Laser-Scribed Graphene for Human Health Monitoring: From Biophysical Sensing to Biochemical Sensing
Abstract
:1. Introduction
2. Key Influencing Factors to Properties of LSG
2.1. Carbon Precursor Species
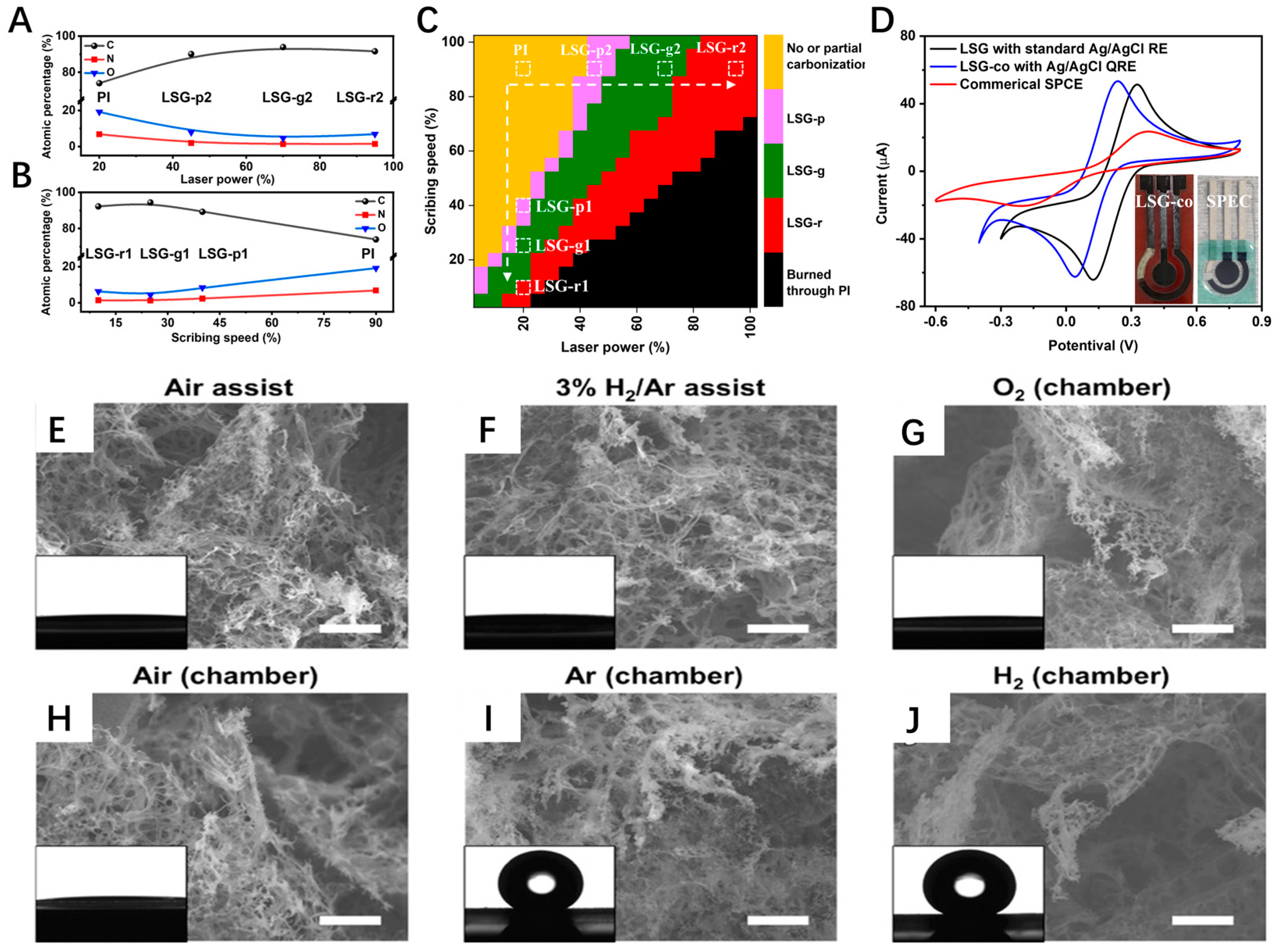
2.2. Processing Parameters and Conditions
3. Modification of LSG
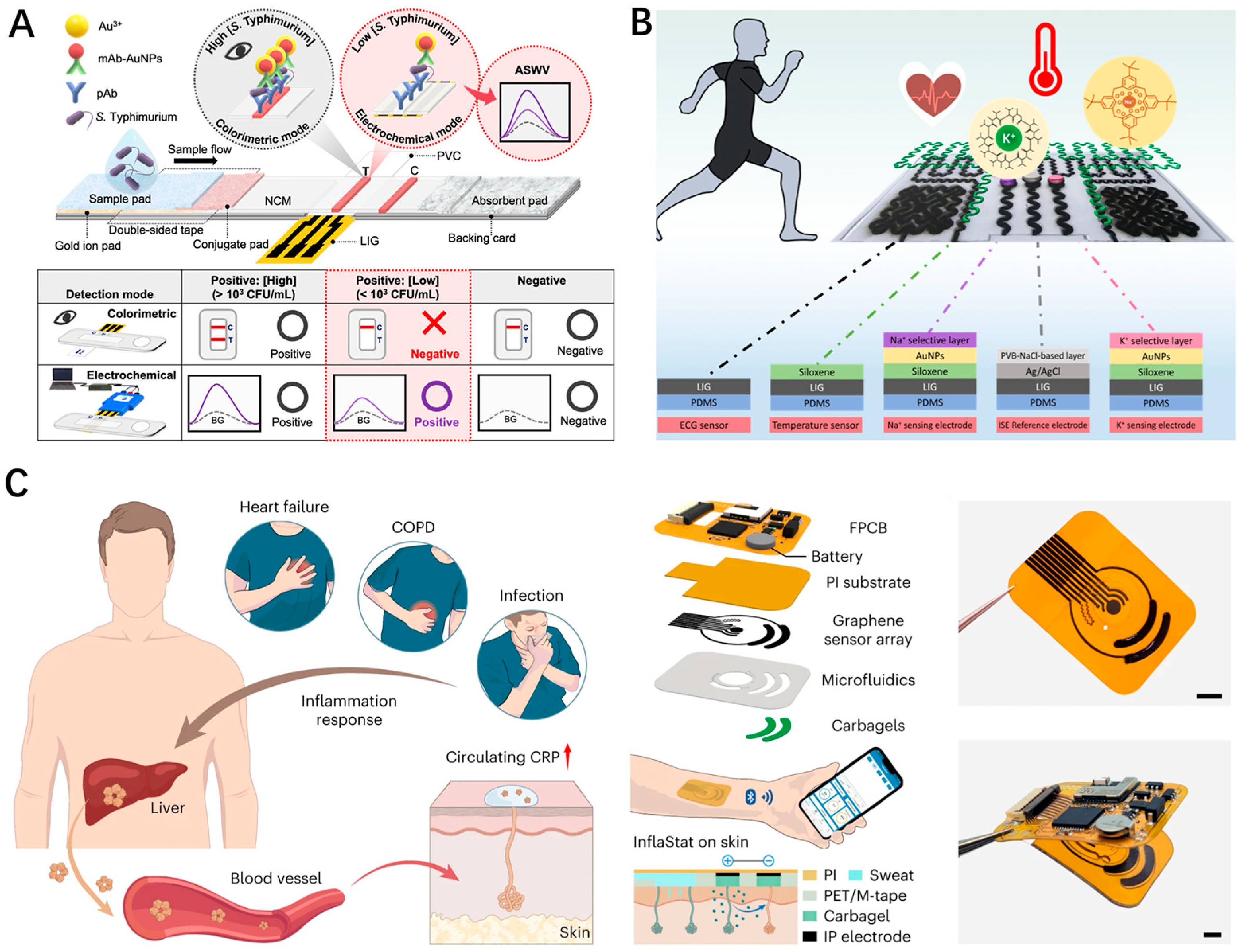
4. LEG-Based Biophysical Sensing
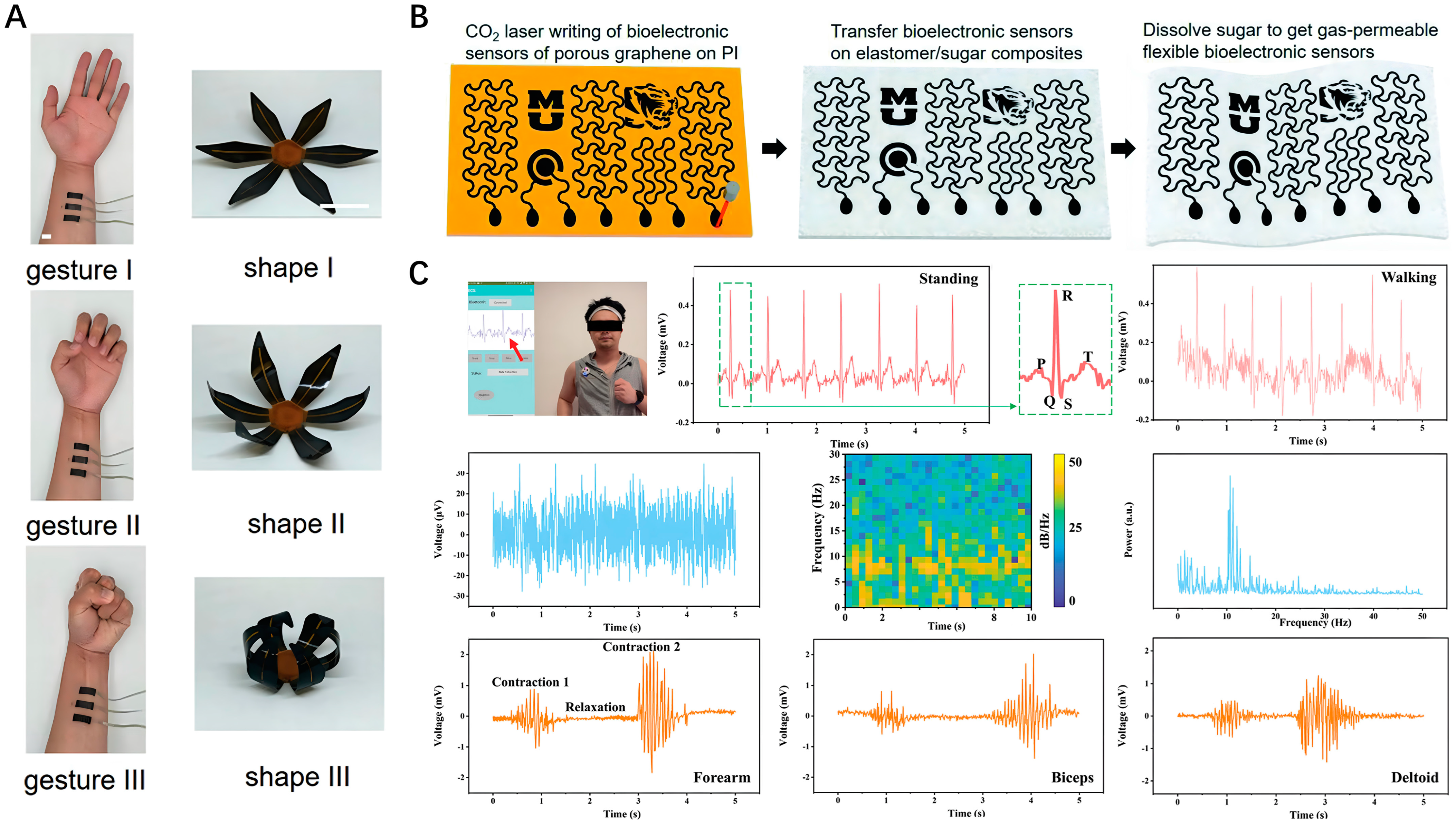
4.1. Electrophysiological Monitoring
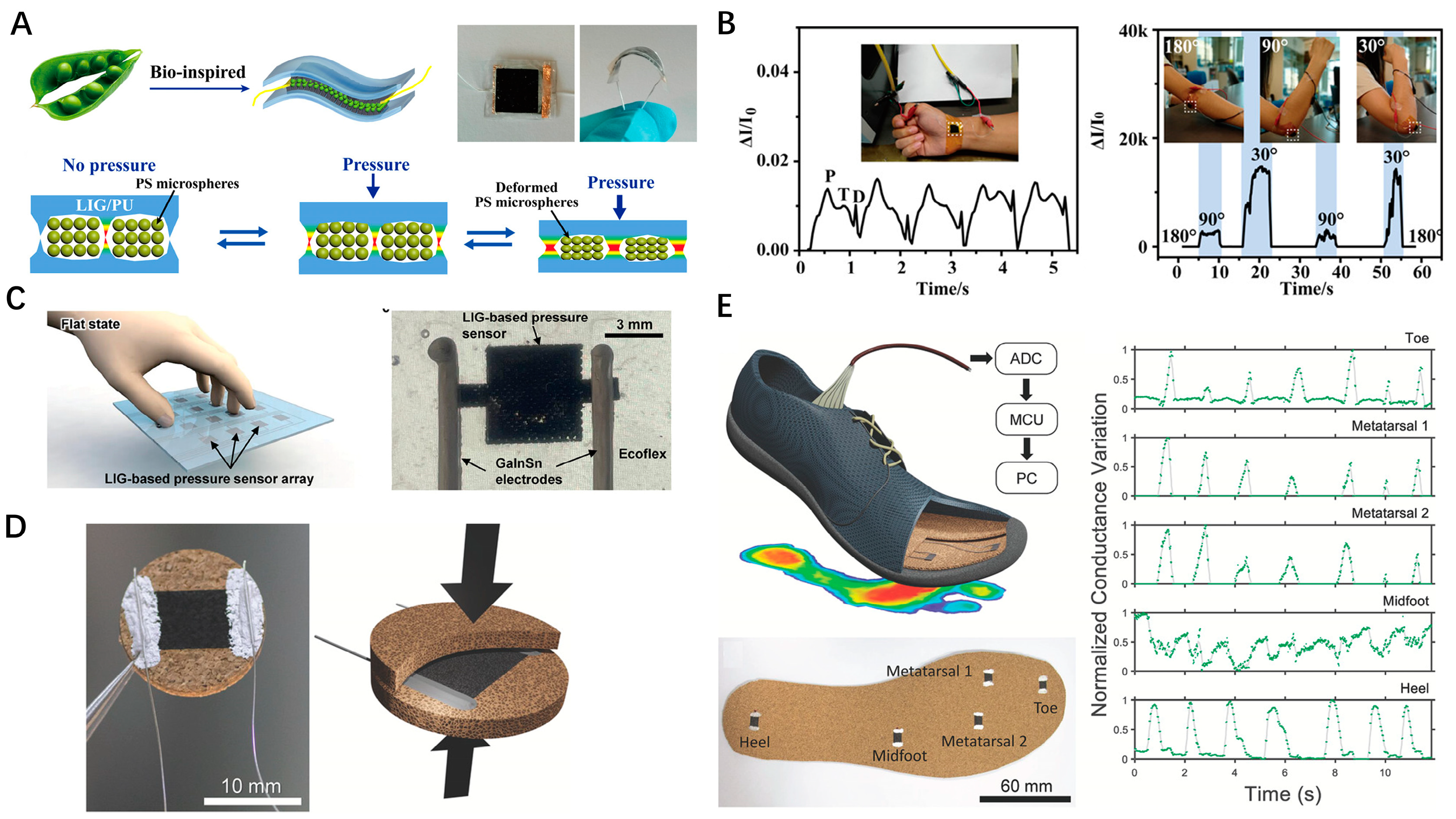
4.2. Motion Monitoring
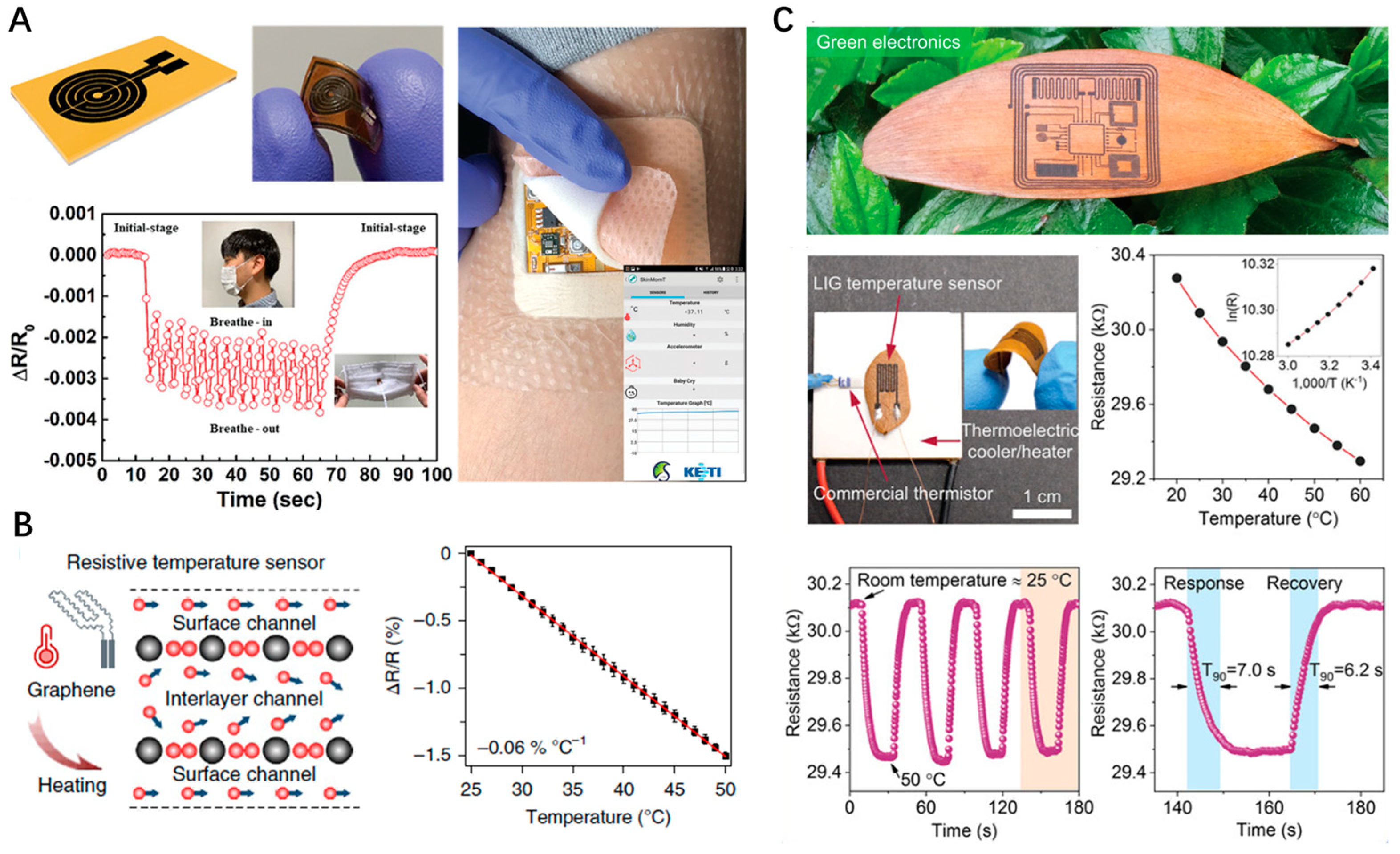
4.3. Body Temperature Monitoring
5. LEG-Based Biochemical Sensing
6. LSG-Based Multi-Modal Sensing Integration
7. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.; Xiang, Z.; Zhao, P.; Wan, J.; Miao, L.; Guo, H.; Xu, C.; Zhao, W.; Han, M.; Zhang, H. Double-Sided Wearable Multifunctional Sensing System with Anti-interference Design for Human–Ambience Interface. ACS Nano 2022, 16, 14679–14692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lai, J.; Zheng, B.; Jin, X.; Zhao, G.; Liu, H.; Chen, W.; Ma, A.; Li, X.; Wu, Y. From glutinous-rice-inspired adhesive organohydrogels to flexible electronic devices toward wearable sensing, power supply, and energy storage. Adv. Funct. Mater. 2022, 32, 2108423. [Google Scholar] [CrossRef]
- Weiss, N.O.; Zhou, H.; Liao, L.; Liu, Y.; Jiang, S.; Huang, Y.; Duan, X. Graphene: An emerging electronic material. Adv. Mater. 2012, 24, 5782–5825. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.; Mitra, A.; Mukhopadhyay, S.C. Graphene and its sensor-based applications: A review. Sens. Actuators A 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Pinheiro, T.; Correia, R.; Morais, M.; Coelho, J.; Fortunato, E.; Sales, M.G.F.; Marques, A.C.; Martins, R. Water peel-off transfer of electronically enhanced, paper-based laser-induced graphene for wearable electronics. ACS Nano 2022, 16, 20633–20646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, H.; Zhang, W.; Zhang, Z.; Lu, J.; Xu, K.; Liu, Y.; Saetang, V. A review of laser-induced graphene: From experimental and theoretical fabrication processes to emerging applications. Carbon 2023, 214, 118356. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-induced graphene. Acc. Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef]
- Cui, T.R.; Li, D.; Hirtz, T.; Shao, W.C.; Zhou, Z.B.; Ji, S.R.; Li, X.; Xu, J.D.; Jian, J.M.; Chen, Z.K.; et al. Laser-induced graphene for multifunctional and intelligent wearable systems: For health care and human–computer interaction. Appl. Sci. 2023, 13, 4688. [Google Scholar] [CrossRef]
