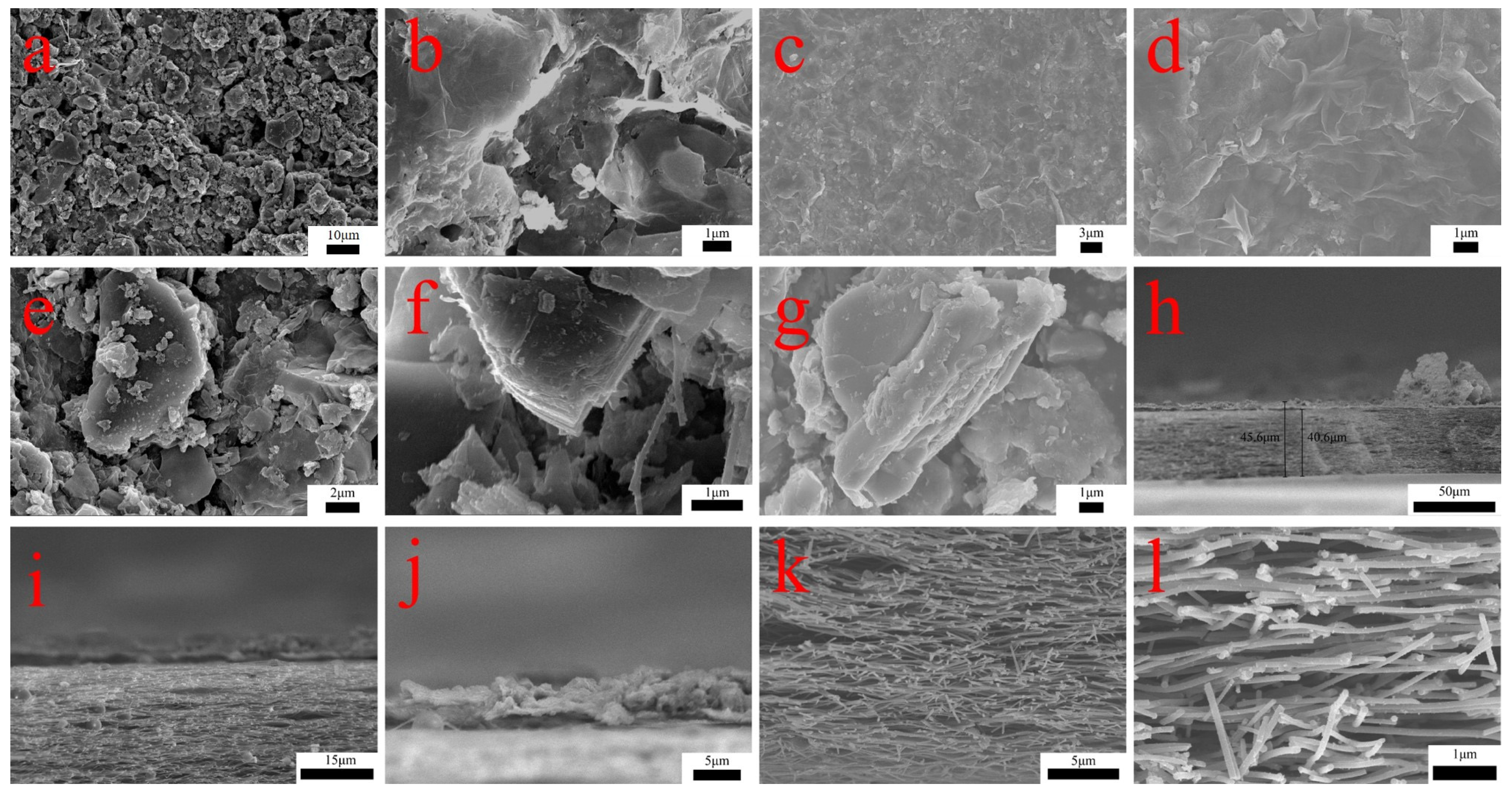
3.1. SEM Analysis
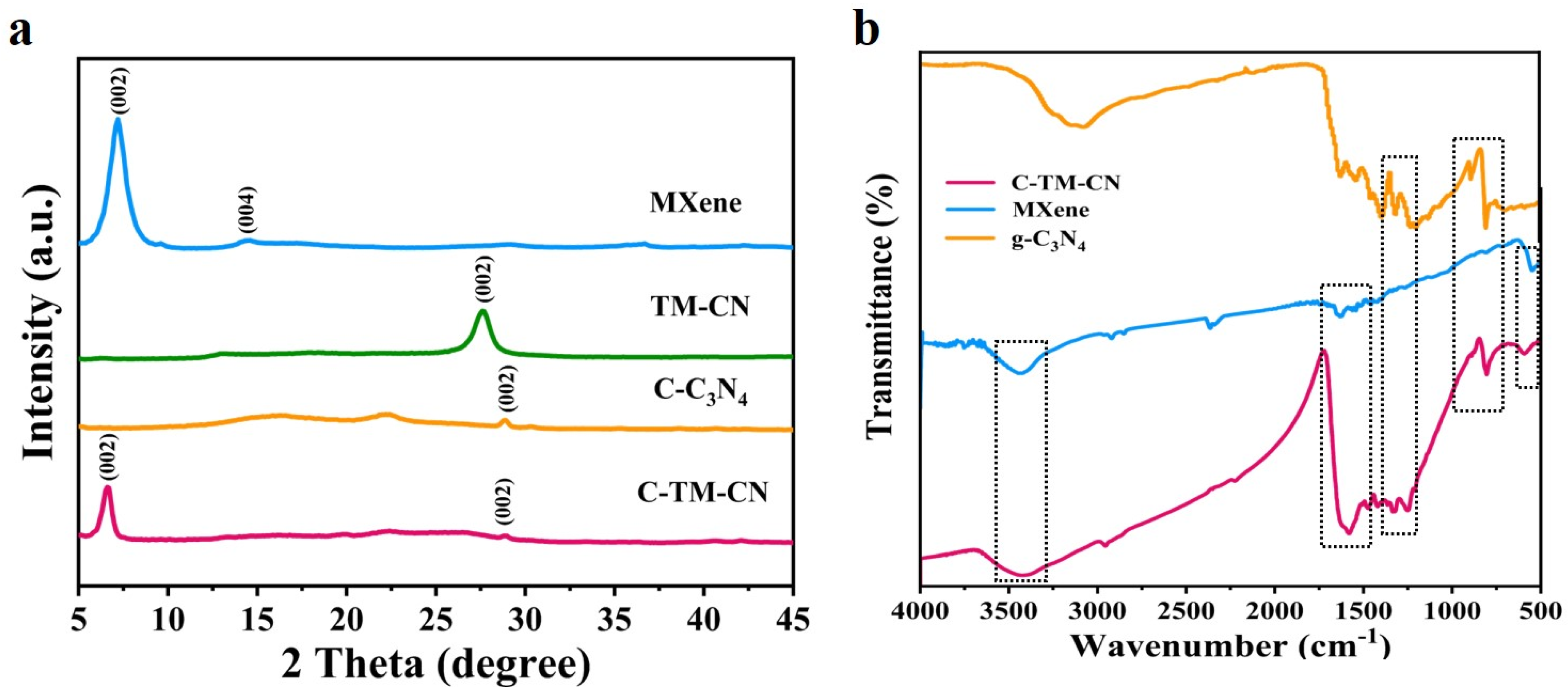
3.2. XRD Pattern Analysis and FTIR Spectra Analysis
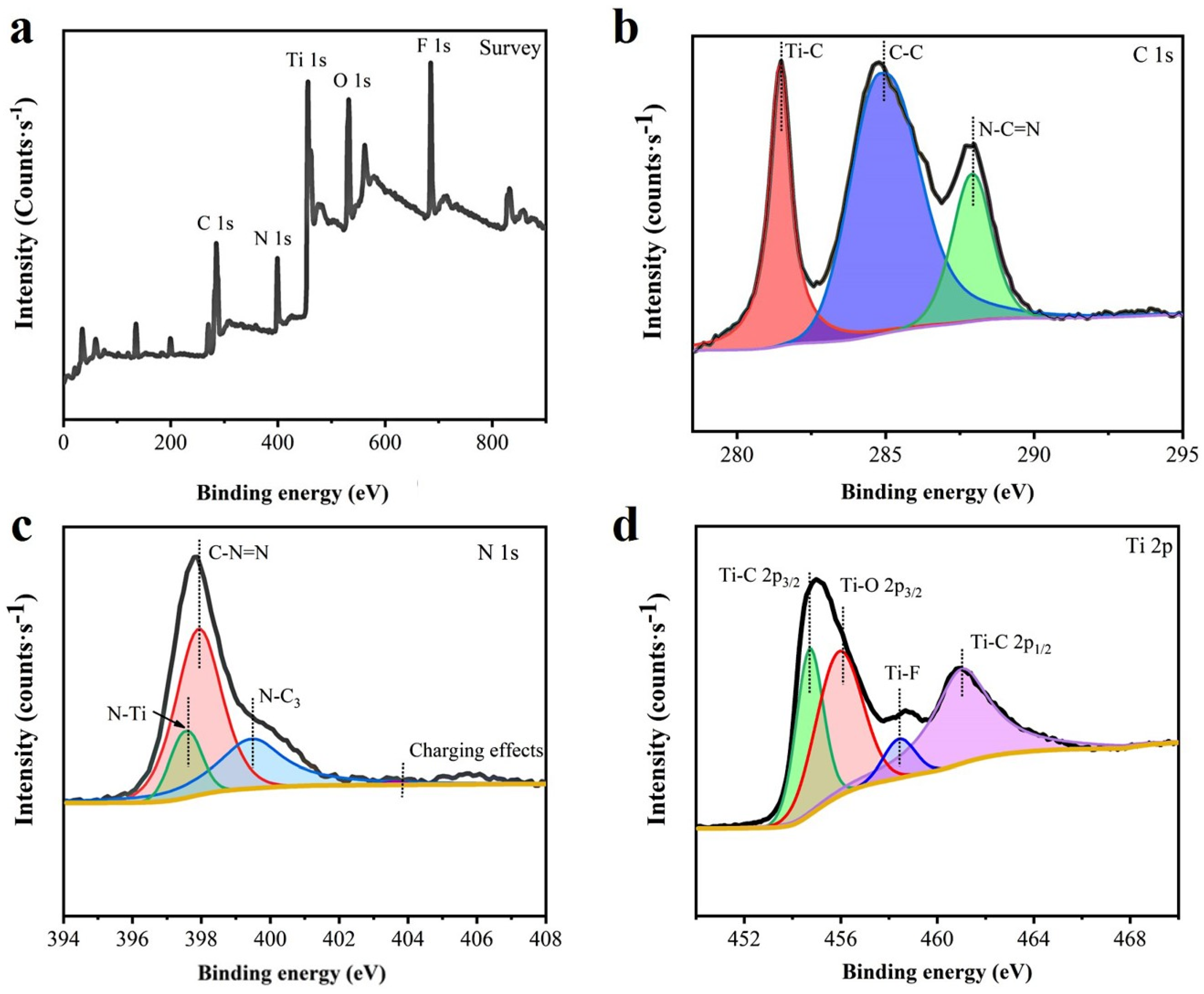
3.3. XPS Analysis
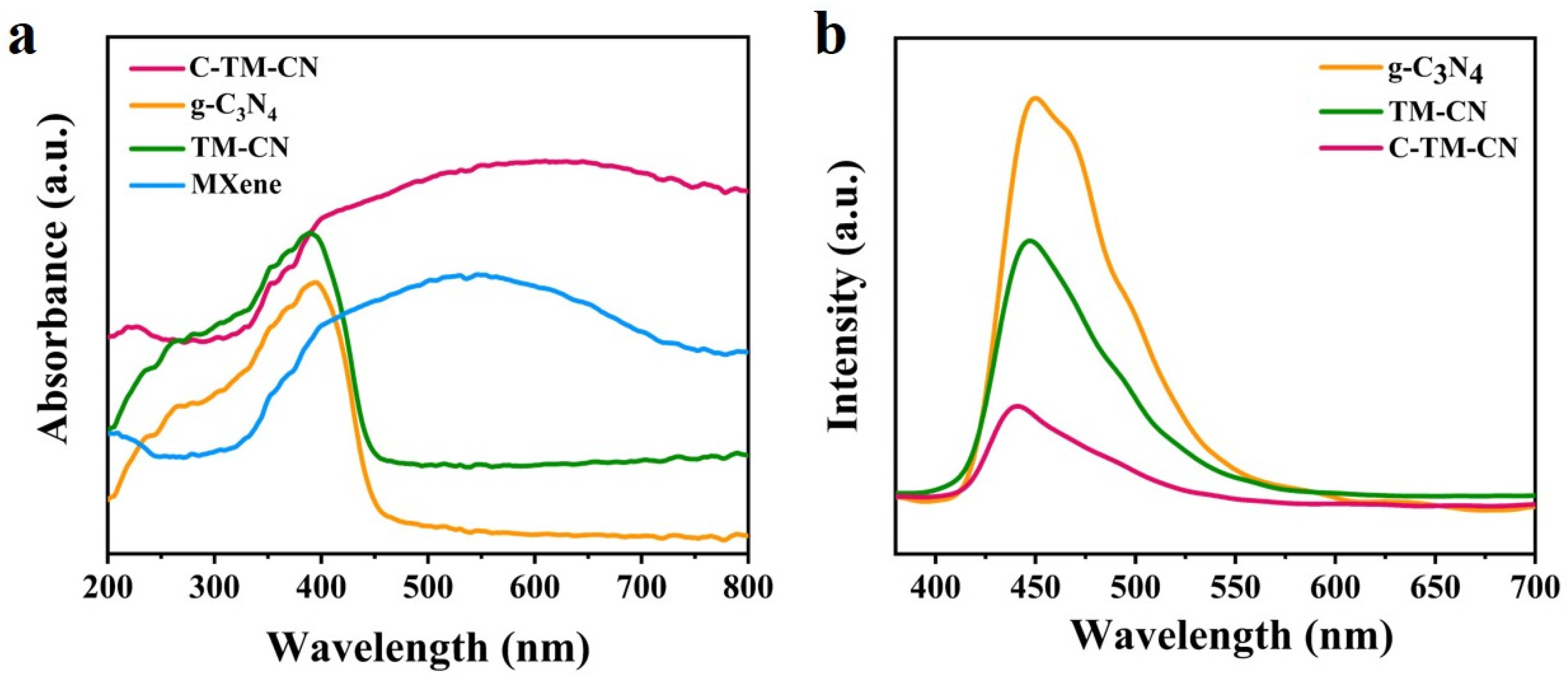
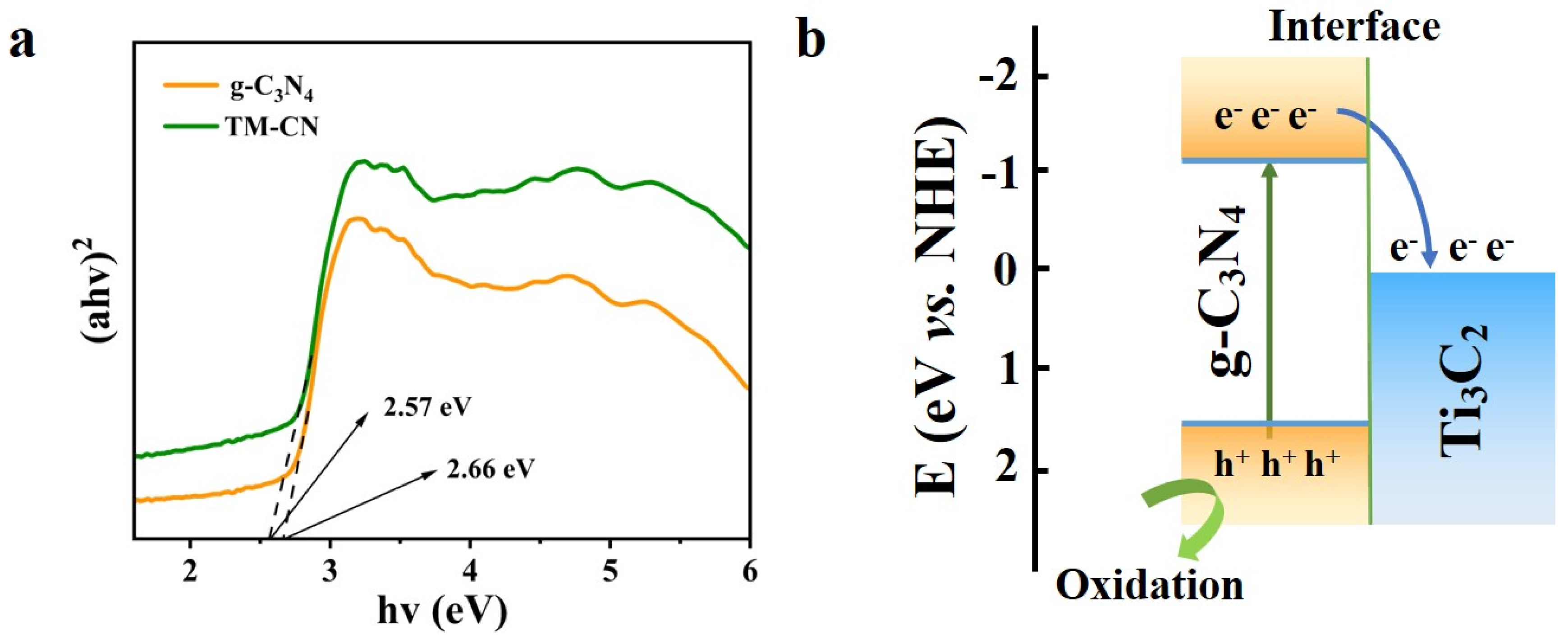
3.4. UV–Vis Diffuse Reflection and PL Photoluminescence
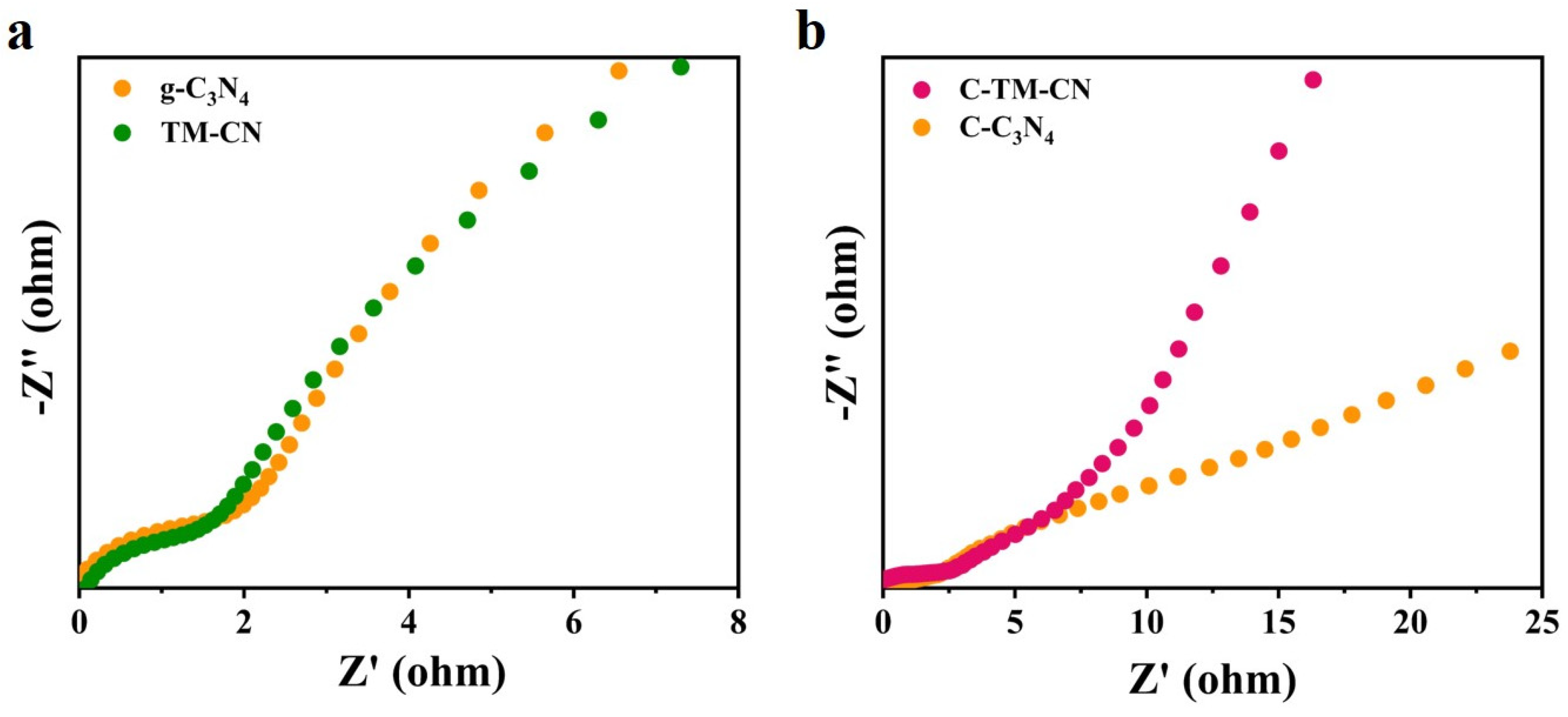
3.5. Electrochemical Impedance
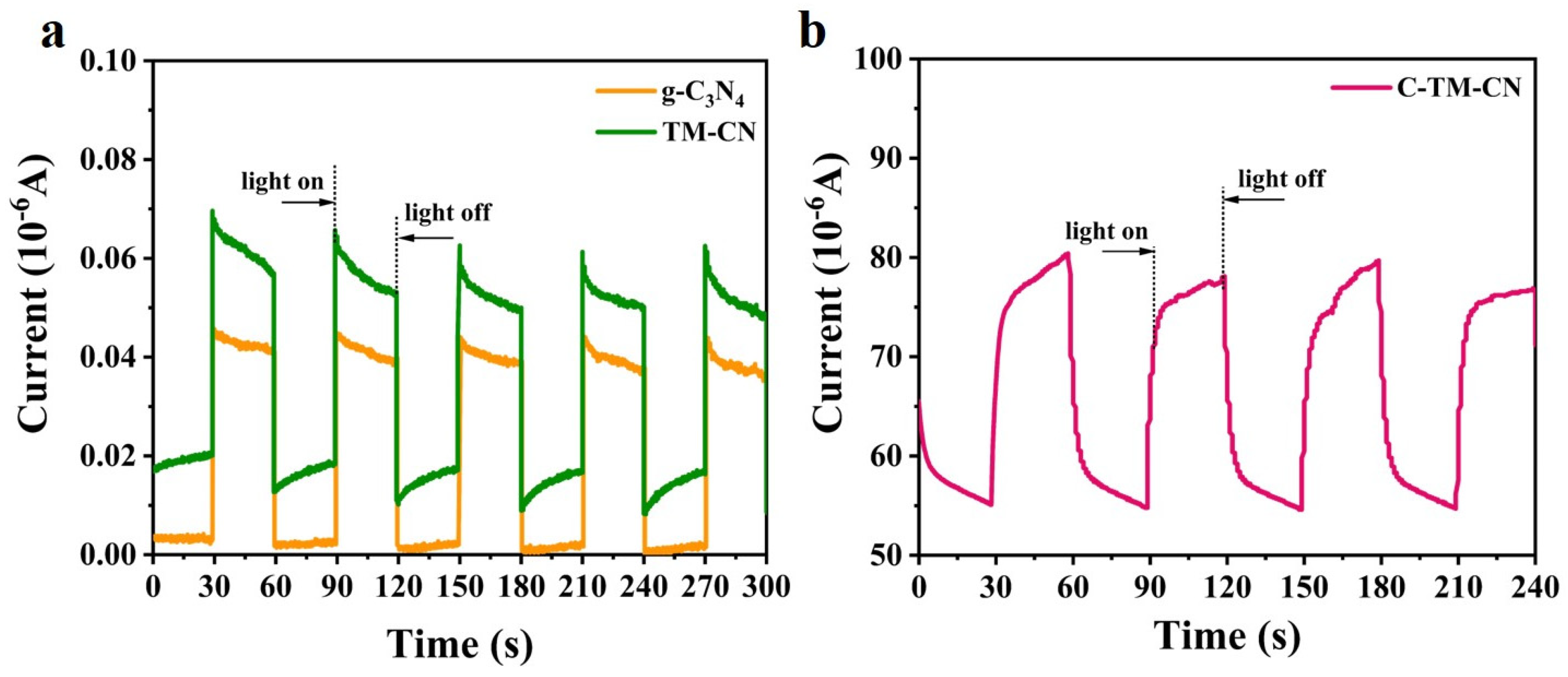
3.6. Photocurrent Response
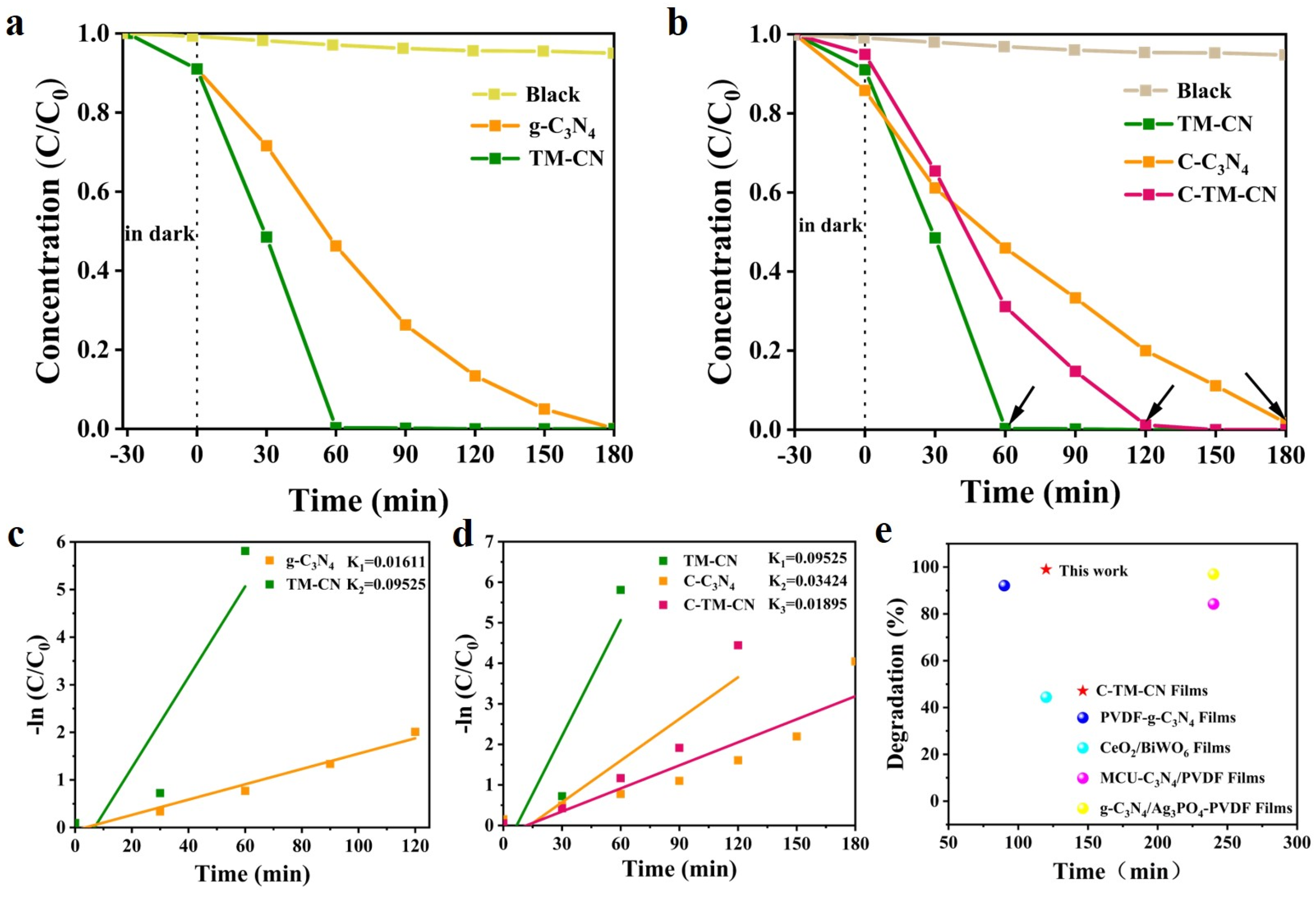
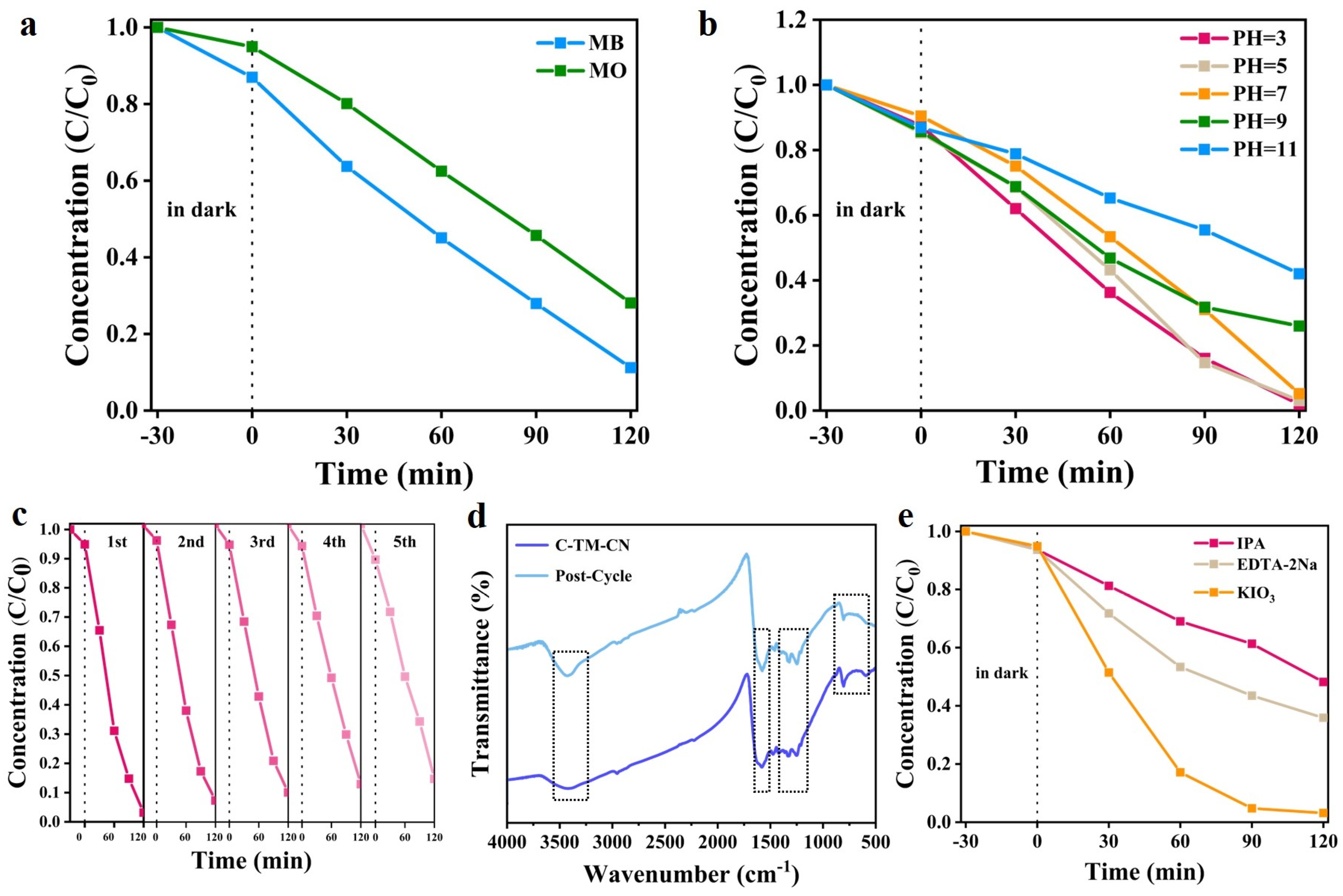
3.7. Photocatalytic Properties and Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rai, H.S.; Bhattacharyya, M.S.; Singh, J.; Bansal, T.K.; Vats, P.; Banerjee, U.C. Removal of dyes from the effluent of textile and dyestuff manufacturing industry: A review of emerging techniques with reference to biological treatment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 219–238. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Khan, T.A.; Asim, M. Removal of Arsenic from Water by Electrocoagulation and Electrodialysis Techniques. Sep. Purif. Rev. 2011, 40, 25–42. [Google Scholar] [CrossRef]
- Beluci, N.; Mateus, G.; Miyashiro, C.S.; Homem, N.; Gomes, R.G.; Fagundes-Klen, M.R.; Bergamasco, R.; Vieira, A.M.S. Hybrid treatment of coagulation/flocculation process followed by ultrafiltration in TIO2-modified membranes to improve the removal of reactive black 5 dye. Sci. Total Environ. 2019, 664, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.-Q.; Jiang, Y.-Y.; Wang, T.; Wu, L.-G.; Yu, X.-Y.; Lin, J.-Z.; Shi, S.-X. Enhanced performance of polyimide hybrid membranes for benzene separation by incorporating three-dimensional silver–graphene oxide. J. Colloid Interface Sci. 2016, 478, 145–154. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J. Hazard. Mater. 2011, 185, 17–23. [Google Scholar] [CrossRef]
