Termiticidal Effects and Morpho-Histological Alterations in the Subterranean Termite (Odontotermes formosanus) Induced by Biosynthesized Zinc Oxide, Titanium Dioxide, and Chitosan Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gathering and Raising Termite Colonies
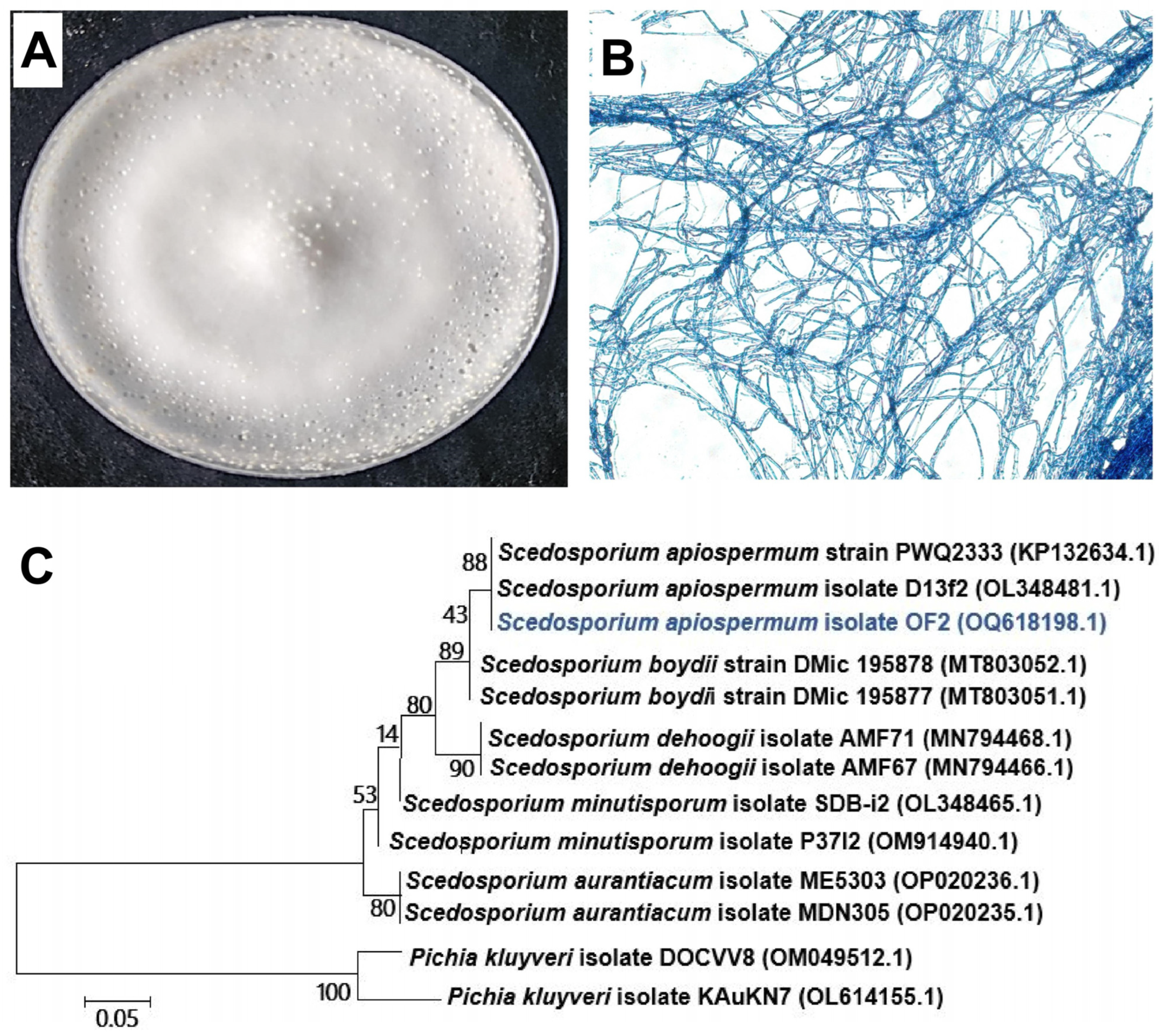
2.3. Isolation of Rhizosphere Fungi
2.4. Identification of Rhizosphere Fungi
2.5. Preparation of the Culture Filtrate
2.6. Biosynthesis of Nanoparticles
2.6.1. Biosynthesis of ZnONPs and TiO2NPs
2.6.2. Biosynthesis of CsNPs
2.7. Characterization of Biosynthesized Nanoparticles
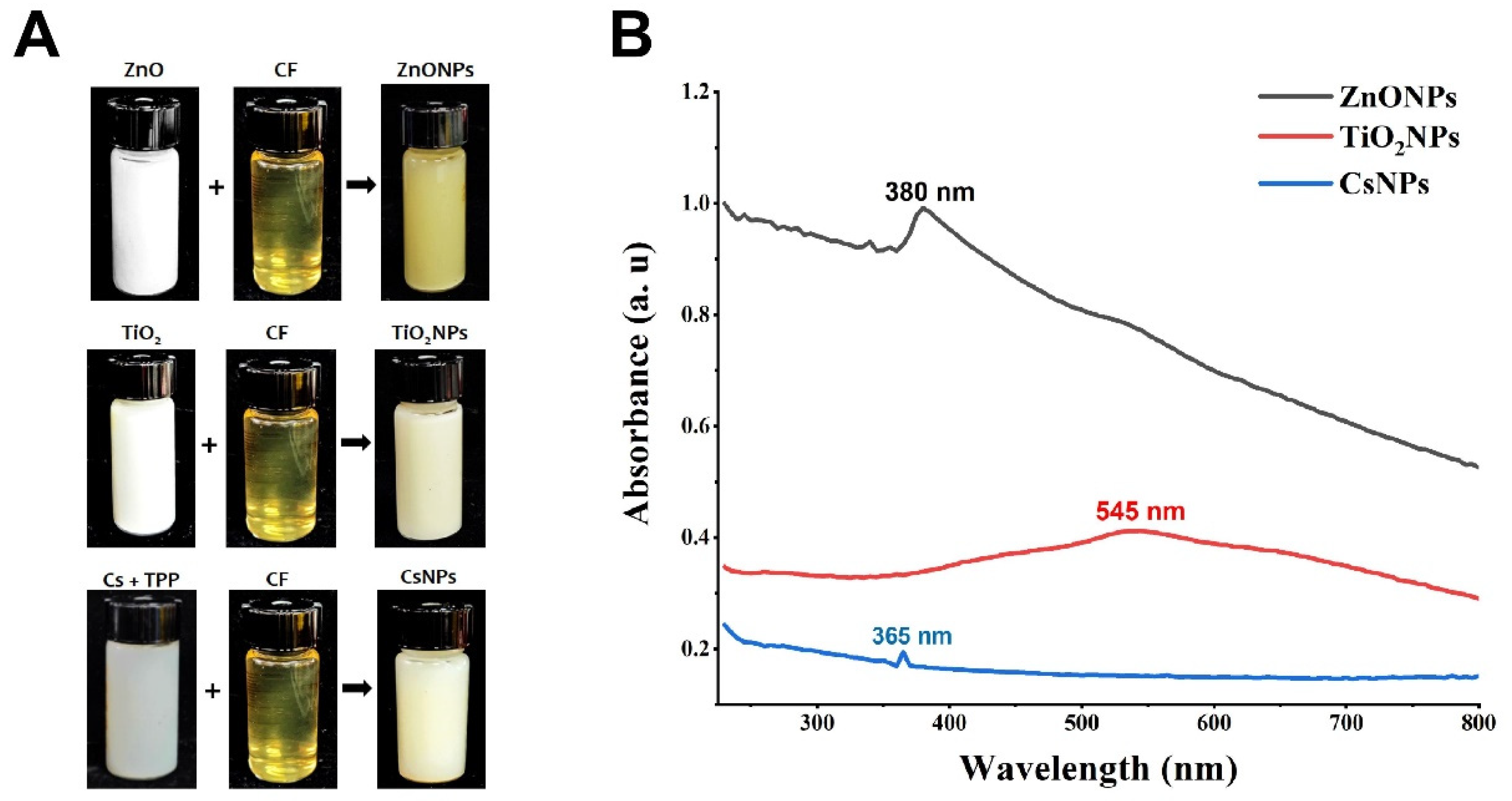
2.7.1. UV-Visible Spectrum
2.7.2. Fourier Transform Infrared (FTIR) Measurements
2.7.3. X-ray Diffraction (XRD) Measurements
2.7.4. SEM and EDS Measurements
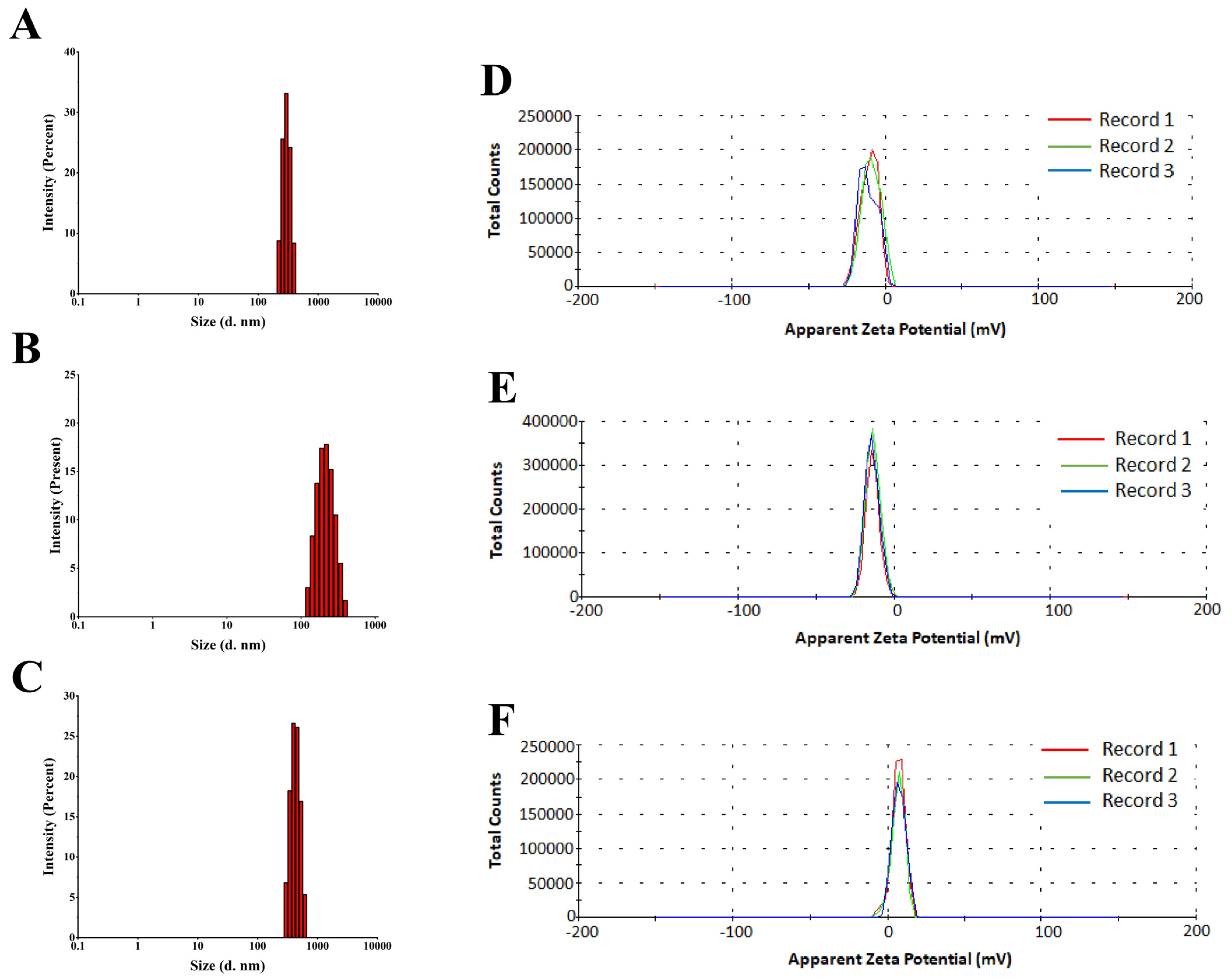
2.7.5. Dynamic Light Scattering (DLS) and Zeta Potential (ZP)
2.8. Termiticidal Activity
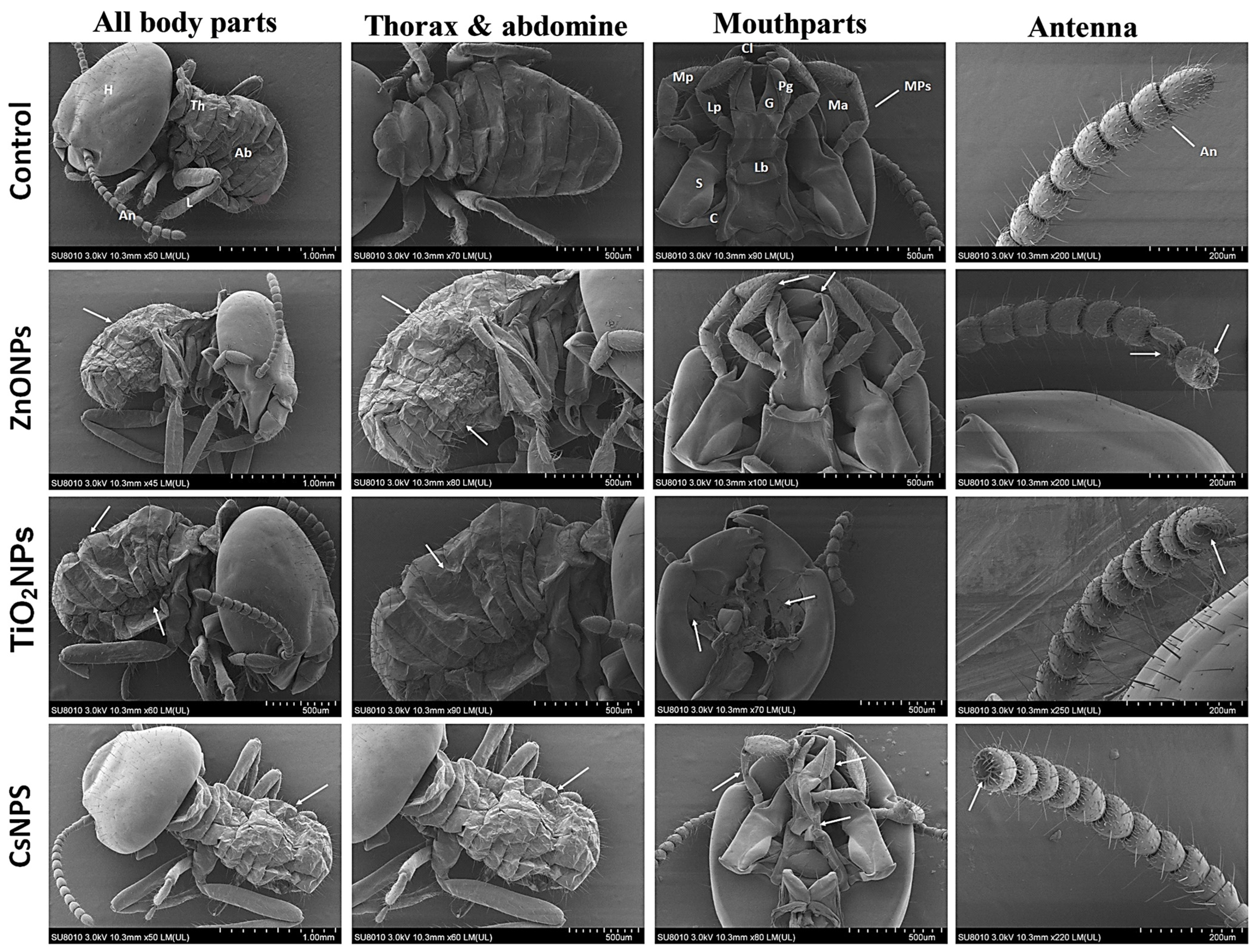
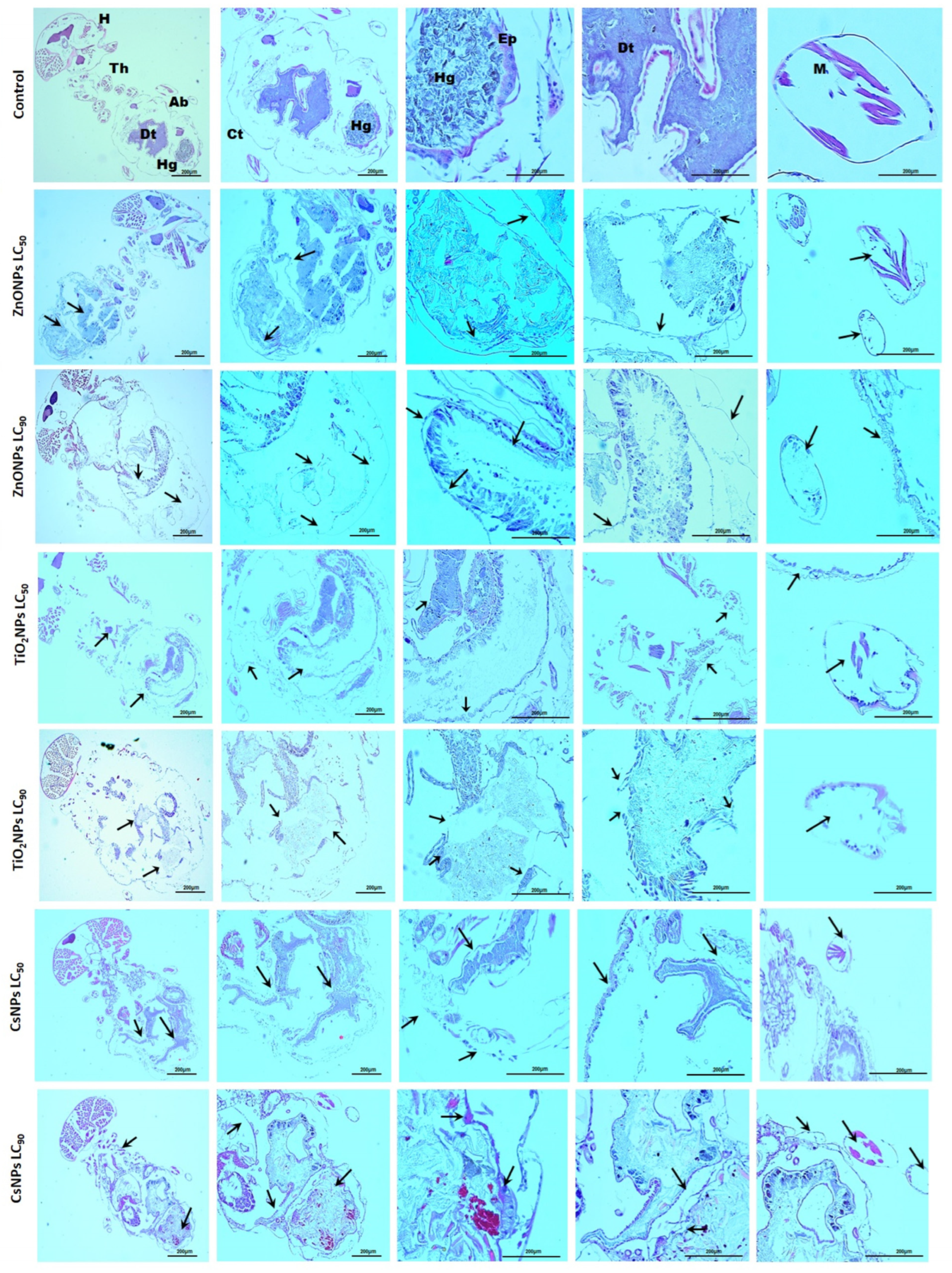
2.9. Morphological and Histological Observations Post-Exposure to Biosynthesized Nanoparticles
2.10. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Rhizosphere Fungi
3.2. Biosynthesis of Nanoparticles
3.3. Characterization of Nanoparticles
3.3.1. FTIR
3.3.2. XRD
3.3.3. SEM and EDS Measurements
3.3.4. DLS and ZP Measurements
3.4. Termiticidal Activity
3.5. Morphological Observations Post-Exposure to Biosynthesized Nanoparticles
3.6. Histological Observations Post-Exposure to Biosynthesized Nanoparticles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Han, H.; Li, B.; Zhang, D.; Zhang, Z.; Xie, Y. Fumigant toxicity and physiological effects of spearmint (Mentha spicata, Lamiaceae) essential oil and its major constituents against Reticulitermes dabieshanensis. Ind. Crop Prod. 2021, 171, 113894. [Google Scholar] [CrossRef]
- Verma, M.; Sharma, S.; Prasad, R. Biological alternatives for termite control: A review. Int. Biodeterior. Biodegrad. 2009, 63, 959–972. [Google Scholar] [CrossRef]
- Soleymaninejadian, E.; Ji, B.Z.; Liu, S.W.; Yang, J.J.; Zhang, X.W.; Ding, F. Polyethism in the case of feeding, construction, and defense behaviors of Odontotermes formosanus Shiraki. Int. J. Agric. Innov. Res. 2014, 3, 1–6. [Google Scholar]
- Vidkjær, N.H.; Schmidt, S.; Hu, H.; Bodawatta, K.H.; Beemelmanns, C.; Poulsen, M. Species- and Caste-Specific Gut Metabolomes in Fungus-Farming Termites. Metabolites 2021, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Su, N.-Y. Novel technologies for subterranean termite control. Sociobiology 2002, 40, 95–102. [Google Scholar]
