A Uni-Micelle Approach for the Controlled Synthesis of Monodisperse Gold Nanocrystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Precursor Solution
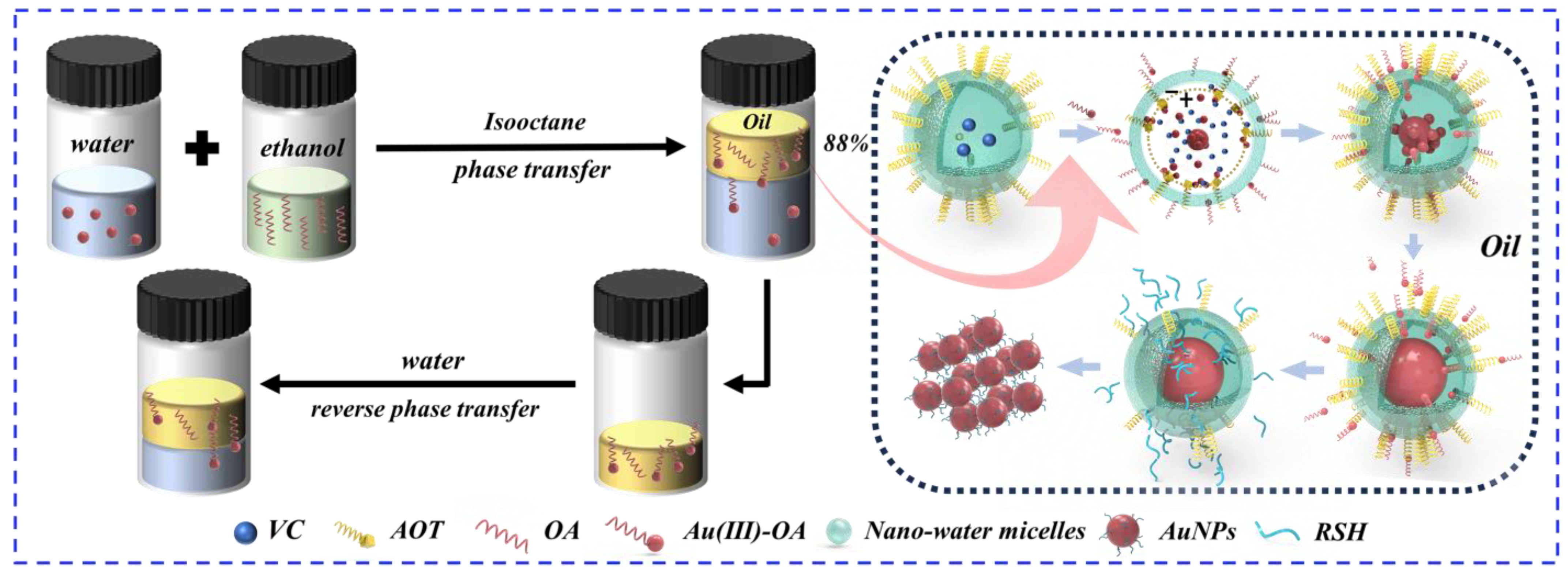
2.3. Preparation of Reversed-Phase Water Micelles
2.4. Synthesis of Nanoparticles
2.5. Material Characterization
2.6. Heating Effect Test
3. Results
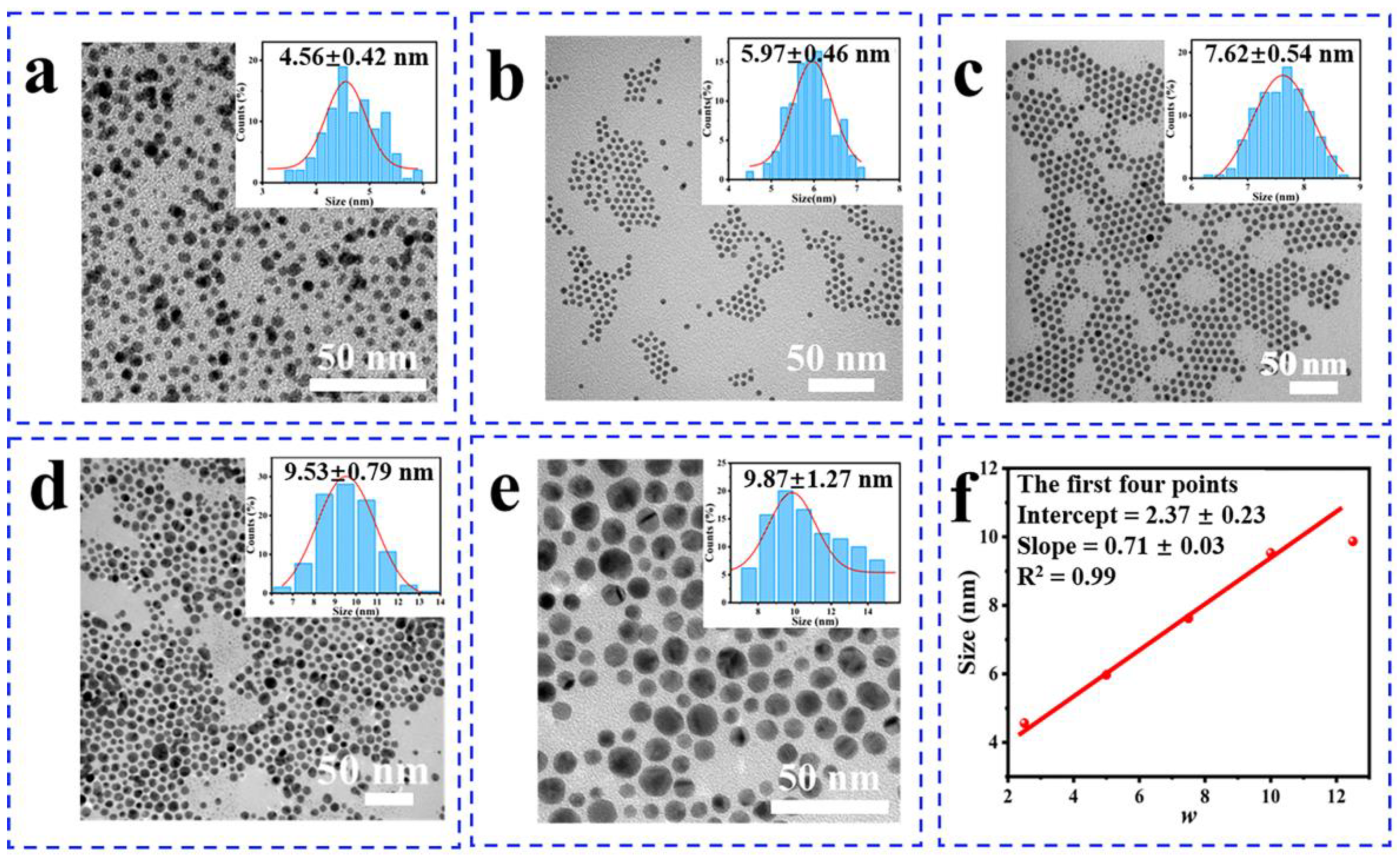
3.1. The Structure of Gold Nanoparticles
3.2. The Mechanism of the Method
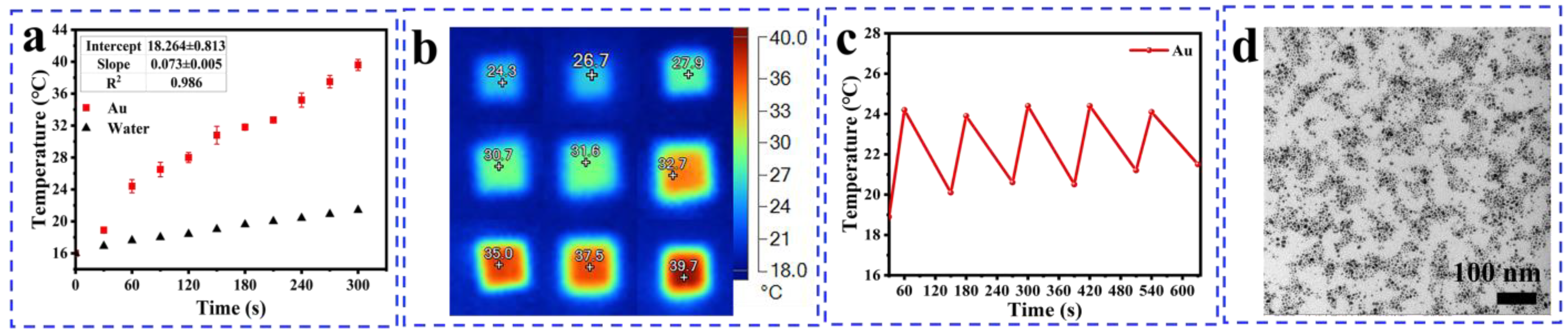
3.3. Result of Heating Effect
3.4. Formatting of Mathematical Components
- The calculation of the ratio w of water to AOT is as follows:where is the density of water, is the volume of water added, is the concentration of AOT isooctane solution, is the volume of AOT isooctane solution added, and is the molecular weight of water.
- The standard deviation (σ) is as follows:
- 3.
- The yield of the obtained AuNPs is as follows:
- 4.
- The optical band gap () of the synthesized AuNPs is as follows:where α is the absorption coefficient, is Planck’s constant, is frequency, and is a proportionality constant.
- 5.
- The synthesis mechanism of AuNPs is as follows:AuCl4−(aq) + RNH3+(org) → RNH3+AuCl4−
mRNH3+AuCl4− + nC20H37NaO7S → (C20H37O7S−)n(Au3+)m↓ + mRNH3+ + 4mCl− + nNa+
C6H8O6 → C6H6O6 + 2e− + 2H+
Au3+ + 3e− → Au
xAu + yRSH → Aux(RSH)y
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, T.; Cai, H.; Hu, Y.; Zhang, Z.; Huang, M.; Peng, J.; Lai, W. Molecular Engineering and Confinement Effect Powered Ultrabright Nanoparticles for Improving Sensitivity of Lateral Flow Immunoassay. ACS Nano 2024, 18, 2346–2354. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, K.; Xie, T.-Q.; Jin, X.-K.; Zhang, C.; Li, Q.-R.; Feng, J.; Liu, C.-J.; Zhang, X.-Z. Bioorthogonal “Click and Release” Reaction-Triggered Aggregation of Gold Nanoparticles Combined with Released Lonidamine for Enhanced Cancer Photothermal Therapy. Angew. Chem. Int. Ed. 2024, 63, e202318539. [Google Scholar] [CrossRef] [PubMed]