- Gupta, V.; Nayak, A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem. Eng. J. 2012, 180, 81–90. [Google Scholar] [CrossRef]
- Homem, N.C.; Beluci, N.d.C.L.; Amorim, S.; Reis, R.; Vieira, A.M.S.; Vieira, M.F.; Bergamasco, R.; Amorim, M.T.P. Surface modification of a polyethersulfone microfiltration membrane with graphene oxide for reactive dyes removal. Appl. Surf. Sci. 2019, 486, 499–507. [Google Scholar] [CrossRef]
- Shao, F.; Dong, L.; Dong, H.; Zhang, Q.; Zhao, M.; Yu, L.; Pang, B.; Chen, Y. Graphene oxide modified polyamide reverse osmosis membranes with enhanced chlorine resistance. J. Membr. Sci. 2017, 525, 9–17. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhang, T.; Xu, P.; Zhang, L.; Liu, J.; Chen, Z. Growth of C3N4 nanosheets on carbon-fiber cloth as flexible and macroscale filter-membrane-shaped photocatalyst for degrading the flowing wastewater. Appl. Catal. B Environ. 2017, 219, 425–431. [Google Scholar] [CrossRef]
- Sundar, K.P.; Kanmani, S. Progression of Photocatalytic reactors and it’s comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, M.L. Prediction of New Low Compressibility Solids. Science 1989, 245, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Teter, D.M.; Hemley, R.J. Low-Compressibility Carbon Nitrides. Science 1996, 271, 53–55. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Safaei-Ghomi, J.; Akbarzadeh, Z.; Teymuri, R. ZnS nanoparticlesimmobilized on graphitic carbon nitride as a recyclable and environmentally friendly catalyst for synthesis of 3-cinnamoyl coumarins. Res. Chem. Intermed. 2019, 45, 3425–3439. [Google Scholar] [CrossRef]
- Wei, B.; Wang, C.; He, Y.; Ran, G.; Song, Q. A novel FeS2@g-C3N4 composite with enhanced photo-Fenton catalytic activity for pollutant degradation. Compos. Commun. 2021, 24, 100652. [Google Scholar] [CrossRef]
