Emerging Trends in Nanotechnology for Endometriosis: Diagnosis to Therapy
Abstract
1. Introduction
2. Nanotechnology in Cancer and Endometriosis
3. Overview of Endometriosis
3.1. Conventional Techniques
3.2. Challenges
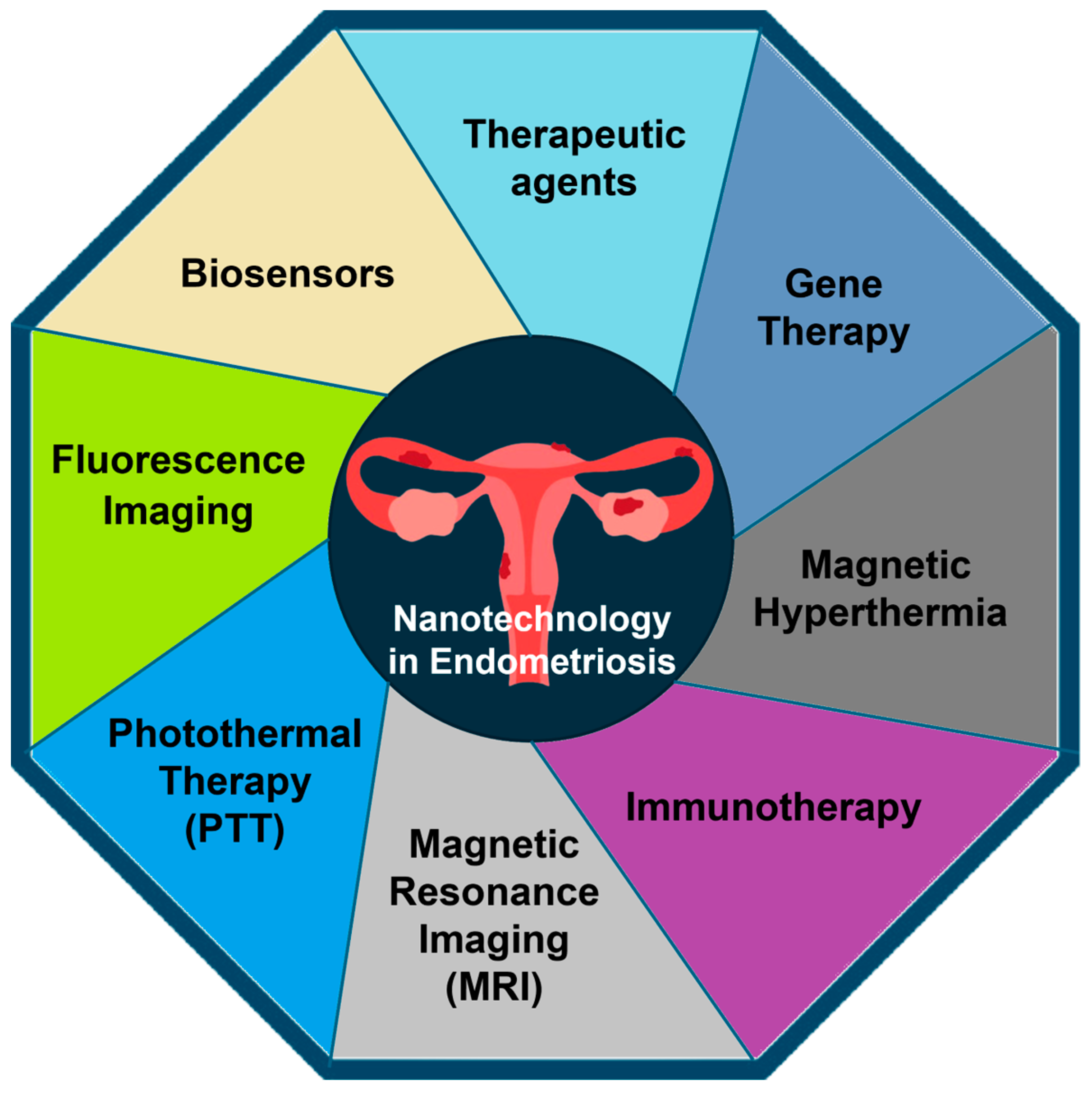
4. Applications of Nanotechnology in Endometriosis
4.1. Diagnosis
4.1.1. Fluorescence Imaging
4.1.2. Magnetic Resonance Imaging (MRI)
4.1.3. Biosensors
4.2. Treatment
4.2.1. Nanomaterials and Therapeutic Agents
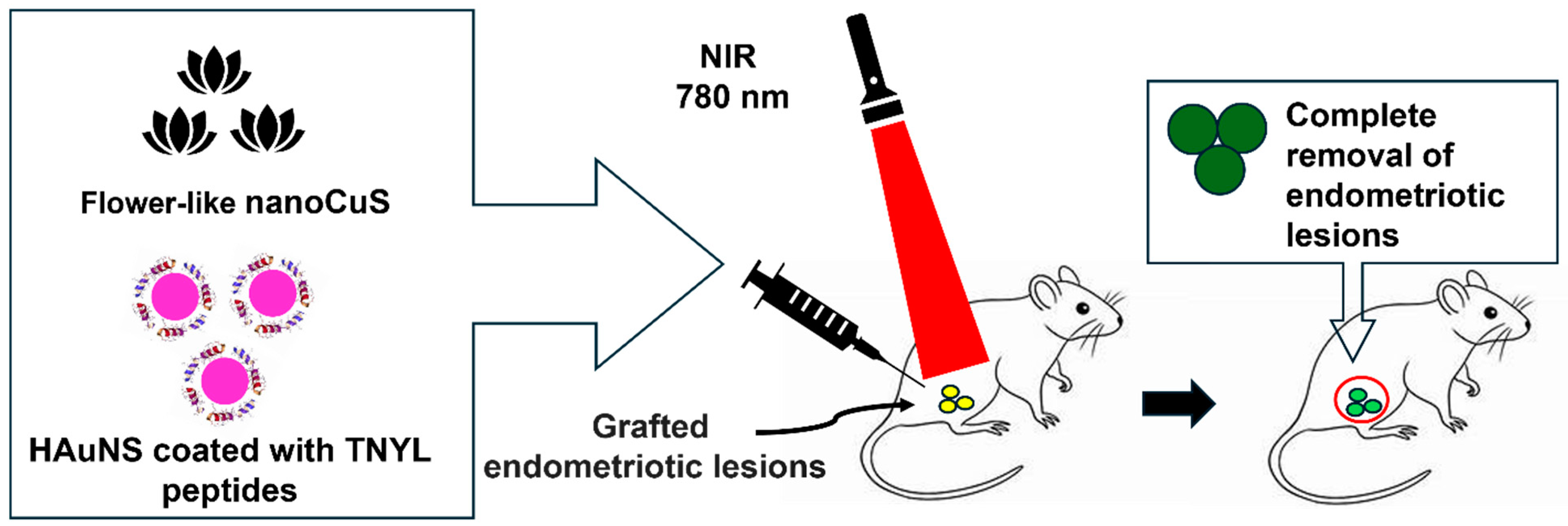
4.2.2. NP-Mediated Photothermal Therapy
4.2.3. Immunotherapy
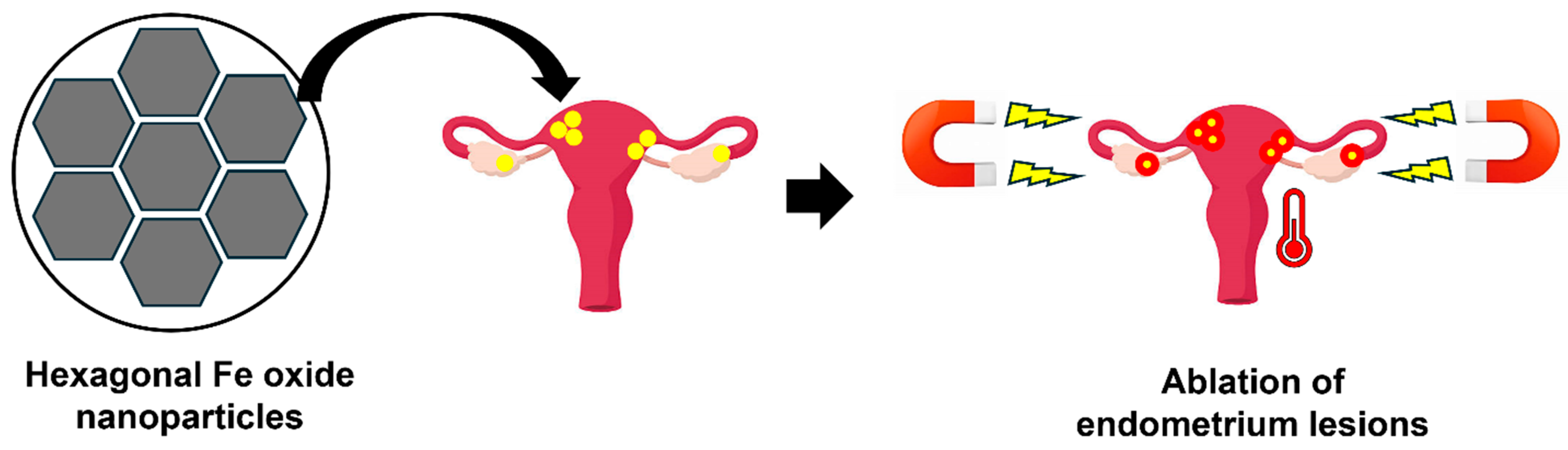
4.2.4. Magnetic Hyperthermia
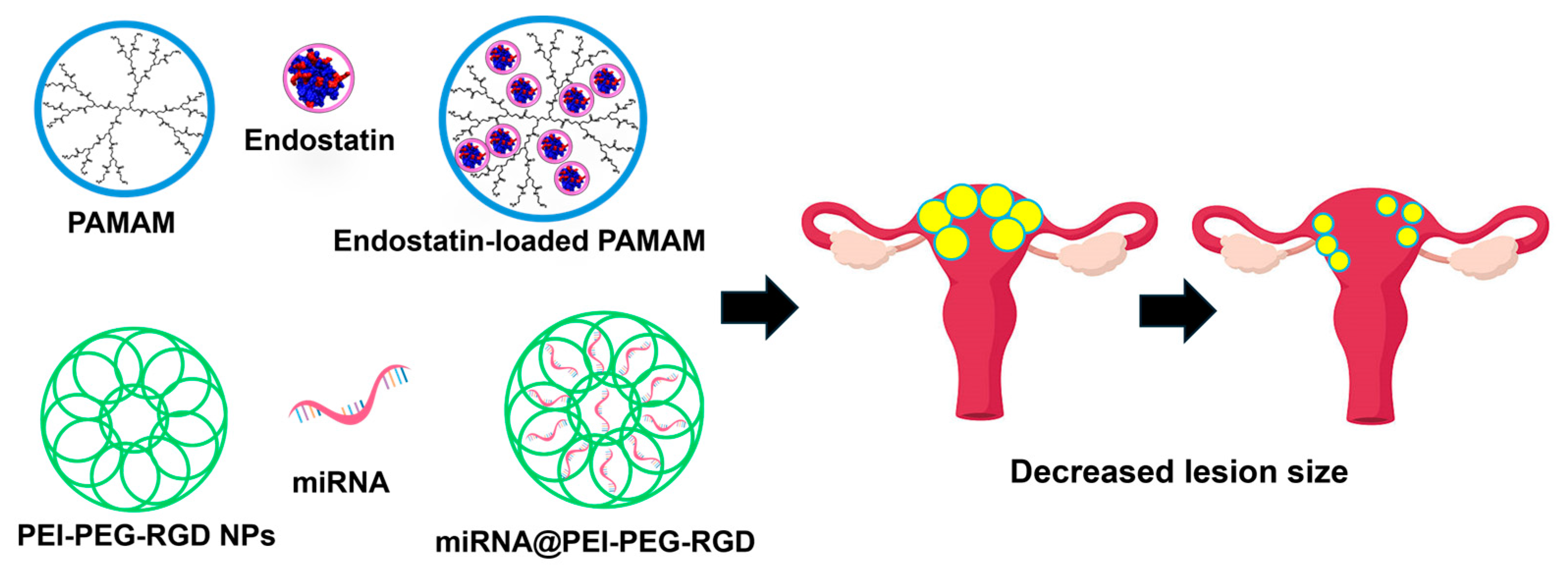
4.2.5. Gene Therapy
5. Conclusions
6. Future Scope
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPs | Nanoparticles |
| MRI | Magnetic resonance imaging |
| PTT | Photothermal therapy |
| GnRH | Gonadotropin-releasing hormone |
| LH | Luteinizing hormone |
| FSH | Follicle-stimulating hormone |
| ROS | Reactive oxygen species |
| PPIX | Protoporphyrin IX |
| ALA | 5-aminolevulinic acid |
| NIR | Near-infrared |
| FDA | Food and Drug Administration |
| ICG | Indocyanine green |
| SiNc-NP | Silicon naphthalocyanine-loaded polyethylene glycol-polycaprolactone NP |
| USPIO | Ultrasmall superparamagnetic iron oxide |
| HA | Hyaluronic acid |
| VEGFR-2 | Vascular endothelial growth factor receptor-2 |
| CA125 | Cancer antigen 125 |
| MWCNT | Multiwalled carbon nanotubes |
| CS | Chitosan |
| CA 19-9 | Cancer antigen 19-9 |
| AuNPs | Gold NPs |
| GO | Graphene oxide |
| GCE | Glassy carbon electrodes |
| HP | Haptoglobin |
| CNP | Cerium oxide NPs |
| OS | Oxidative stress |
| EGCG | Epigallocatechin gallate |
| DOX | Doxycycline |
| PLGA | Poly (lactic-co-glycolic acid) |
| MMP | Matrix metalloproteinase |
| CPO | Copaiba oleoresin |
| PCL | Poly ε-caprolactone |
| PEG | Polyethylene glycol |
| PDT | Photodynamic therapy |
| HAuNS | Hollow gold nanoshells |
| CuS | Copper sulfide |
| CTLA-4 | Cytotoxic T lymphocyte antigen 4 |
| PLGA | Poly (lactic-co-glycolic acid) |
| UNPs | Unmodified mesoporous silica NPs |
| AMNPs | Aminopropyl-modified silica NPs |
| GMDP | Glucosaminyl muramyl dipeptide |
| MMP-9 | Matrix metalloproteinase 9 |
| M1NVs | M1-like macrophages |
| EVs | Extracellular vesicles |
| AMF | Alternating magnetic field |
| MNs | Magnetic NPs |
| DNA | Deoxyribonucleic acid |
| CSO-SA/PEDF | Chitosan micelles/pigment epithelium-derived factor |
| PAMAM | Polyamidoamine |
| GFP | Green fluorescent protein |
| PEI-PEG-RGD | Polyethylenimine-polyethylene glycol-arginine-glycine-aspartic acid |
| miRNA@PEI-PEG-RGD | miR-200c mimic RNA Polyethylenimine -polyethylene glycol-arginine-glycine-aspartic acid |
