SnS Quantum Dots Enhancing Carbon-Based Hole Transport Layer-Free Visible Photodetectors
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Materials and Precursors
2.2. Device Fabrication
2.3. Characterization and Device Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koppens, F.H.; Mueller, T.; Avouris, P.; Ferrari, A.C.; Vitiello, M.S.; Polini, M. Photodetectors based on graphene, other two-dimensional materials and hybrid systems. Nat. Nanotechnol. 2014, 9, 780–793. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, J.; Wu, X.; Zhang, G.Q.; Chu, Y.L.; Liu, D.P.; Zhao, Y.X.; Liang, Z.Q.; Jia, H. Ultrasensitive photodetectors based on island structured CH3NH3PbI3 thin films. ACS Appl. Mater. Interfaces 2015, 7, 21634–21642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Y.; Wang, Y.; Zhang, X.; Zuo, C.; Shen, L.; Ding, L. Lead-free perovskite photodetectors: Progress, challenges, and opportunities. Adv. Mater. 2021, 33, 2006691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, H.; Chen, Y.; Zeng, Z.; Huang, L.; Wang, C.; Dong, K.; Hu, Z.; Ke, W.; Fang, G. Spontaneous crystallization of strongly confined CsSnxPb1-xI3 perovskite colloidal quantum dots at room temperature. Nat. Commun. 2024, 15, 1609. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Ma, Q.L.; Zhai, B.G. Development of porous silicon based visible light photodetectors. Key Eng. Mater. 2013, 538, 341–344. [Google Scholar] [CrossRef]
- Chen, Z.L.; Li, C.L.; Zhumekenov, A.A.; Zheng, X.P.; Yang, C.; Yang, H.Z.; He, Y.; Turedi, B.; Mohammed, O.F.; Shen, L.; et al. Solution-processed visible-blind ultraviolet photodetectors with nanosecond response time and high detectivity. Adv. Opt. Mater. 2019, 7, 1900506. [Google Scholar] [CrossRef]
- Dias, S.; Kumawat, K.L.; Krupanidhi, S.B. CH3NH3PbBr3 quantum dots for visible wavelength photodetector applications. J. Mater. Sci. Mater. Electron. 2019, 30, 3061–3068. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Huang, L.; Sun, W. Research progress of high-sensitivity perovskite photodetectors: A review of photodetectors: Noise, structure, and materials. ACS Appl. Electron. Mater. 