- Govorushko, S. Economic and ecological importance of termites: A global review. Entomol. Sci. 2019, 22, 21–35. [Google Scholar] [CrossRef]
- Ahmad, F.; Fouad, H.; Liang, S.Y.; Hu, Y.; Mo, J.C. Termites and Chinese agricultural system: Applications and advances in integrated termite management and chemical control. Insect Sci. 2021, 28, 2–20. [Google Scholar] [CrossRef]
- Govindarajan, M.; Sivakumar, R.; Rajeswari, M.; Yogalakshmi, K. Chemical composition and larvicidal activity of essential oil from Mentha spicata (Linn.) against three mosquito species. Parasitol. Res. 2012, 110, 2023–2032. [Google Scholar] [CrossRef]
- Frima, H.J.; Gabellieri, C.; Nilsson, M.-I. Drug delivery research in the european Union’s Seventh Framework Programme for Research. J. Control Release 2012, 161, 409–415. [Google Scholar] [CrossRef]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 2020, 6, e04896. [Google Scholar] [CrossRef]
- Tso, C.-P.; Zhung, C.-M.; Shih, Y.-H.; Tseng, Y.-M.; Wu, S.-C.; Doong, R.-A. Stability of metal oxide nanoparticles in aqueous solutions. Water Sci. Technol. 2010, 61, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Din, M.I.; Rani, A.; Hussain, Z.; Khalid, R.; Aihetasham, A.; Mukhtar, M. Biofabrication of size-controlled ZnO nanoparticles using various capping agents and their cytotoxic and antitermite activity. Int. J. Environ. Anal. Chem. 2021, 101, 821–837. [Google Scholar] [CrossRef]
- Miao, W.; Zhu, B.; Xiao, X.; Li, Y.; Dirbaba, N.B.; Zhou, B.; Wu, H. Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat. Toxicol. 2015, 161, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Soni, R.; Tripathi, V.; Mishra, A.K.; Kumar, P. Nanotechnological interventions for plant health improvement and sustainable agriculture. 3Biotech 2020, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, K.; Karthika, V.; Sundaravadivelan, C.; Gowri, S.; Arumugam, A. Mycogenesis of cerium oxide nanoparticles using Aspergillus niger culture filtrate and their applications for antibacterial and larvicidal activities. J. Nanostruct. Chem. 2015, 5, 295–303. [Google Scholar] [CrossRef]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. 2016, 23, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Fouda, A.; Abdel-Rahman, M.A.; Salem, S.S.; Elsaied, A.; Oelmüller, R.; Hijri, M.; Bhowmik, A.; Elkelish, A.; Hassan, S.E.-D. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: An overview. Plants 2021, 10, 935. [Google Scholar] [CrossRef]
