Comparative Study of Callistemon citrinus (Bottlebrush) and Punica granatum (Pomegranate) Extracts for Sustainable Synthesis of Silver Nanoparticles and Their Oral Antimicrobial Efficacy
Abstract
1. Introduction
2. Materials and Methods
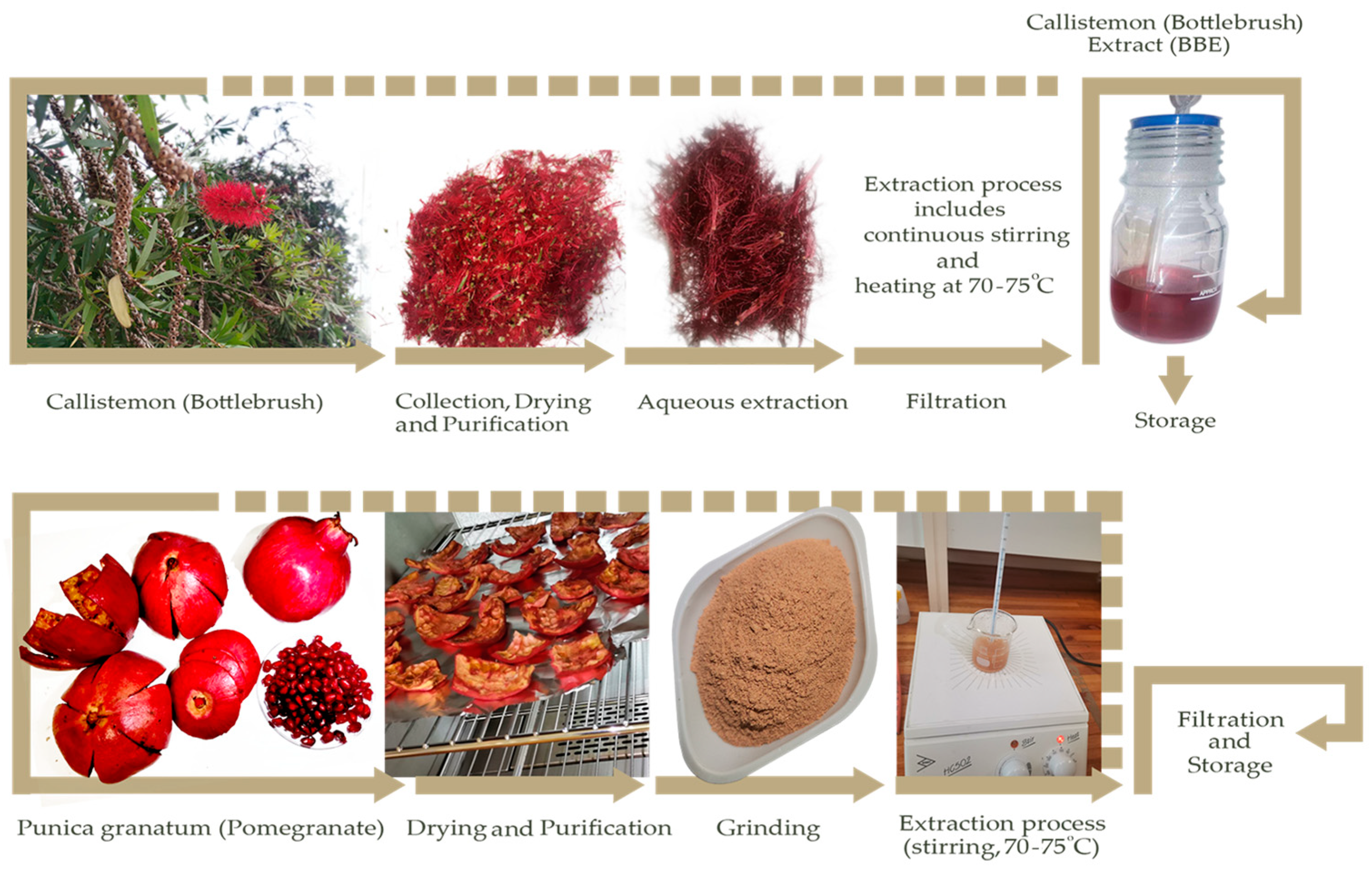
2.1. Extraction Process
2.2. Plant-Mediated Synthesis of Silver Nanoparticles
- (a)
- Plant concentration: Different serial dilutions of PPE were applied.
- (b)
- Volume ratio: three different ratios of extract: Ag precursor (1:1, 1:2, 2:1) were applied.
- (c)
- Reaction time: Different time intervals, including 0 h, 0.5 h, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 3.5 h, and 4 h were applied. The selected optimal conditions for the green synthesis of Ag NPs using PPE were then applied for Ag NP synthesis using BBE. The green-synthesized silver nanoparticles, Ag-BBE and Ag-PPE, were then analyzed using different characterization techniques to analyze their different characteristics and properties. The optical properties of the synthesized samples were evaluated using UV-Vis spectroscopy (SPECTROstar Nano 2450, BMG LABTECH, Offenburg, Germany). Their crystallographic properties were studied using an x-ray diffractometer (Rigaku Smartlab, Tokyo, Japan) at 1.5406 Å, Cu Kα1 line). The morphological properties were studied through a high-resolution transmission electron microscope (HRTEM, JOEL-JEM 2100, Tokyo, Japan). Furthermore, the vibrational studies of the green-synthesized Ag NPs were examined using XploRA Plus Raman spectroscopy (Jobin–Yvon T64000, Palaiseau, France) via an Argon ion laser line of 514.5 nm. The antimicrobial properties were evaluated using an antimicrobial activity test and statistical analysis.