2022, 4, 1485–1505. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, F.; Kim, H.B.; Wang, C.F.; Cho, E.; Konefal, R.; Puttisong, Y.; Terado, K.; Gao, F. Ion-modulated radical doping of spiro-OMeTAD for more efficient and stable perovskite solar cells. Science 2022, 377, 495–501. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Guo, X.; Lu, C.; Wei, J.; Fang, J. Constructing heterojunctions by surface sulfidation for efficient inverted perovskite solar cells. Science 2022, 375, 434–437. [Google Scholar] [CrossRef]
- Wu, S.; Chen, R.; Zhang, S.; Babu, B.H.; Yue, Y.F.; Zhu, H.M.; Yang, Z.C.; Chen, C.L.; Chen, W.T.; Huang, Y.Q.; et al. A chemically inert bismuth interlayer enhances long-term stability of inverted perovskite solar cells. Nat. Commun. 2019, 10, 1161. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Yavuz, I.; De Marco, N.; Huang, T.Y.; Lee, S.; Choi, C.S.; Wang, M.H.; Nuryyeva, S.; Wang, R.; Zhao, Y.P.; et al. Steric impediment of ion migration contributes to improved operational stability of perovskite solar cells. Adv. Mater. 2020, 32, 1906995. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, A.; Yao, K.; Jen, A.K.Y. Toward perovskite solar cell commercialization: A perspective and research roadmap based on interfacial engineering. Adv. Mater. 2018, 30, 1800455. [Google Scholar] [CrossRef] [PubMed]
- Ku, Z.; Rong, Y.; Xu, M.; Liu, T.; Han, H. Full printable processed mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells with carbon counter electrode. Sci. Rep. 2013, 3, 3132. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Mei, A.; Liu, S.; Duan, M.; Jiang, P.; Tian, C.B.; Ong, Y.; Rong, Y.G.; Han, H.W.; Hu, T. Mixed (5-AVA)xMA1−xPbI3−y(BF4)y perovskites enhance the photovoltaic performance of hole- conductor-free printable mesoscopic solar cells. J. Mater. Chem. A 2018, 6, 2360–2364. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y.; Lan, K.; Mei, A.; Sheng, Y.; Zhao, D.; Han, H. Fully printable hole-conductor-free mesoscopic perovskite solar cells based on mesoporous anatase single crystals. New J. Chem. 2018, 42, 2669–2674. [Google Scholar] [CrossRef]
