Simple Synthesis of Cellulose-Based Nanocomposites as SERS Substrates for In Situ Detection of Thiram
Abstract
:1. Introduction
2. Materials and Methods
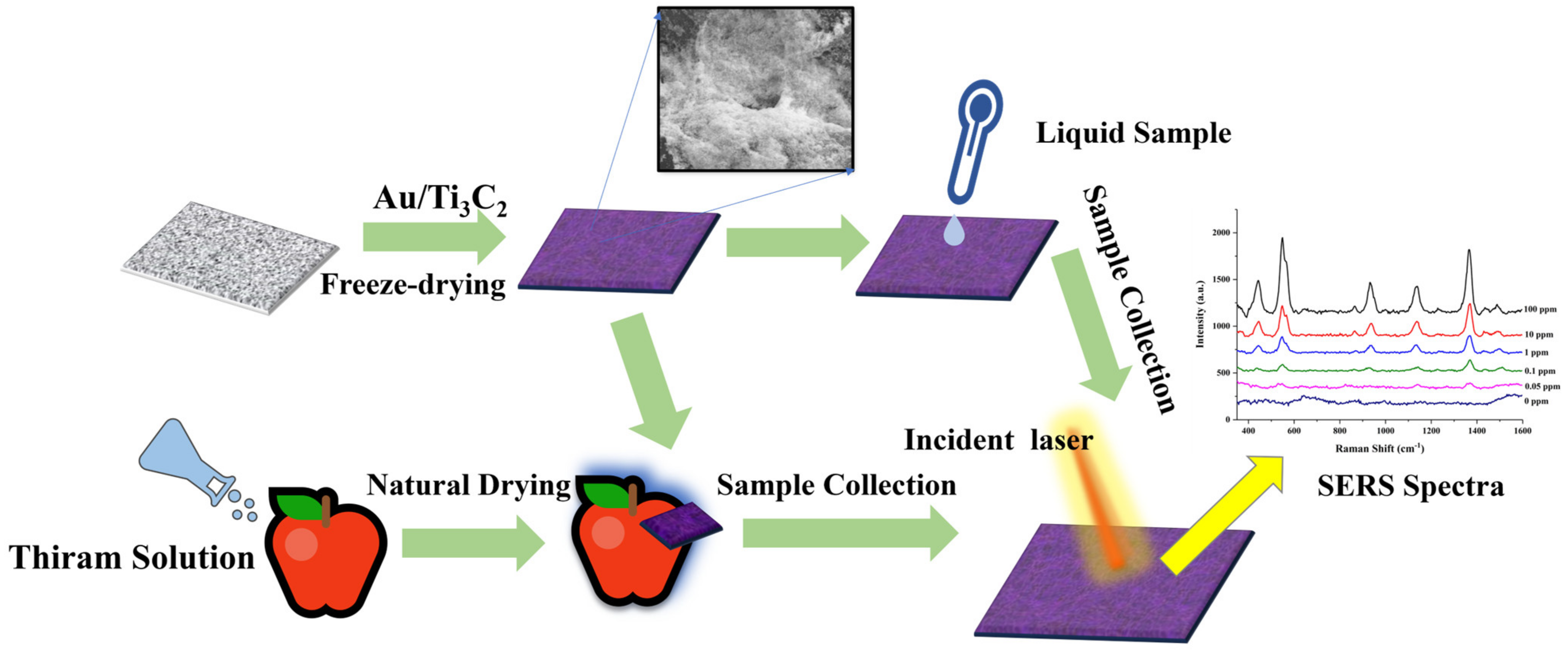
2.1. Synthesis of CS/Au/Ti3C2
2.2. SERS Measurement
3. Results and Discussions
3.1. Characterization of CS/Au/Ti3C2
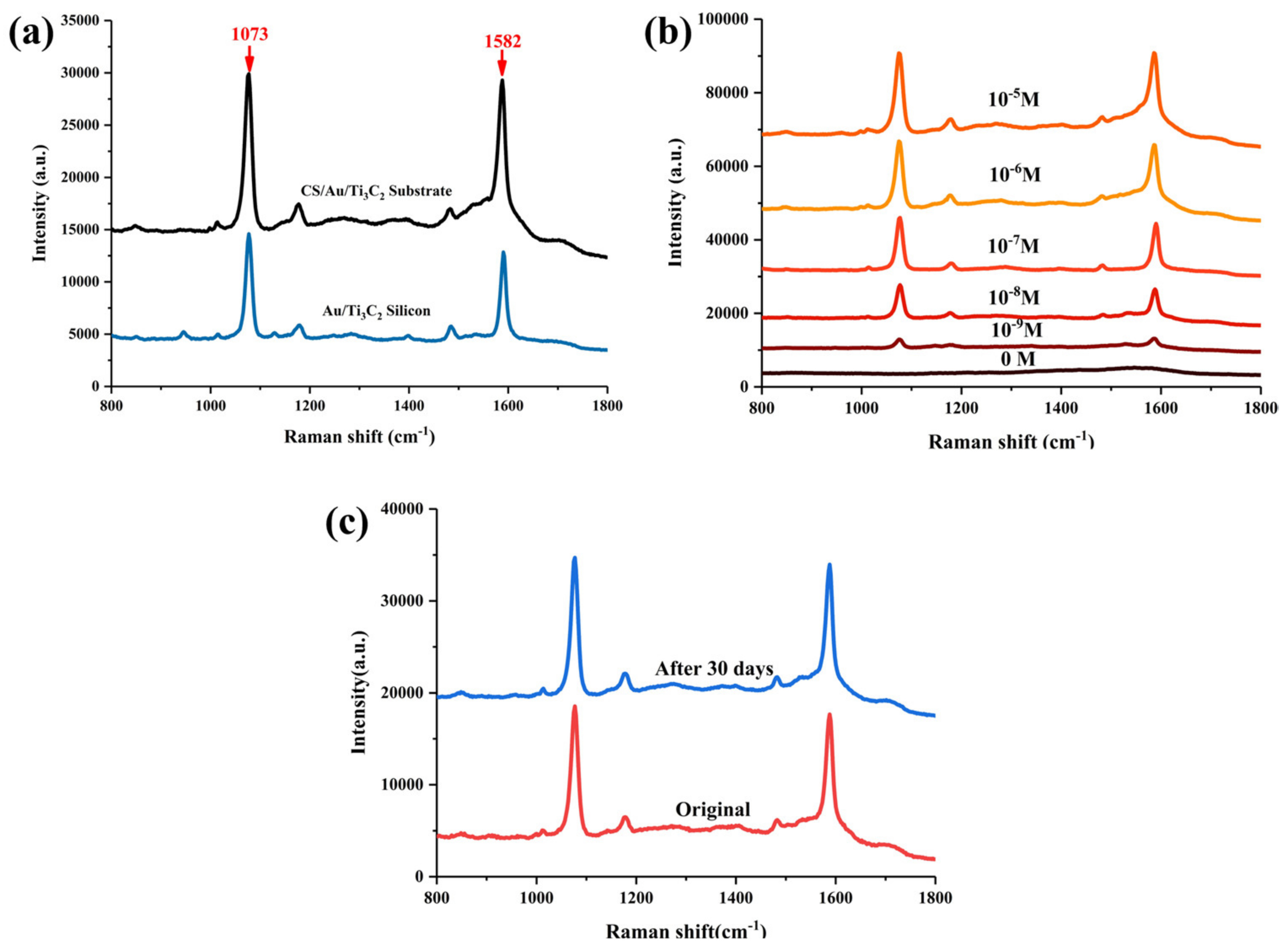
3.2. SERS Properties of CS/Au/Ti3C2
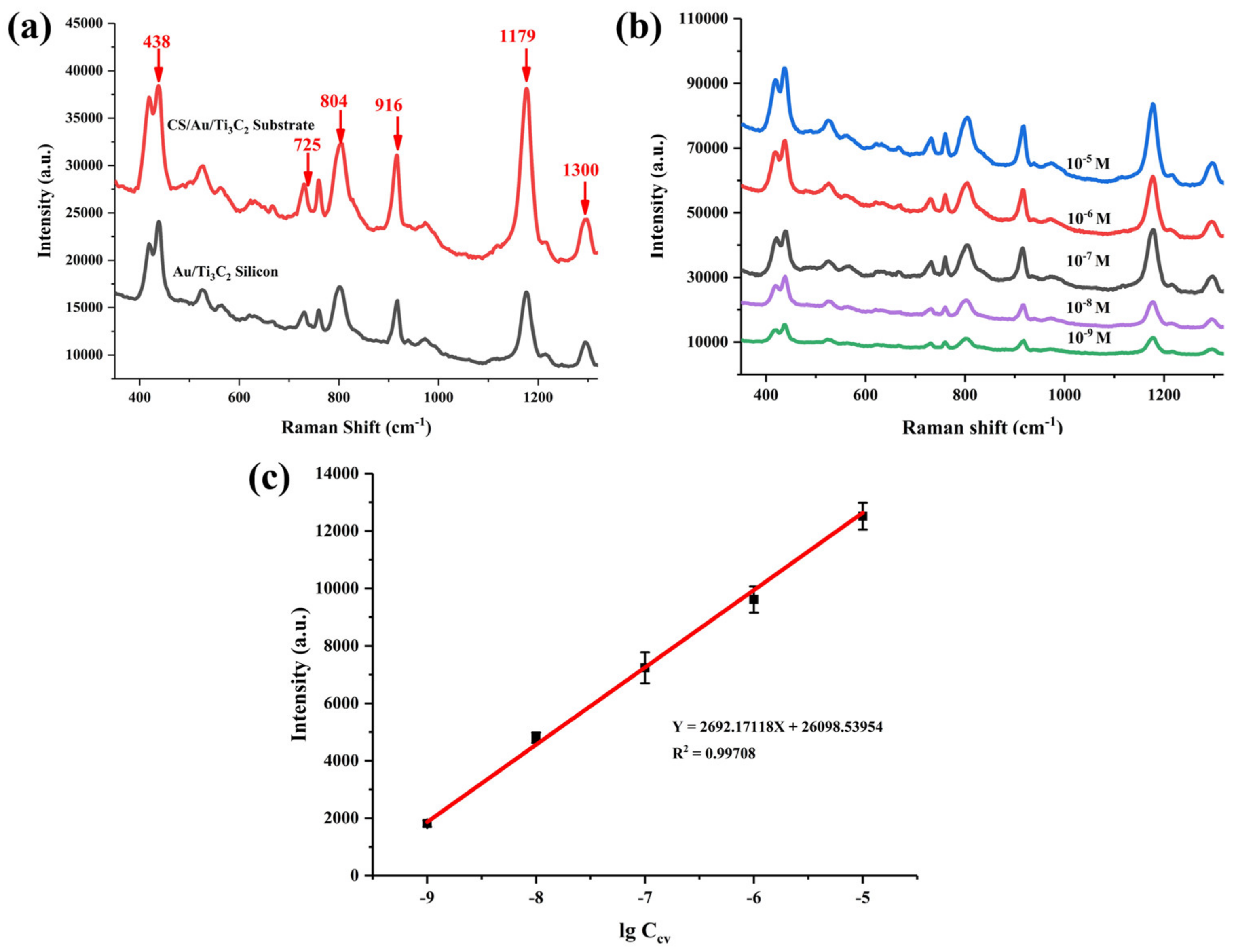
3.3. SERS Detection of CV
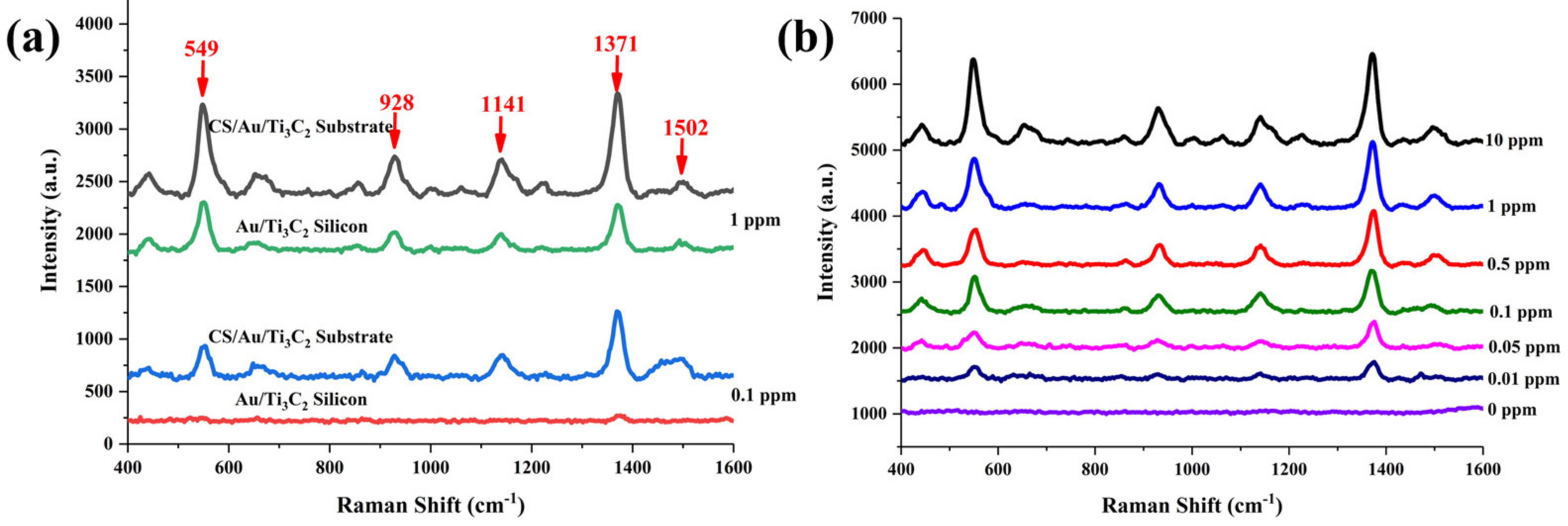
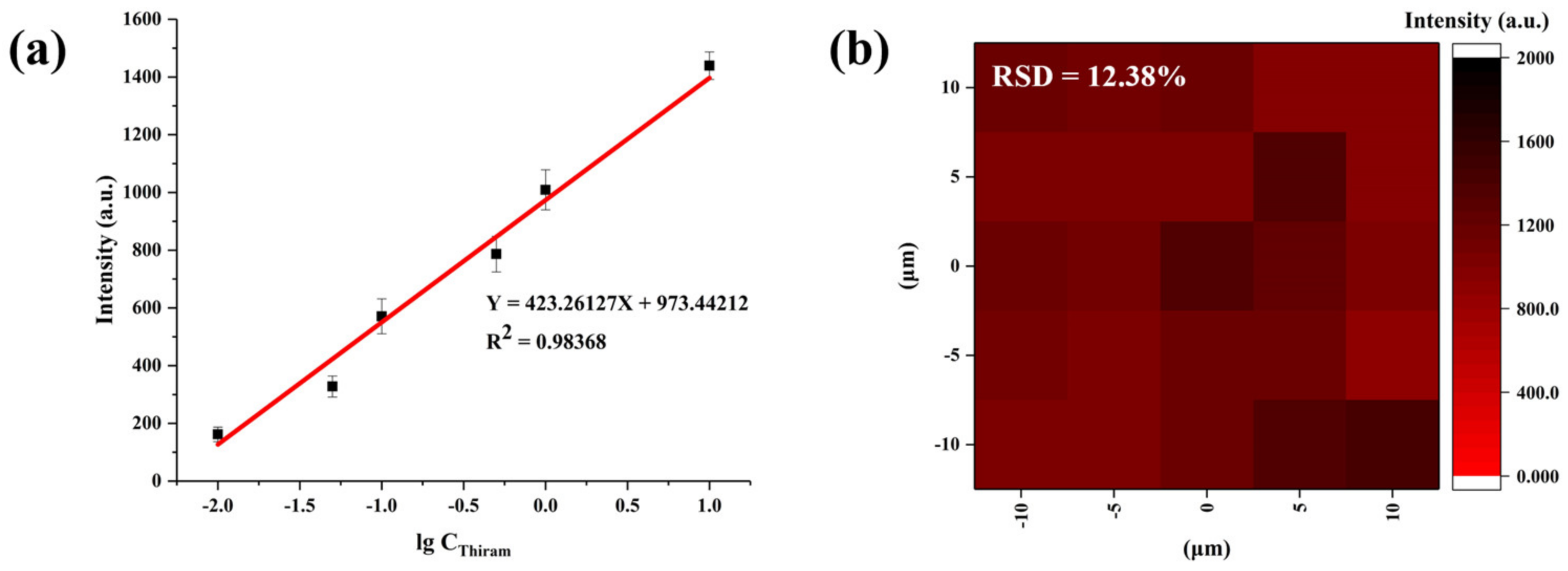
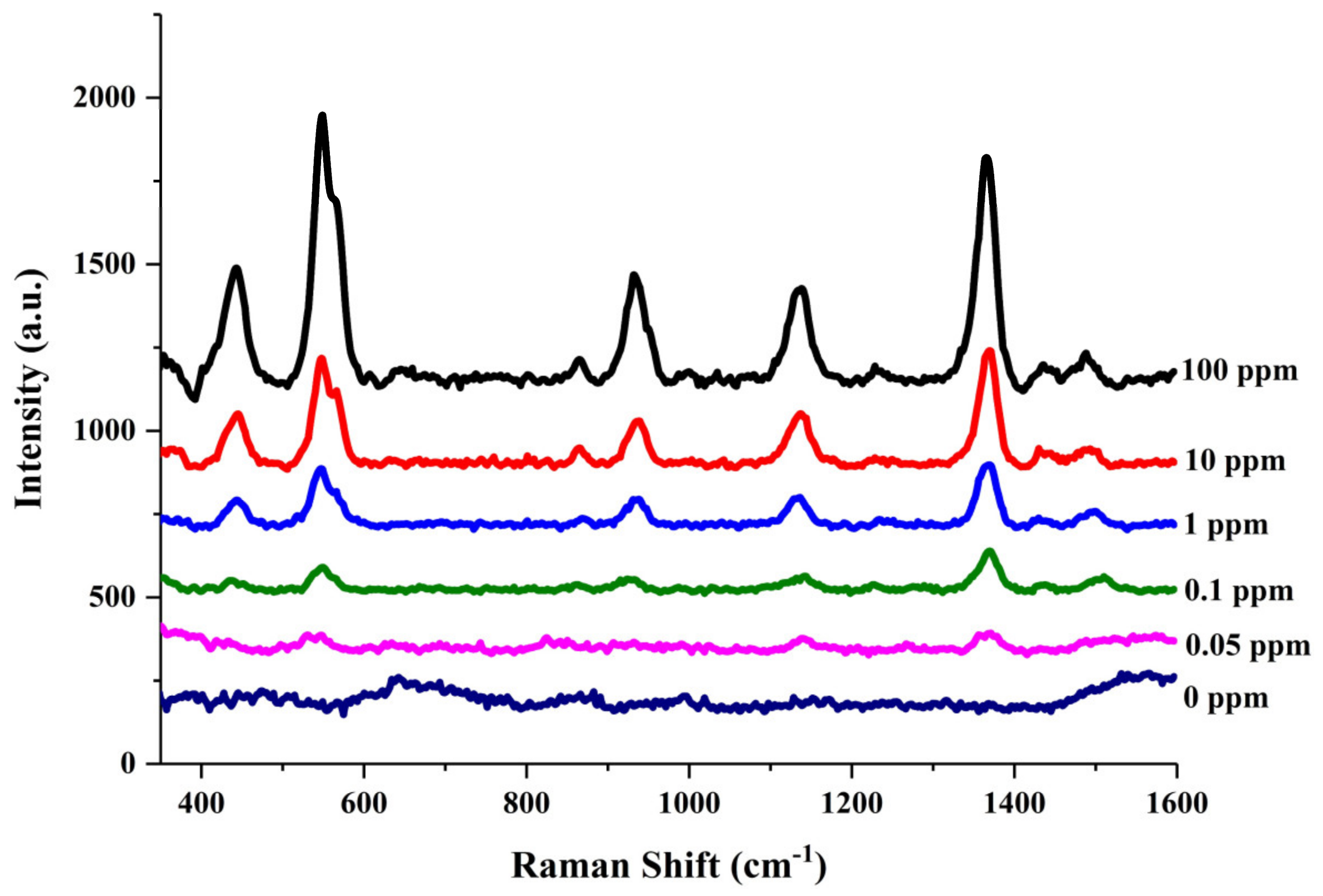
3.4. SERS Detection of Thiram