- Kroke, E.; Schwarz, M.; Horath-Bordon, E.; Kroll, P.; Noll, B.; Norman, A.D. Tri-s-triazine derivatives. Part I. From trichloro-tri-s-triazine to graphitic C3N4 structures. New J. Chem. 2002, 26, 508–512. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Wang, X.; Liu, E.; Ye, J.; Wang, D. Boosting the Photocatalytic Activity of P25 for Carbon Dioxide Reduction by using a Surface-Alkalinized Titanium Carbide MXene as Cocatalyst. ChemSusChem 2018, 11, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Wei, P.; Li, X.; Liu, Y.; Cao, Y.; Wang, H.; Yu, H.; Peng, F.; Zhang, L.; Zhang, B.; et al. High efficiency photocatalytic hydrogen production over ternary Cu/TiO2@Ti3C2Tx enabled by low-work-function 2D titanium carbide. Nano Energy 2018, 53, 97–107. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Liu, W.; Sun, M.; Ding, Z.; Gao, B.; Ding, W. Ti3C2 MXene embellished g-C3N4 nanosheets for improving photocatalytic redox capacity. J. Alloys Compd. 2021, 877, 160223. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, Y.; Meng, X. Fabrication of TiO2 nanofibers/MXene Ti3C2 nanocomposites for photocatalytic H2 evolution by electrostatic self-assembly. Appl. Surf. Sci. 2019, 496, 143647. [Google Scholar] [CrossRef]
- Attanayake, N.H.; Abeyweera, S.C.; Thenuwara, A.C.; Anasori, B.; Gogotsi, Y.; Sun, Y.; Strongin, D.R. Vertically aligned MoS2 on Ti3C2 (MXene) as an improved HER catalyst. J. Mater. Chem. A 2018, 6, 16882–16889. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Yuan, X.; Zeng, G.; Zhou, J.; Wang, X.; Chew, J.W. Clay-inspired MXene-based electrochemical devices and photo-electrocatalyst: State-of-the-art progresses and challenges. Adv. Mater. 2018, 30, 1704561. [Google Scholar] [CrossRef]