- Velgosova, O.; Dolinská, S.; Mražíková, A.; Briančin, J. Effect of P. kessleri extracts treatment on AgNPs synthesis. Inorg. Nano-Met. Chem. 2020, 50, 842–852. [Google Scholar] [CrossRef]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.-C.; Al Khulaifi, M.M.; Al-Shwaiman, H.A.; Elgorban, A.M.; Syed, A. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J. Biol. Sci. 2020, 27, 706–712. [Google Scholar] [CrossRef]
- Velgosova, O.; Mudra, E.; Vojtko, M. Preparing, characterization and anti-biofilm activity of polymer fibers doped by green synthesized AgNPs. Polymers 2021, 13, 605. [Google Scholar] [CrossRef] [PubMed]
- Spagnoletti, F.N.; Spedalieri, C.; Kronberg, F.; Giacometti, R. Extracellular biosynthesis of bactericidal Ag/AgCl nanoparticles for crop protection using the fungus Macrophomina phaseolina. J. Environ. Manag. 2019, 231, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, M.M.; George, E. Scedosporium apiospermum: An emerging opportunistic pathogen that must be distinguished from Aspergillus and other hyalohyphomycetes. J. Cutan. Pathol. 2009, 36 (Suppl. S1), 39–41. [Google Scholar] [CrossRef] [PubMed]
- Linares, M.J.; Charriel, G.; Solís, F.; Rodriguez, F.; Ibarra, A.; Casal, M. Susceptibility of filamentous fungi to voriconazole tested by two microdilution methods. J. Clin. Microbiol. 2005, 43, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.; Ahmad, A.A.; Abdo, E.-S.; Bakr, M.A.; Khalil, M.A.; Abdallah, Y.; Ogunyemi, S.O.; Mohany, M.; Al-Rejaie, S.S.; Shou, L.; et al. Suppression of Root Rot Fungal Diseases in Common Beans (Phaseolus vulgaris L.) through the Application of Biologically Synthesized Silver Nanoparticles. Nanomaterials 2024, 14, 710. [Google Scholar] [CrossRef] [PubMed]
- Ru, Z.; Di, W. Trichoderma spp. from rhizosphere soil and their antagonism against Fusarium sambucinum. Afr. J. Biotechnol. 2012, 11, 4180–4186. [Google Scholar]
- Stracquadanio, C.; Quiles, J.M.; Meca, G.; Cacciola, S.O. Antifungal activity of bioactive metabolites produced by Trichoderma asperellum and Trichoderma atroviride in liquid medium. J. Fungi 2020, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 1, 2–3. [Google Scholar] [CrossRef]