- You, S.; Wang, H.; Bi, S.Q.; Zhou, J.Y.; Qin, L.; Qiu, X.H.; Zhao, Z.Q.; Xu, Y.; Zhang, Y.; Shi, X.H.; et al. A Biopolymer Heparin Sodium Interlayer Anchoring TiO2 and MAPbI3 Enhances Trap Passivation and Device Stability in Perovskite Solar Cells. Adv. Mater. 2018, 30, 1706924. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Lu, X.Y.; Mei, Y.T.; Dong, C.; Gangadharan, D.T.; Liu, K.; Wang, Z.J.; Qu, S.C.; Saidaminov, M.I.; Zhang, W.F. Blade-coated carbon electrode perovskite solar cells to exceed 20% efficiency through protective buffer layers. Adv. Funct. Mater. 2023, 33, 2301920. [Google Scholar] [CrossRef]
- Chen, K.; Xiao, X.F.; Liu, J.L.; Qi, J.H.; Gao, Q.J.; Ma, Y.M.; Cheng, Y.J.; Mei, A.Y.; Han, H.W. Record-efficiency printable hole-conductor-free mesoscopic perovskite solar cells enabled by the multifunctional schiff base derivative. Adv. Mater. 2024, 3, 2401319. [Google Scholar] [CrossRef]
- Binyamin, T.; Etgar, L. Ways to improve the performance of triple-mesoscopic hole-conductor-free perovskite-based solar cells. Sol. RRL 2022, 6, 2200295. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Mo, H.; Cheng, D.; Liu, X.; Liu, W.; Jacobs, J.; Thomas, A.G.; Liu, Z.; Curry, R.J. In situ laser generation of NiOx nanoparticles embedded in graphene flakes for ambient-processed hole-transport-layer-free perovskite solar cells. Carbon 2023, 214, 118360–118370. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wu, Z.; Zhang, R.; Hong, J.; Zhang, J.; Rao, H.; Pan, Z.; Zhong, X. Self-driven prenucleation-induced perovskite crystallization enables efficient perovskite solar cells. Angew. Chem. Int. Ed. 2023, 62, 202302342. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yong, K.T.; Roy, I.; Ohulchanskyy, T.Y.; Bergey, E.J.; Lee, H.H.; Tramposch, K.M.; He, S.L.; Maitra, A.; Prasad, P.N. Imaging pancreatic cancer using surface-functionalized quantum dots. J. Phys. Chem. B 2007, 111, 6969–6972. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Kim, J.Y.; Seo, H.W.; Lim, K.M.; Ko, M.J.; Seong, T.Y.; Kim, B.S. Characteristics of gradient-interface-structured ZnCdSSe quantum dots with modified interface and its application to quantum-dot-sensitized solar cells. Appl. Surf. Sci. 2018, 429, 16–22. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Li, S.; Li, T.; Chen, P.; Zhao, Q.; Li, G. Quantum dot bridges enabling highly efficient carbon-based hole transport material-free perovskite solar cells. Sol. RRL 2023, 7, 2300606. [Google Scholar] [CrossRef]
- Zhou, H.P.; Chen, M.W.; Liu, C.G.; Zhang, R.; Li, J.; Liao, S.N.; Lu, H.F.; Yang, Y.P. Interfacial passivation of CsPbI3 quantum dots improves the performance of hole-transport-layer-free perovskite photodetectors. Discover Nano 2023, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, S.N.; Wall, J.; Li, L.; Xiong, G.; Zhang, Q.; Yan, F. Low-temperature processed highly efficient hole transport layer free carbon-based planar perovskite solar cells with SnO2 quantum dot electron transport layer. Mater. Today Phys. 2020, 13, 100204. [Google Scholar] [CrossRef]
- Qi, X.; Wang, J.; Tan, F.; Dong, C.; Liu, K.; Li, X.; Zhang, L.; Wu, H.; Wang, H.L.; Qu, S.; et al. Quantum dot interface-mediated CsPbIBr2 film growth and passivation for efficient carbon-based solar cells. ACS Appl. Mater. Interfaces 2021, 13, 55349–55357. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; He, B.; Ti, J.; Sun, S.; Jiao, W.; Chen, H.; Duan, Y.; Yang, P.; Tang, Q. Sulfur vacancy defects healing of WS2 quantum dots boosted hole extraction for all-inorganic perovskite solar cells. Chem. Eng. J. 2023, 455, 140728. [Google Scholar] [CrossRef]
- Xu, Y.; Al-Salim, N.; Bumby, C.W.; Tilley, R.D. Synthesis of SnS Quantum Dots. J. Am. Chem. Soc. 2009, 131, 15990–15999. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, Z.; Zhou, X.Q. Synthesis and photoluminescence of ZnS quantum dots. Nanosci. Nanotechnol. Lett. 2008, 8, 1312–1315. [Google Scholar]
- Li, Y.; Wang, Z.W.; Ren, D.; Liu, Y.; Zheng, A.; Zakeeruddin, S.M.; Dong, X.; Hagfeldt, A.; Gratzel, M. SnS quantum dots as hole transporter of perovskite solar cells. ACS Appl. Electron. Mater. 2019, 2, 3822–3829. [Google Scholar] [CrossRef]