- Huang, K.; Li, C.; Li, H.; Ren, G.; Wang, L.; Wang, W.; Meng, X. Photocatalytic applications of two-dimensional Ti3C2 MXenes: A review. ACS Appl. Nano Mater. 2020, 3, 9581–9603. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.-T.; Ma, T.-Y.; Du, A.; Qiao, S.-Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, A.S.; Mubarak, N.M.; Hashmi, Z.; Solangi, N.H.; Karri, R.R.; Hua, T.Y.; Mazari, S.A.; Koduru, J.R.; Alfantazi, A. New insights into MXene applications for sustainable environmental remediation. Chemosphere 2023, 313, 137497. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yuan, C.; Lv, H.; Zhang, K.; Chen, X.; Zhang, Y. Hierarchical Ti3C2 MXene/Zn3In2S6 schottky junction for efficient visible-light-driven Cr(VI) photoreduction. Ceram. Int. 2022, 48, 11320–11329. [Google Scholar] [CrossRef]
- Hu, J.; Ding, J.; Zhong, Q. Ultrathin 2D Ti3C2 MXene Co-catalyst anchored on porous g-C3N4 for enhanced photocatalytic CO2 reduction under visible-light irradiation. J. Colloid Interface Sci. 2021, 582, 647–657. [Google Scholar] [CrossRef]
- Yang, S.; Wang, K.; Chen, Q.; Wu, Y. Enhanced photocatalytic hydrogen production of S-scheme TiO2/g-C3N4 heterojunction loaded with single-atom Ni. J. Mater. Sci. Technol. 2024, 175, 104–114. [Google Scholar] [CrossRef]
- Shi, W.N.; Fang, W.X.; Wang, J.C.; Qiao, X.; Wang, B.B.; Guo, X.W. pH-controlled mechanism of photocatalytic RhB degradation over g-C3N4 under sunlight irradiation. Photochem. Photobiol. Sci. 2021, 20, 303–313. [Google Scholar] [CrossRef]
- Le, C.; Chen, T.Q.; Liang, T.; Zhang, P.; MacMillan, D.W.C. A radical approach to the copper oxidative addition problem: Trifluoromethylation of bromoarenes. Science 2018, 360, 1010–1014. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Yang, C.; Yang, L.; Jiang, Q.; Huang, H. Constructing 3D interweaved MXene/graphitic carbon nitride nanosheets/graphene nanoarchitectures for promoted electrocatalytic hydrogen evolution. J. Energy Chem. 2022, 67, 483–491. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.-L.; Wang, G.-G.; Zhang, H.-Y.; Zhang, B.; Li, G.-Z.; Wu, Z.-P.; Dang, L.-Y.; Han, J.-C. Few-layered Ti3C2Tx MXenes coupled with Fe2O3 nanorod arrays grown on carbon cloth as anodes for flexible asymmetric supercapacitors. J. Mater. Chem. A 2019, 7, 22631–22641. [Google Scholar] [CrossRef]