- Kurra, N.; Jiang, Q.; Nayak, P.; Alshareef, H.N. Laser-derived graphene: A three-dimensional printed graphene electrode and its emerging applications. Nano Today 2019, 24, 81–102. [Google Scholar] [CrossRef]
- Vivaldi, F.M.; Dallinger, A.; Bonini, A.; Poma, N.; Sembranti, L.; Biagini, D.; Salvo, P.; Greco, F.; Di Francesco, F. Three-dimensional (3D) laser-induced graphene: Structure, properties, and application to chemical sensing. ACS Appl. Mater. Interfaces 2021, 13, 30245–30260. [Google Scholar] [CrossRef]
- Le, T.S.D.; Phan, H.P.; Kwon, S.; Park, S.; Jung, Y.; Min, J.; Chun, B.J.; Yoon, H.; Ko, S.H.; Kim, S.W.; et al. Recent advances in laser-induced graphene: Mechanism, fabrication, properties, and applications in flexible electronics. Adv. Funct. Mater. 2022, 32, 2205158. [Google Scholar] [CrossRef]
- Liu, J.; Ji, H.; Lv, X.; Zeng, C.; Li, H.; Li, F.; Qu, B.; Cui, F.; Zhou, Q. Laser-induced graphene (LIG)-driven medical sensors for health monitoring and diseases diagnosis. Microchim. Acta 2022, 189, 54. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Chyan, Y.; Zhang, J.; Li, Y.; Han, X.; Kittrell, C.; Tour, J.M. Laser-induced graphene formation on wood. Adv. Mater. 2017, 29, 1702211. [Google Scholar] [CrossRef] [PubMed]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-induced graphene by multiple lasing: Toward electronics on cloth, paper, and food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, S.; Xiang, X.; Shu, J.; Fei, J. Fabricating process-electrochemical property correlation of laser-scribed graphene and smartphone-based electrochemical platform for portable and sensitive biosensing. Biosens. Bioelectron. 2023, 237, 115525. [Google Scholar] [CrossRef] [PubMed]
- Duy, L.X.; Peng, Z.; Li, Y.; Zhang, J.; Ji, Y.; Tour, J.M. Laser-induced graphene fibers. Carbon 2018, 126, 472–479. [Google Scholar] [CrossRef]
- Santos, N.F.; Pereira, S.O.; Moreira, A.; Girão, A.V.; Carvalho, A.F.; Fernandes, A.J.; Costa, F.M. IR and UV Laser-Induced Graphene: Application as Dopamine Electrochemical Sensors. Adv. Mater. Technol. 2021, 6, 2100007. [Google Scholar] [CrossRef]
- Le, T.S.D.; Park, S.; An, J.; Lee, P.S.; Kim, Y.J. Ultrafast laser pulses enable one-step graphene patterning on woods and leaves for green electronics. Adv. Funct. Mater. 2019, 29, 1902771. [Google Scholar] [CrossRef]
