Er-Doped BiVO4/BiFeO3 Nanocomposites Synthesized via Sonochemical Process and Their Piezo-Photocatalytic Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. BiFeO3 Preparation
2.2. BiVO4/BiFeO3 Composite Preparation
2.3. Erbium Doped BiVO4/BiFeO3 Composite Powder Preparation
2.4. Composite Film Preparation
2.5. Characterizations
3. Results and Discussion
3.1. Crystalline Structure
3.2. Optical Property
3.3. Chemical Bonding
3.4. Surface Morphology
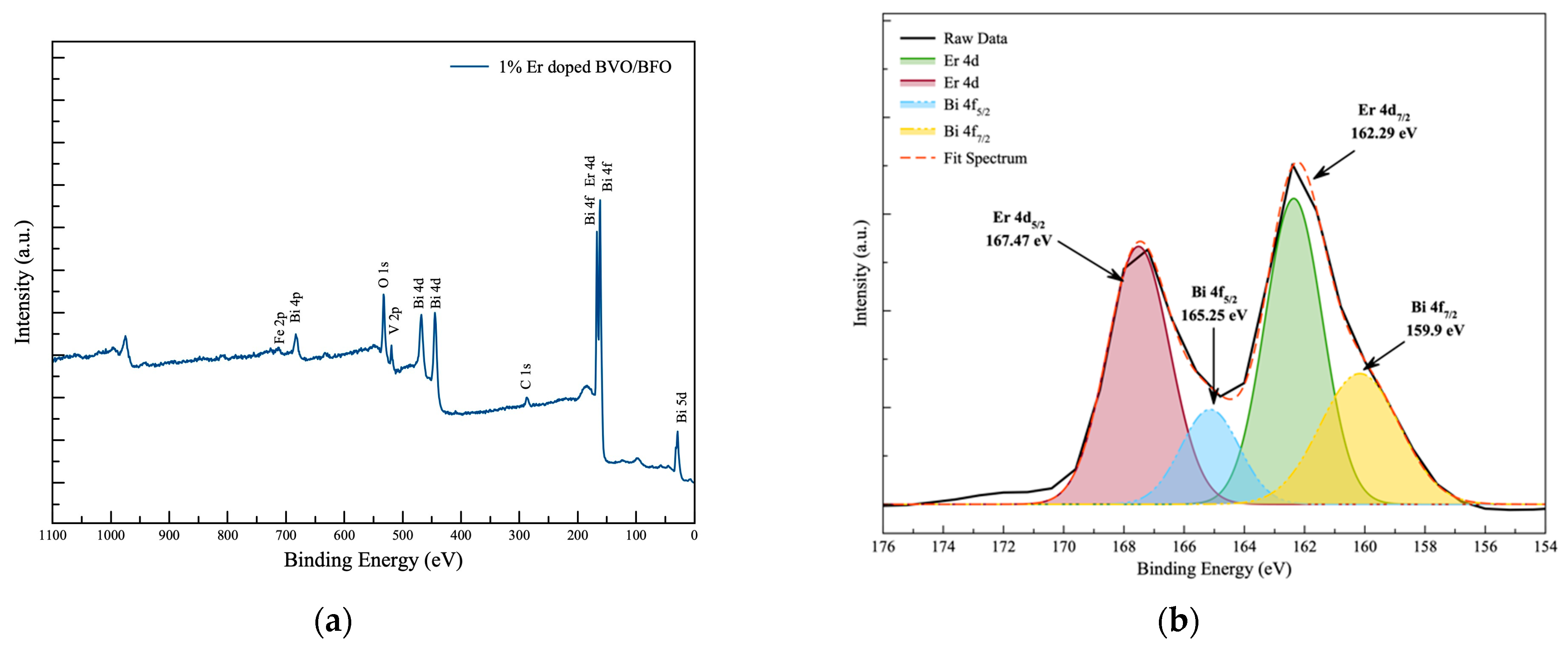
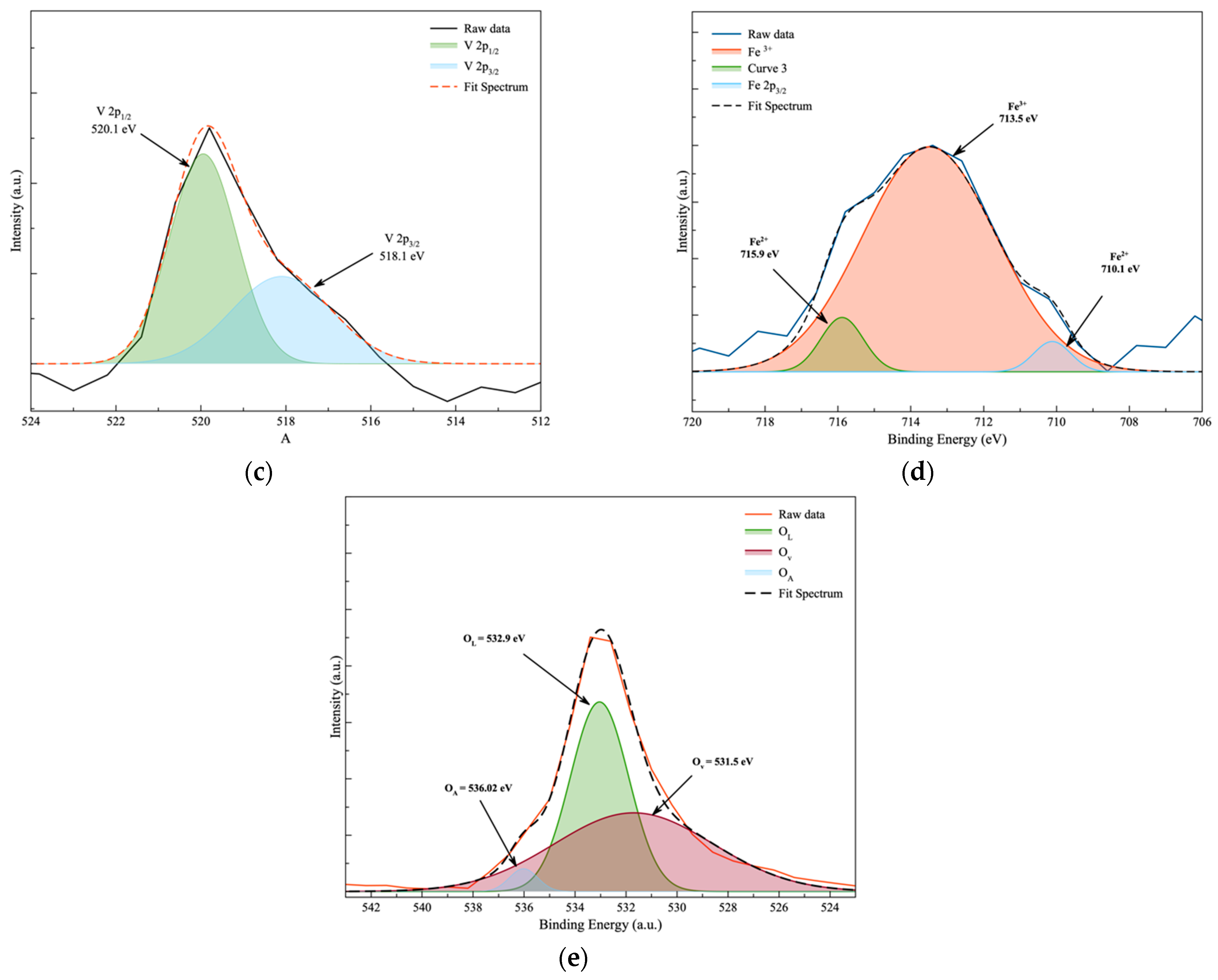
3.5. Surface Chemical Analysis
3.6. Piezo-Photocatalytic Performance
3.7. Scavenger Testing
3.8. Impedance Property
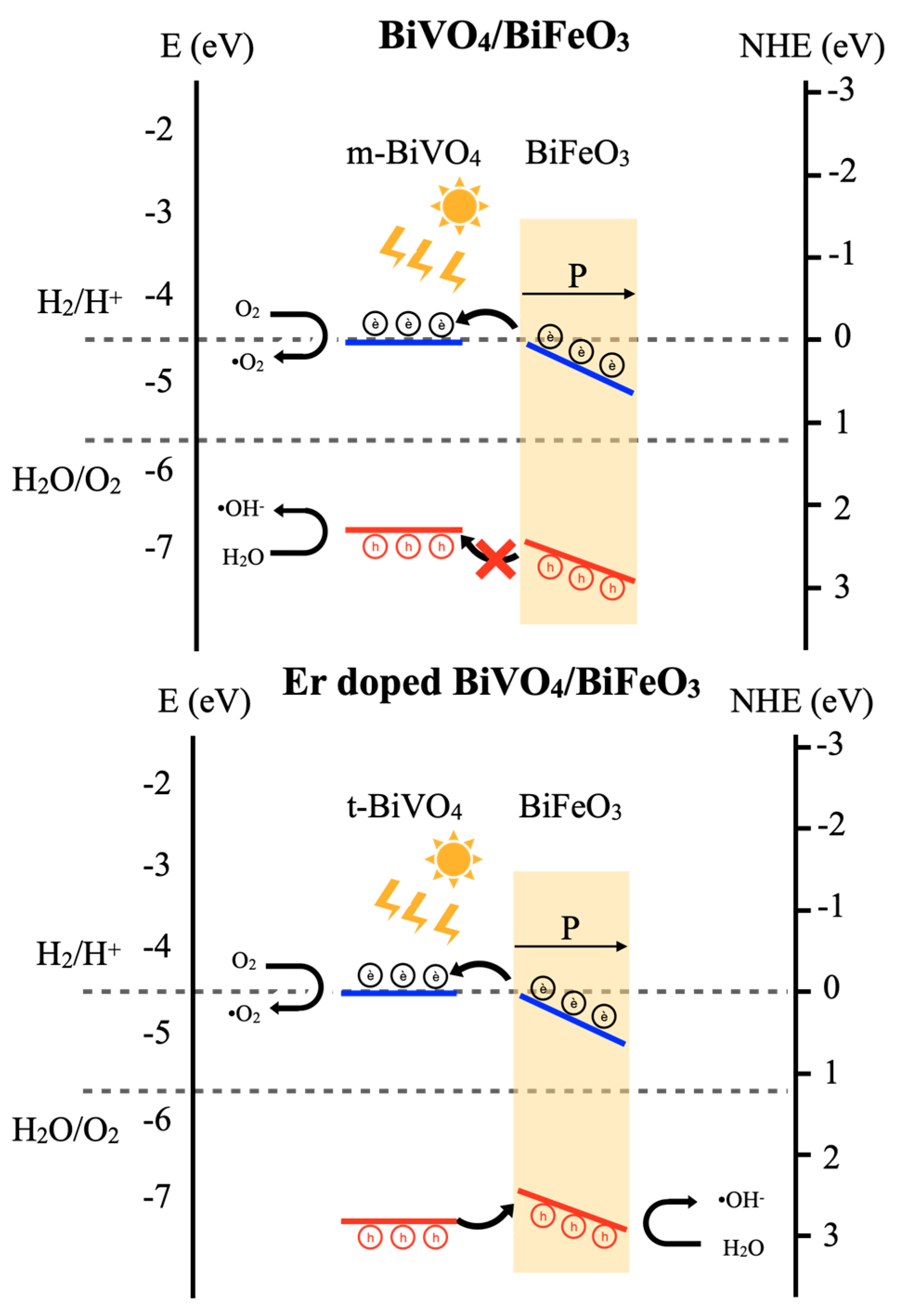
3.9. Piezo-Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Hone, F.G. Experimental and computational study of metal oxide nanoparticles for the photocatalytic degradation of organic pollutants: A review. RSC Adv. 2023, 13, 18404–18442. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Lu, Y.N.; Tao, F.F.; Liang, P.F.; Zhang, P.A. ZnO nanoparticles modified by carbon quantum dots for the photocatalytic removal of synthetic pigment pollutants. ACS Omega 2013, 8, 7845–7857. [Google Scholar] [CrossRef] [PubMed]
- Kalaycioglu, Z.; Ozugur Uysal, B.; Pekcan, O.; Erim, F.B. Efficient photocatalytic degradation of methylene blue dye from aqueous solution with cerium oxide nanoparticles and graphene oxide-doped polyacrylamide. ACS Omega 2023, 8, 13004–13015. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, R.; Zhu, C.; Pei, J. Preparation, Characterization and photocatalytic degradation efficacy of bismuth oxide under visible and ultraviolet light. J. Mater. Res. 2021, 36, 2936–2949. [Google Scholar] [CrossRef]
- Noor, M.; Sharmin, F.; Mamun, M.A.A.; Hasan, S.; Hakim, M.A.; Basith, M.A. Effect of Gd and Y co-doping in BiVO4 photocatalyst for enhanced degradation of methylene blue dye. J. Alloys Compd. 2022, 895, 162639. [Google Scholar] [CrossRef]
- Xu, H.; Wu, C.; Li, H.; Chu, J.; Sun, G.; Xu, Y.; Yan, Y. Synthesis, characterization, and photocatalytic activities of rare earth-loaded BiVO4 catalysts. Appl. Surf. Sci. 2009, 256, 597–602. [Google Scholar] [CrossRef]
- Xian, T.; Ma, X.; Sun, X.; Sun, C.; Wang, H.; Di, L.; Ma, K.; Yang, H. Construction of p-n type AgCl/BiFeO3 heterojunction with promising photocatalytic and piezo-photocatalytic water purification. Opt. Mater. 2024, 149, 115054. [Google Scholar] [CrossRef]
- Sun, X.; Xu, T.; Xian, T.; Yi, Z.; Liu, G.; Dai, J.; Yang, H. Insight on the enhanced piezo-photocatalytic mechanism of In2O3/BiFeO3 heterojunctions for degradation of tetracycline hydrochloride. App. Surf. Sci. 2023, 640, 158408. [Google Scholar] [CrossRef]
- McDonnell, K.A.; Wadnerkar, N.; English, N.J.; Rahman, M.; Dowling, D. Photo-active and optical properties of bismuth ferrite (BiFeO3): An experimental and theoretical study. Chem. Phys. Lett. 2013, 572, 78–84. [Google Scholar] [CrossRef]