- Mehravani, B.; Ribeiro, A.I.; Zille, A. Gold Nanoparticles Synthesis and Antimicrobial Effect on Fibrous Materials. Nanomaterials 2021, 11, 1067. [Google Scholar] [CrossRef]
- Du, Y.; Sheng, H.; Astruc, D.; Zhu, M. Atomically Precise Noble Metal Nanoclusters as Efficient Catalysts: A Bridge between Structure and Properties. Chem. Rev. 2020, 120, 526–622. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakov, V.; Denisov, S.A.; Mostafavi, M. Selective Oxidation of Transient Organic Radicals in the Presence of Gold Nanoparticles. Nanomaterials 2021, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Cherian, T.; Maity, D.; Rajendra Kumar, R.T.; Balasubramani, G.; Ragavendran, C.; Yalla, S.; Mohanraju, R.; Peijnenburg, W.J.G.M. Green Chemistry Based Gold Nanoparticles Synthesis Using the Marine Bacterium Lysinibacillus odysseyi PBCW2 and Their Multitudinous Activities. Nanomaterials 2022, 12, 2940. [Google Scholar] [CrossRef]
- Mosquera-Romero, S.; Anaya-Garzon, J.; Garcia-Timermans, C.; Van Dorpe, J.; Hoorens, A.; Commenges-Bernole, N.; Verbeken, K.; Rabaey, K.; Varia, J. Combined Gold Recovery and Nanoparticle Synthesis in Microbial Systems Using Fractional Factorial Design. Nanomaterials 2023, 13, 83. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Zhang, H.; Wan, Y.; Luo, J. Biomimetic-photo-coupled catalysis for boosting H2O2 production. Chem. Eng. J. 2024, 483, 149183. [Google Scholar] [CrossRef]
- Yañez-Aulestia, A.; Gupta, N.K.; Hernández, M.; Osorio-Toribio, G.; Sánchez-González, E.; Guzmán-Vargas, A.; Rivera, J.L.; Ibarra, I.A.; Lima, E. Gold nanoparticles: Current and upcoming biomedical applications in sensing, drug, and gene delivery. Chem. Commun. 2022, 58, 10886–10895. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, Y.; Zhao, L.; Ji, C.; Chen, D.; Nie, L. Applications of Gold Nanoparticles in Non-Optical Biosensors. Nanomaterials 2018, 8, 977. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Win, K.Y.; Yao, X.; Yang, W.; Han, M.-Y. Plasmonically Modulated Gold Nanostructures for Photothermal Ablation of Bacteria. Adv. Healthc. Mater. 2021, 10, 2001158. [Google Scholar] [CrossRef]