- Li, Y.; Luong, D.X.; Zhang, J.; Tarkunde, Y.R.; Kittrell, C.; Sargunaraj, F.; Ji, Y.; Arnusch, C.J.; Tour, J.M. Laser-induced graphene in controlled atmospheres: From superhydrophilic to superhydrophobic surfaces. Adv. Mater. 2017, 29, 1700496. [Google Scholar] [CrossRef]
- Ye, R.; Peng, Z.; Wang, T.; Xu, Y.; Zhang, J.; Li, Y.; Nilewski, L.G.; Lin, J.; Tour, J.M. In situ formation of metal oxide nanocrystals embedded in laser-induced graphene. ACS Nano 2015, 9, 9244–9251. [Google Scholar] [CrossRef]
- Ge, L.; Hong, Q.; Li, H.; Li, F. A laser-induced TiO2-decorated graphene photoelectrode for sensitive photoelectrochemical biosensing. Chem. Commun. 2019, 55, 4945–4948. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Hong, Q.; Li, H.; Liu, C.; Li, F. Direct-laser-writing of metal sulfide-graphene nanocomposite photoelectrode toward sensitive photoelectrochemical sensing. Adv. Funct. Mater. 2019, 29, 1904000. [Google Scholar] [CrossRef]
- Nam, K.H.; Abdulhafez, M.; Castagnola, E.; Tomaraei, G.N.; Cui, X.T.; Bedewy, M. Laser direct write of heteroatom-doped graphene on molecularly controlled polyimides for electrochemical biosensors with nanomolar sensitivity. Carbon 2022, 188, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Umer, M.; Lobino, M.; Thiel, D.; Nguyen, N.T.; Trinchi, A.; Shiddiky, M.J.; Gao, Y.; Li, Q. Laser induced self-N-doped porous graphene as an electrochemical biosensor for femtomolar miRNA detection. Carbon 2020, 163, 385–394. [Google Scholar] [CrossRef]
- Chen, Y.; Long, J.; Xie, B.; Kuang, Y.; Chen, X.; Hou, M.; Gao, J.; Liu, H.; He, Y.; Wong, C.P. One-Step Ultraviolet laser-induced fluorine-doped graphene achieving superhydrophobic properties and its application in deicing. ACS Appl. Mater. Interfaces 2022, 14, 4647–4655. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Y.; Gao, W. Laser-engraved graphene for flexible and wearable electronics. Trends Chem. 2021, 3, 969–981. [Google Scholar] [CrossRef]
- Ling, Y.; Pang, W.; Li, X.; Goswami, S.; Xu, Z.; Stroman, D.; Liu, Y.; Fei, Q.; Xu, Y.; Zhao, G.; et al. Laser-induced graphene for electrothermally controlled, mechanically guided, 3D assembly and human–soft actuators interaction. Adv. Mater. 2020, 32, 1908475. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elbaki, M.K.; Ragab, T.M.; Ismael, N.E.; Khalil, A.S. Robust, self-adhesive and anti-bacterial silk-based LIG electrodes for electrophysiological monitoring. RSC Adv. 2023, 13, 31704–31719. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; McCay, R.N.; Goswami, S.; Xu, Y.; Zhang, C.; Ling, Y.; Lin, J.; Yan, Z. Gas-permeable, multifunctional on-skin electronics based on laser-induced porous graphene and sugar-templated elastomer sponges. Adv. Mater. 2018, 30, 1804327. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, M.; Liu, X.; Cui, Y.; Hu, H.; Li, Q.; Jin, M.; Xian, J.; Nie, Z.; Zhang, C. Three-in-one portable electronic sensory system based on low-impedance laser-induced graphene on-skin electrode sensors for electrophysiological signal monitoring. Adv. Mater. Interfaces 2023, 10, 2201735. [Google Scholar] [CrossRef]
- Huang, L.; Wang, H.; Zhan, D.; Fang, F. Flexible capacitive pressure sensor based on laser–induced graphene and polydimethylsiloxane foam. IEEE Sens. J. 2021, 21, 12048–12056. [Google Scholar] [CrossRef]
- Tian, Q.; Yan, W.; Li, Y.; Ho, D. Bean pod-inspired ultrasensitive and self-healing pressure sensor based on laser-induced graphene and polystyrene microsphere sandwiched structure. ACS Appl. Mater. Interfaces 2020, 12, 9710–9717. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Matsumura, G.; Xuan, Y.; Honda, S.; Takei, K. Stretchable Electronic Skin using Laser-Induced Graphene and Liquid Metal with an Action Recognition System Powered by Machine Learning. Adv. Funct. Mater. 2024, 2313824. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Fernandes, A.J.; Leitão, C.; Deuermeier, J.; Marques, A.C.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-induced graphene strain sensors produced by ultraviolet irradiation of polyimide. Adv. Funct. Mater. 2018, 28, 1805271. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Fernandes, A.J.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-induced graphene piezoresistive sensors synthesized directly on cork insoles for gait analysis. Adv. Mater. Technol. 2020, 5, 2000630. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Wang, M.; Tu, J.; Kogan, A.; Zhang, H.; et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kulyk, B.; Silva, B.F.; Carvalho, A.F.; Silvestre, S.; Fernandes, A.J.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-induced graphene from paper for mechanical sensing. ACS Appl. Mater. Interfaces 2021, 13, 10210–10221. [Google Scholar] [CrossRef]