- Su, P.; Zhang, D.; Yao, X.; Liang, T.; Yang, N.; Zhang, D.; Pu, X.; Liu, J.; Cai, P.; Li, Z. Enhanced piezo-photocatalytic performance in ZnIn2S4/BiFeO3 heterojunction stimulated by solar and mechanical energy for efficient hydrogen evolution. J. Colloid. Interf. Sci. 2024, 662, 276–288. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Long, K.; Yuan, H.; Sun, X. Activities of BiFeO3/carbon-dots catalysts in piezo-photocatalytic degradation of ciprofloxacin upon light/ultrasonic excitation. Ultrason. Sonochem. 2024, 103, 106770. [Google Scholar] [CrossRef] [PubMed]
- Wechprasit, T.; Bootchanont, A.; Sailuam, W.; Kansaard, T.; Noinonmueng, T.; Mekprasart, W.; Boonyarattanakalin, K.; Chirawatkul, P.; Jayasankar, C.K.; Pecharapa, W. Structural and photocatalytic properties and X-ray absorption spectroscopic study of BiVO4 nanoparticles incorporated with Fe synthesized by sonochemical method. Radiat. Phys. Chem. 2022, 201, 110480. [Google Scholar] [CrossRef]
- Obregón, S.; Colón, G. Excellent photocatalytic activity of Yb3+, Er3+ co-doped BiVO4 Photocatalyst. Appl. Catal. B Environ. 2014, 152, 328–334. [Google Scholar] [CrossRef]