- Mishra, M.; Paliwal, J.S.; Singh, S.K.; Selvarajan, E.; Subathradevi, C.; Mohanasrinivasan, V. Studies on the inhibitory activity of biologically synthesized and characterized zinc oxide nanoparticles using Lactobacillus sporogens against Staphylococcus aureus. J. Pure Appl. Microbiol. 2013, 7, 1263–1268. [Google Scholar]
- Nagaonkar, D.; Gaikwad, S.; Rai, M. Catharanthus roseus leaf extract-synthesized chitosan nanoparticles for controlled in vitro release of chloramphenicol and ketoconazole. Colloid. Polym. Sci. 2015, 293, 1465–1473. [Google Scholar] [CrossRef]
- Javed, B.; Raja, N.I.; Nadhman, A.; Mashwani, Z.-u.-R. Understanding the potential of bio-fabricated non-oxidative silver nanoparticles to eradicate Leishmania and plant bacterial pathogens. Appl. Nanosci. 2020, 10, 2057–2067. [Google Scholar] [CrossRef]
- Fouad, H.; Yang, G.; El-Sayed, A.A.; Mao, G.; Khalafallah, D.; Saad, M.; Ga’al, H.; Ibrahim, E.; Mo, J. Green synthesis of AgNP–ligand complexes and their toxicological effects on Nilaparvata lugens. J. Nanobiotechnol. 2021, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Dinesh, D.; Paulpandi, M.; Althbyani, A.D.M.; Subramaniam, J.; Madhiyazhagan, P.; Wang, L.; Suresh, U.; Kumar, P.M.; Mohan, J. Nanoparticles in the fight against mosquito-borne diseases: Bioactivity of Bruguiera cylindrica-synthesized nanoparticles against dengue virus DEN-2 (in vitro) and its mosquito vector Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2015, 114, 4349–4361. [Google Scholar] [CrossRef] [PubMed]
- Neves Filho, R.A.W.; da SILVA, C.A.; da SILVA, C.S.B.; Brustein, V.P.; Navarro, D.M.d.A.F.; dos SANTOS, F.A.B.; Alves, L.C.; dos Santos Cavalcanti, M.G.; Srivastava, R.M.; das Graças Carneiro-Da-Cunha, M. Improved microwave-mediated synthesis of 3-(3-aryl-1,2,4-oxadiazol-5-yl) propionic acids and their larvicidal and fungal growth inhibitory properties. Chem. Pharm. Bull. 2009, 57, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Al-Mehmadi, R.M.; Al-Khalaf, A.A. Larvicidal and histological effects of Melia azedarach extract on Culex quinquefasciatus Say larvae (Diptera: Culicidae). J. King Saud. Univ. Sci. 2010, 22, 77–85. [Google Scholar] [CrossRef]
- Nasser, R.; Ibrahim, E.; Fouad, H.; Ahmad, F.; Li, W.; Zhou, Q.; Yu, T.; Chidwala, N.; Mo, J. Termiticidal, biochemical, and morpho-histological effects of botanical based nanoemulsion against a subterranean termite, Odontotermes formosanus Shiraki. Front. Plant Sci. 2024, 14, 1292272. [Google Scholar] [CrossRef] [PubMed]