- Chhetry, A.; Sharifuzzaman, M.; Yoon, H.; Sharma, S.; Xuan, X.; Park, J.Y. MoS2-decorated laser-induced graphene for a highly sensitive, hysteresis-free, and reliable piezoresistive strain sensor. ACS Appl. Mater. Interfaces 2019, 11, 22531–22542. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Bai, R.; Gao, Y.; Wu, R.; Ju, K.; Tan, J.; Xuan, F. Laser direct writing of flexible sensor arrays based on carbonized carboxymethylcellulose and its composites for simultaneous mechanical and thermal stimuli detection. ACS Appl. Mater. Interfaces 2021, 13, 10171–10180. [Google Scholar] [CrossRef]
- Chhetry, A.; Sharma, S.; Barman, S.C.; Yoon, H.; Ko, S.; Park, C.; Yoon, S.; Kim, H.; Park, J.Y. Black phosphorus@ laser-engraved graphene heterostructure-based temperature–strain hybridized sensor for electronic-skin applications. Adv. Funct. Mater. 2021, 31, 2007661. [Google Scholar] [CrossRef]
- Raza, T.; Tufail, M.K.; Ali, A.; Boakye, A.; Qi, X.; Ma, Y.; Ali, A.; Qu, L.; Tian, M. Wearable and flexible multifunctional sensor based on laser-induced graphene for the sports monitoring system. ACS Appl. Mater. Interfaces 2022, 14, 54170–54181. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, D.; Liu, C.; Wang, Q.; Wang, H. Full-Range On-Body Strain Sensor of Laser-Induced Graphene Embedded in Thermoplastic Elastomer via Hot Pressing Transfer for Monitoring of the Physiological Signals. Adv. Mater. Technol. 2024, 9, 2301658. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Liu, P.; Guo, X. A soft and stretchable electronics using laser-induced graphene on polyimide/PDMS composite substrate. npj Flex. Electron. 2022, 6, 26. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, W.; Wang, S.; Chen, Q.; Liu, X. Direct Conversion of Liquid Organic Precursor into 3D Laser-Induced Graphene Materials. Adv. Mater. 2023, 35, 2209545. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Chen, G.; Yu, W.; Liu, J.; Wang, Y.; Zhao, W.; Liu, X. In Situ Growth of Laser-Induced Graphene on Flexible Substrates for Wearable Sensors. ACS Appl. Nano Mater. 2024, 7, 3279–3288. [Google Scholar] [CrossRef]
- Gandla, S.; Naqi, M.; Lee, M.; Lee, J.J.; Won, Y.; Pujar, P.; Kim, J.; Lee, S.; Kim, S. Highly linear and stable flexible temperature sensors based on laser-induced carbonization of polyimide substrates for personal mobile monitoring. Adv. Mater. Technol. 2020, 5, 2000014. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Yu, C.; Li, Y.; Guo, X.; Zhang, Y.; Chen, C.; Cao, L. High-linearity graphene-based temperature sensor fabricated by laser writing. J. Mater. Sci. Mater. Electron. 2024, 35, 109. [Google Scholar] [CrossRef]