- Shan, L.; Liu, Y. Er3+, Yb3+ doping induced core–shell structured BiVO4 and near-infrared photocatalytic properties. J. Mol. Catal. A Chem. 2016, 416, 1–9. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, G.; Dong, G.; Zhang, L.; Huang, J.; Yang, W.; Zhao, C.; Ren, H. Structural transformation of Sm3+ doped BiVO4 with high photocatalytic activity under simulated sun-light. Appl. Surf. Sci. 2015, 324, 505–511. [Google Scholar] [CrossRef]
- Luo, W.; Wang, J.; Zhao, X.; Zhao, Z.; Lia, Z.; Zou, Z. Formation energy and photoelectrochemical properties of BiVO4 after doping at Bi3+ or V5+ sites with higher valence metal ions. Phys. Chem. Chem. Phys. 2013, 15, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Pellicer-Porres, J.; Vázquez-Socorro, D.; López-Moreno, S.; Muñoz, A.; Rodríguez-Hernández, P.; Martínez-García, D.; Achary, S.N.; Rettie, A.J.E.; Mullins, C.B. Phase transition systematics in BiVO4 by means of high-pressure–high-temperature Raman experiments. Phys. Rev. B 2018, 98, 214109. [Google Scholar] [CrossRef]
- Obregón, S.; Lee, S.W.; Colón, G. Exalted photocatalytic activity of tetragonal BiVO4 by Er3+ doping through a luminescence cooperative mechanism. Dalton Trans. 2014, 43, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tan, G.; Zhao, C.; Xu, C.; Su, Y.; Wang, Y.; Ren, H.; Xia, A.; Shao, D.; Yan, S. Enhanced photocatalytic mechanism of the Nd-Er co-doped tetragonal BiVO4 photocatalysts. Appl. Catal. B Environ. 2017, 15, 87–96. [Google Scholar] [CrossRef]
- Regmi, C.; Kshetri, Y.K.; Ray, S.K.; Pandey, R.P.; Lee, S.W. Utilization of visible to NIR light energy by Yb+3, Er+3 and Tm+3 doped BiVO4 for the photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2017, 392, 61–70. [Google Scholar] [CrossRef]