| HESC’s | Human endometriotic stromal cells |
| ZEB1 | Zinc finger E-box-binding homeobox 1 |
| ZEB2 | Zinc finger E-box-binding homeobox 2 |
| qRT-PCR | Real-Time Quantitative Reverse Transcription PCR |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
References
- Bai, J.W.; Qiu, S.Q.; Zhang, G.J. Molecular and functional imaging in cancer-targeted therapy: Current applications and future directions. Signal Transduct. Target. Ther. 2023, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Demessie, A.A.; Luo, A.; Taratula, O.R.; Moses, A.S.; Do, P.; Campos, L.; Jahangiri, Y.; Wyatt, C.R.; Albarqi, H.A.; et al. Targeted Nanoparticles with High Heating Efficiency for the Treatment of Endometriosis with Systemically Delivered Magnetic Hyperthermia. Small 2022, 18, e2107808. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S. Emerging therapies for endometriosis. Fertil. Steril. 2021, 115, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Moses, A.S.; Demessie, A.A.; Taratula, O.; Korzun, T.; Slayden, O.D.; Taratula, O. Nanomedicines for Endometriosis: Lessons Learned from Cancer Research. Small 2021, 17, e2004975. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.L.; Reis, F.M.; Taylor, R.N. Angiogenesis and endometriosis. Obstet. Gynecol. Int. 2013, 2013, 859619. [Google Scholar] [CrossRef] [PubMed]
- Groothuis, P.G.; Nap, A.W.; Winterhager, E.; Grummer, R. Vascular development in endometriosis. Angiogenesis 2005, 8, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Yuxue, J.; Ran, S.; Minghui, F.; Minjia, S. Applications of nanomaterials in endometriosis treatment. Front. Bioeng. Biotechnol. 2023, 11, 1184155. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt-Hawes, E.M.; Campbell, N.; Maley, P.E.; Won, H.; Hooshmand, D.; Henry, A.; Ledger, W.; Abbott, J.A. The Surgical Treatment of Severe Endometriosis Positively Affects the Chance of Natural or Assisted Pregnancy Postoperatively. Biomed. Res. Int. 2015, 2015, 438790. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Warner, M.L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. N. Am. 1997, 24, 235–258. [Google Scholar] [CrossRef]
- Rowlands, I.J.; Abbott, J.A.; Montgomery, G.W.; Hockey, R.; Rogers, P.; Mishra, G.D. Prevalence and incidence of endometriosis in Australian women: A data linkage cohort study. BJOG 2021, 128, 657–665. [Google Scholar] [CrossRef]
- Jacobson, T.Z.; Duffy, J.M.; Barlow, D.; Koninckx, P.R.; Garry, R. Laparoscopic surgery for pelvic pain associated with endometriosis. Cochrane Database Syst. Rev. 2009, CD001300. [Google Scholar] [CrossRef] [PubMed]