- Pinheiro, T.; Silvestre, S.; Coelho, J.; Marques, A.C.; Martins, R.; Sales, M.G.F.; Fortunato, E. Laser-induced graphene on paper toward efficient fabrication of flexible, planar electrodes for electrochemical sensing. Adv. Mater. Interfaces 2021, 8, 2101502. [Google Scholar] [CrossRef]
- Nayak, P.; Kurra, N.; Xia, C.; Alshareef, H.N. Highly efficient laser scribed graphene electrodes for on-chip electrochemical sensing applications. Adv. Electron. Mater. 2016, 2, 1600185. [Google Scholar] [CrossRef]
- de Araujo, W.R.; Frasson, C.M.; Ameku, W.A.; Silva, J.R.; Angnes, L.; Paixão, T.R. Single-step reagentless laser scribing fabrication of electrochemical paper-based analytical devices. Angew. Chem. 2017, 129, 15309–15313. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, L.; Li, J.; Cui, C.; Zhou, Z.; Wen, L. Surface engineering of laser-induced graphene enables long-term monitoring of on-body uric acid and pH simultaneously. Nano Lett. 2022, 22, 5451–5458. [Google Scholar] [CrossRef] [PubMed]
- Adiraju, A.; Jalasutram, A.; Wang, J.; Tegenkamp, C.; Kanoun, O. Electrodeposited Silver Dendrites on Laser-Induced Graphene for Electrochemical Detection of Nitrate with Tunable Sensor Properties. Adv. Mater. Interfaces 2024, 2400102. [Google Scholar] [CrossRef]
- Samoson, K.; Soleh, A.; Saisahas, K.; Promsuwan, K.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Chang, K.H.; Abdullah, A.F.L.; Tayayuth, K.; et al. Facile fabrication of a flexible laser induced gold nanoparticle/chitosan/porous graphene electrode for uric acid detection. Talanta 2022, 243, 123319. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhang, X.; Sun, Z.; Chen, H.; Fu, J.; Si, H.; Ge, C.; Lin, S. Laser-induced graphene-based wearable epidermal ion-selective sensors for noninvasive multiplexed sweat analysis. Biosensors 2022, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, I.S.; Sanborn, D.; Chen, B.; Garland, N.; Serhan, M.; Forzani, E.; Gomes, C.; Claussen, J.C. Ion-selective sensors based on laser-induced graphene for evaluating human hydration levels using urine samples. Adv. Mater. Technol. 2020, 5, 1901037. [Google Scholar] [CrossRef]
- Lee, C.W.; Jeong, S.Y.; Kwon, Y.W.; Lee, J.U.; Cho, S.C.; Shin, B.S. Fabrication of laser-induced graphene-based multifunctional sensing platform for sweat ion and human motion monitoring. Sens. Actuators A 2022, 334, 113320. [Google Scholar] [CrossRef]