- He, S.; Zhan, Y.; Hu, J.; Zhang, G.; Zhao, S.; Feng, Q.; Yang, W. Chemically stable two-dimensional MXene@UIO-66-(COOH)2 composite lamellar membrane for multi-component pollutant-oil-water emulsion separation. Compos. Part B Eng. 2020, 197, 108188. [Google Scholar] [CrossRef]
- Tu, W.; Liu, Y.; Chen, M.; Ma, L.; Li, L.; Yang, B. Photocatalytic self-cleaning graphene oxide membrane coupled with carbon nitride and Ti3C2-Mxene for enhanced wastewater purification. Sep. Purif. Technol. 2022, 296, 121398. [Google Scholar] [CrossRef]
- Ren, H.-T.; Li, D.-S.; Cai, C.-C.; Li, T.-T.; Lou, C.-W.; Lin, J.-H.; Han, X. Facile Photopolymerization of High-Molecular-Weight Polyaniline Composites Induced by g-C3N4 at Room Temperature for Trace Fe Ion Sensors. Macromolecules 2023, 56, 9509–9522. [Google Scholar] [CrossRef]
- Xiao, R.; Zhao, C.; Zou, Z.; Chen, Z.; Tian, L.; Xu, H.; Tang, H.; Liu, Q.; Lin, Z.; Yang, X. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 268, 118382. [Google Scholar] [CrossRef]
- Zheng, D.; Pang, C.; Liu, Y.; Wang, X. Shell-engineering of hollow g-C3N4 nanospheres via copolymerization for photocatalytic hydrogen evolution. Chem. Commun. 2015, 51, 9706–9709. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.J.; Yu, D.S.; Hu, F.; Huang, S.C.; Song, J.N.; Chen, H.Y.; Li, L.L.; Peng, S.J. Clusters Induced Electron Redistribution to Tune Oxygen Reduction Activity of Transition Metal Single-Atom for Metal-Air Batteries. Angew. Chem. Int. Ed. 2022, 134, e202116068. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, R.; Huang, J.; Jiang, Y.; Zhao, C.; Yang, X. In situ construction of protonated g-C3N4/Ti3C2 MXene Schottky heterojunctions for efficient photocatalytic hydrogen production. Chin. J. Catal. 2021, 42, 107–114. [Google Scholar] [CrossRef]