- Kho, R.M.; Andres, M.P.; Borrelli, G.M.; Neto, J.S.; Zanluchi, A.; Abrao, M.S. Surgical treatment of different types of endometriosis: Comparison of major society guidelines and preferred clinical algorithms. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Han, E. Endometriosis and Female Pelvic Pain. Semin. Reprod. Med. 2018, 36, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Tsamantioti, E.S.; Mahdy, H. Endometriosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Brown, J.; Crawford, T.J.; Datta, S.; Prentice, A. Oral contraceptives for pain associated with endometriosis. Cochrane Database Syst. Rev. 2018, 5, CD001019. [Google Scholar] [CrossRef] [PubMed]
- Magon, N. Gonadotropin releasing hormone agonists: Expanding vistas. Indian J. Endocrinol. Metab. 2011, 15, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.S.; Vitale, S.G.; Trovato, M.A.; Palmara, V.I.; Rapisarda, A.M.; Granese, R.; Sturlese, E.; De Dominici, R.; Alecci, S.; Padula, F.; et al. Full-Thickness Excision versus Shaving by Laparoscopy for Intestinal Deep Infiltrating Endometriosis: Rationale and Potential Treatment Options. Biomed. Res. Int. 2016, 2016, 3617179. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.H.; Min, H.S.; Li, L.; Tran, T.H.; Lee, Y.K.; Kwon, I.C.; Choi, K.; Kim, K.; Huh, K.M. Cancer cell-specific photoactivity of pheophorbide a-glycol chitosan nanoparticles for photodynamic therapy in tumor-bearing mice. Biomaterials 2013, 34, 6454–6463. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Khachikyan, I.; Stratton, P. Invasive and noninvasive methods for the diagnosis of endometriosis. Clin. Obstet. Gynecol. 2010, 53, 413–419. [Google Scholar] [CrossRef]
- Murphy, A.A.; Green, W.R.; Bobbie, D.; dela Cruz, Z.C.; Rock, J.A. Unsuspected endometriosis documented by scanning electron microscopy in visually normal peritoneum. Fertil. Steril. 1986, 46, 522–524. [Google Scholar] [CrossRef]
- Stummer, W.; Stocker, S.; Wagner, S.; Stepp, H.; Fritsch, C.; Goetz, C.; Goetz, A.E.; Kiefmann, R.; Reulen, H.J. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 1998, 42, 518–525; discussion 525–526. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; Van Dijk-Smith, J.P.; Van Vugt, D.A.; Kennedy, J.C.; Reid, R.L. Fluorescence and photosensitization of experimental endometriosis in the rat after systemic 5-aminolevulinic acid administration: A potential new approach to the diagnosis and treatment of endometriosis. Am. J. Obstet. Gynecol. 1996, 174, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Hillemanns, P.; Weingandt, H.; Stepp, H.; Baumgartner, R.; Xiang, W.; Korell, M. Assessment of 5-aminolevulinic acid-induced porphyrin fluorescence in patients with peritoneal endometriosis. Am. J. Obstet. Gynecol. 