- Zhai, Y.; DuChene, J.S.; Wang, Y.-C.; Qiu, J.; Johnston-Peck, A.C.; You, B.; Guo, W.; DiCiaccio, B.; Qian, K.; Zhao, E.W.; et al. Polyvinylpyrrolidone-induced anisotropic growth of gold nanoprisms in plasmon-driven synthesis. Nat. Mater. 2016, 15, 889–895. [Google Scholar] [CrossRef]
- Pang, X.; Zhao, L.; Han, W.; Xin, X.; Lin, Z. A general and robust strategy for the synthesis of nearly monodisperse colloidal nanocrystals. Nat. Nanotechnol. 2013, 8, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiao, X.; Chen, J. Synthesis of silver nanoparticles—Effects of concerned parameters in water/oil microemulsion. Mater. Sci. Eng. B 2007, 142, 1–15. [Google Scholar] [CrossRef]
- Rivera-Rangel, R.D.; González-Muñoz, M.P.; Avila-Rodriguez, M.; Razo-Lazcano, T.A.; Solans, C. Green synthesis of silver nanoparticles in oil-in-water microemulsion and nano-emulsion using geranium leaf aqueous extract as a reducing agent. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 60–67. [Google Scholar] [CrossRef]
- Ahmad, T.; Iqbal, J.; Bustam, M.A.; Irfan, M.; Anwaar Asghar, H.M. A critical review on phytosynthesis of gold nanoparticles: Issues, challenges and future perspectives. J. Clean. Prod. 2021, 309, 127460. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Lee, S.; Sim, K.; Moon, S.Y.; Choi, J.; Jeon, Y.; Nam, J.-M.; Park, S.-J. Controlled Assembly of Plasmonic Nanoparticles: From Static to Dynamic Nanostructures. Adv. Mater. 2021, 33, 2007668. [Google Scholar] [CrossRef]
- King, S.R.; Shimmon, S.; Totonjian, D.D.; McDonagh, A.M. Influence of Bound versus Non-Bound Stabilizing Molecules on the Thermal Stability of Gold Nanoparticles. J. Phys. Chem. C 2017, 121, 13944–13951. [Google Scholar] [CrossRef]