- Li, K.; Lu, X.; Zhang, Y.; Liu, K.; Huang, Y.; Liu, H. Bi3TaO7/Ti3C2 heterojunctions for enhanced photocatalytic removal of water-borne contaminants. Environ. Res. 2020, 185, 109409. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, Y.; Shi, X.; Huang, J.D.; Zhang, K.; Wang, R.; Ye, L.Q. Mesoporous g-C3N4/MXene (Ti3C2Tx) heterojunction as a 2D electronic charge transfer for efficient photocatalytic CO2 reduction. Appl. Surf. Sci. 2021, 546, 149111. [Google Scholar] [CrossRef]
- Tian, X.; Wu, H.; Hu, X.; Wang, Z.; Ren, C.; Cheng, Z.; Dou, L.; Lin, Y.-W. Enhanced photocatalytic performance of ZnO/AgCl composites prepared by high-energy mechanical ball milling. New J. Chem. 2022, 46, 9155–9171. [Google Scholar] [CrossRef]
- Zarezadeh, S.; Habibi-Yangjeh, A.; Mousavi, M.; Ghosh, S. Novel ZnO/Ag3PO4/AgI photocatalysts: Preparation, characterization, and the excellent visible-light photocatalytic performances. Mater. Sci. Semicond. Process. 2020, 119, 105229. [Google Scholar] [CrossRef]
- Ding, Q.; Zou, X.; Ke, J.; Dong, Y.; Cui, Y.; Ma, H. Enhanced artificial nitrogen fixation efficiency induced by construction of ternary TiO2/MIL-88A(Fe)/g-C3N4 Z-scheme heterojunction. J. Colloid Interface Sci. 2023, 649, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Rong, J.; Zhang, Y.; Zheng, X.; Li, X.; Xu, S.; Li, Z. An S-scheme CeO2/foveolate g-C3N4 composite with horseradish peroxidase activity for photo-enzyme synergistic catalytic degradation of phenanthrene. Appl. Catal. B Environ. Energy 2023, 337, 12300. [Google Scholar] [CrossRef]
