Effect of Nanoporous Molecular Sieves TS-1 on Electrical Properties of Crosslinked Polyethylene Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
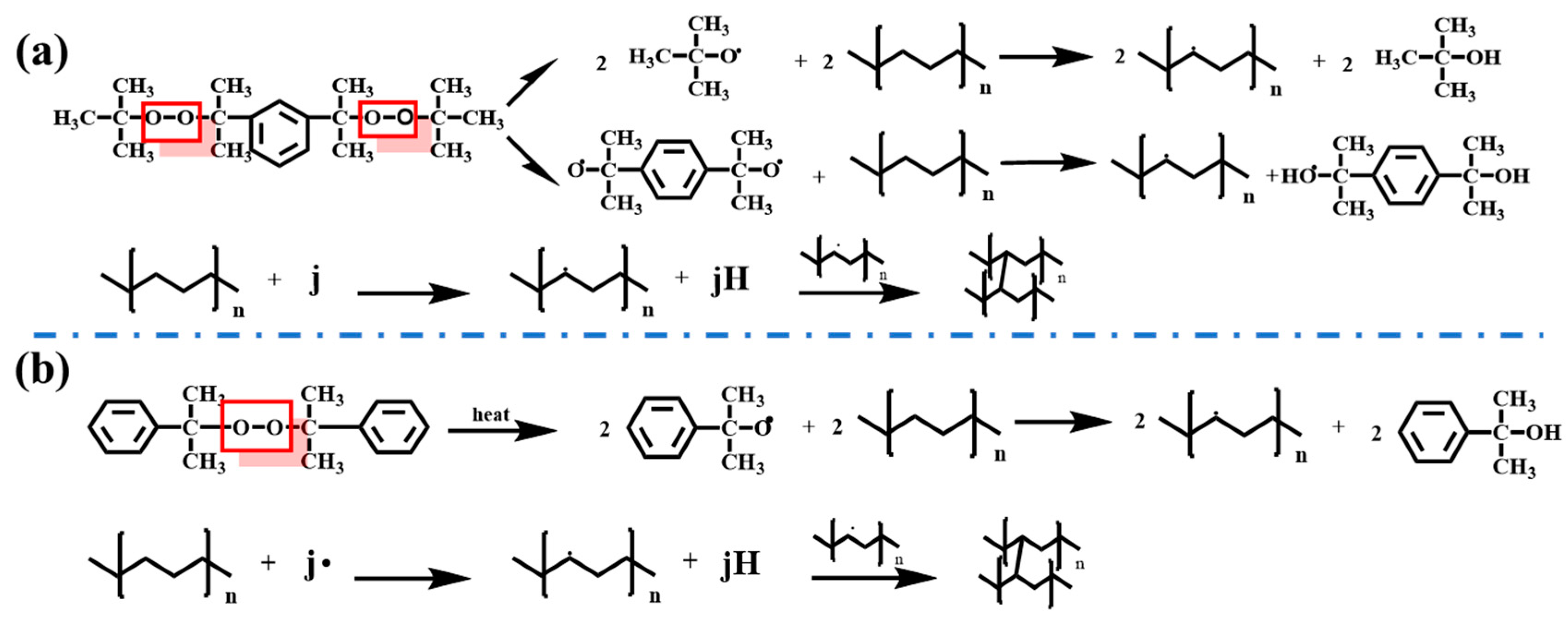
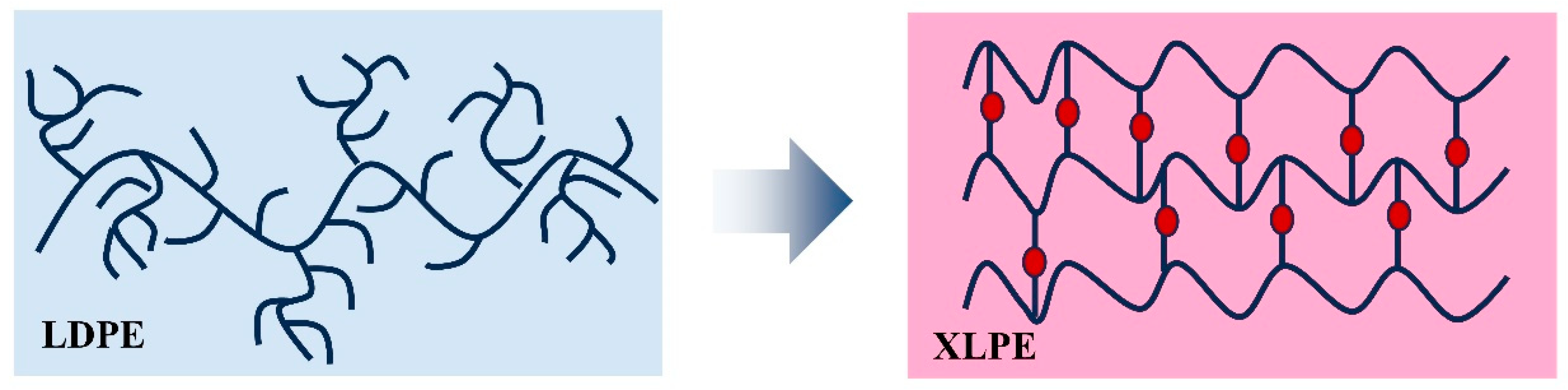
2.1. Synthesis of XLPE
2.2. Synthesis of TS-1
2.3. Preparation of TS-1/XLPE Nanocomposites
3. Results and Discussion
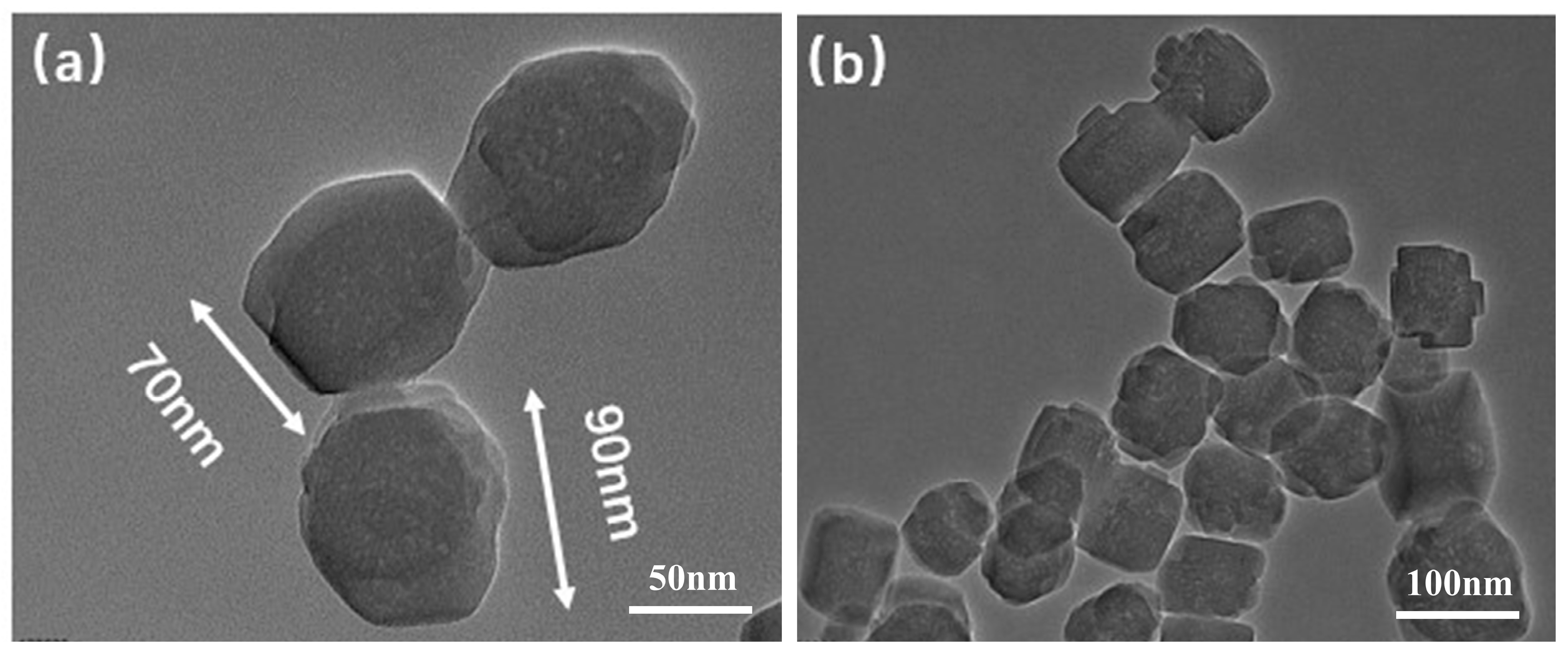
3.1. TEM
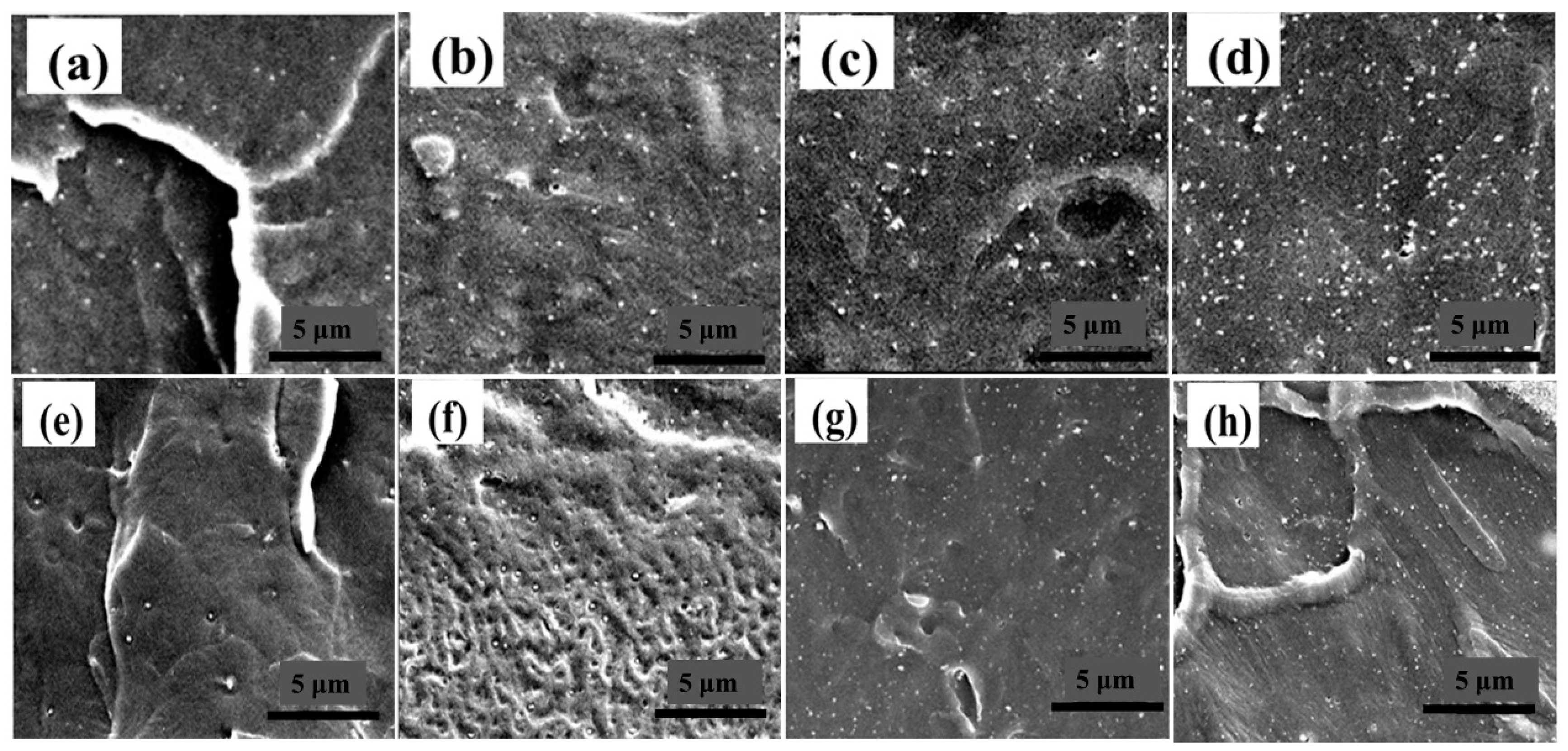
3.2. SEM
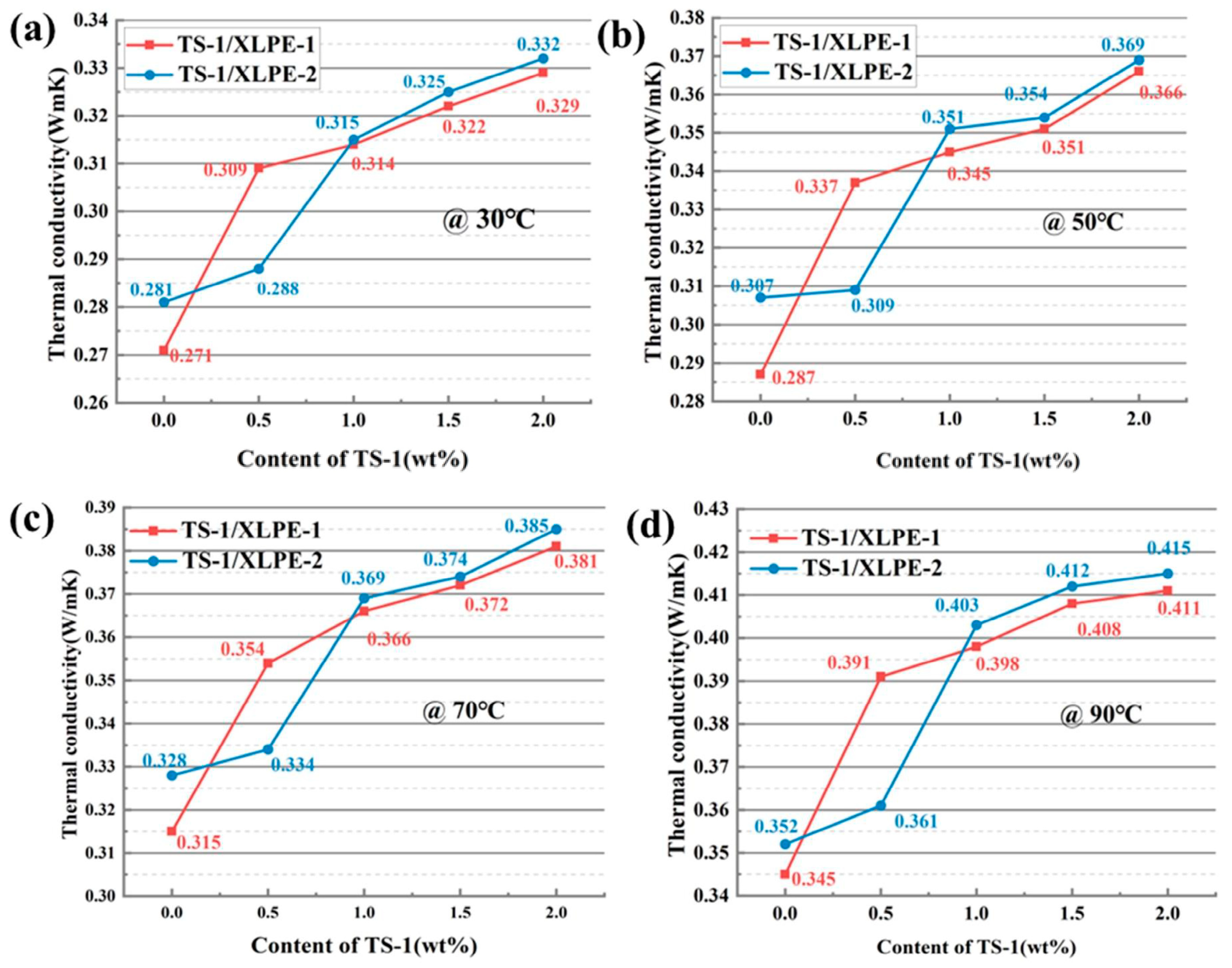
3.3. Thermal Conductivity Analysis (TC)
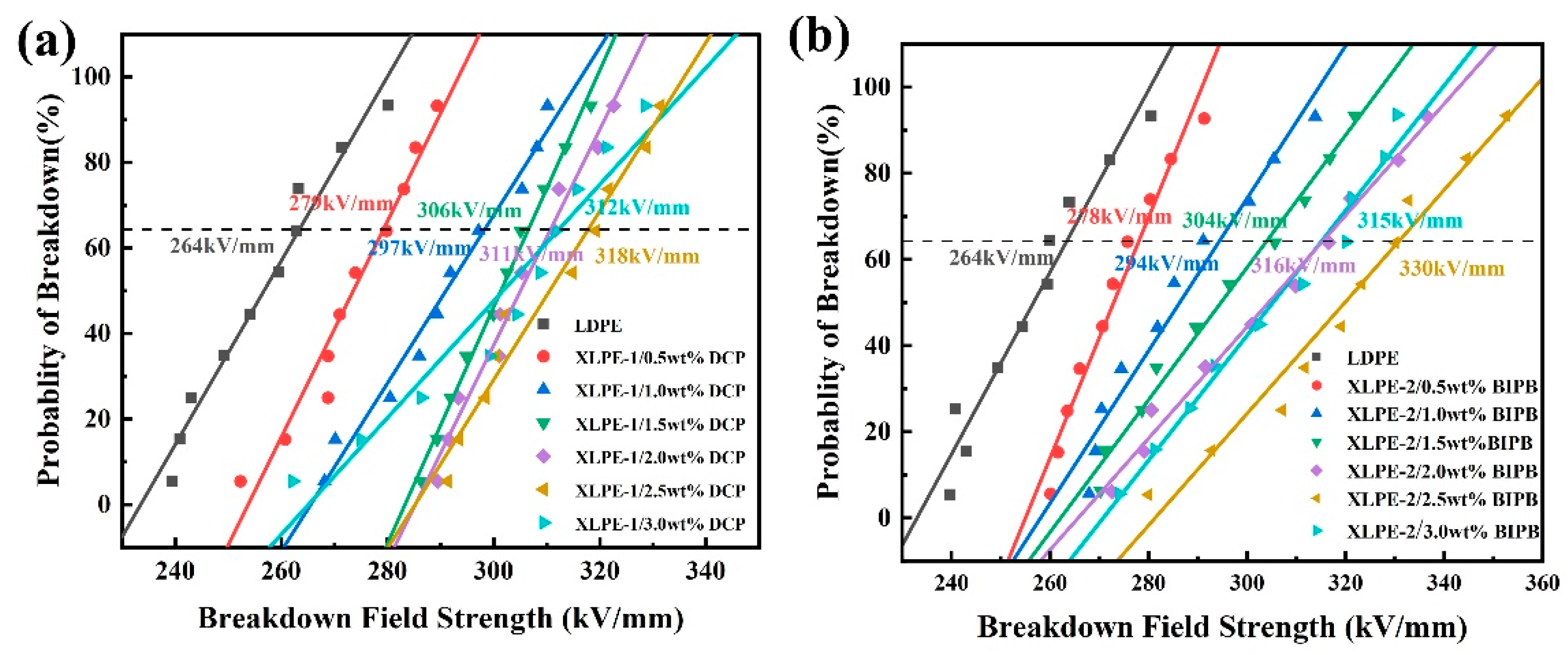
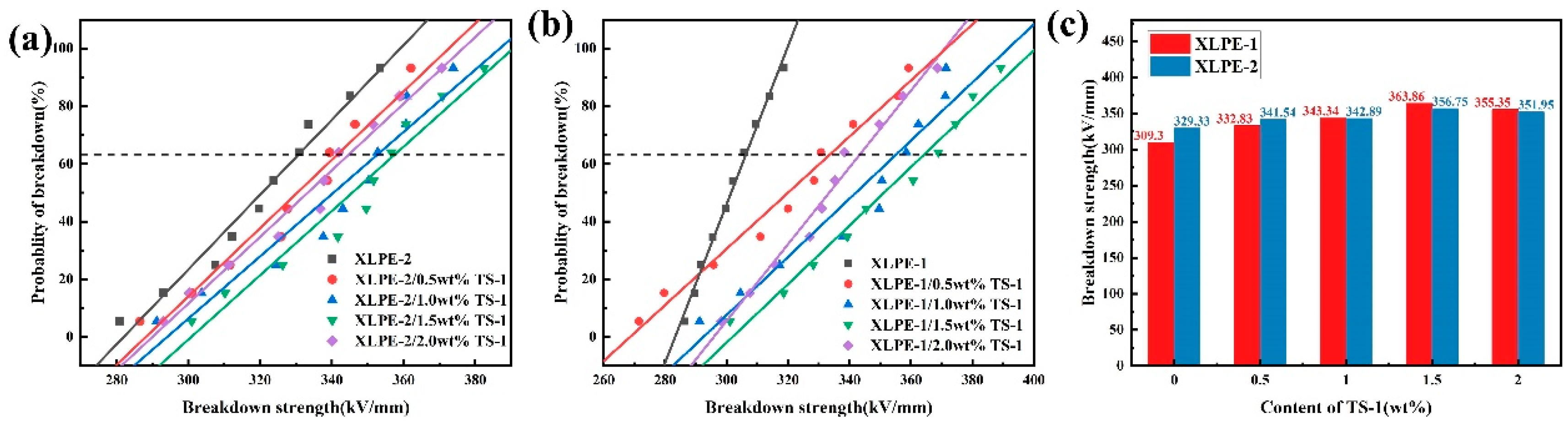
3.4. Breakdown Strength (Eb)
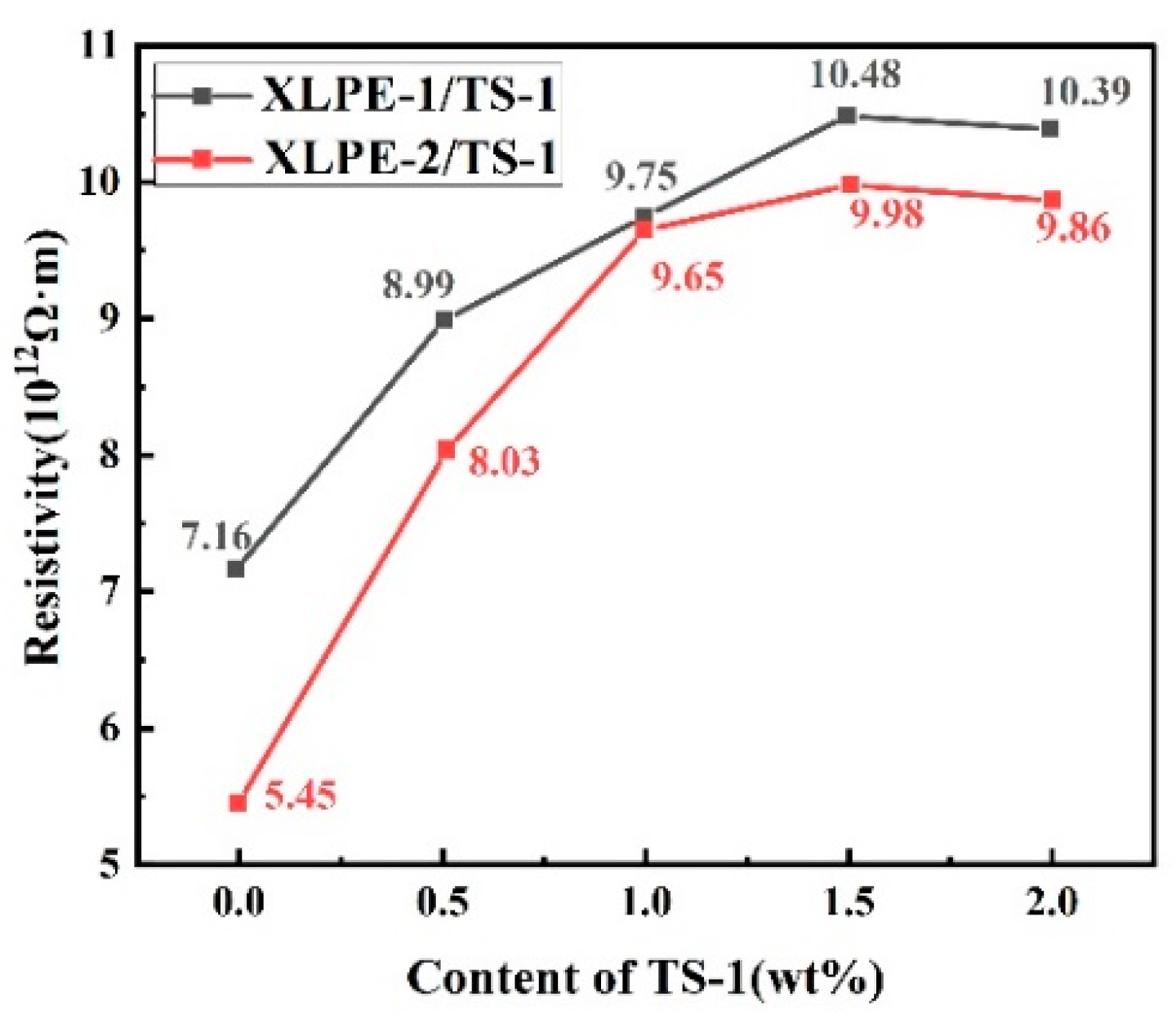
3.5. Resistivity (Rv)
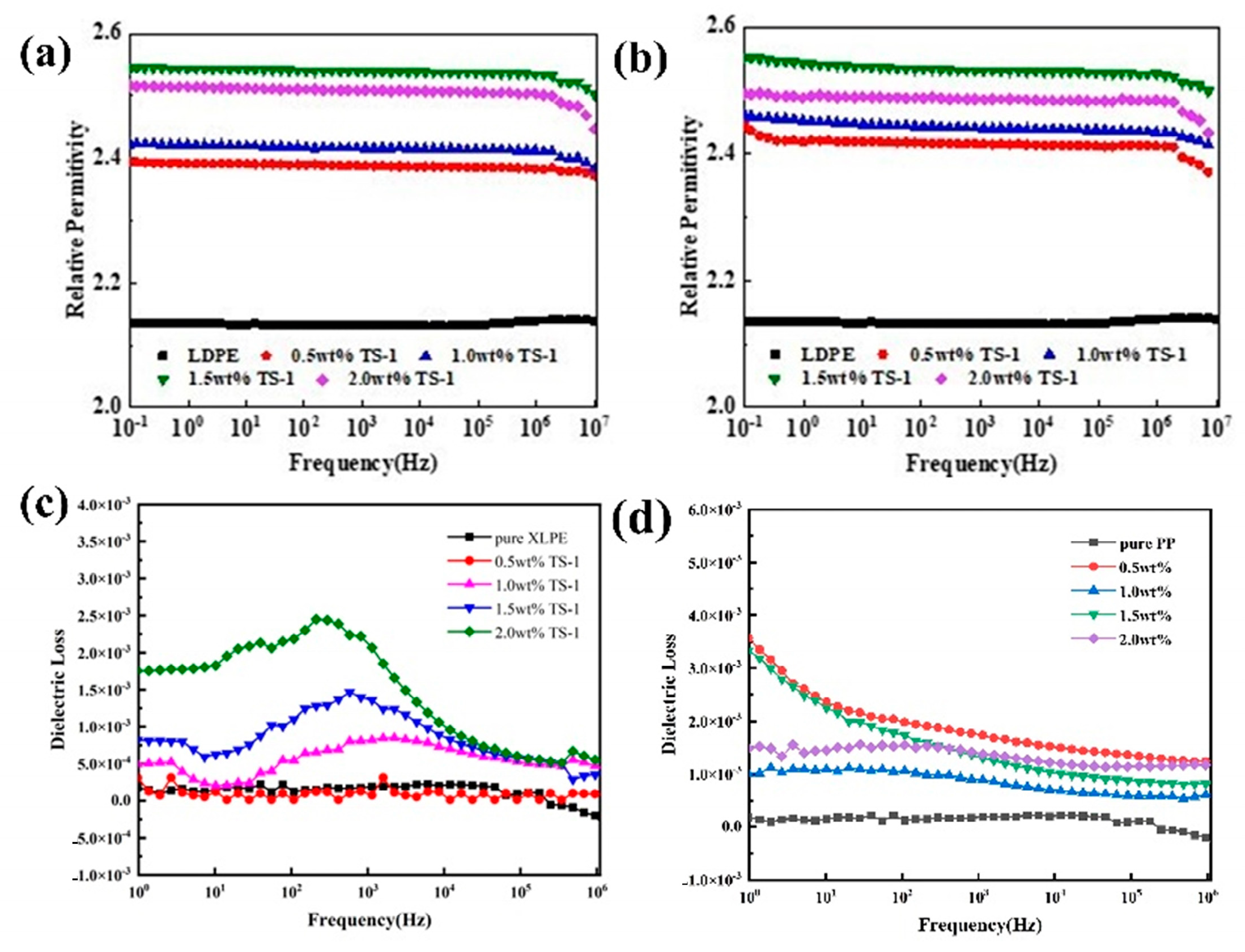
3.6. Dielectric Constant
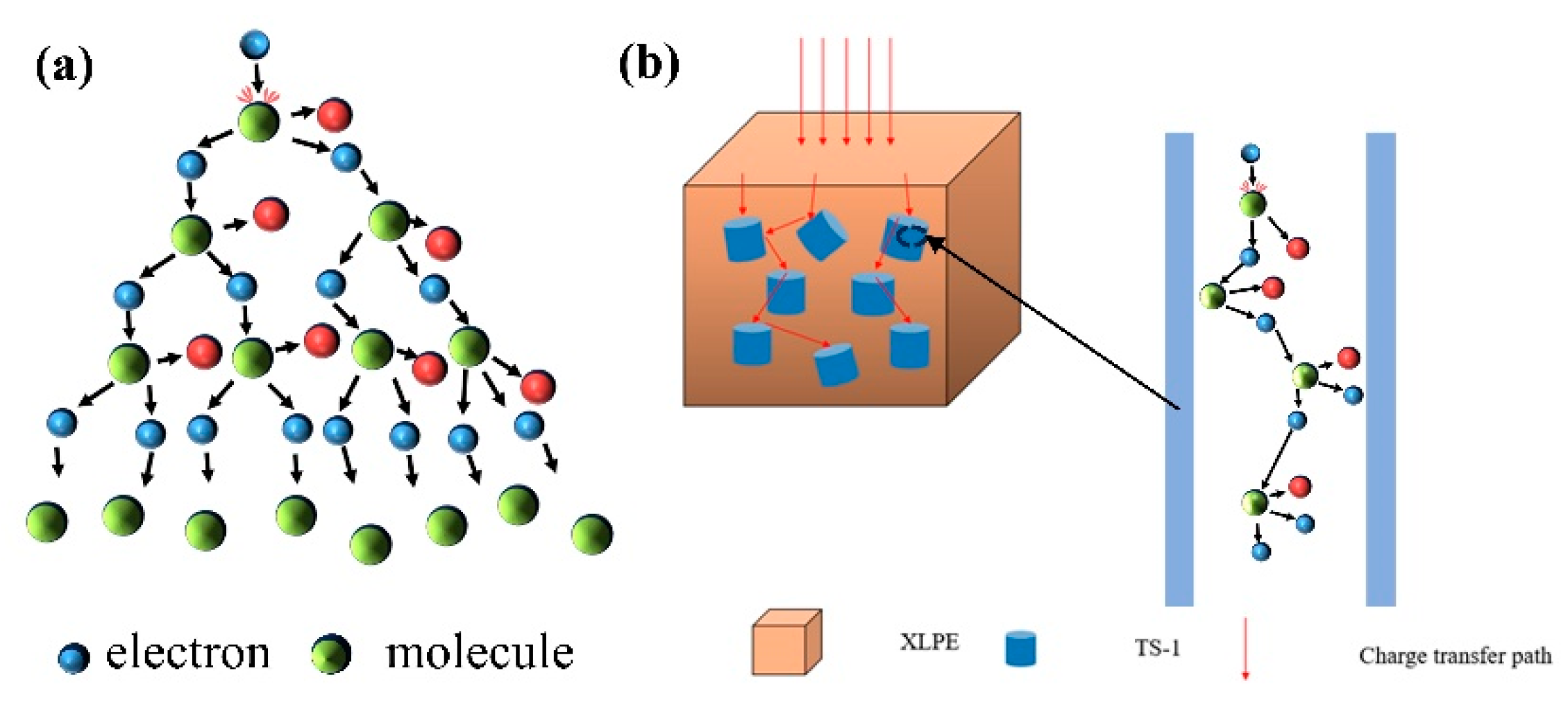
3.7. Space Charge Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, Y.-H.; Mu, H.-B.; Deng, J.-B.; Zhang, G.-J. Effect of Space Charge on Breakdown Characteristics of Aged Oil-paper Insulation under DC Voltage. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3143–3150. [Google Scholar] [CrossRef]
- Said, A.R.; Nawar, A.G.; Elsayed, A.E.; Abd-Allah, M.A.; Kamel, S. Enhancing Electrical, Thermal, and Mechanical Properties of HV Cross-Linked Polyethylene Insulation Using Silica Nanofillers. J. Mater. Eng. Perform. 2021, 30, 1796–1807. [Google Scholar] [CrossRef]