- Soltani, T.; Tayyebi, A.; Lee, B.K. BiFeO3/BiVO4 p-n heterojunction for efficient and stable photocatalytic and photoelectrochemical water splitting under visible-light irradiation. Catal. Today 2020, 340, 188–196. [Google Scholar] [CrossRef]
- Di, L.; Yang, H.; Xian, T.; Chen, X. Enhanced Photocatalytic Activity of NaBH4 Reduced BiFeO3 Nanoparticles for Rhodamine B Decolorization. Materials 2017, 10, 1118. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Gong, S.; Zubairu, S.M.; Liu, L.; Xu, Y.; Guo, L.; Dang, R.; Zhu, G. Fabrication of Er3+/Yb3+ Co-Doped Bi5O7I Microsphere with Upconversion Luminescence and Enhanced Photocatalytic Activity for Bisphenol A Degradation. Front. Chem. 2020, 8, 773. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Cheng, S.; Hao, X.; Wang, Z.; Ma, N.; Du, P. Effect of Ag doping on the formation and properties of percolative Ag/BiFeO3 composite thin film by sol–gel method. Appl. Phys. A 2017, 123, 289. [Google Scholar] [CrossRef]
- Navjot; Tovstolytkin, A.; Lotey, G.S. Plasmonic Enhanced Photocatalytic Activity of Ag Nanospheres Decorated BiFeO3 Nanoparticles. Catal. Lett. 2017, 147, 1640–1645. [Google Scholar] [CrossRef]
- Chen, F.; Her, J.; Shao, Y.; Matsuda, Y.H.; Pan, T. Structural and electrical characteristics of high-K Er2O3 and Er2TiO5 gate dielectrics for a-IGZO thin-film transistors. Nanoscale Res. Lett. 2013, 8, 18. [Google Scholar] [PubMed]