2.3. Antimicrobial Activity Test
3. Results and Discussion
3.1. Screening Step and Optimization
3.2. UV-Vis Spectroscopy Analysis (Optical Properties)
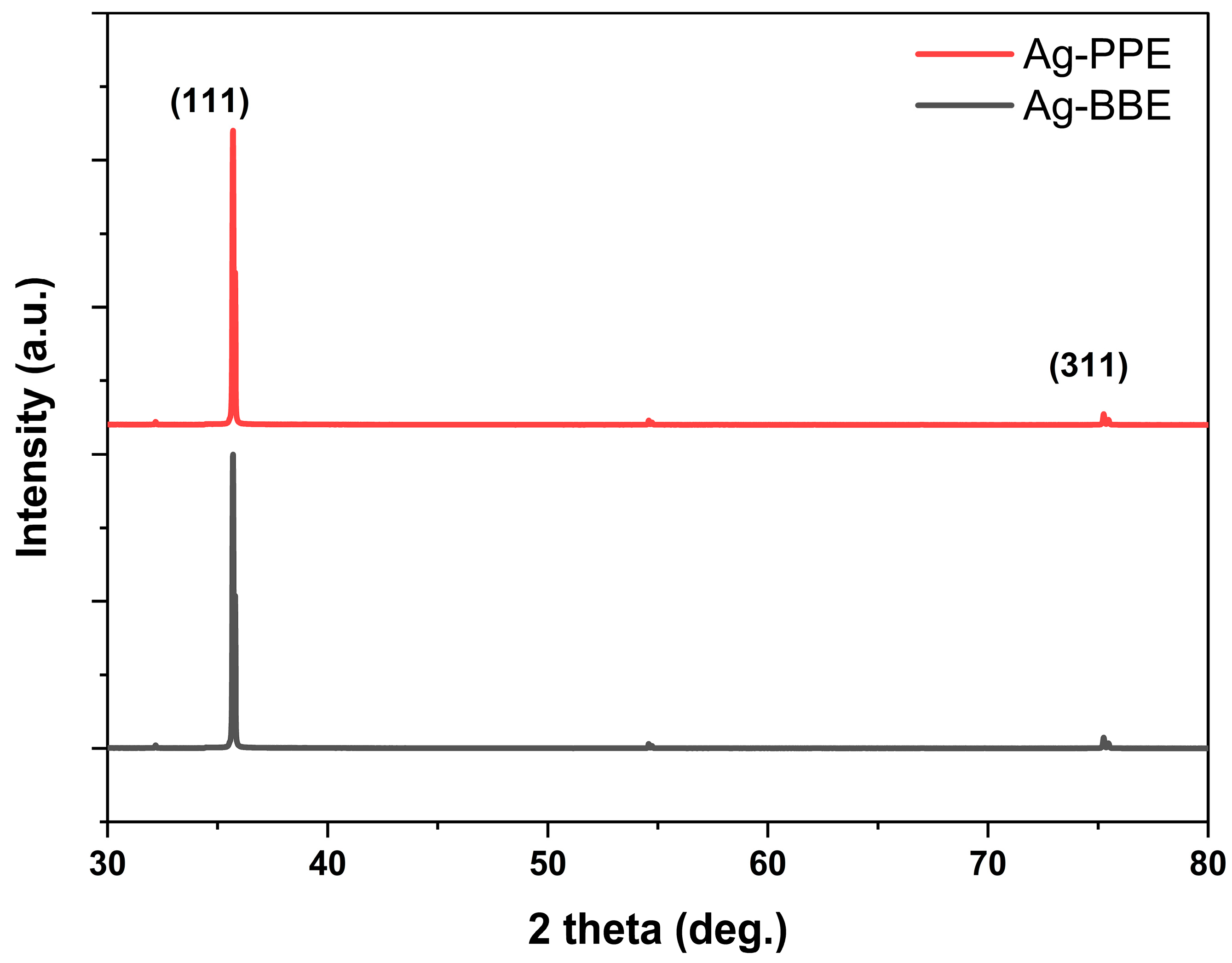
3.3. X-ray Diffraction (XRD) Analysis (Crystallographic Properties)
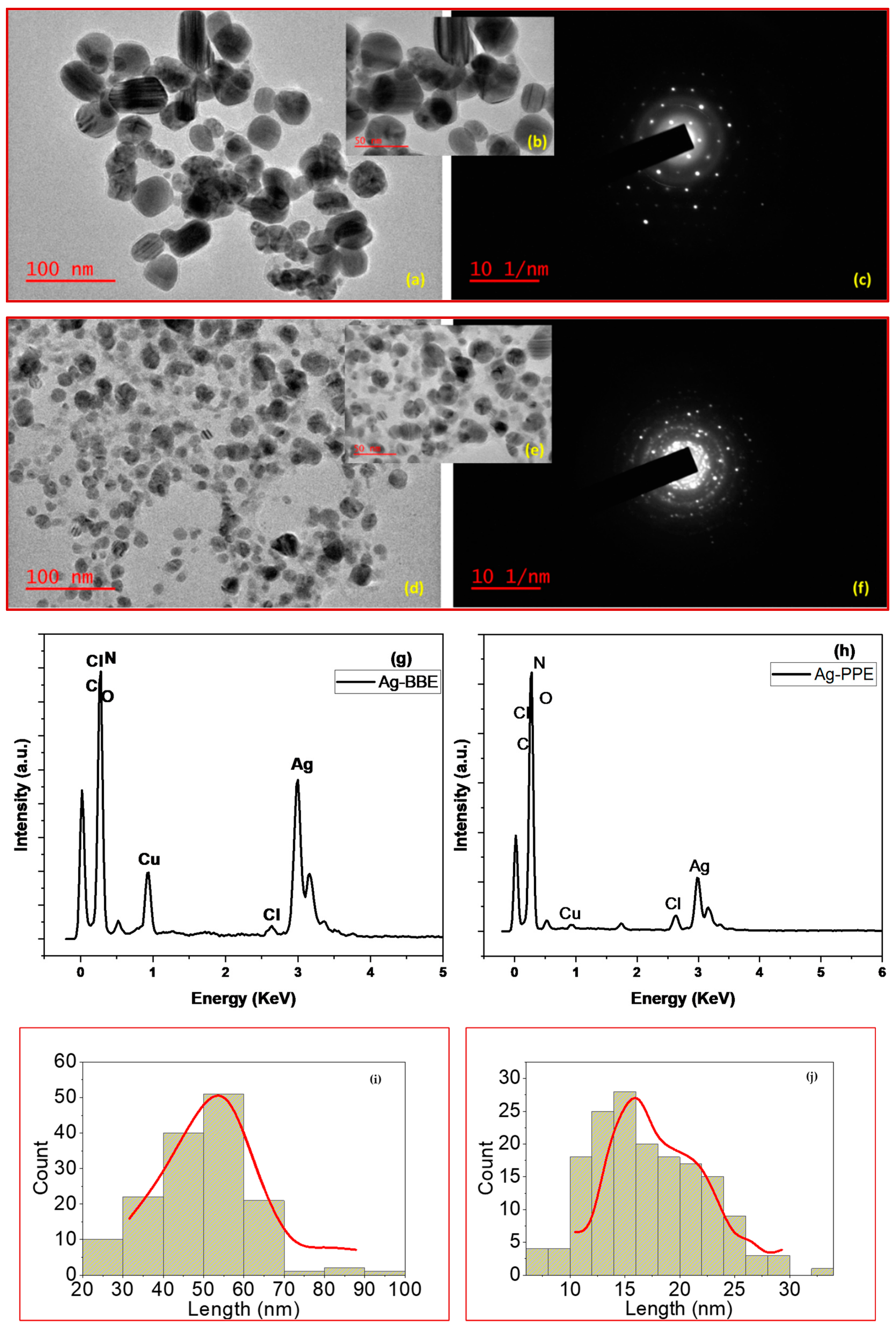
3.4. Transmission Electron Microscope (TEM): Morphological Properties
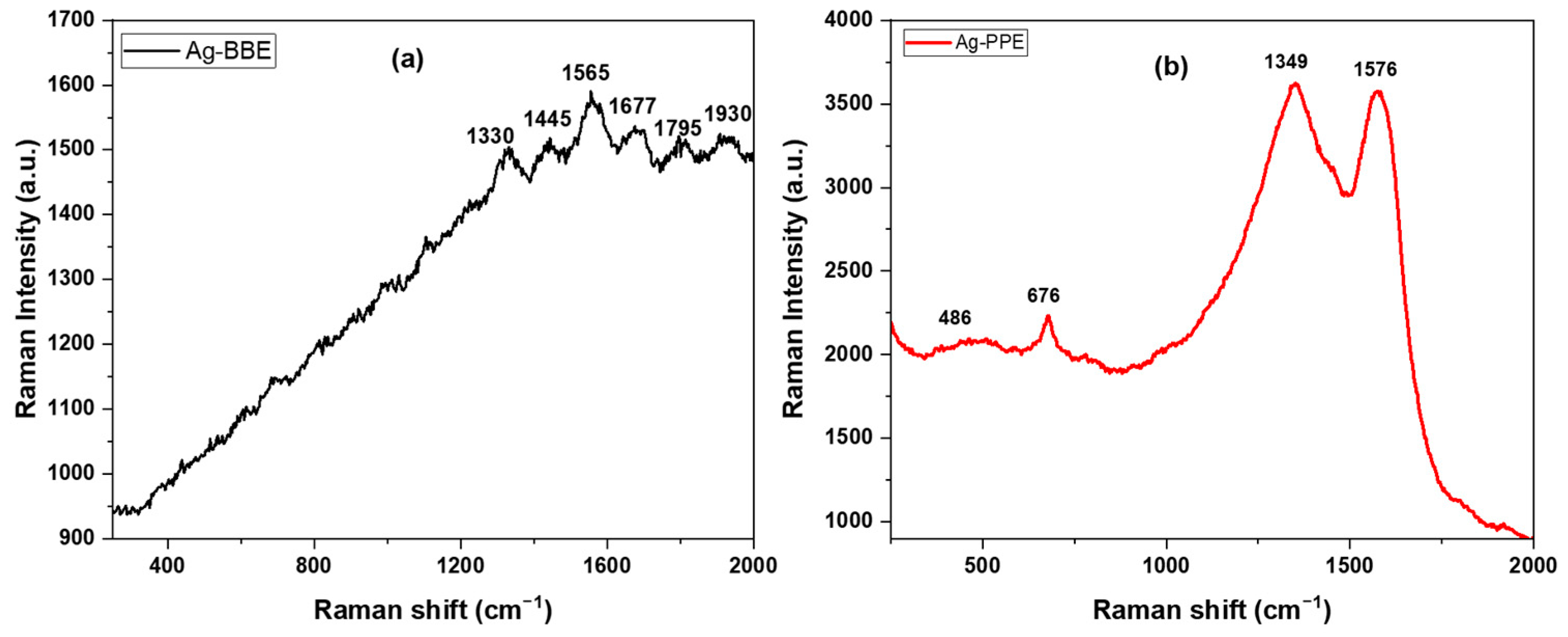
3.5. Raman Spectroscopy (Vibrational Properties)
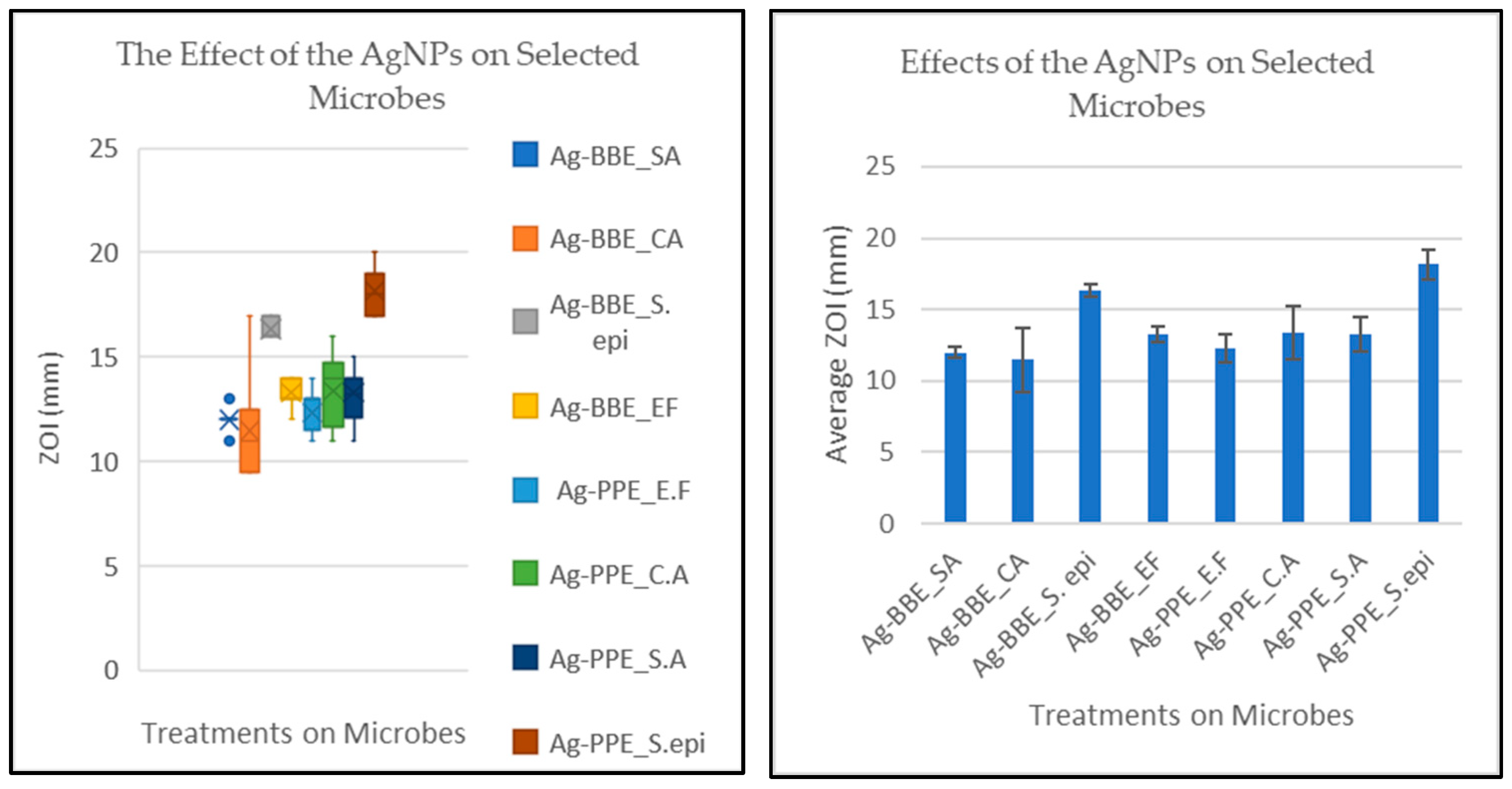
3.6. Antimicrobial Activity and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s global oral health status report 2022: Actions, discussion and implementation. Oral Dis. 2024, 30, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Winning, L.; Lundy, F.T.; Blackwood, B.; McAuley, D.F.; El Karim, I. Oral health care for the critically ill: A narrative review. Crit. Care 2021, 25, 353. [Google Scholar] [CrossRef] [PubMed]
- Kanjevac, T.; Taso, E.; Stefanovic, V.; Petkovic-Curcin, A.; Supic, G.; Markovic, D.; Djukic, M.; Djuran, B.; Vojvodic, D.; Sculean, A.; et al. Estimating the Effects of Dental Caries and Its Restorative Treatment on Periodontal Inflammatory and Oxidative Status: A Short Controlled Longitudinal Study. Front. Immunol. 2021, 12, 716359. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Corbella, S.; Taschieri, S.; Francetti, L.; Fakhruddin, K.S.; Samaranayake, L.P. Fungal species in endodontic infections: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0255003. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Lang, N.P. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol. 2000 2010, 53, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Poeschl, P.W.; Crepaz, V.; Russmueller, G.; Seemann, R.; Hirschl, A.M.; Ewers, R. Endodontic pathogens causing deep neck space infections: Clinical impact of different sampling techniques and antibiotic susceptibility. J. Endod. 2011, 37, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Passariello, C.; Puttini, M.; Iebba, V.; Pera, P.; Gigola, P. Influence of oral conditions on colonization by highly toxigenic Staphylococcus aureus strains. Oral Dis. 2012, 18, 402–409. [Google Scholar] [CrossRef]
- Alghamdi, F.; Shakir, M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus 2020, 12, e7257. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Yah, C.S.; Simate, G.S. Nanoparticles as potential new generation broad spectrum antimicrobial agents. DARU J. Pharm. Sci. 2015, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, M.; Rudayni, H.A.; Assiri, R.A.; Bepari, A.; Basavarajappa, D.S.; Nagaraja, S.K.; Chakraborty, B.; Swamy, P.S.; Agadi, S.N.; Niazi, S.K.; et al. Plumeria alba-Mediated Green Synthesis of Silver Nanoparticles Exhibits Antimicrobial Effect and Anti-Oncogenic Activity against Glioblastoma U118 MG Cancer Cell Line. Nanomaterials 2022, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomedicine 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Noronha, V.T.; Paula, A.J.; Durán, G.; Galembeck, A.; Cogo-Müller, K.; Franz-Montan, M.; Durán, N. Silver nanoparticles in dentistry. Dent. Mater. 2017, 33, 1110–1126. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, A.S.; Monteiro, D.R.; Gorup, L.F.; Silva, E.A.; de Camargo, E.R.; Gomes-Filho, J.E.; de Oliveira, S.H.P.; Barbosa, D.B. Biocompatible silver nanoparticles incorporated in acrylic resin for dental application inhibit Candida albicans biofilm. Mater. Sci. Eng. C 2021, 118, 111341. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.; Karobari, M.I.; Heboyan, A.; Mohamed, R.N.; Mustafa, M.; Basheer, S.N.; Desai, V.; Batool, S.; Ahmed, N.; Zeshan, B. Synthesis of Silver Nanoparticles from Extracts of Wild Ginger (Zingiber zerumbet) with Antibacterial Activity against Selective Multidrug Resistant Oral Bacteria. Molecules 2022, 27, 2007. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz Öztürk, B.; Yenice Gürsu, B.; Dağ, İ. Antibiofilm and antimicrobial activities of green synthesized silver nanoparticles using marine red algae Gelidium corneum. Process Biochem. 2020, 89, 208–219. [Google Scholar] [CrossRef]
- Rauwel, P.; Küünal, S.; Ferdov, S.; Rauwel, E. A review on the green synthesis of silver nanoparticles and their morphologies studied via TEM. Adv. Mater. Sci. Eng. 2015, 2015, 682749. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Rafique, M.; Rafique, M.S.; Kalsoom, U.; Afzal, A.; Butt, S.H.; Usman, A. Laser ablation synthesis of silver nanoparticles in water and dependence on laser nature. Opt. Quantum Electron. 2019, 51, 179. [Google Scholar] [CrossRef]
- Gharibshahi, L.; Saion, E.; Gharibshahi, E.; Shaari, A.H.; Matori, K.A. Structural and Optical Properties of Ag Nanoparticles Synthesized by Thermal Treatment Method. Materials 2017, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Meader, V.K.; John, M.G.; Frias Batista, L.M.; Ahsan, S.; Tibbetts, K.M. Radical Chemistry in a Femtosecond Laser Plasma: Photochemical Reduction of Ag+ in Liquid Ammonia Solution. Molecules 2018, 23, 532. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver nanoparticles: Synthesis, investigation techniques, and properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef] [PubMed]
- Natsuki, J. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325. [Google Scholar] [CrossRef]
- Rahimi, H.-R.; Doostmohammadi, M. Nanoparticle synthesis, applications, and toxicity. Appl. Nanobiotechnol. 2019, 10, 3–18. [Google Scholar]
- Sharma, A.; Kumar, S. Synthesis and Green Synthesis of Silver Nanoparticles. Eng. Mater. 2021, 1, 25–64. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Badeggi, U.M.; Badmus, J.A.; Botha, S.S.; Ismail, E.; Marnewick, J.L.; Africa, C.W.J.; Hussein, A.A. Biosynthesis, characterization, and biological activities of procyanidin capped silver nanoparticles. J. Funct. Biomater. 2020, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vargas, J.M.; Echeverry-Cardona, L.M.; Moreno-Montoya, L.E.; Restrepo-Parra, E. Evaluation of Antifungal Activity of Ag Nanoparticles Synthetized by Green Chemistry against Fusarium solani and Rhizopus stolonifera. Nanomaterials 2023, 13, 548. [Google Scholar] [CrossRef]
- Arif, R.; Uddin, R. A review on recent developments in the biosynthesis of silver nanoparticles and its biomedical applications. Med. Devices Sens. 2021, 4, e10158. [Google Scholar] [CrossRef]
- Essghaier, B.; Hannachi, H.; Nouir, R.; Mottola, F.; Rocco, L. Green Synthesis and Characterization of Novel Silver Nanoparticles Using Achillea maritima subsp. maritima Aqueous Extract: Antioxidant and Antidiabetic Potential and Effect on Virulence Mechanisms of Bacterial and Fungal Pathogens. Nanomaterials 2023, 13, 1964. [Google Scholar] [CrossRef] [PubMed]
- Balciunaitiene, A.; Januskevice, V.; Saunoriute, S.; Raubyte, U.; Viskelis, J.; Memvanga, P.B.; Viskelis, P. Antimicrobial Antioxidant Polymer Films with Green Silver Nanoparticles from Symphyti radix. Polymers 2024, 16, 317. [Google Scholar] [CrossRef] [PubMed]