- Saha, T.; Purkait, P. Investigation of polarization and depolarization current measurements for the assessment of oil-paper insulation of aged transformers. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 144–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Lewiner, J.; Alquie, C.; Hampton, N. Evidence of strong correlation between space-charge buildup and breakdown in cable insulation. IEEE Trans. Dielectr. Electr. Insul. 1996, 3, 778–783. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Xiao, K. Investigation of the electrical properties of XLPE/SiC nanocomposites. Polym. Test. 2016, 50, 145–151. [Google Scholar] [CrossRef]
- Kochetov, R.; Tsekmes, I.A.; Morshuis, P.H.F. Electrical conductivity, dielectric response and space charge dynamics of an electroactive polymer with and without nanofiller reinforcement. Smart Mater. Struct. 2015, 24, 7. [Google Scholar] [CrossRef]
- Morshuis, P.; Jeroense, M. Space charge measurements on impregnated paper: A review of the PEA method and a discussion of results. IEEE Electr. Insul. Mag. 1997, 13, 26–35. [Google Scholar] [CrossRef]
- Mizutani, T. Space Charge Measurement Techniques and Space Charge in Pol yet hylene. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 923–933. [Google Scholar] [CrossRef]
- Pleşa, I.; Noţingher, P.V.; Stancu, C.; Wiesbrock, F.; Schlögl, S. Polyethylene Nanocomposites for Power Cable Insulations. Polymers 2018, 11, 24. [Google Scholar] [CrossRef]