- Deka, S.; Devi, M.B.; Khan, M.R.; Keerthana; Venimadhav, A.; Choudhury, B. Piezo-Photocatalytic and Photocatalytic Bismuth Vanadate Nanorods with Antibacterial Property. ACS Appl. Nano Mater. 2022, 5, 10724–10734. [Google Scholar] [CrossRef]
- Jing, Q.; Liu, Z.; Cheng, X.; Li, C.; Ren, P.; Guo, K.; Yue, H.; Xie, B.; Li, T.; Wang, Z.; et al. Boosting piezo-photocatalytic activity of BiVO4/BiFeO3 heterojunctions through built-in polarization field tailoring carrier transfer performances. Chem. Eng. J. 2023, 464, 142617. [Google Scholar]
- Ma, B.C.; Ghasimi, S.; Landfester, K.; Vilela, F.; Zhang, K.A.I. Conjugated microporous polymer nanoparticles with enhanced dispersibility and water compatibility for photocatalytic applications. J. Mater. Chem. A 2015, 3, 16064–16071. [Google Scholar] [CrossRef]
- Meng, N.; Liu, W.; Jiang, R.; Zhang, Y.; Dunn, S.; Wu, J.; Yan, H. Fundamentals, advances and perspectives of piezocatalysis: A marriage of solid-state physics and catalytic chemistry. Prog. Mater. Sci. 2023, 138, 101161. [Google Scholar] [CrossRef]
- Wang, K.; Han, C.; Li, J.; Qiu, J.; Sunarso, J.; Liu, S. The Mechanism of Piezocatalysis: Energy Band Theory or Screening Charge Effect? Angew. Chem. Int. Ed. 2022, 61, e202110429. [Google Scholar] [CrossRef] [PubMed]