- Taran, M.; Rad, M.; Alavi, M. Biosynthesis of TiO2 and ZnO nanoparticles by Halomonas elongata IBRC-M 10214 in different conditions of medium. BioImpacts BI 2018, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.-A.; Saber, W.I.; Zweil, A.M.; Bashir, S.I. An innovative green synthesis approach of chitosan nanoparticles and their inhibitory activity against phytopathogenic Botrytis cinerea on strawberry leaves. Sci. Rep. 2022, 12, 3515. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cell Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef]
- Abdallah, Y.; Liu, M.; Ogunyemi, S.O.; Ahmed, T.; Fouad, H.; Abdelazez, A.; Yan, C.; Yang, Y.; Chen, J.; Li, B. Bioinspired green synthesis of chitosan and zinc oxide nanoparticles with strong antibacterial activity against rice pathogen Xanthomonas oryzae pv. oryzae. Molecules 2020, 25, 4795. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Alzohairy, M.A.; Alomary, M.N.; Jalal, M.; AlYahya, S.; Asiri, S.M.M.; Khan, H.M. Effect of biosynthesized ZnO nanoparticles on multi-drug resistant Pseudomonas aeruginosa. Antibiotics 2020, 9, 260. [Google Scholar] [CrossRef]
- Alarif, W.M.; Shaban, Y.A.; Orif, M.I.; Ghandourah, M.A.; Turki, A.J.; Alorfi, H.S.; Tadros, H.R. Green Synthesis of TiO2 Nanoparticles Using Natural Marine Extracts for Antifouling Activity. Mar. Drugs 2023, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Al Masoudi, L.M.; Alqurashi, A.S.; Abu Zaid, A.; Hamdi, H. Characterization and Biological Studies of Synthesized Titanium Dioxide Nanoparticles from Leaf Extract of Juniperus phoenicea (L.) Growing in Taif Region, Saudi Arabia. Processes 2023, 11, 272. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Shiha, A.M.; Mahrous, H.; Mohammed, A.A. Green synthesis of chitosan nanoparticles, optimization, characterization and antibacterial efficacy against multi drug resistant biofilm-forming Acinetobacter baumannii. Sci. Rep. 2022, 12, 19869. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Roopan, S.M.; Khanna, V.G.; Elango, G.; Kamaraj, C.; Zahir, A.A.; Velayutham, K. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim. Acta Pt. A-Mol. Biomol. Spectrosc. 2012, 91, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Eppler, A.S.; Zhu, J.; Anderson, E.A.; Somorjai, G.A. Model catalysts fabricated by electron beam lithography: AFM and TPD surface studies and hydrogenation/dehydrogenation of cyclohexene+ H2 on a Pt nanoparticle array supported by silica. Top. Catal. 2000, 13, 33–41. [Google Scholar] [CrossRef]