- Rath, J.K.; Prastani, C.; Nanu, D.E.; Nanu, N.; Schropp, R.; Vetushka, A.; Hyvl, M.; Fejfar, A. Fabrication of SnS quantum dots for solar-cell applications: Issues of capping and doping. Phys. Status Solidi B 2014, 251, 1309–1321. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Gui, P.B.; Grice, C.R.; Song, Z.H.; Wang, H.; Fang, G.J. Ga-doped ZnO nanorod scaffold for high-performance, hole-transport-layer free, self-powered CH3NH3PbI3 perovskite photodetectors. Sol. Energy Mater. Sol. Cells 2019, 193, 246–252. [Google Scholar] [CrossRef]
- Zhang, T.; Ren, Z.Y.; Guo, S.Y.; Zhang, G.J.; Wang, S.F.; Qiao, S. Broadband Self-Powered CdS ETL-Based MAPbI3 Heterojunction Photodetector Induced by a Photovoltaic−Pyroelectric−Thermoelectric Effect. ACS Appl. Mater. Interfaces 2023, 15, 44444–44455. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Liu, X.Y.; Sun, B.; Tan, X.H.; Ye, H.B.; Zhou, J.X.; Tang, Z.R.; Shi, T.L.; Liao, G.L. A Cu-doping strategy to enhance photoelectric performance of self-powered hole-conductor-free perovskite photodetector for optical communication applications. Adv. Mater. Technol. 2020, 5, 2000260. [Google Scholar] [CrossRef]
- Li, Z.B.; Li, H.N.; Jiang, K.; Ding, D.; Li, J.N.; Ma, C.; Jiang, S.C.; Wang, Y.; Anthopoulos, T.D.; Shi, Y.M. Self-powered perovskite/CdS heterostructure photodetectors. ACS Appl. Mater. Interfaces 2019, 11, 40204–40213. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.; Madhusudanan, S.; Pathak, A.; Jayasankar, P.M.; Batabyal, S.K. Carbon assisted methylammonium lead iodide microcrystalline device for an inexpensive self-powered photodetector. Mater. Lett. 2019, 254, 428–432. [Google Scholar] [CrossRef]
- Luo, X.H.; He, Z.Y.; Meng, R.W.; Zhang, C.; Chen, M.; Lu, H.; Yang, Y. SnS quantum dots with different sizes in active layer for enhancing the performance of perovskite solar cells. Appl. Phys. A 2021, 127, 8396–8417. [Google Scholar] [CrossRef]
- Burton, L.A.; Walsh, A. Phase stability of the earth-abundant tin sulfides SnS, SnS2, and Sn2S3. J. Phys. Chem. C 2012, 116, 24262. [Google Scholar] [CrossRef]