- Șuică-Bunghez, I.R.; Senin, R.M.; Sorescu, A.A.; Ganciarov, M.; Răut, I.; Firincă, C.; Constantin, M.; Gifu, I.C.; Stoica, R.; Fierăscu, I.; et al. Application of Lavandula angustifolia Mill. Extracts for the Phytosynthesis of Silver Nanoparticles: Characterization and Biomedical Potential. Plants 2024, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.K.; Farooq, U.; Shakeel, Q.; Ali, S.; Ashiq, S.; Shahzad, S.; Tariq, M.; Seleiman, M.F.; Jamal, A.; Saeed, M.F.; et al. The Green Synthesis of Silver Nanoparticles from Avena fatua Extract: Antifungal Activity against Fusarium oxysporum f.sp. lycopersici. Pathogens 2023, 12, 1247. [Google Scholar] [CrossRef] [PubMed]
- Ajaykumar, A.P.; Sabira, O.; Binitha, V.S.; Varma, S.R.; Mathew, A.; Jayaraj, K.N.; Janish, P.A.; Zeena, K.V.; Sheena, P.; Venugopal, V.; et al. Bio-Fabricated Silver Nanoparticles from the Leaf Extract of the Poisonous Plant, Holigarna arnottiana: Assessment of Antimicrobial, Antimitotic, Anticancer, and Radical-Scavenging Properties. Pharmaceutics 2023, 15, 2468. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Abduljabbar, B.T.; El-Zayat, M.M.; Sarker, T.C.; Abd-ElGawad, A.M. Synthesis of Metal Nanoparticles via Pulicaria undulata and an Evaluation of Their Antimicrobial, Antioxidant, and Cytotoxic Activities. Chemistry 2023, 5, 2075–2093. [Google Scholar] [CrossRef]
- Habibipour, R.; Moradi-Haghgou, L.; Farmany, A. Green synthesis of AgNPs@PPE and its Pseudomonas aeruginosa biofilm formation activity compared to pomegranate peel extract. Int. J. Nanomed. 2019, 14, 6891–6899. [Google Scholar] [CrossRef] [PubMed]
- Abdel Moneim, A.E. Evaluating the potential role of pomegranate peel in aluminum-induced oxidative stress and histopathological alterations in brain of female rats. Biol. Trace Elem. Res. 2012, 150, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Gullon, B.; Pintado, M.E.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing factors affecting the phytochemical and nutritional properties of pomegranate (punica granatum L.) peel waste: A review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef] [PubMed]
- Türkyılmaz, M.; Tağı, Ş.; Dereli, U.; Özkan, M. Effects of various pressing programs and yields on the antioxidant activity, antimicrobial activity, phenolic content and colour of pomegranate juices. Food Chem. 2013, 138, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gupta, A.; Singh, A.K.; Bishayee, A.; Pandey, A.K. The Antioxidant and Antihyperglycemic Activities of Bottlebrush Plant (Callistemon lanceolatus) Stem Extracts. Medicines 2020, 7, 11. [Google Scholar] [CrossRef]
- Abdelhady, M.I.S.; Motaal, A.A.; Beerhues, L. Total Phenolic Content and Antioxidant Activity of Standardized Extracts from Leaves and Cell Cultures of Three Callistemon Species. Am. J. Plant Sci. 2011, 02, 847–850. [Google Scholar] [CrossRef]
- Oyedeji, O.O.; Lawal, O.A.; Shode, F.O.; Oyedeji, A.O. Chemical Composition and Antibacterial Activity of the Essential Oils of Callistemon citrinus and Callistemon viminalis from South Africa. Molecules 2009, 14, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Mabhiza, D.; Chitemerere, T.; Mukanganyama, S. Antibacterial Properties of Alkaloid Extracts from Callistemon citrinus and Vernonia adoensis against Staphylococcus aureus and Pseudomonas aeruginosa. Int. J. Med. Chem. 2016, 2016, 6304163. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Ali, H.M.; El-Shanhorey, N.A.; Abdel-Megeed, A. Evaluation of extracts and essential oil from Callistemon viminalis leaves: Antibacterial and antioxidant activities, total phenolic and flavonoid contents. Asian Pac. J. Trop. Med. 2013, 6, 785–791. [Google Scholar] [CrossRef]