- Zheng, C.; Ling, Y.; Chen, J.; Yuan, X.; Li, S.; Zhang, Z. Design of a versatile and selective electrochemical sensor based on dummy molecularly imprinted PEDOT/laser-induced graphene for nitroaromatic explosives detection. Environ. Res. 2023, 236, 116769. [Google Scholar] [CrossRef] [PubMed]
- Bossard, B.; Grothe, R.A.; Martins, A.B.; Lobato, A.; Tasić, N.; Paixao, T.R.; Gonçalves, L.M. Nanographene laser-pyrolyzed paper electrodes for the impedimetric detection of D-glucose via a molecularly imprinted polymer. Monatshefte Chem.-Chem. Mon. 2022, 153, 1129–1135. [Google Scholar] [CrossRef]
- Beduk, T.; Gomes, M.; De Oliveira Filho, J.I.; Shetty, S.S.; Khushaim, W.; Garcia-Ramirez, R.; Durmus, C.; Ait Lahcen, A.; Salama, K.N. A portable molecularly imprinted sensor for on-site and wireless environmental bisphenol A monitoring. Front. Chem. 2022, 10, 833899. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, R.; Chen, J.; Li, S.; Ling, Y.; Zhang, Z. Development of a selective electrochemical microsensor based on molecularly imprinted polydopamine/ZIF-67/laser-induced graphene for point-of-care determination of 3-nitrotyrosine. Biosens. Bioelectron. 2024, 255, 116246. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A wearable electrochemical biosensor for the monitoring of metabolites and nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, J.; Ding, Z.; Wang, H.; Huang, L.; Feng, X. Laser-Induced Graphene Arrays-Based Three-Phase Interface Enzyme Electrode for Reliable Bioassays. Biomimetics 2023, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, H.; Yuan, M.; Yu, J.; Wang, Z.; Chen, X. One-step laser synthesis platinum nanostructured 3D porous graphene: A flexible dual-functional electrochemical biosensor for glucose and pH detection in human perspiration. Talanta 2023, 257, 124362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Liu, C.; Dong, N.; Liu, D.; You, T. Laser induced graphene electrochemical aptasensor based on tetrahedral DNA for ultrasensitive on-site detection of microcystin-LR. Biosens. Bioelectron. 2023, 239, 115610. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Rodríguez, R.M.; Lukas, H.; Tu, J.; Min, J.; Yang, Y.; Xu, C.; Rossiter, H.B.; Gao, W. SARS-CoV-2 RapidPlex: A graphene-based multiplexed telemedicine platform for rapid and low-cost COVID-19 diagnosis and monitoring. Matter 2020, 3, 1981–1998. [Google Scholar] [CrossRef] [PubMed]