- Kansaard, T.; Ishihara, K.N.; Pecharapa, W. Structural, optical and photo-induced catalytic properties of derived-Leucoxene/BiVO4 composite prepared by sonochemical process. Optik 2022, 267, 169665. [Google Scholar] [CrossRef]
- Lee, M.G.; Park, J.S.; Jang, H.W. Solution-Processed Metal Oxide Thin Film Nanostructures for Water Splitting Photoelectrodes: A Review. J. Korean Ceram. Soc. 2018, 55, 185–202. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016, 52, 35–59. [Google Scholar] [CrossRef] [PubMed]
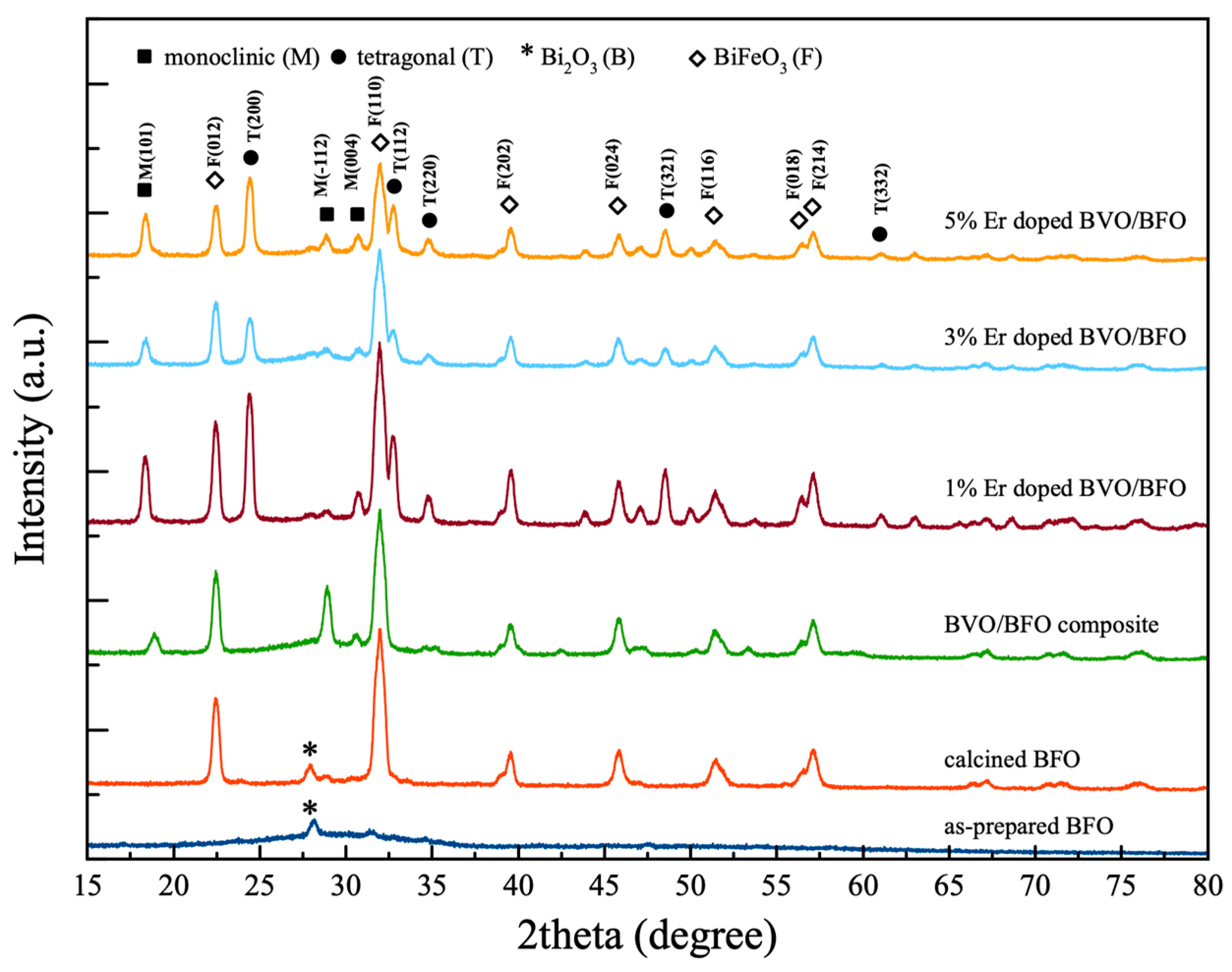
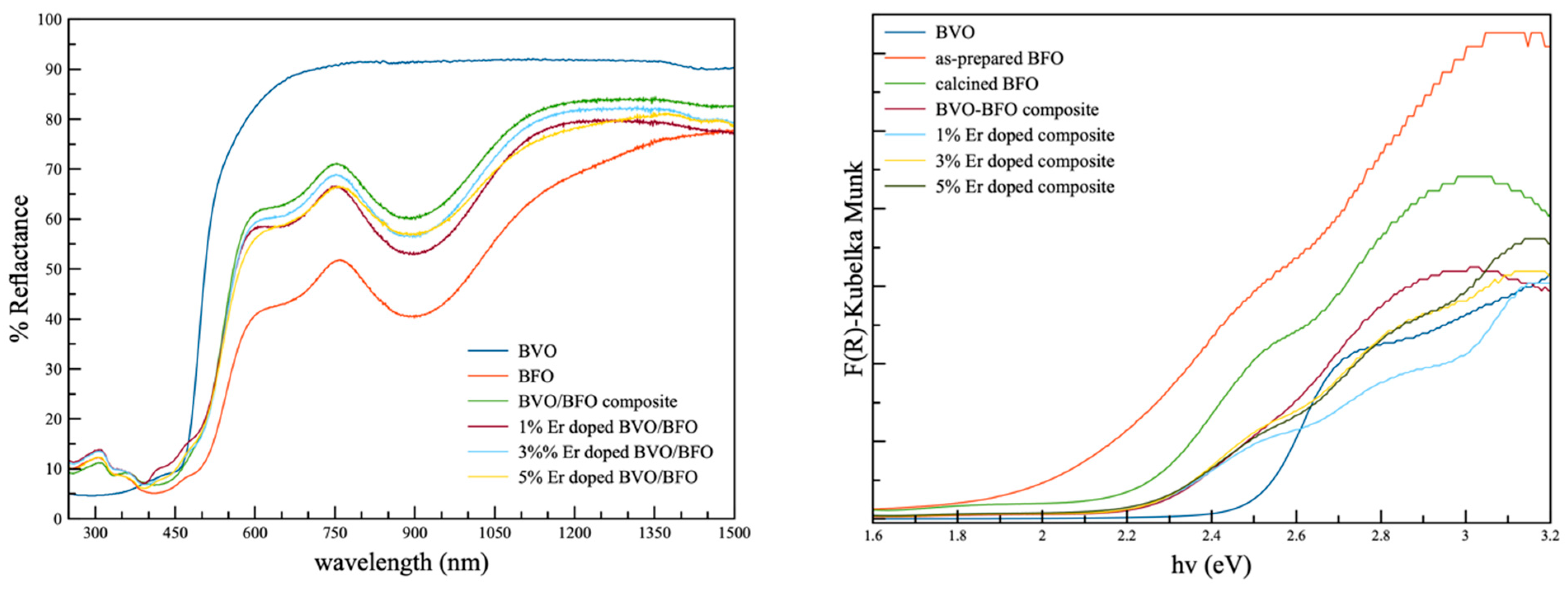
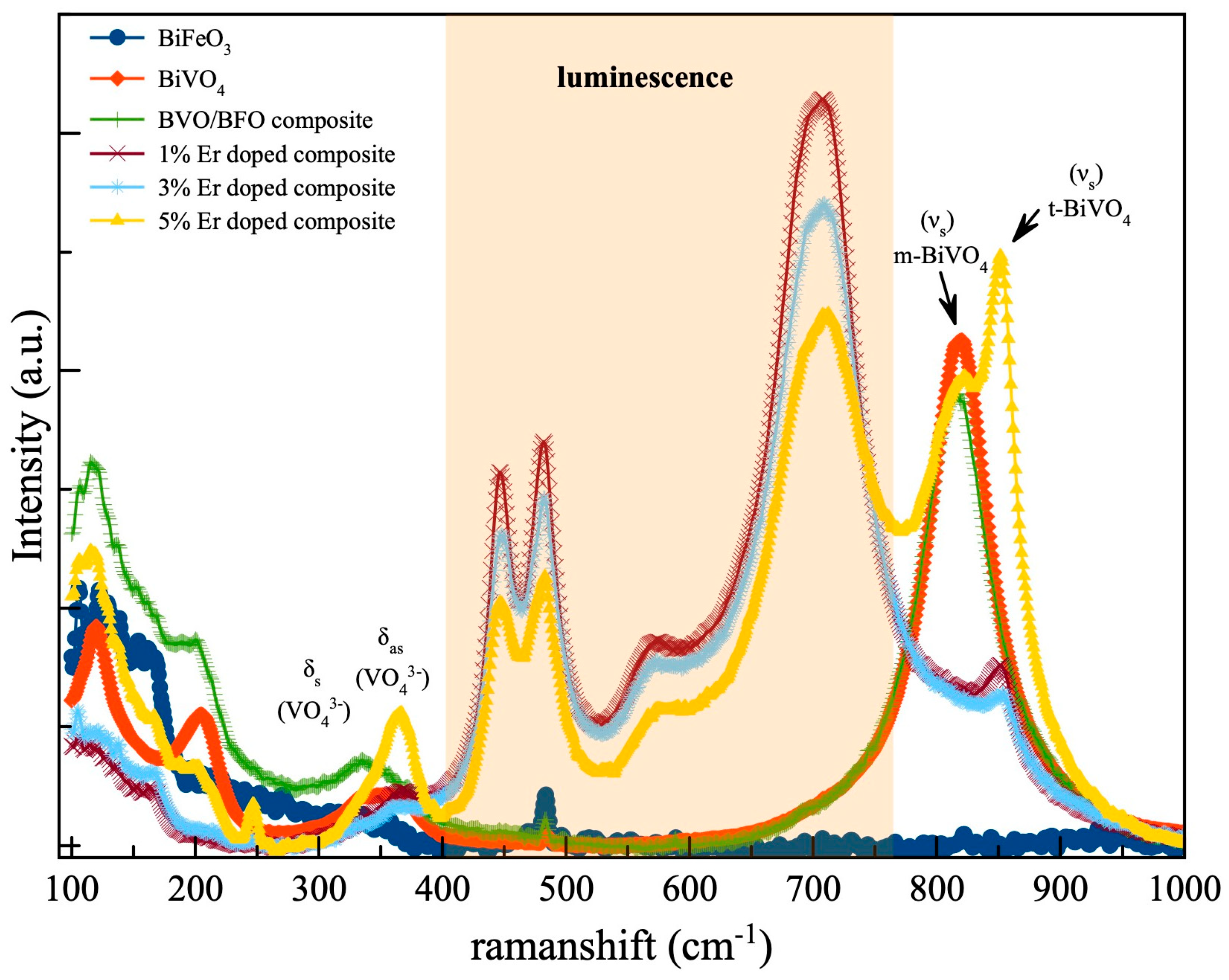
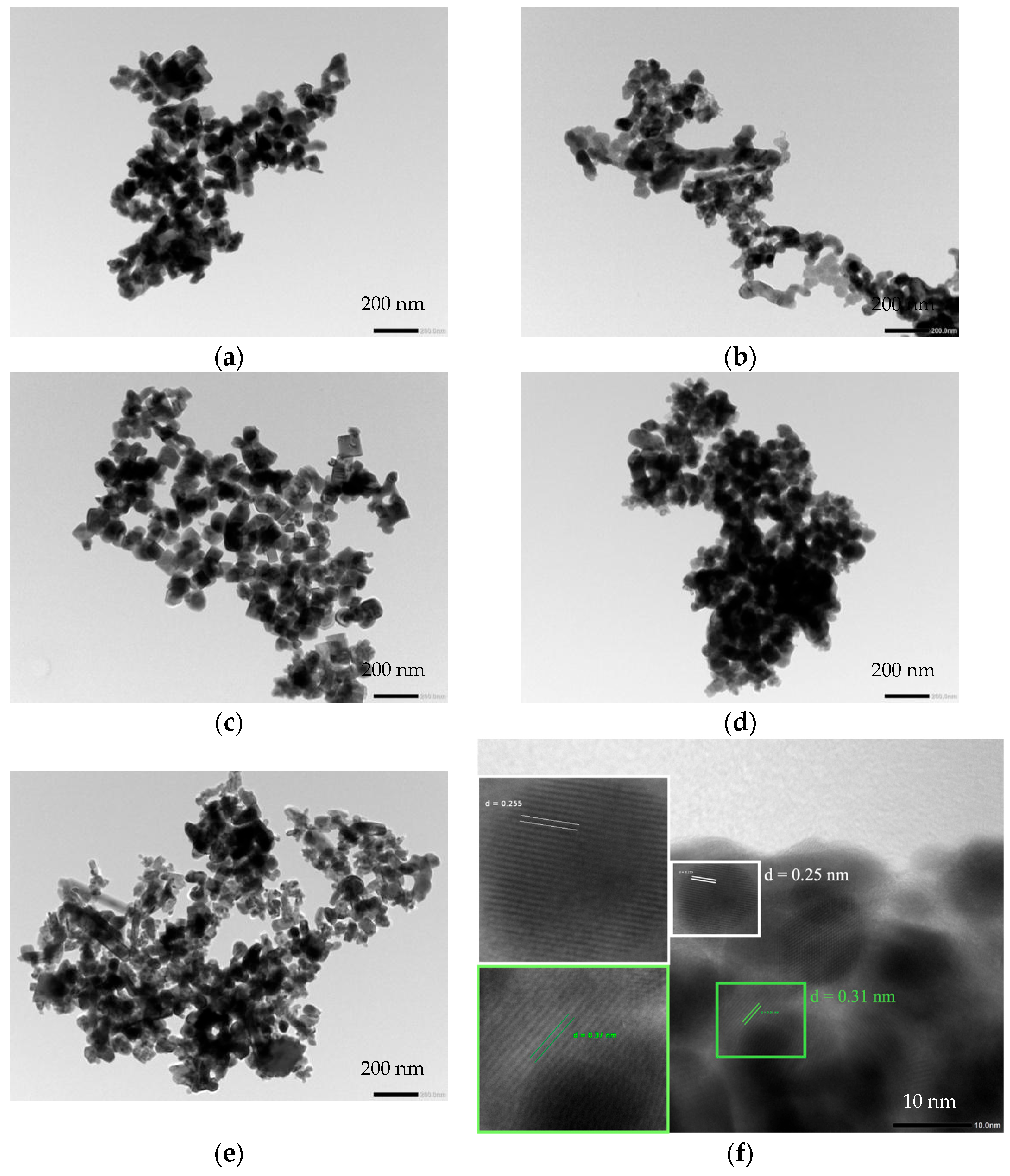



| Samples | Crystalline Size (nm) | Cell Parameters | |||||
|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | α (°) | β (°) | Strain (ε) | ||
| BFO (trigonal) | 16.05 | 3.12 | 3.12 | 3.12 | 156.76 | 156.76 | 1.403 |
| BVO/BFO (trigonal) | 13.81 | 3.11 | 3.11 | 3.11 | 157.26 | 157.26 | 0.085 |
| 1% Er-BVO/BFO (Tetragonal) | 19.21 | 7.29 | 7.29 | 6.44 | 90.00 | 90.00 | 0.281 |
| 3% Er-BVO/BFO (Tetragonal) | 15.83 | 7.29 | 7.29 | 6.44 | 90.00 | 90.00 | 1.983 |
| 5% Er-BVO/BFO (Tetragonal) | 18.59 | 7.31 | 7.31 | 6.42 | 90.00 | 90.00 | 0.764 |
| Sample | Surface Area (m2/g) | Piezocatalyst | Photocatalyst | Piezo-Photocatalyst | |||
|---|---|---|---|---|---|---|---|
| k (min−1) | R2 | k (min−1) | R2 | k (min−1) | R2 | ||
| BFO | 38.41 | 0.0036 | 0.836 | 0.0049 | 0.997 | 0.0117 | 0.961 |