- Hassan, D.; Khalil, A.T.; Saleem, J.; Diallo, A.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush): Their physical properties and novel biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Monika, P.; Chandraprabha, M.N.; Hari Krishna, R.; Vittal, M.; Likhitha, C.; Pooja, N.; Chaudhary, V.; Manjunatha, C. Recent advances in pomegranate peel extract mediated nanoparticles for clinical and biomedical applications. Biotechnol. Genet. Eng. Rev. 2022, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Rotimi, L.; Ojemaye, M.O.; Okoh, O.O.; Sadimenko, A.; Okoh, A.I. Synthesis, characterization, antimalarial, antitrypanocidal and antimicrobial properties of gold nanoparticle. Green Chem. Lett. Rev. 2019, 12, 61–68. [Google Scholar] [CrossRef]
- Ismail, E.; Mohamed, A.; Maboza, E.; Dhlamini, M.S.; Adam, R.Z. Callistemon citrinus: A plant-mediated synthesis of sustainable Rhodium nanoparticles and their antimicrobial activity. Appl. Res. 2024, e202300130. [Google Scholar] [CrossRef]
- Aly, A.A.; Fahmy, H.M.; Abou-Okeil, A. Green synthesis of silver nanoparticles using commercially available starch products. Egypt. J. Chem. 2023, 67, 309–317. [Google Scholar] [CrossRef]
- Gou, Y.; Zhou, R.; Ye, X.; Gao, S.; Li, X. Highly efficient in vitro biosynthesis of silver nanoparticles using Lysinibacillus sphaericus MR-1 and their characterization. Sci. Technol. Adv. Mater. 2015, 16, 15004. [Google Scholar] [CrossRef] [PubMed]
- Mehata, M.S. Green route synthesis of silver nanoparticles using plants/ginger extracts with enhanced surface plasmon resonance and degradation of textile dye. Mater. Sci. Eng. B 2021, 273, 115418. [Google Scholar] [CrossRef]
- Ganaie, S.A.; Zahoor, I.; Singh, R. Prunella vulgaris leaf extract assisted green synthesis of silver nanoparticles: Antimicrobial activity. Mater. Today Proc. 2022, 79, 107–112. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Bhui, D.K.; Bar, H.; Sarkar, P.; Sahoo, G.P.; De, S.P.; Misra, A. Synthesis and UV-vis spectroscopic study of silver nanoparticles in aqueous SDS solution. J. Mol. Liq. 2009, 145, 33–37. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, J.; Long, X.; Pan, C.; Liu, G.; Peng, J. Green Synthesis of Silver Nanoparticles Using Jasminum nudiflorum Flower Extract and Their Antifungal and Antioxidant Activity. Nanomaterials 2023, 13, 2558. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Singh, A.; Ahmad, W. Microwave Assisted Green Synthesis of Silver Nanoparticles and Its Application: A Review. J. Inorg. Organomet. Polym. Mater. 2023, 33, 663–672. [Google Scholar] [CrossRef]
- Orbaek, A.W.; McHale, M.M.; Barron, A.R. Synthesis and characterization of silver nanoparticles for an undergraduate laboratory. J. Chem. Educ. 2015, 92, 339–344. [Google Scholar] [CrossRef]
- Pandey, S.; Mewada, A.; Thakur, M.; Shinde, S.; Shah, R.; Oza, G.; Sharon, M. Rapid Biosynthesis of Silver Nanoparticles by Exploiting the Reducing Potential of Trapa bispinosa Peel Extract. J. Nanosci. 2013, 2013, 516357. [Google Scholar] [CrossRef]