- Beduk, T.; Beduk, D.; de Oliveira Filho, J.I.; Zihnioglu, F.; Cicek, C.; Sertoz, R.; Arda, B.; Goksel, T.; Turhan, K.; Salama, K.N.; et al. Rapid point-of-care COVID-19 diagnosis with a gold-nanoarchitecture-assisted laser-scribed graphene biosensor. Anal. Chem. 2021, 93, 8585–8594. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Han, Y.; He, Q.; Liu, H.; Zhang, Y.; Han, L. Ultrasensitive and Reliable SERS Chip Based on Facile Assembly of AgNPs on Porous LIG to Enhance the Local Electromagnetic Field. J. Phys. Chem. C 2023, 127, 4195–4202. [Google Scholar] [CrossRef]
- Chen, L.; Hou, T.; Tan, Y.; Guo, C.; Wang, B.; Ge, L.; Li, F. Laser-induced N-and B-codoped graphene nanozymes with intrinsic peroxidase-like activities for bactericidal application. ACS Sustain. Chem. Eng. 2022, 10, 2750–2760. [Google Scholar] [CrossRef]
- Preechakasedkit, P.; Panphut, W.; Lomae, A.; Wonsawat, W.; Citterio, D.; Ruecha, N. Dual Colorimetric/Electrochemical Detection of Salmonella typhimurium Using a Laser-Induced Graphene Integrated Lateral Flow Immunoassay Strip. Anal. Chem. 2023, 95, 13904–13912. [Google Scholar] [CrossRef]
- Song, Y.; Tay, R.Y.; Li, J.; Xu, C.; Min, J.; Shirzaei Sani, E.; Kim, G.; Heng, W.; Kim, I.; Gao, W. 3D-printed epifluidic electronic skin for machine learning–powered multimodal health surveillance. Sci. Adv. 2023, 9, eadi6492. [Google Scholar] [CrossRef]
- Hui, X.; Asaduzzaman, M.; Zahed, M.A.; Sharma, S.; Jeong, S.; Song, H.; Faruk, O.; Park, J.Y. Multifunctional Siloxene-Decorated Laser-Inscribed Graphene Patch for Sweat Ion Analysis and Electrocardiogram Monitoring. ACS Appl. Mater. Interfaces 2024, 16, 9725–9735. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Min, J.; Song, Y.; Xu, C.; Li, J.; Moore, J.; Hanson, J.; Hu, E.; Parimon, T.; Wang, T.Y.; et al. A wireless patch for the monitoring of C-reactive protein in sweat. Nat. Biomed. Eng. 2023, 7, 1293–1306. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, Y.; Wu, S.; Wu, X.; Shu, J. Laser-Scribed Graphene for Human Health Monitoring: From Biophysical Sensing to Biochemical Sensing. Nanomaterials 2024, 14, 942. https://doi.org/10.3390/nano14110942
Li Y, Li Y, Wu S, Wu X, Shu J. Laser-Scribed Graphene for Human Health Monitoring: From Biophysical Sensing to Biochemical Sensing. Nanomaterials. 2024; 14(11):942. https://doi.org/10.3390/nano14110942
Chicago/Turabian StyleLi, Yakang, Yaxin Li, Sirui Wu, Xuewen Wu, and Jian Shu. 2024. "Laser-Scribed Graphene for Human Health Monitoring: From Biophysical Sensing to Biochemical Sensing" Nanomaterials 14, no. 11: 942. https://doi.org/10.3390/nano14110942