- Lee, U.G.; Kim, W.B.; Han, D.H.; Chung, H.S. A modified equation for thickness of the film fabricated by spin coating. Symmetry 2019, 11, 1183. [Google Scholar] [CrossRef]
- Emslie, A.G.; Bonner, F.T.; Peck, L.G. Flow of a viscous liquid on a rotating disk. J. Appl. Phys. 1958, 29, 858–862. [Google Scholar] [CrossRef]
- Luo, X.H. Study on the Effect of SnS Quantum Dots Doped Active Layer on the Photovoltaic Performance of Photovoltaic Cells. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2021. [Google Scholar]
- Shin, D.H.; Shin, S.H.; Lee, S.G.; Kim, S.; Choi, S.H. High-detectivity/-speed flexible and self-powered graphene quantum dots/perovskite photodiodes. ACS Sustain. Chem. Eng. 2019, 7, 19961–19968. [Google Scholar] [CrossRef]
- Rakstys, K.; Abate, A.; Dar, M.I.; Gao, P.; Jankauskas, V.; Jacopin, G.; Kamarauskas, E.; Kazim, S.; Ahmad, S.; Gratzel, M.; et al. Triazatruxene-based hole transporting materials for highly efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 16172–16178. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Duan, C.; Du, H.; Zeng, S.; Kong, A.; Chen, Y.; Peng, Y.; Cheng, Y.B.; Ku, Z. A stress relaxation strategy for preparing high-quality organic–inorganic perovskite thin films via a vapor–solid reaction. J. Mater. Chem. A 2023, 11, 23387–23396. [Google Scholar] [CrossRef]
- Gao, Z.W.; Wang, Y.; Choy, W.C.H. Buried Interface Modification in Perovskite Solar Cells: A Materials Perspective. Adv. Energy Mater. 2022, 12, 2104030. [Google Scholar] [CrossRef]
- Park, Y.D.; Lim, J.A.; Lee, H.S.; Cho, K. Interface engineering in organic transistors. Mater. Today 2007, 10, 46–54. [Google Scholar] [CrossRef]
- Ravi, V.K.; Markad, G.B.; Nag, A. Band edge energies and excitonic transition probabilities of colloidal CsPbX3 (X = Cl, Br, I) perovskite nanocrystals. ACS Energy Lett. 2016, 1, 665–671. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xu, J.L.; Chen, T.; Gao, X.; Wang, S.D. Toward broadband imaging: Surface-engineered PbS quantum dot/perovskite composite integrated ultrasensitive photodetectors. ACS Appl. Mater. Interfaces 2019, 11, 44430–44437. [Google Scholar] [CrossRef]