- Kirichkov, M.V.; Guda, A.A.; Budnyk, A.P.; Lastovina, T.A.; Bugaev, A.L.; Shapovalov, V.V.; Rusalev, Y.V.; Chernyshev, A.V.; Soldatov, A.V. In situ analysis of the formation steps of gold nanoparticles by oleylamine reduction. J. Struct. Chem. 2017, 58, 1403–1410. [Google Scholar] [CrossRef]
- Pileni, M.-P. Zinc porphyrin sensitized redox processes in microemulsions. Chem. Phys. Lett. 1980, 75, 540–544. [Google Scholar] [CrossRef]
- Tianimoghadam, S.; Salabat, A. A microemulsion method for preparation of thiol-functionalized gold nanoparticles. Particuology 2018, 37, 33–36. [Google Scholar] [CrossRef]
- Kapil, N.; Cardinale, F.; Weissenberger, T.; Trogadas, P.; Nijhuis, T.A.; Nigra, M.M.; Coppens, M.-O. Gold nanoparticles with tailored size through ligand modification for catalytic applications. Chem. Commun. 2021, 57, 10775–10778. [Google Scholar] [CrossRef]
- Perez-Coronado, A.M.; Calvo, L.; Alonso-Morales, N.; Heras, F.; Rodriguez, J.J.; Gilarranz, M.A. Multiple approaches to control and assess the size of Pd nanoparticles synthesized via water-in-oil microemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 28–34. [Google Scholar] [CrossRef]
- Suherman, A.L.; Zampardi, G.; Amin, H.M.A.; Young, N.P.; Compton, R.G. Tannic acid capped gold nanoparticles: Capping agent chemistry controls the redox activity. Phys. Chem. Chem. Phys. 2019, 21, 4444–4451. [Google Scholar] [CrossRef]
- Eastoe, J.; Robinson, B.H.; Visser, A.J.W.G.; Steytler, D.C. Rotation Dynamics of AOT Reversed Micelles in Near-critical and Supercritical Alkanes. J. Chem. Soc. Faraday Trans. 1991, 87, 1899–1903. [Google Scholar] [CrossRef]
- Khan, A.W.; Lali, N.S.; Sabei, F.Y.; Irfan, M.I.; Naeem-ul-Hassan, M.; Sher, M.; Safhi, A.Y.; Alsalhi, A.; Albariqi, A.H.; Kamli, F.; et al. Sunlight-assisted green synthesis of gold nanocubes using horsetail leaf extract: A highly selective colorimetric sensor for Pb2+, photocatalytic and antimicrobial agent. J. Environ. Chem. Eng. 2024, 12, 112576. [Google Scholar] [CrossRef]