- Sangermano, M.; Deorsola, F.; Fabiani, D.; Montanari, G.; Rizza, G. Epoxy-boehmite nanocomposites as new insulating materials. J. Appl. Polym. Sci. 2009, 114, 2541–2546. [Google Scholar] [CrossRef]
- Sarathi, R.; Sahu, R.K.; Rajeshkumar, P. Understanding the thermal, mechanical and electrical properties of epoxy nanocomposites. Mater. Sci. Eng. A 2007, 445–446, 567–578. [Google Scholar] [CrossRef]
- Thelakkadan, A.; Coletti, G.; Guastavino, F.; Fina, A. Thermomechanical and electrical characterization of epoxy-organoclay nanocomposites. Polym. Eng. Sci. 2011, 52, 1037–1046. [Google Scholar] [CrossRef]
- Thelakkadan, A.; Coletti, G.; Guastavino, F.; Fina, A. Effect of the nature of clay on the thermo-mechanodynamical and electrical properties of epoxy/clay nanocomposites. Polym. Compos. 2011, 32, 1499–1504. [Google Scholar] [CrossRef]
- Roy, M.; Nelson, J.K.; MacCrone, R.K.; Schadler, L.S. Candidate mechanisms controlling the electrical characteristics of silica/XLPE nanodielectrics. J. Mater. Sci. 2007, 42, 3789–3799. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Jee, Y.-S.; Yoon, H.-J.; Kim, S. Comparison of general and epidural anesthesia in elective cesarean section for placenta previa totalis: Maternal hemodynamics, blood loss and neonatal outcome. Int. J. Obstet. Anesthesia 2003, 12, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jiang, P.; Liu, F.; Hyon, S.; Ri, M.-G.; Yu, Y.; Ho, M. Investigation on dielectric breakdown behavior of thermally aged cross-linked polyethylene cable insulation. Polym. Test. 2019, 80, 106045. [Google Scholar] [CrossRef]