- Pandit, S.; Bhowal, A.C.; Kundu, S. Green synthesis of silver nanoparticles and its potential applications. Appl. Silver Nanoparticles 2023, 5, 41–74. [Google Scholar]
- Sadeq, M.S.; Elzwawy, A.; El Awady, M.E.; Hamed, A.A.; Sedqy, E.M.; Ibrahim, S.; Baki, M.A. Influence of ZnO on the structural, optical, ligand field and antibacterial characteristics of sodium borosilicate glasses containing minor Cr2O3 additions. Phys. Scr. 2023, 98, 055933. [Google Scholar] [CrossRef]
- Singh, C.; Anand, S.K.; Upadhyay, R.; Pandey, N.; Kumar, P.; Singh, D.; Tiwari, P.; Saini, R.; Tiwari, K.N.; Mishra, S.K.; et al. Green synthesis of silver nanoparticles by root extract of Premna integrifolia L. and evaluation of its cytotoxic and antibacterial activity. Mater. Chem. Phys. 2023, 297, 127413. [Google Scholar] [CrossRef]
- Ali, I.A.M.; Ahmed, A.B.; Al-Ahmed, H.I. Green synthesis and characterization of silver nanoparticles for reducing the damage to sperm parameters in diabetic compared to metformin. Sci. Rep. 2023, 13, 2256. [Google Scholar] [CrossRef]
- Rashid, T.M.; Nayef, U.M.; Jabir, M.S. Nano-ZnO decorated on gold nanoparticles as a core-shell via pulse laser ablation in liquid. Optik 2021, 248, 168164. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J.; Debnath (Das), M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Al-Khedhairy, A.A.; Wahab, R. Silver Nanoparticles: An Instantaneous Solution for Anticancer Activity against Human Liver (HepG2) and Breast (MCF-7) Cancer Cells. Metals 2022, 12, 148. [Google Scholar] [CrossRef]
- Bhat, R.S.; Almusallam, J.; Daihan, S.A.; Al-Dbass, A. Biosynthesis of silver nanoparticles using Azadirachta indica leaves: Characterisation and impact on Staphylococcus aureus growth and glutathione-S-transferase activity. IET Nanobiotechnol. 2019, 13, 42–46. [Google Scholar] [CrossRef]
- Ijaz, I.; Bukhari, A.; Gilani, E.; Nazir, A.; Zain, H.; Saeed, R. Green synthesis of silver nanoparticles using different plants parts and biological organisms, characterization and antibacterial activity. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100704. [Google Scholar] [CrossRef]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, L.; Tkáčiková, L. Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, M.J. Quantitative analysis using Raman spectrometry. Appl. Spectrosc. 2003, 57, 20A–42A. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sharma, N.; Sharma, V.K.; Mishra, N.D.; Joshi, C.S. Green synthesis, characterisation and biological studies of AgNPs prepared using Shivlingi (Bryonia laciniosa) seed extract. IET Nanobiotechnol. 2018, 12, 371–375. [Google Scholar] [CrossRef]
- Joshi, N.; Jain, N.; Pathak, A.; Singh, J.; Prasad, R.; Upadhyaya, C.P. Biosynthesis of silver nanoparticles using Carissa carandas berries and its potential antibacterial activities. J. Sol-Gel Sci. Technol. 2018, 86, 682–689. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Chang, H.-T.; Tan, W. Cancer Cell Targeting Using Multiple Aptamers Conjugated on Nanorods. Anal. Chem. 2008, 80, 567–572. [Google Scholar] [CrossRef]