- Bao, C.; Yang, J.; Bai, S.; Xu, W.; Yan, Z.; Xu, Q.; Liu, J.; Zhang, W.; Gao, F. High performance and stable all-inorganic metal halide perovskite-based photodetectors for optical communication applications. Adv. Mater. 2018, 30, 1803422. [Google Scholar] [CrossRef]
- Sulaman, M.; Yang, S.; Bukhtiar, A.; Tang, P.Y.; Zhang, Z.H.; Song, Y.; Imran, A.; Jiang, Y.R.; Cui, Y.Y.; Tang, L.B.; et al. Hybrid bulk-heterojunction of colloidal quantum dots and mixed-halide perovskite nanocrystals for high performance self-powered broadband photodetectors. Adv. Funct. 2022, 32, 2201527. [Google Scholar] [CrossRef]
- Poli, I.; Liang, X.; Baker, R.; Eslava, S.; Cameron, P. Enhancing the hydrophobicity of perovskite solar cells using C18 capped CH3NH3PbI3 nanocrystals. J. Phys. Chem. C 2018, 6, 7149–7156. [Google Scholar] [CrossRef]
- Spalatu, N.; Hiie, J.; Kaupmees, R.; Volobujeva, O.; Krustok, J.; Acik, I.O.; Krunks, M. Postdeposition processing of SnS thin films and solar cells: Prospective strategy to obtain large, sintered, and doped SnS grains by recrystallization in the presence of a metal halide flux. ACS Appl. Mater. Interfaces 2019, 11, 17539–17554. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Qi, Y.; Zhang, Q.; Box, D.; Williams, K.; Tatum, J.; Das, P.; Ranjan, N.; Dai, Q. Enhanced Moisture and Water Resistance in Inverted Perovskite Solar Cells by Poly(3-hexylthiophene). ACS Appl. Energy Mater. 2021, 4, 1815–1823. [Google Scholar] [CrossRef]
- Ghosh, J.; Natu, G.; Giri, P.K. Plasmonic hole-transport-layer enabled self-powered hybrid perovskite photodetector using a modifed perovskite deposition method in ambient air. Org. Electron. 2019, 71, 175–184. [Google Scholar] [CrossRef]
- Yeddu, V.; Seo, G.; Bamidele, M.; Jung, H.; Yu, H.; Lee, J.W.; Lee, J.; Kim, D.Y. High-detectivity UV−VIS−NIR broadband perovskite photodetector using a mixed Pb−Sn narrow-band-gap absorber and a NiOx electron blocker. ACS Appl. Electron. Mater. 2022, 4, 1206–1213. [Google Scholar] [CrossRef]