| BVO/BFO composite | 42.35 | 0.0160 | 0.924 | 0.0033 | 0.994 | 0.0201 | 0.993 |
| 1% Er-doped BVO/BFO | 54.97 | 0.0049 | 0.934 | 0.0180 | 0.917 | 0.0484 | 0.824 |
| 3% Er-doped BVO/BFO | 40.21 | 0.0114 | 0.980 | 0.0316 | 0.986 | 0.0332 | 0.963 |
| 5% Er-doped BVO/BFO | 48.32 | 0.0037 | 0.986 | 0.0151 | 0.993 | 0.0202 | 0.977 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kansaard, T.; Songpanit, M.; Noonuruk, R.; Wattanawikkam, C.; Mekprasart, W.; Boonyarattanakalin, K.; Jayasankar, C.K.; Pecharapa, W. Er-Doped BiVO4/BiFeO3 Nanocomposites Synthesized via Sonochemical Process and Their Piezo-Photocatalytic Application. Nanomaterials 2024, 14, 954. https://doi.org/10.3390/nano14110954
Kansaard T, Songpanit M, Noonuruk R, Wattanawikkam C, Mekprasart W, Boonyarattanakalin K, Jayasankar CK, Pecharapa W. Er-Doped BiVO4/BiFeO3 Nanocomposites Synthesized via Sonochemical Process and Their Piezo-Photocatalytic Application. Nanomaterials. 2024; 14(11):954. https://doi.org/10.3390/nano14110954
Chicago/Turabian StyleKansaard, Thanaphon, Maneerat Songpanit, Russameeruk Noonuruk, Chakkaphan Wattanawikkam, Wanichaya Mekprasart, Kanokthip Boonyarattanakalin, Chalicheemalapalli Kulala Jayasankar, and Wisanu Pecharapa. 2024. "Er-Doped BiVO4/BiFeO3 Nanocomposites Synthesized via Sonochemical Process and Their Piezo-Photocatalytic Application" Nanomaterials 14, no. 11: 954. https://doi.org/10.3390/nano14110954