- Khamhaengpol, A.; Siri, S. Green synthesis of silver nanoparticles using tissue extract of weaver ant larvae. Mater. Lett. 2017, 192, 72–75. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Diaf, H.; Pereira, A.; Melinon, P.; Blanchard, N.; Bourquard, F.; Garrelie, F.; Donnet, C. Discrimination of different amorphous carbon by low fluence laser irradiation. Carbon Trends 2022, 9, 100195. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, C.X.; Ma, C.L.; Zheng, X.X.; Lv, X.Y.; Lv, G.D.; Tang, J.; Wu, G.H. Raman spectroscopic study of cervical precancerous lesions and cervical cancer. Lasers Med. Sci. 2021, 36, 1855–1864. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Panayotov, D.A.; Mihaylov, M.Y.; Ivanova, E.Z.; Chakarova, K.K.; Andonova, S.M.; Drenchev, N.L. Power of Infrared and Raman Spectroscopies to Characterize Metal-Organic Frameworks and Investigate Their Interaction with Guest Molecules. Chem. Rev. 2021, 121, 1286–1424. [Google Scholar] [CrossRef]
- Dirks, T.; Dumas, T.; Solari, P.L.; Charbonnel, M.C. Ruthenium Nitrosyl Structure in Solvent Extraction Systems: A Comparison of Tributyl Phosphate, Tetrabutyl Urea, N-Methyl, N-Octyl Ethylhexanamide, and N, N, N′, N′-Tetraoctyl Diglycolamide. Ind. Eng. Chem. Res. 2019, 58, 14938–14946. [Google Scholar] [CrossRef]
- Campos, L.; Seixas, L.; Henriques, M.H.F.; Peres, A.M.; Veloso, A.C.A. Pomegranate Peels and Seeds as a Source of Phenolic Compounds: Effect of Cultivar, By-Product, and Extraction Solvent. Int. J. Food Sci. 2022, 2022, 9189575. [Google Scholar] [CrossRef]
- Badeggi, U.M.; Ismail, E.; Adeloye, A.O.; Botha, S.; Badmus, J.A.; Marnewick, J.L.; Cupido, C.N.; Hussein, A.A. Green synthesis of gold nanoparticles capped with procyanidins from leucosidea sericea as potential antidiabetic and antioxidant agents. Biomolecules 2020, 10, 452. [Google Scholar] [CrossRef]
- Viršilė, A.; Samuolienė, G.; Laužikė, K.; Šipailaitė, E.; Balion, Z.; Jekabsone, A. Species-Specific Plant-Derived Nanoparticle Characteristics. Plants 2022, 11, 3139. [Google Scholar] [CrossRef]
- Tao, R.; You, C.; Qu, Q.; Zhang, X.; Deng, Y.; Ma, W.; Huang, C. Recent advances in the design of controlled- and sustained-release micro/nanocarriers of pesticide. Environ. Sci. Nano 2023, 10, 351–371. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Oriola, A.O.; Onwudiwe, D.C. Plant Extracts Mediated Metal-Based Nanoparticles: Synthesis and Biological Applications. Biomolecules 2022, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Tolaymat, T.M.; El Badawy, A.M.; Genaidy, A.; Scheckel, K.G.; Luxton, T.P.; Suidan, M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: A systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total Environ. 2010, 408, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Niu, B.; Zhang, G. Effects of Different Nanoparticles on Microbes. Microorganisms 2023, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.; Sibuyi, N.R.S.; Fadaka, A.O.; Madiehe, M.A.; Maboza, E.; Meyer, M.; Geerts, G. Plant Extract-Synthesized Silver Nanoparticles for Application in Dental Therapy. Pharmaceutics 2022, 14, 380. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef]
- Vanotterloo, L.M.; Trent, M.S. Microbial Primer: Lipopolysaccharide—A remarkable component of the Gram-negative bacterial surface. Microbiology 2024, 170, 1439. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 551136. [Google Scholar] [CrossRef] [PubMed]
- Afhkami, F.; Ahmadi, P.; Chiniforush, N.; Sooratgar, A. Effect of different activations of silver nanoparticle irrigants on the elimination of Enterococcus faecalis. Clin. Oral Investig. 2021, 25, 6893–6899. [Google Scholar] [CrossRef] [PubMed]


| Silver NPs | λmax (nm) | A at λmax | FWHM (nm) | FWHM Range (nm) | Energy Gap (eV) |
|---|---|---|---|---|---|