- Zhao, Z.; Zou, Y.; Liu, P.; Lai, Z.; Wen, L.; Jin, Y. EIS equivalent circuit model prediction using interpretable machine learning and parameter identification using global optimization algorithms. Electrochim. Acta 2022, 418, 140350. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.; Sun, G.; Xia, J.; Wu, C.; Ye, Z.; Zhang, Q. Photocatalytic activity of La2O3-modified silver vanadates catalyst for Rhodamine B dye degradation under visible light irradiation. Chem. Eng. J. 2010, 160, 33–41. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, L.; Meng, M.; Zhang, Q.; Li, B.; Wu, Y.; Zhang, Y.; Lang, J.; Li, C. Facile preparation of antifouling g-C3N4/Ag3PO4 nanocomposite photocatalytic polyvinylidene fluoride membranes for effective removal of rhodamine B. Korean J. Chem. Eng. 2019, 36, 236–247. [Google Scholar] [CrossRef]
- Huang, J.; Hu, J.; Shi, Y.; Zeng, G.; Cheng, W.; Yu, H.; Gu, Y.; Shi, L.; Yi, K. Evaluation of self-cleaning and photocatalytic properties of modified g-C3N4 based PVDF membranes driven by visible light. J. Colloid Interface Sci. 2019, 541, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Issarapanacheewin, S.; Wetchakun, K.; Phanichphant, S.; Kangwansupamonkon, W.; Wetchakun, N. Efficient photocatalytic degradation of Rhodamine B by a novel CeO2/Bi2WO6 composite film. Catal. Today 2016, 278, 280–290. [Google Scholar] [CrossRef]
- Kolesnyk, I.; Kujawa, J.; Bubela, H.; Konovalova, V.; Burban, A.; Cyganiuk, A.; Kujawski, W. Photocatalytic properties of PVDF membranes modified with g-C3N4 in the process of Rhodamines decomposition. Sep. Purif. Technol. 2020, 250, 117231. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, J.; Liu, H. Ultra-high Rhodamine B adsorption capacities from an aqueous solution by activated carbon derived from Phragmites australis doped with organic acid by phosphoric acid activation. RSC Adv. 2016, 6, 40818–40827. [Google Scholar] [CrossRef]
- Wei, Z.; Spinney, R.; Ke, R.; Yang, Z.; Xiao, R. Effect of pH on the sonochemical degradation of organic pollutants. Environ. Chem. Lett. 2016, 14, 163–182. [Google Scholar] [CrossRef]