- Xin, M.; Chang, Z.; Luo, H.; Zeng, R.; Zhang, G.; Lei, Q. An electrical super-insulator prototype of 1D gas-solid Al2O3 nanocell. Nano Energy 2017, 39, 95–100. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Q.; Zhou, X.; Xing, Z.; Hao, C. Effect of Different TS-1 on the Electrical Properties of Cross-Linked Polyethylene. ECS J. Solid State Sci. Technol. 2023, 12, 123003. [Google Scholar] [CrossRef]
- Yujia, L.; Yu, L.; Qingyui, W.; Chuncheng, H.; Qingquan, L.; Xiangjin, G. Effect of the cross-linking agent on the cross-linking degree and electrical properties of cross-linked polyethylene. Mater. Res. Express 2023, 10, 055301. [Google Scholar] [CrossRef]
- Wang, C.-T.; Wu, C.-L.; Chen, I.-C.; Huang, Y.-H. Humidity sensors based on silica nanoparticle aerogel thin films. Sensors Actuators B Chem. 2005, 107, 402–410. [Google Scholar] [CrossRef]
- Sudhakar, K.; Reddy, N.N.; Jayaramudu, T.; Jayaramudu, J.; Reddy, A.B.; Manjula, B.; Sadiku, E.R. Aerogels and Foamed Nanostructured Polymer Blends. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 75–99. [Google Scholar] [CrossRef]
- Sinkó, K. Influence of Chemical Conditions on the Nanoporous Structure of Silicate Aerogels. Materials 2010, 3, 704–740. [Google Scholar] [CrossRef]
- Bi, Y.-T.; Li, Z.-J.; Liu, T.-S. Preparation and characterization of Octa(aminophenyl)silsesquioxane–aldehyde organic/inorganic hybrid aerogel. Eur. Polym. J. 2014, 58, 201–206. [Google Scholar] [CrossRef]