2000, 183, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.A.; Lee, S.; Choi, J.; Henary, M.; Choi, H.S. NIR fluorescent small molecules for intraoperative imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Schumann, C.; Naleway, M.A.; Pang, A.J.; Chon, K.J.; Taratula, O. A multifunctional theranostic platform based on phthalocyanine-loaded dendrimer for image-guided drug delivery and photodynamic therapy. Mol. Pharm. 2013, 10, 3946–3958. [Google Scholar] [CrossRef] [PubMed]
- Cosco, E.D.; Lim, I.; Sletten, E.M. Photophysical Properties of Indocyanine Green in the Shortwave Infrared Region. ChemPhotoChem 2021, 5, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Starosolski, Z.; Bhavane, R.; Ghaghada, K.B.; Vasudevan, S.A.; Kaay, A.; Annapragada, A. Indocyanine green fluorescence in second near-infrared (NIR-II) window. PLoS ONE 2017, 12, e0187563. [Google Scholar] [CrossRef] [PubMed]
- Muckle, T.J. Plasma proteins binding of indocyanine green. Biochem. Med. 1976, 15, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Moses, A.S.; Taratula, O.R.; Lee, H.; Luo, F.; Grenz, T.; Korzun, T.; Lorenz, A.S.; Sabei, F.Y.; Bracha, S.; Alani, A.W.G.; et al. Nanoparticle-Based Platform for Activatable Fluorescence Imaging and Photothermal Ablation of Endometriosis. Small 2020, 16, e1906936. [Google Scholar] [CrossRef]
- Li, X.; Schumann, C.; Albarqi, H.A.; Lee, C.J.; Alani, A.W.G.; Bracha, S.; Milovancev, M.; Taratula, O.; Taratula, O. A Tumor-Activatable Theranostic Nanomedicine Platform for NIR Fluorescence-Guided Surgery and Combinatorial Phototherapy. Theranostics 2018, 8, 767–784. [Google Scholar] [CrossRef]
- Cosentino, F.; Vizzielli, G.; Turco, L.C.; Fagotti, A.; Cianci, S.; Vargiu, V.; Zannoni, G.F.; Ferrandina, G.; Scambia, G. Near-Infrared Imaging with Indocyanine Green for Detection of Endometriosis Lesions (Gre-Endo Trial): A Pilot Study. J. Minim. Invasive Gynecol. 2018, 25, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, F.; Knabben, L.; Mohr, S.; Nirgianakis, K.; Imboden, S.; Mueller, M.D. Visualization of endometriosis with laparoscopy and near-infrared optics with indocyanine green. Acta Obstet. Gynecol. Scand. 2020, 99, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Ianieri, M.M.; Della Corte, L.; Campolo, F.; Cosentino, F.; Catena, U.; Bifulco, G.; Scambia, G. Indocyanine green in the surgical management of endometriosis: A systematic review. Acta Obstet. Gynecol. Scand. 2021, 100, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.; Munro, D.; Clarke, J. Endometriosis Is Undervalued: A Call to Action. Front. Glob. Womens Health 2022, 3, 902371. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, H.J.; Lee, J.M.; Chang, Y.; Woo, S.T. Ultrasmall superparamagnetic iron oxides enhanced MR imaging in rats with experimentally induced endometriosis. Magn. Reson. Imaging 2012, 30, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Mohamed, A.S.R.; Ding, Y.; Wang, J.; Lai, S.Y.; Fuller, C.D.; Shah, R.; Butler, R.T.; Weber, R.S. Ultra-small superparamagnetic iron oxide (USPIO) magnetic resonance imaging in benign mixed tumor of the parotid gland. Clin. Case Rep. 2021, 9, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Sun, W.; Hu, Y.; Zhang, G.; Shen, M.; Shi, X. Hyaluronic acid-modified magnetic iron oxide nanoparticles for MR imaging of surgically induced endometriosis model in rats. PLoS ONE 2014, 9, e94718. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, T.; Sangili, A.; Nanda, A.; Prakash, S.; Kaushik, A.; Kumar Jana, S. Bio-nanocomposite based highly sensitive and label-free electrochemical immunosensor for endometriosis diagnostics application. Bioelectrochemistry 2021, 139, 107740. [Google Scholar] [CrossRef] [PubMed]
- Xavier, P.; Beires, J.; Belo, L.; Rebelo, I.; Martinez-de-Oliveira, J.; Lunet, N.; Barros, H. Are we employing the most effective CA 125 and CA 19-9 cut-off values to detect endometriosis? Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 123, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Tuten, A.; Kucur, M.; Imamoglu, M.; Kaya, B.; Acikgoz, A.S.; Yilmaz, N.; Ozturk, Z.; Oncul, M. Copeptin is associated with the severity of endometriosis. Arch. Gynecol. Obstet. 2014, 290, 75–82. [Google Scholar] [CrossRef]
- Harada, T.; Kubota, T.; Aso, T. Usefulness of CA19-9 versus CA125 for the diagnosis of endometriosis. Fertil. Steril. 2002, 78, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, A.; Fang, X.; Han, X.; Zhang, Y. An ultrasensitive supersandwich electrochemical DNA biosensor based on gold nanoparticles decorated reduced graphene oxide. Anal. Biochem. 2015, 469, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Sangili, A.; Kalyani, T.; Chen, S.M.; Nanda, A.; Jana, S.K. Label-Free Electrochemical Immunosensor Based on One-Step Electrochemical Deposition of AuNP-RGO Nanocomposites for Detection of Endometriosis Marker CA 125. ACS Appl. Bio Mater. 2020, 3, 7620–7630. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, K.; Babu, K.N.; Singh, A.K.; Das, S.; Kumar, A.; Seal, S. Mitigation of endometriosis using regenerative cerium oxide nanoparticles. Nanomedicine 2013, 9, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, A.; Krajcir, N.; Alvarez, J.G. Role of oxidative stress in endometriosis. Reprod. Biomed. Online 2006, 13, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.; Chereau, C.; Nicco, C.; Weill, B.; Chapron, C.; Batteux, F. Reactive oxygen species controls endometriosis progression. Am. J. Pathol. 