- He, Z.; Yang, Y.; Liu, J.; Li, J.; Lu, X.; Zou, J.; Yang, S.; Zou, Y. Antisolvent-treated MAPbI3 and PbS quantum dots for high performance broadband photodetectors. ACS Appl. Nano Mater. 2023, 6, 1119–1128. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, C.; Zhang, X.; Wang, K.; Fu, J.; Guo, J.; Wang, S.; Zhao, Q.; Ma, W.; Yuan, J. Controllable colloidal synthesis of MAPbI3 perovskite nanocrystals for dual-mode optoelectronic applications. Nano Lett. 2023, 23, 9143–9150. [Google Scholar] [CrossRef]
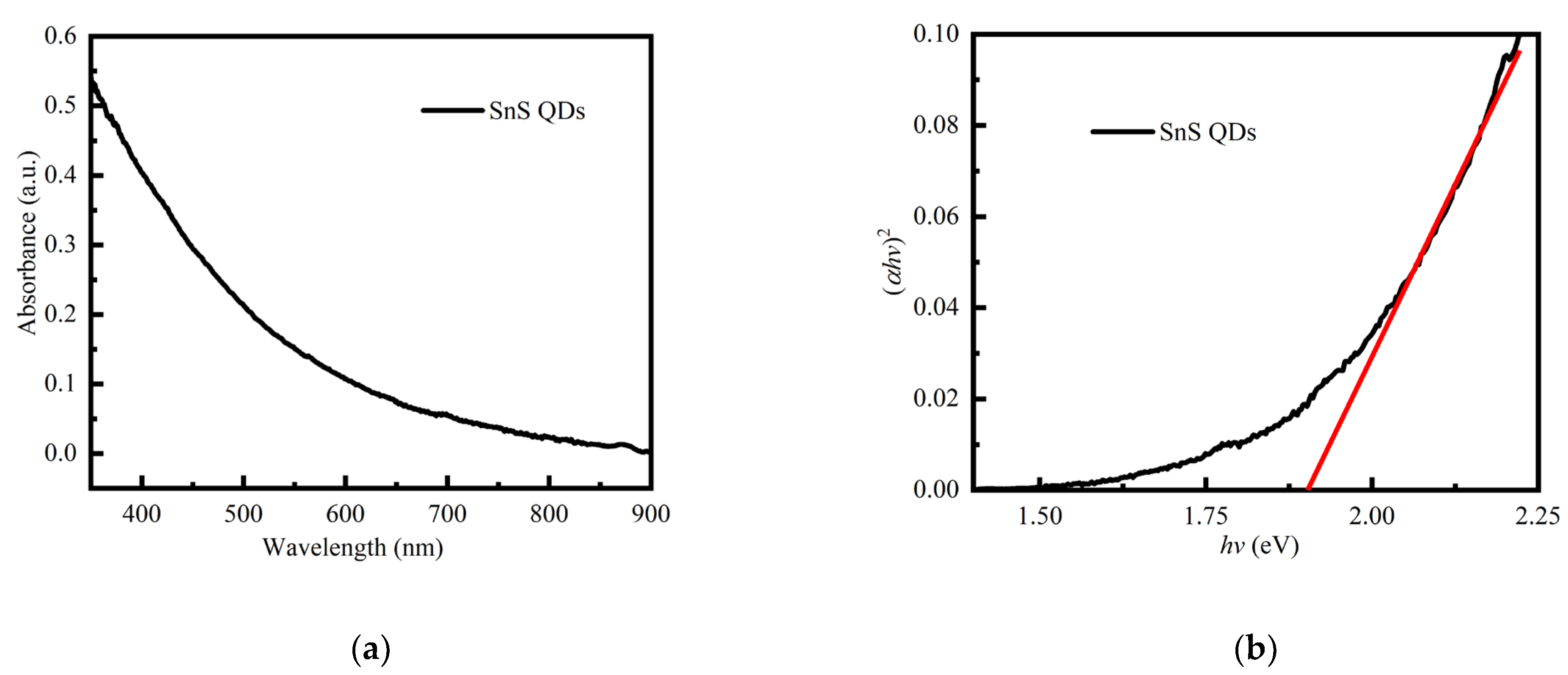
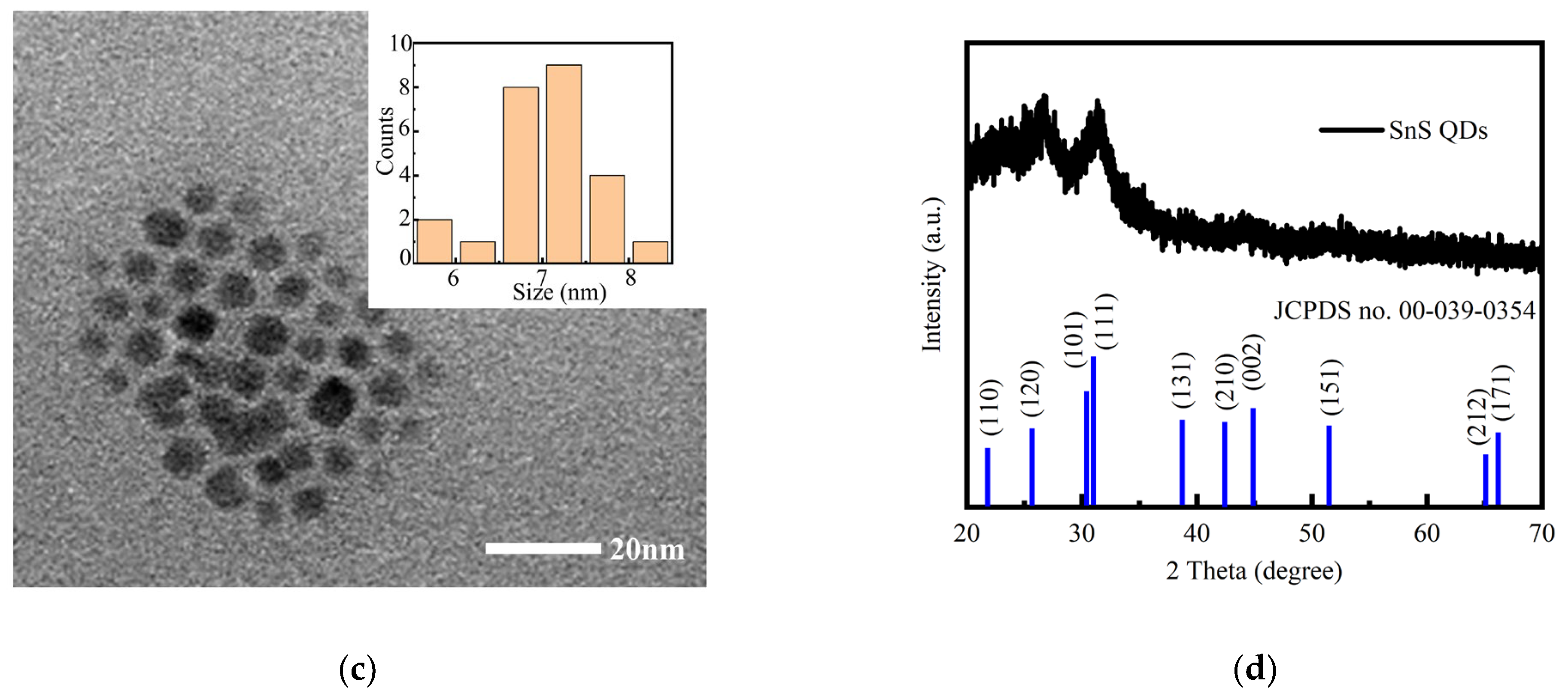
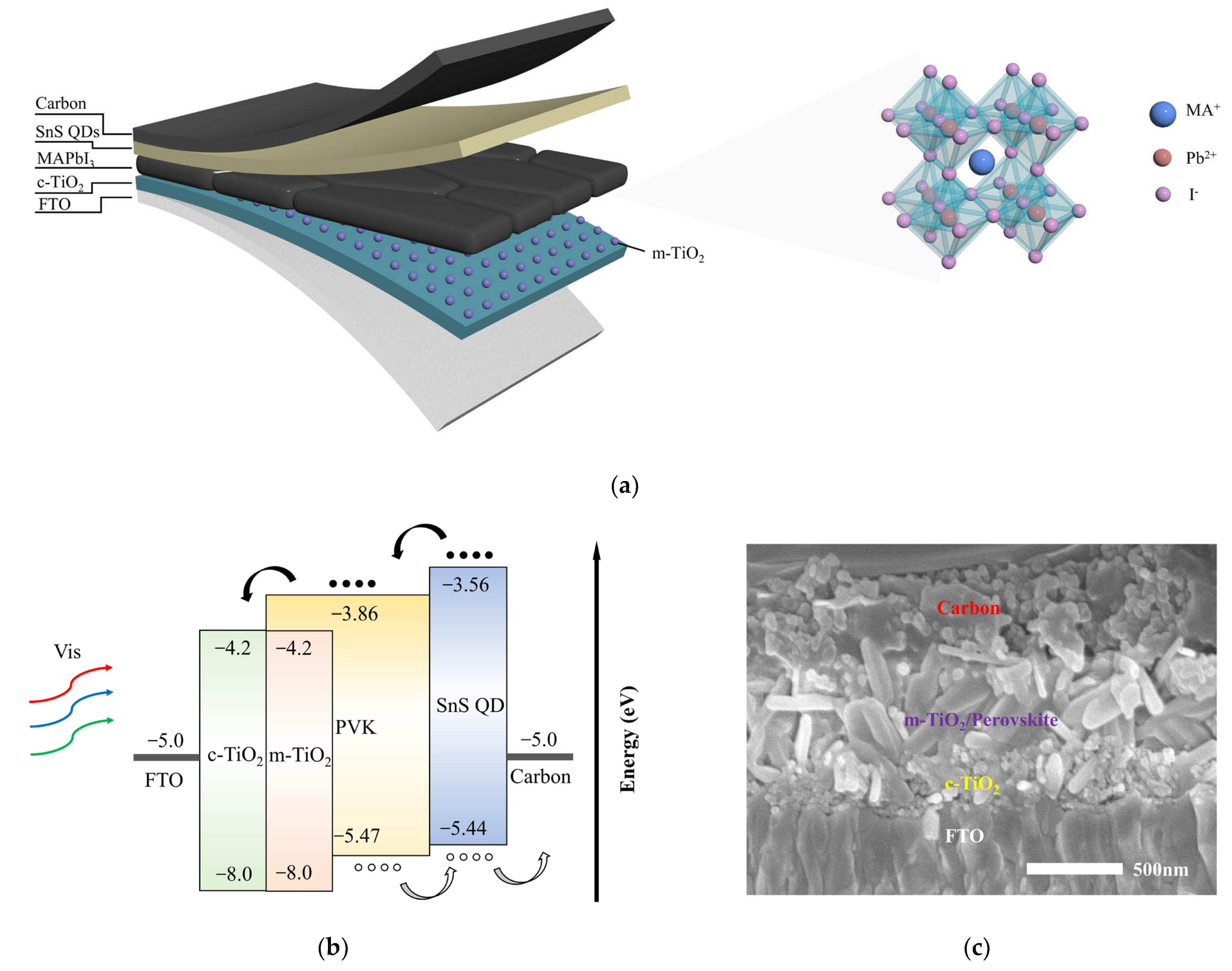
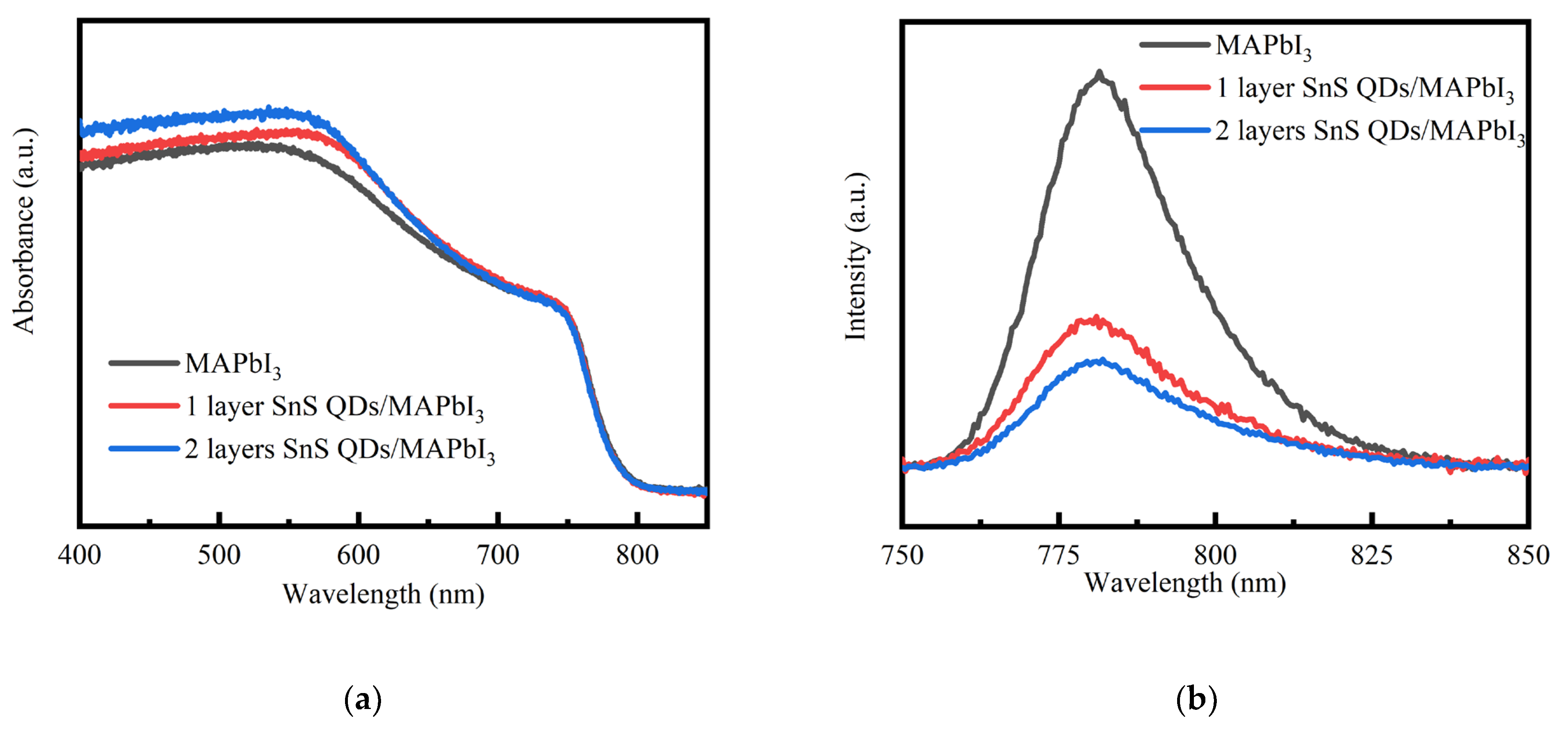
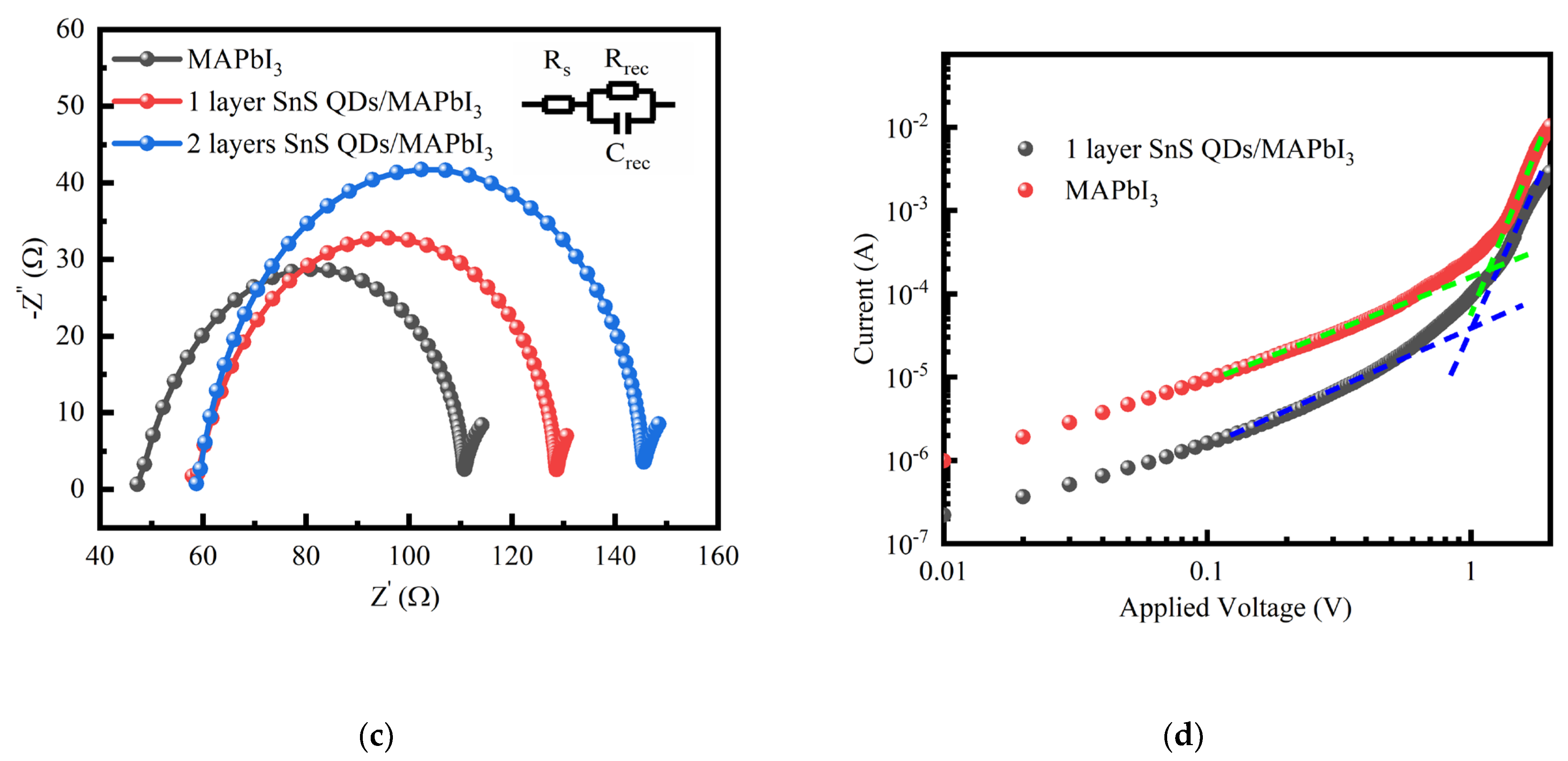
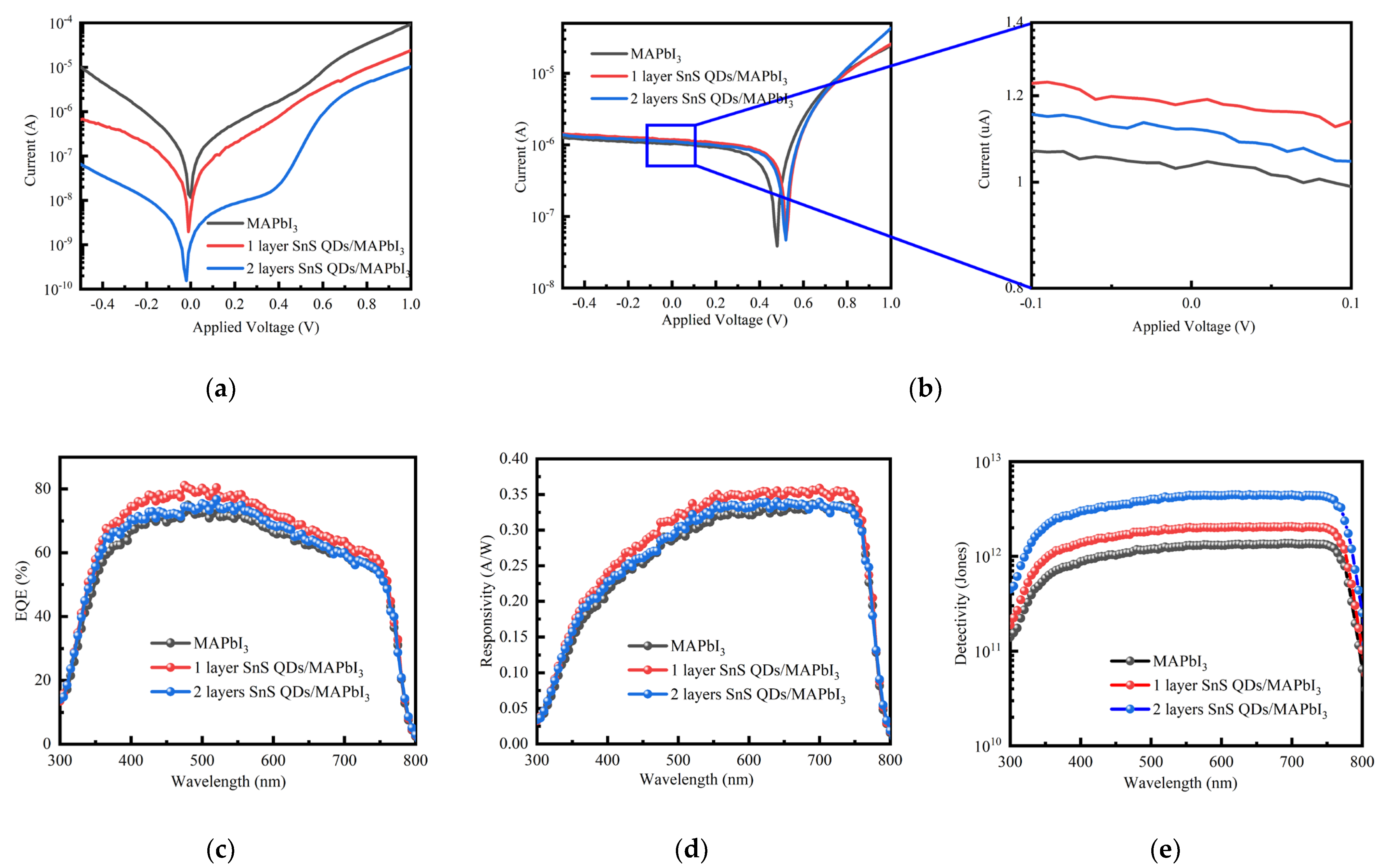

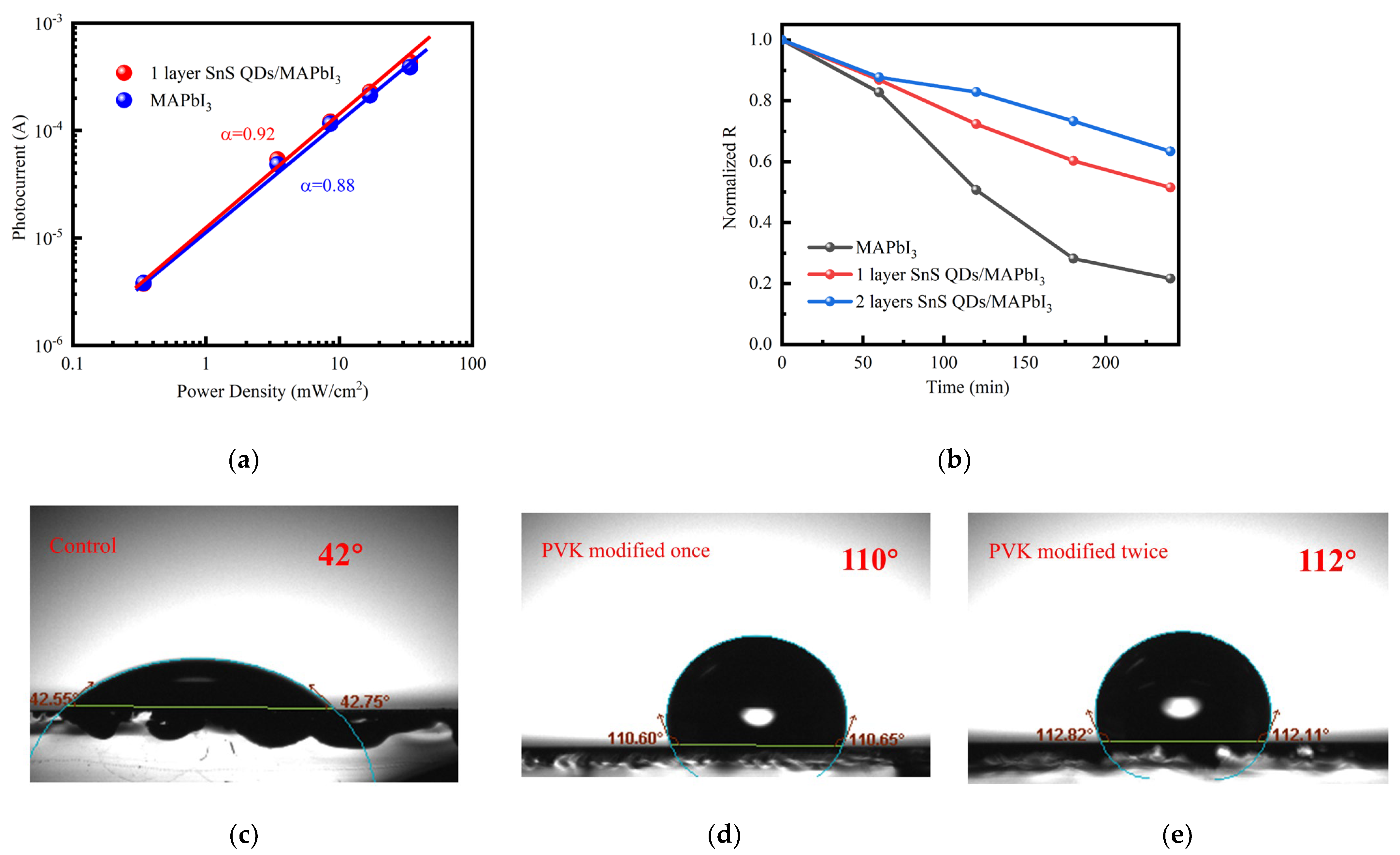
| Device Structure | R(A/W) 500 nm | D* (Jones) | τrise/τfall | Ref. |
|---|---|---|---|---|
| FTO/PEDOT: PSS + Ag NPs/MAPbI3/Al | 0.25 | 1.5 × 1011 | 110 ms/72 ms | [56] |
| ITO/NiOx/Pb-Sn Perovskite/C60/TmPyPB/Ag | 0.32 | >1 × 1012 | 14 μs/14 μs | [57] |
| ITO/NiOx/PbS QDs/MAPbI3/PC61BM/Ag | 0.34 | 5.6 × 1011 | 3.5 μs/35 μs | [58] |
| FTO/TiO2/MAPbI3 PNCs/PTAA/MoO3/Ag | 0.27 | 2 × 1011 | 46 ms/47 ms | [59] |
| FTO/TiO2/m-TiO2/MAPbI3/SnS QDs/Carbon | 0.32 | 1.9 × 1012 | 47 ms/43 ms | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Li, J.; Liao, S.; Huang, S.; Shen, C.; Chen, M.; Yang, Y. SnS Quantum Dots Enhancing Carbon-Based Hole Transport Layer-Free Visible Photodetectors. Nanomaterials 2024, 14, 956. https://doi.org/10.3390/nano14110956
Zhang R, Li J, Liao S, Huang S, Shen C, Chen M, Yang Y. SnS Quantum Dots Enhancing Carbon-Based Hole Transport Layer-Free Visible Photodetectors. Nanomaterials. 2024; 14(11):956. https://doi.org/10.3390/nano14110956
Chicago/Turabian StyleZhang, Rui, Jing Li, Sainan Liao, Shuxin Huang, Chenguang Shen, Mengwei Chen, and Yingping Yang. 2024. "SnS Quantum Dots Enhancing Carbon-Based Hole Transport Layer-Free Visible Photodetectors" Nanomaterials 14, no. 11: 956. https://doi.org/10.3390/nano14110956