- Madyan, O.A.; Fan, M.; Feo, L.; Hui, D. Physical properties of clay aerogel composites: An overview. Compos. Part B Eng. 2016, 102, 29–37. [Google Scholar] [CrossRef]
- Wei, L.; Hu, N.; Zhang, Y. Synthesis of Polymer—Mesoporous Silica Nanocomposites. Materials 2010, 3, 4066–4079. [Google Scholar] [CrossRef]
- Zhang, H.; Qiao, Y.; Zhang, X.; Fang, S. Structural and thermal study of highly porous nanocomposite SiO2-based aerogels. J. Non-Crystalline Solids 2010, 356, 879–883. [Google Scholar] [CrossRef]
- Ai, X.; Wang, D.; Li, X.; Pan, H.; Kong, J.; Yang, H.; Zhang, H.; Dong, L. The properties of chemical cross-linked poly(lactic acid) by bis(tert-butyl dioxy isopropyl) benzene. Polym. Bull. 2018, 76, 575–594. [Google Scholar] [CrossRef]
- Qiu, G.; Ma, W.; Jiao, Y.; Wu, L. Low-dielectric-constant aromatic homopolyimide and copolyimide derived from pyromellitic dianhydride, 4,4′-oxydianiline, 2,2-bis[4-(4-aminephenoxy)phenyl]propane, 1,4-bis(4-aminophenoxy)benzene, or 1,3-bis(4-aminophenoxy)benzene. J. Appl. Polym. Sci. 2019, 136, 47405. [Google Scholar] [CrossRef]
- Wang, W.; Chen, G.; Fang, X. 1,4-Bis(2,3-dicarboxyl-phenoxy)benzene dianhydride-based phenylethynyl terminated thermoset oligoimides for resin transfer molding applications. J. Appl. Polym. Sci. 2019, 136, 47967. [Google Scholar] [CrossRef]