2009, 175, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum. Reprod. 2014, 29, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Chakravarty, B.; Chaudhury, K. Nanoparticle-Assisted Combinatorial Therapy for Effective Treatment of Endometriosis. J. Biomed. Nanotechnol. 2015, 11, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Henriques da Silva, J.; Borges, V.R.; Pereira Lda, C.; Ferrari, R.; de Mattos, R.M.; Barros, E.G.; Palmero, C.Y.; Fernandes, P.D.; de Carvalho, P.R.; Pereira de Sousa, V.; et al. The oil-resin of the tropical rainforest tree Copaifera langsdorffii reduces cell viability, changes cell morphology and induces cell death in human endometriotic stromal cultures. J. Pharm. Pharmacol. 2015, 67, 1744–1755. [Google Scholar] [CrossRef]
- de Almeida Borges, V.R.; Tavares, M.R.; da Silva, J.H.; Tajber, L.; Boylan, F.; Ribeiro, A.F.; Nasciutti, L.E.; Cabral, L.M.; de Sousa, V.P. Development and characterization of poly(lactic-co-glycolic) acid nanoparticles loaded with copaiba oleoresin. Pharm. Dev. Technol. 2018, 23, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, S.; Hosseini, S.; Pashandi, Z.; Faridi-Majidi, R.; Salehi, M. Curcumin-loaded nanofibers for targeting endometriosis in the peritoneum of a mouse model. J. Mater. Sci. Mater. Med. 2019, 31, 8. [Google Scholar] [CrossRef] [PubMed]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Z.; Huang, D.; Liu, Z.; Guo, X.; Zhong, H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials 2011, 32, 8555–8561. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Cheng, Y.J.; Zhang, X.Z. Recent advances in nanomaterials for enhanced photothermal therapy of tumors. Nanoscale 2018, 10, 22657–22672. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef] [PubMed]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Guo, X.; Li, W.; Zhou, J.; Hou, W.; Wen, X.; Zhang, H.; Kong, F.; Luo, L.; Li, Q.; Du, Y.; et al. Specific Photothermal Ablation Therapy of Endometriosis by Targeting Delivery of Gold Nanospheres. Small 2017, 13, 1603270. [Google Scholar] [CrossRef]
- Tian, Q.; Tang, M.; Sun, Y.; Zou, R.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv. Mater. 2011, 23, 3542–3547. [Google Scholar] [CrossRef]