- Niu, W.; Xu, G. Crystallographic control of noble metal nanocrystals. Nano Today 2011, 6, 265–285. [Google Scholar] [CrossRef]
- Kumar, S.; Sindhu, A.; Venkatesu, P. Ionic Liquid-Modified Gold Nanoparticles for Enhancing Antimicrobial Activity and Thermal Stability of Enzymes. ACS Appl. Nano Mater. 2021, 4, 3185–3196. [Google Scholar] [CrossRef]
- Salabat, A.; Mirhoseini, F. A novel and simple microemulsion method for synthesis of biocompatible functionalized gold nanoparticles. J. Mol. Liq. 2018, 268, 849–853. [Google Scholar] [CrossRef]
- He, K.; Tan, Y.; Zhao, Z.; Chen, H.; Liu, J. Weak Anchoring Sites of Thiolate-Protected Luminescent Gold Nanoparticles. Small 2021, 17, 2102481. [Google Scholar] [CrossRef]
- Reimers, J.R.; Ford, M.J.; Marcuccio, S.M.; Ulstrup, J.; Hush, N.S. Competition of van der Waals and chemical forces on gold–sulfur surfaces and nanoparticles. Nat. Rev. Chem. 2017, 1, 0017. [Google Scholar] [CrossRef]
- Fajstavr, D.; Karasová, A.; Michalcová, A.; Ulbrich, P.; Slepičková Kasálková, N.; Siegel, J.; Švorčík, V.; Slepička, P. PEGylated Gold Nanoparticles Grafted with N-Acetyl-L-Cysteine for Polymer Modification. Nanomaterials 2021, 11, 1434. [Google Scholar] [CrossRef]
- Pérez-Bitrián, A.; Alvarez, S.; Baya, M.; Echeverría, J.; Martín, A.; Orduna, J.; Menjón, B. Terminal Au−N and Au−O Units in Organometallic Frames. Chem.—A Eur. J. 2023, 29, e202203181. [Google Scholar] [CrossRef]
- Crudden, C.M.; Horton, J.H.; Ebralidze, I.I.; Zenkina, O.V.; McLean, A.B.; Drevniok, B.; She, Z.; Kraatz, H.-B.; Mosey, N.J.; Seki, T.; et al. Ultra stable self-assembled monolayers of N-heterocyclic carbenes on gold. Nat. Chem. 2014, 6, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.-T.; Yang, Z.; Lee, C.-Y.; Yang, H.-Y.; Wang, C.R.C. Aqueous−Organic Phase Transfer of Gold Nanoparticles and Gold Nanorods Using an Ionic Liquid. J. Am. Chem. Soc. 2004, 126, 5036–5037. [Google Scholar] [CrossRef] [PubMed]