- Conley, M.L.; Mohammed, F.S.; Winslow, C.; Eldridge, H.; Cogen, J.M.; Chaudhary, B.I.; Pollet, P.; Liotta, C.L. Mechanism of Acid-Catalyzed Decomposition of Dicumyl Peroxide in Dodecane: Intermediacy of Cumene Hydroperoxide. Ind. Eng. Chem. Res. 2016, 55, 5865–5873. [Google Scholar] [CrossRef]
- D’Elia, R.; Dusserre, G.; Del Confetto, S.; Eberling-Fux, N.; Descamps, C.; Cutard, T. Effect of dicumyl peroxide concentration on the polymerization kinetics of a polysilazane system. Polym. Eng. Sci. 2017, 58, 859–869. [Google Scholar] [CrossRef]
- Liu, L.; Hou, J.; Wang, L.; Zhang, J.; Duan, Y. Role of Dicumyl Peroxide on Toughening PLLA via Dynamic Vulcanization. Ind. Eng. Chem. Res. 2016, 55, 9907–9914. [Google Scholar] [CrossRef]
- Sari, N.H.; Sanjay, M.; Arpitha, G.; Pruncu, C.I.; Siengchin, S. Synthesis and properties of pandanwangi fiber reinforced polyethylene composites: Evaluation of dicumyl peroxide (DCP) effect. Compos. Commun. 2019, 15, 53–57. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, R.; Ruan, K.; Ma, T.; Guo, Y.; Gu, J. Improvement of thermal conductivities and simulation model for glass fabrics reinforced epoxy laminated composites via introducing hetero-structured BNN-30@BNNS fillers. J. Mater. Sci. Technol. 2021, 82, 239–249. [Google Scholar] [CrossRef]
- Nazeran, N.; Moghaddas, J. Synthesis and characterization of silica aerogel reinforced rigid polyurethane foam for thermal insulation application. J. Non-Crystalline Solids 2017, 461, 1–11. [Google Scholar] [CrossRef]