- Fu, L.; Fu, Z. Plectranthus amboinicus leaf extract–assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram. Int. 2015, 41, 2492–2496. [Google Scholar] [CrossRef]
- Thamilarasan, V.; Sethuraman, V.; Gopinath, K.; Balalakshmi, C.; Govindarajan, M.; Mothana, R.A.; Siddiqui, N.A.; Khaled, J.M.; Benelli, G. Single step fabrication of chitosan nanocrystals using Penaeus semisulcatus: Potential as new insecticides, antimicrobials and plant growth promoters. J. Clust. Sci. 2018, 29, 375–384. [Google Scholar] [CrossRef]
- Ali, J.; Irshad, R.; Li, B.; Tahir, K.; Ahmad, A.; Shakeel, M.; Khan, N.U.; Khan, Z.U.H. Synthesis and characterization of phytochemical fabricated zinc oxide nanoparticles with enhanced antibacterial and catalytic applications. J. Photochem. Photobiol. B-Biol. 2018, 183, 349–356. [Google Scholar] [CrossRef]
- Murugan, K.; Jaganathan, A.; Rajaganesh, R.; Suresh, U.; Madhavan, J.; Senthil-Nathan, S.; Rajasekar, A.; Higuchi, A.; Kumar, S.S.; Alarfaj, A.A. Poly (styrene sulfonate)/poly (allylamine hydrochloride) encapsulation of TiO2 nanoparticles boosts their toxic and repellent activity against Zika virus mosquito vectors. J. Clust. Sci. 2018, 29, 27–39. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.E.; Valverde-Aguilar, G.; Plascencia-Jatomea, M.; Correa-Pacheco, Z.; Jiménez-Aparicio, A.; Solorza-Feria, J.; Barrera-Necha, L.; Bautista-Baños, S. Characterization of chitosan nanoparticles added with essential oils: In vitro effect on Pectobacterium carotovorum. Rev. Mex. Ing. Quim. 2015, 14, 589–599. [Google Scholar]
- Pinto, I.; Buss, A. ζ Potential as a Measure of Asphalt Emulsion Stability. Energy Fuels 2020, 34, 2143–2151. [Google Scholar] [CrossRef]
- Lian, J.; Cheng, L.; Wang, X.; Chen, Y.; Deng, C.; Wang, Y.; Pan, J.; Shohag, M.J.I.; Xin, X.; He, Z.; et al. Bespoke ZnO NPs Synthesis Platform to Optimize Their Performance for Improving the Grain Yield, Zinc Biofortification, and Cd Mitigation in Wheat. ACS Sustain. Chem. Eng. 2024, 12, 716–727. [Google Scholar] [CrossRef]
- Gutiérrez-Ramírez, J.A.; Betancourt-Galindo, R.; Aguirre-Uribe, L.A.; Cerna-Chávez, E.; Sandoval-Rangel, A.; Ángel, E.C.-d.; Chacón-Hernández, J.C.; García-López, J.I.; Hernández-Juárez, A. Insecticidal effect of zinc oxide and titanium dioxide nanoparticles against Bactericera cockerelli Sulc. (Hemiptera: Triozidae) on tomato Solanum lycopersicum. Agronomy 2021, 11, 1460. [Google Scholar] [CrossRef]