- Podgaec, S.; Rizzo, L.V.; Fernandes, L.F.; Baracat, E.C.; Abrao, M.S. CD4+ CD25high Foxp3+ cells increased in the peritoneal fluid of patients with endometriosis. Am. J. Reprod. Immunol. 2012, 68, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Cousins, F.L.; Murray, A.; Esnal-Zufiaurre, A.; Fassbender, A.; Horne, A.W.; Saunders, P.T. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am. J. Pathol. 2014, 184, 1930–1939. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, P.; Liu, L.; Ma, G.; Ma, J.; Liu, X.; Liu, Y.; Lin, W.; Zhu, Y. Evaluation of PLGA containing anti-CTLA4 inhibited endometriosis progression by regulating CD4+CD25+Treg cells in peritoneal fluid of mouse endometriosis model. Eur. J. Pharm. Sci. 2017, 96, 542–550. [Google Scholar] [CrossRef]
- Antsiferova, Y.; Sotnikova, N.; Parfenyuk, E. Different effects of the immunomodulatory drug GMDP immobilized onto aminopropyl modified and unmodified mesoporous silica nanoparticles upon peritoneal macrophages of women with endometriosis. Biomed. Res. Int. 2013, 2013, 924362. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yuan, M.; Jiao, X.; Huang, Y.; Li, J.; Li, D.; Ji, M.; Wang, G. M1 Macrophage-Derived Nanovesicles Repolarize M2 Macrophages for Inhibiting the Development of Endometriosis. Front. Immunol. 2021, 12, 707784. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol. J. 2011, 6, 1342–1347. [Google Scholar] [CrossRef]
- Redolfi Riva, E.; Sinibaldi, E.; Grillone, A.F.; Del Turco, S.; Mondini, A.; Li, T.; Takeoka, S.; Mattoli, V. Enhanced In Vitro Magnetic Cell Targeting of Doxorubicin-Loaded Magnetic Liposomes for Localized Cancer Therapy. Nanomaterials 2020, 10, 2104. [Google Scholar] [CrossRef]
- Que-Gewirth, N.S.; Sullenger, B.A. Gene therapy progress and prospects: RNA aptamers. Gene Ther. 2007, 14, 283–291. [Google Scholar] [CrossRef]
- Chen, S.; Shimoda, M.; Chen, J.; Matsumoto, S.; Grayburn, P.A. Transient overexpression of cyclin D2/CDK4/GLP1 genes induces proliferation and differentiation of adult pancreatic progenitors and mediates islet regeneration. Cell Cycle 2012, 11, 695–705. [Google Scholar] [CrossRef]
- Zhao, M.D.; Sun, Y.M.; Fu, G.F.; Du, Y.Z.; Chen, F.Y.; Yuan, H.; Zheng, C.H.; Zhang, X.M.; Hu, F.Q. Gene therapy of endometriosis introduced by polymeric micelles with glycolipid-like structure. Biomaterials 2012, 33, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, B.; Liang, L.; Wu, Y.; Xie, H.; Huang, J.; Guo, X.; Tan, J.; Zhan, X.; Liu, Y.; et al. Antiangiogenesis therapy of endometriosis using PAMAM as a gene vector in a noninvasive animal model. Biomed. Res. Int. 2014, 2014, 546479. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, Y.; Zhao, Y.; Xu, C.; Zhang, A.; Zhang, Q.; Wang, D.; He, J.; Hua, W.; Duan, P. miR-200c suppresses endometriosis by targeting MALAT1 in vitro and in vivo. Stem Cell Res. Ther. 2017, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.D.; Cheng, J.L.; Yan, J.J.; Chen, F.Y.; Sheng, J.Z.; Sun, D.L.; Chen, J.; Miao, J.; Zhang, R.J.; Zheng, C.H.; et al. Hyaluronic acid reagent functional chitosan-PEI conjugate with AQP2-siRNA suppressed endometriotic lesion formation. Int. J. Nanomed. 2016, 11, 1323–1336. [Google Scholar] [CrossRef]