- Wen, X. One-pot route to graft long-chain polymer onto silica nanoparticles and its application for high-performance poly(l-lactide) nanocomposites. RSC Adv. 2019, 9, 13908–13915. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Feng, H.; Wei, Y.; Li, G.; Zhu, Y.; Hao, C.; Lei, Q.; Li, S. Effect of barium strontium titanate modification on the dielectric-breakdown properties of phenyl-vinyl binary composite silicone rubber. Ceram. Int. 2023, 49, 14057–14063. [Google Scholar] [CrossRef]
- Goc, K.; Gaska, K.; Klimczyk, K.; Wujek, A.; Prendota, W.; Jarosinski, L.; Rybak, A.; Kmita, G.; Kapusta, C. Influence of magnetic field-aided filler orientation on structure and transport properties of ferrite filled composites. J. Magn. Magn. Mater. 2016, 419, 345–353. [Google Scholar] [CrossRef]
- Huang, L.; Lv, X.; Tang, Y.; Ge, G.; Zhang, P.; Li, Y. Effect of Alumina Nanowires on the Thermal Conductivity and Electrical Performance of Epoxy Composites. Polymers 2020, 12, 2126. [Google Scholar] [CrossRef]
- Jarosinski, L.; Rybak, A.; Gaska, K.; Kmita, G.; Porebska, R.; Kapusta, C. Enhanced thermal conductivity of graphene nanoplatelets epoxy composites. Mater. Sci. 2017, 35, 382–389. [Google Scholar] [CrossRef]
- Ren, L.; Pashayi, K.; Fard, H.R.; Kotha, S.P.; Borca-Tasciuc, T.; Ozisik, R. Engineering the coefficient of thermal expansion and thermal conductivity of polymers filled with high aspect ratio silica nanofibers. Compos. Part B Eng. 2014, 58, 228–234. [Google Scholar] [CrossRef]