- Zaki, A.; Zaki, A.; Farghali, A.; Abdel-Rahim, E.F. Sodium titanate-bacillus as a new nanopesticide for cotton leaf-worm. J. Pure Appl. Microbiol. 2017, 11, 725–732. [Google Scholar] [CrossRef]
- Asghar, M.S.; Sarwar, Z.M.; Almadiy, A.A.; Shami, A.; El Hadi Mohamed, R.A.; Ahmed, N.; Waghulade, M.S.; Alam, P.; Abd Al Galil, F.M. Toxicological Effects of Silver and Zinc Oxide Nanoparticles on the Biological and Life Table Parameters of Helicoverpa armigera (Noctuidae: Lepidoptera). Agriculture 2022, 12, 1744. [Google Scholar] [CrossRef]
- Santos, T.S.; de Souza Varize, C.; Sanchez-Lopez, E.; Jain, S.A.; Souto, E.B.; Severino, P.; Mendonça, M.d.C. Entomopathogenic fungi-mediated AgNPs: Synthesis and insecticidal effect against Plutella xylostella (Lepidoptera: Plutellidae). Materials 2022, 15, 7596. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Du, C.; Zhang, J.; Yang, B.; Cuthbertson, A.G.; Ali, S. Synthesis of Metarhizium anisopliae–chitosan nanoparticles and their pathogenicity against Plutella xylostella (Linnaeus). Microorganisms 2021, 10, 1. [Google Scholar] [CrossRef]
- Alfy, H.; Ghareeb, R.Y.; Soltan, E.; Farag, D.A. Impact of chitosan nanoparticles as insecticide and nematicide against Spodoptera littoralis, Locusta migratoria, and Meloidogyne incognita. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 126–140. [Google Scholar]
- Shahzad, K.; Manzoor, F. Nanoformulations and their mode of action in insects: A review of biological interactions. Drug Chem. Toxicol. 2021, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lade, B.D.; Gogle, D.P. Nano-biopesticides: Synthesis and applications in plant safety. In Nanobiotechnology Applications in Plant Protection; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2, pp. 169–189. [Google Scholar]
- Stadler, T.; Buteler, M.; Valdez, S.R.; Gitto, J.G. Particulate nanoinsecticides: A new concept in insect pest management. In Insecticides—Agriculture and Toxicology; InTech Open: London, UK, 2018; p. 83. [Google Scholar]
- Sutherland, P.; Burgess, E.; Philip, B.; McManus, M.; Watson, L.; Christeller, J. Ultrastructural changes to the midgut of the black field cricket (Teleogryllus commodus) following ingestion of potato protease inhibitor II. J. Insect Physiol. 2002, 48, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Enain, I.M.; Elqady, E.M.; El-said, E.; Salem, H.H.; Badr, N.F.; Abd-Allah, G.E.; Rezk, M.M. Biosynthesized silver nanoparticles (Ag NPs) from isolated actinomycetes strains and their impact on the black cutworm, Agrotis ipsilon. Pestic. Biochem. Phys. 2023, 194, 105492. [Google Scholar] [CrossRef] [PubMed]