- Suherman, A.L.; Zampardi, G.; Kuss, S.; Tanner, E.E.L.; Amin, H.M.A.; Young, N.P.; Compton, R.G. Understanding gold nanoparticle dissolution in cyanide-containing solution via impact-chemistry. Phys. Chem. Chem. Phys. 2018, 20, 28300–28307. [Google Scholar] [CrossRef]
- Hong, J.; Chen, M.; Xian, J.; Li, C.; Zheng, X.; Deng, Q.; Yin, X.; Li, M. Preparation of Au-based hybrid nanoflowers as efficient photothermal agents for antibacterial application. Mater. Lett. 2022, 317, 132034. [Google Scholar] [CrossRef]

| Transfer Rate | Hexylamin | Octylamine | Decylamine | Dodecylamine | Oleylamine |
|---|---|---|---|---|---|
| Au(III) | 36% | 46% | 64% | 60% | 88% |
| Ion Specie | C0 (mM) | Coil (mM) | Caq (mM) | K1 | C’oil (mM) | C’aq (mM) | K2 |
|---|---|---|---|---|---|---|---|
| Au(III) | 10.0 | 7.94 | 1.14 | 6.95 | 6.67 | 0.96 | 6.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, L.; Wang, W.; Qian, L.; Tang, J.; Liu, J. A Uni-Micelle Approach for the Controlled Synthesis of Monodisperse Gold Nanocrystals. Nanomaterials 2024, 14, 900. https://doi.org/10.3390/nano14110900
Shan L, Wang W, Qian L, Tang J, Liu J. A Uni-Micelle Approach for the Controlled Synthesis of Monodisperse Gold Nanocrystals. Nanomaterials. 2024; 14(11):900. https://doi.org/10.3390/nano14110900
Chicago/Turabian StyleShan, Liangang, Wenchao Wang, Lei Qian, Jianguo Tang, and Jixian Liu. 2024. "A Uni-Micelle Approach for the Controlled Synthesis of Monodisperse Gold Nanocrystals" Nanomaterials 14, no. 11: 900. https://doi.org/10.3390/nano14110900
APA StyleShan, L., Wang, W., Qian, L., Tang, J., & Liu, J. (2024). A Uni-Micelle Approach for the Controlled Synthesis of Monodisperse Gold Nanocrystals. Nanomaterials, 14(11), 900. https://doi.org/10.3390/nano14110900