- Li, S.; Yu, S.; Feng, Y. Progress in and prospects for electrical insulating materials. High Volt. 2016, 1, 122–129. [Google Scholar] [CrossRef]
- Topkan, E.; Somay, E. In reply to Chatzopoulos et al. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101458. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, L.; Zhang, C.; Xing, Z.; Kang, Y.; Han, W.; Xin, M.; Hao, C. Effect of Nanoporous Molecular Sieves TS-1 on Electrical Properties of Crosslinked Polyethylene Nanocomposites. Nanomaterials 2024, 14, 985. https://doi.org/10.3390/nano14110985
Shi L, Zhang C, Xing Z, Kang Y, Han W, Xin M, Hao C. Effect of Nanoporous Molecular Sieves TS-1 on Electrical Properties of Crosslinked Polyethylene Nanocomposites. Nanomaterials. 2024; 14(11):985. https://doi.org/10.3390/nano14110985
Chicago/Turabian StyleShi, Lirui, Chong Zhang, Zhaoliang Xing, Yuanyi Kang, Weihua Han, Meng Xin, and Chuncheng Hao. 2024. "Effect of Nanoporous Molecular Sieves TS-1 on Electrical Properties of Crosslinked Polyethylene Nanocomposites" Nanomaterials 14, no. 11: 985. https://doi.org/10.3390/nano14110985
APA StyleShi, L., Zhang, C., Xing, Z., Kang, Y., Han, W., Xin, M., & Hao, C. (2024). Effect of Nanoporous Molecular Sieves TS-1 on Electrical Properties of Crosslinked Polyethylene Nanocomposites. Nanomaterials, 14(11), 985. https://doi.org/10.3390/nano14110985