- Zheng, D.; Huo, M.; Li, B.; Wang, W.; Piao, H.; Wang, Y.; Zhu, Z.; Li, D.; Wang, T.; Liu, K. The Role of Exosomes and Exo-somal MicroRNA in Cardiovascular Disease. Front. Cell Dev. Biol. 2020, 8, 616161. [Google Scholar] [CrossRef]

| Application | Nanomaterial | Cargo | References |
|---|---|---|---|
| MRI | Iron oxide NPs | N/A | Lee et al., 2012 [36] |
| Zhang et al., 2014 [38] | |||
| Fluorescence imaging | PEG-PCL polymeric NPs | NIR dye (SiNc) | Moses et al., 2020 [30] |
| Photothermal therapy | Hollow gold nanospheres | N/A | Guo et al., 2017 [61] |
| PEG-PCL polymeric NPs | NIR dye (SiNc) | Moses et al., 2020 [30] | |
| Therapeutic agent | Nanoceria | Chaudhury et al., 2013 [45] | |
| Drug Carrying NPs | PLGA | Epigallocatechin gallate and doxycycline | Singh et al., 2015 [50] |
| PLGA | Copaiba oleoresin | de Almeida Borges et al., 2018 [52] | |
| PEG and PCL nanofibers | Curcumin | Boroumand et al., 2019 [53] | |
| Immunotherapy | PLGA | Anti-CTLA-4 | Liu et al., 2017 [65] |
| M1NVs (nanovesicles derived from M1 macrophages) | Li et al., 2021 [67] | ||
| Aminopropyl modified silica NPs (AMNPs) | GMDP (glucosaminyl muramyl dipeptide) | Y. Antsiferova et al., 2013 [66] | |
| Magnetic hyperthermia | Hexagonal iron oxide NPs coated by PEG-PCL | KDR | Park et al., 2022 [2] |
| Gene Therapy | CSO-SA | PEDF | Zhao et al., 2012 [72] |
| (CSO-PEI) HA | AQP2-siRNA | Zhao et al., 2016 [75] | |
| PAMAM | Endostatin | Wang et al., 2014 [73] | |
| PEI–PEG–RGD | MiR-200c | Liang et al., 2017 [74] | |
| Exosome | miR-21-3p | Zhang et al., 2021 [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talukdar, S.; Singh, S.K.; Mishra, M.K.; Singh, R. Emerging Trends in Nanotechnology for Endometriosis: Diagnosis to Therapy. Nanomaterials 2024, 14, 976. https://doi.org/10.3390/nano14110976
Talukdar S, Singh SK, Mishra MK, Singh R. Emerging Trends in Nanotechnology for Endometriosis: Diagnosis to Therapy. Nanomaterials. 2024; 14(11):976. https://doi.org/10.3390/nano14110976
Chicago/Turabian StyleTalukdar, Souvanik, Santosh K. Singh, Manoj K. Mishra, and Rajesh Singh. 2024. "Emerging Trends in Nanotechnology for Endometriosis: Diagnosis to Therapy" Nanomaterials 14, no. 11: 976. https://doi.org/10.3390/nano14110976
APA StyleTalukdar, S., Singh, S. K., Mishra, M. K., & Singh, R. (2024). Emerging Trends in Nanotechnology for Endometriosis: Diagnosis to Therapy. Nanomaterials, 14(11), 976. https://doi.org/10.3390/nano14110976