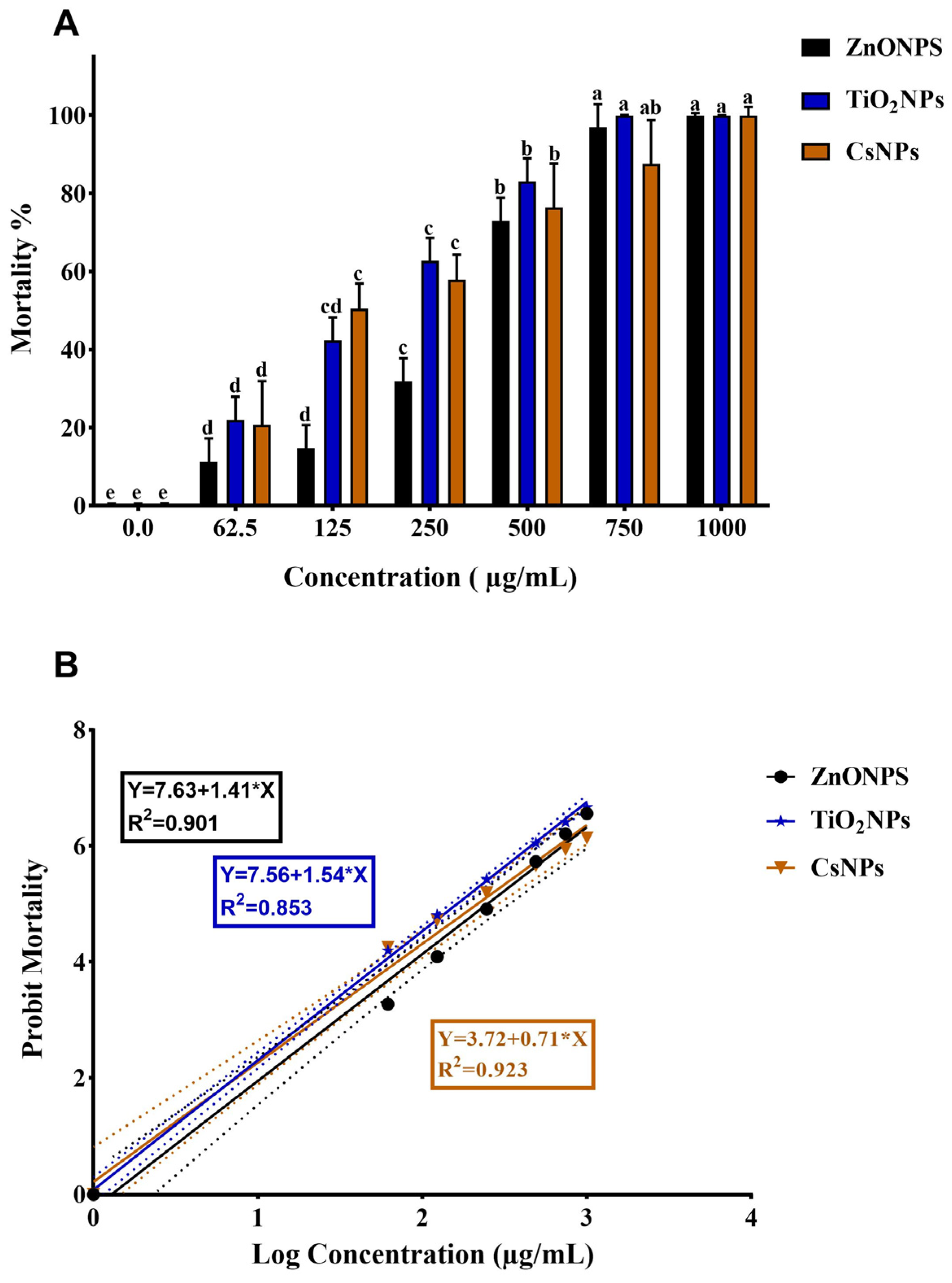
| Treatment | No. | Concentrations (µg/mL) | Mortality (%) ± SD | LC50 (95% LCL-UCL) | LC90 (95% LCL-UCL) | X2 |
|---|---|---|---|---|---|---|
| ZnONPs | 1 | 0.00 | 0.00 ± 0.00 e | 260.90 (179.90–366.41) | 724.50 (491.00–1505.81) | 3.05 |
| 2 | 62.5 | 11.30 ± 5.93 d | ||||
| 3 | 125 | 14.73 ± 5.93 d | ||||
| 4 | 250 | 31.86 ± 5.93 c | ||||
| 5 | 500 | 72.97 ± 5.93 b | ||||
| 6 | 750 | 96.95 ± 5.93 a | ||||
| 7 | 1000 | 100.00 ± 0.00 a | ||||
| TiO2NPs | 1 | 0.00 | 0.00 ± 0.00 e | 147.50 (84.60–217.90) | 512.92 (328.91–1279.10) | 1.53 |
| 2 | 62.5 | 22.04 ± 5.87 d | ||||
| 3 | 125 | 42.38 ± 5.87 cd | ||||
| 4 | 250 | 62.73 ± 5.87 c | ||||
| 5 | 500 | 83.07 ± 5.87 b | ||||
| 6 | 750 | 100.00 ± 0.00 a | ||||
| 7 | 1000 | 100.00 ± 0.00 a | ||||
| CsNPs | 1 | 0.00 | 0.00 ± 0.00 e | 183.51 (78.23–308.90) | 1220.30 (614.90–8865.2) | 1.11 |
| 2 | 62.5 | 20.29 ± 10.86 d | ||||
| 3 | 125 | 52.89 ± 10.86 c | ||||
| 4 | 250 | 56.52 ± 6.27 c | ||||
| 5 | 500 | 74.63 ± 10.86 b | ||||
| 6 | 750 | 85.51 ± 10.86 ab | ||||
| 7 | 1000 | 97.67 ± 2.23 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, R.; Ibrahim, E.; Fouad, H.; Ahmad, F.; Li, W.; Zhou, Q.; Yu, T.; Chidwala, N.; Mo, J. Termiticidal Effects and Morpho-Histological Alterations in the Subterranean Termite (Odontotermes formosanus) Induced by Biosynthesized Zinc Oxide, Titanium Dioxide, and Chitosan Nanoparticles. Nanomaterials 2024, 14, 927. https://doi.org/10.3390/nano14110927
Nasser R, Ibrahim E, Fouad H, Ahmad F, Li W, Zhou Q, Yu T, Chidwala N, Mo J. Termiticidal Effects and Morpho-Histological Alterations in the Subterranean Termite (Odontotermes formosanus) Induced by Biosynthesized Zinc Oxide, Titanium Dioxide, and Chitosan Nanoparticles. Nanomaterials. 2024; 14(11):927. https://doi.org/10.3390/nano14110927
Chicago/Turabian StyleNasser, Raghda, Ezzeldin Ibrahim, Hatem Fouad, Farhan Ahmad, Wuhan Li, Qihuan Zhou, Ting Yu, Nooney Chidwala, and Jianchu Mo. 2024. "Termiticidal Effects and Morpho-Histological Alterations in the Subterranean Termite (Odontotermes formosanus) Induced by Biosynthesized Zinc Oxide, Titanium Dioxide, and Chitosan Nanoparticles" Nanomaterials 14, no. 11: 927. https://doi.org/10.3390/nano14110927