- Yue, X.; Liu, Z.; Zhang, Q.; Li, X.; Hao, F.; Wei, J.; Guo, W. Oxidative degradation of Rhodamine B in aqueous solution using Fe/PANI nanoparticles in the presence of AQS serving as an electron shuttle. Desalination Water Treat. 2016, 57, 15190–15199. [Google Scholar] [CrossRef]
- Fang, S.; Lv, K.; Li, Q.; Ye, H.; Du, D.; Li, M. Effect of acid on the photocatalytic degradation of rhodamine B over g-C3N4. Appl. Surf. Sci. 2015, 358, 336–342. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, H.; Qian, J.; Xue, B.; Du, H.; Hao, D.; Ji, Y.; Li, Q. Ti3C2-assisted construction of Z-scheme MIL-88A(Fe)/Ti3C2/RF heterojunction: Multifunctional photocatalysis-in-situ-self-Fenton catalyst. J. Mater. Sci. Technol. 2024, 190, 67–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, K.; Yuan, C.; Lv, H.; Yin, H.; Fei, Q.; Xiao, D.; Zhang, Y.; Lau, W. In-situ formation of SrTiO3/Ti3C2 MXene Schottky heterojunction for efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2024, 653, 482–492. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, C.-W.; Xie, M.-M.; Yang, Y.-D.; Wang, H.-Y.; Wang, Z.-K.; Zhang, L.; Hsieh, C.-T.; Liu, L.-Y.; Lin, M.-C.; Li, T.-T. Carbon Nanofiber Membranes Loaded with MXene@g-C3N4: Preparation and Photocatalytic Property. Nanomaterials 2024, 14, 896. https://doi.org/10.3390/nano14100896
Lou C-W, Xie M-M, Yang Y-D, Wang H-Y, Wang Z-K, Zhang L, Hsieh C-T, Liu L-Y, Lin M-C, Li T-T. Carbon Nanofiber Membranes Loaded with MXene@g-C3N4: Preparation and Photocatalytic Property. Nanomaterials. 2024; 14(10):896. https://doi.org/10.3390/nano14100896
Chicago/Turabian StyleLou, Ching-Wen, Meng-Meng Xie, Yan-Dong Yang, Hong-Yang Wang, Zhi-Ke Wang, Lu Zhang, Chien-Teng Hsieh, Li-Yan Liu, Mei-Chen Lin, and Ting-Ting Li. 2024. "Carbon Nanofiber Membranes Loaded with MXene@g-C3N4: Preparation and Photocatalytic Property" Nanomaterials 14, no. 10: 896. https://doi.org/10.3390/nano14100896