| Ag-BBE | 419 | 2.24 | at 1.595 = 61.5 | 333–496 | 2.64 |
| Ag-PPE | 433 | 1.94 | at 1.264 = 99.3 | 331.4–523.5 | 2.51 |
| Reference | Analysis | Bonferroni Post Hoc Test t-Test (Adjusted α = 0.00625) | |||
|---|---|---|---|---|---|
| S. aureus (Ag-BBE) 12 mm | Treatment Interactions | Means (mm Ø) | t Crit. | P (T ≤ t) | Decision |
| C. albicans | Ag-BBE_CA | 11.47 | 1.73 | 0.16699 | No sig. diff. |
| S. epidermidis | Ag-BBE_S. epi | 16.33 | 1.70 | 6.57 × 10−25 | Sig. diff. |
| E. faecalis | Ag-BBE_EF | 13.28 | 1.70 | 5.54 × 10−9 | Sig. diff. |
| E. faecalis | Ag-PPE_EF | 12.31 | 1.73 | 0.12192 | No sig. diff. |
| C. albicans | Ag-PPE_CA | 13.38 | 1.75 | 0.004399 | Sig. diff. |
| S. aureus | Ag-PPE_SA | 13.28 | 1.73 | 0.000264 | Sig. diff. |
| S. epidermidis | Ag-PPE_S. epi | 18.16 | 1.73 | 4.11 × 10−15 | Sig. diff. |
| Reference Microbe | Analysis | Bonferroni Post Hoc Test t-Test (Adjusted α = 0.00625 | |||
|---|---|---|---|---|---|
| Treatment Interactions | Means (mm Ø) | t Crit. | P (T ≤ t) | Decision | |
| C. albicans | Ag-BBE_CA | 11.47 | 1.71 | 0.07989 | No sig. diff. |
| Ag-PPE_EF | 12.31 | ||||
| E. faecalis | Ag-BBE_EF | 12.31 | 1.71 | 0.025312 | No sig. diff. |
| Ag-PPE_CA | 13.38 | ||||
| E. faecalis | Ag-PPE_EF | 12.31 | 1.70 | 0.008358 | No sig. diff. |
| Ag-PPE_SA | 13.28 | ||||
| C. albicans | Ag-PPE_CA | 13.38 | 1.71 | 0.431637 | No sig. diff. |
| Ag-PPE_SA | 13.28 | ||||
| Microbe | Analysis | Bonferroni Post Hoc Test t-Test (Adjusted α = 0.00625 | |||
|---|---|---|---|---|---|
| Treatment Interactions | Means (mm Ø) | t Crit. | P (T ≤ t) | Decision | |
| S. aureus | Ag-BBE_SA | 12 | 1.73 | 0.000264 | Sig. diff. |
| Ag-PPE_SA | 13.28 | ||||
| C. albicans | Ag-BBE_CA | 11.47 | 1.69 | 0.00492 | Sig. diff. |
| Ag-PPE_CA | 13.38 | ||||
| S. epidermidis | Ag-BBE_S. epi | 16.33 | 1.72 | 8.36 × 10−7 | Sig. diff. |
| Ag-PPE_S. epi | 18.16 | ||||
| E. faecalis | Ag-BBE_EF | 13.28 | 1.71 | 0.001054 | Sig. diff. |
| Ag-PPE_EF | 12.31 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, E.; Mohamed, A.; Elzwawy, A.; Maboza, E.; Dhlamini, M.S.; Adam, R.Z. Comparative Study of Callistemon citrinus (Bottlebrush) and Punica granatum (Pomegranate) Extracts for Sustainable Synthesis of Silver Nanoparticles and Their Oral Antimicrobial Efficacy. Nanomaterials 2024, 14, 974. https://doi.org/10.3390/nano14110974
Ismail E, Mohamed A, Elzwawy A, Maboza E, Dhlamini MS, Adam RZ. Comparative Study of Callistemon citrinus (Bottlebrush) and Punica granatum (Pomegranate) Extracts for Sustainable Synthesis of Silver Nanoparticles and Their Oral Antimicrobial Efficacy. Nanomaterials. 2024; 14(11):974. https://doi.org/10.3390/nano14110974
Chicago/Turabian StyleIsmail, Enas, Abubaker Mohamed, Amir Elzwawy, Ernest Maboza, Mokhotjwa Simon Dhlamini, and Razia Z. Adam. 2024. "Comparative Study of Callistemon citrinus (Bottlebrush) and Punica granatum (Pomegranate) Extracts for Sustainable Synthesis of Silver Nanoparticles and Their Oral Antimicrobial Efficacy" Nanomaterials 14, no. 11: 974. https://doi.org/10.3390/nano14110974
APA StyleIsmail, E., Mohamed, A., Elzwawy, A., Maboza, E., Dhlamini, M. S., & Adam, R. Z. (2024). Comparative Study of Callistemon citrinus (Bottlebrush) and Punica granatum (Pomegranate) Extracts for Sustainable Synthesis of Silver Nanoparticles and Their Oral Antimicrobial Efficacy. Nanomaterials, 14(11), 974. https://doi.org/10.3390/nano14110974


_Stamatis.png)