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duan, N.; Yao, T.B.; Li, C.X.; Wang, Z.P.; Wu, S.J. Surface-enhanced Raman spectroscopy relying on bimetallic Au-Ag nanourchins for the detection of the food allergen β-lactoglobulin. Talanta 2022, 245, 8. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.M.; Yang, B.B.; Wen, X.D.; Su, J.N.; Jia, B.Q.; Fu, F.Y.; Zhang, Y.Y.; Yu, Q.L.; Liu, X.D. One-Pot Synthesis of a Three-Dimensional Au-Decorated Cellulose Nanocomposite as a Surface-Enhanced Raman Scattering Sensor for Selective Detection and in Situ Monitoring. ACS Sustain. Chem. Eng. 2021, 9, 3324–3336. [Google Scholar] [CrossRef]
- Queffelec, A.L.; Boisdé, F.; Larue, J.P.; Haelters, J.P.; Corbel, B.; Thouvenot, D.; Nodet, P. Development of an immunoassay (ELISA) for the quantification of thiram in lettuce. J. Agric. Food Chem. 2001, 49, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mehmood, K.; Jiang, X.; Yao, W.Y.; Iqbal, A.; Waqas, M.; Rehman, M.U.; Li, A.Y.; Shen, Y.Q.; Li, J.K. Effect of tetramethyl thiuram disulfide (thiram) in relation to tibial dyschondroplasia in chickens. Environ. Sci. Pollut. Res. 2018, 25, 28264–28274. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zeng, P.; Yang, C.; Gong, J.Y.; Liang, R.Q.; Ou, Q.R.; Zhang, S.Y. Gold-Nanorod-Coated Capillaries for the SERS-Based Detection of Thiram. ACS Appl. Nano Mater. 2019, 2, 598–606. [Google Scholar] [CrossRef]
- Shi, R.Y.; Liu, X.J.; Ying, Y.B. Facing Challenges in Real-Life Application of Surface-Enhanced Raman Scattering: Design and Nanofabrication of Surface-Enhanced Raman Scattering Substrates for Rapid Field Test of Food Contaminants. J. Agric. Food Chem. 2018, 66, 6525–6543. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.D.; Li, S.M.; Petersen, M.; Wang, S.; Lu, X.N. Detection and Characterization of Antibiotic-Resistant Bacteria Using Surface-Enhanced Raman Spectroscopy. Nanomaterials 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, R.; Liu, G.K.; Wang, Y.H.; Liu, J.Y.; Ding, S.Y.; Li, J.F.; Wu, D.Y.; Tian, Z.Q. Surface-enhanced Raman spectroscopy: Bottlenecks and future directions. Chem. Commun. 2018, 54, 10–25. [Google Scholar] [CrossRef]
- Barbillon, G. Applications of Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Photonics 2021, 8, 16. [Google Scholar] [CrossRef]
- Gu, C.Y.; Man, S.Q.; Tang, J.Q.; Zhao, Z.M.; Liu, Z.L.; Zheng, Z.Y. Preparation of a monolayer array of silica@gold core-shell nanoparticles as a SERS substrate. Optik 2020, 221, 8. [Google Scholar] [CrossRef]
- Ma, L.X.; Han, E.; Yin, L.M.; Xu, Q.; Zou, C.X.; Bai, J.W.; Wu, W.; Cai, J.R. Simultaneous detection of mixed pesticide residues based on portable Raman spectrometer and Au@Ag nanoparticles SERS substrate. Food Control 2023, 153, 10. [Google Scholar] [CrossRef]
- Awiaz, G.; Wu, X.X.; Zhang, C.G.; Pan, T.; Xu, X.W.; Lin, J.; Wu, A.G. Au@Ag-Au core@double shell SERS bioprobes for high-resolution tumor cells imaging. Chin. J. Anal. Chem. 2023, 51, 100204. [Google Scholar] [CrossRef]
- Dubkov, S.V.; Savitskiy, A.I.; Trifonov, A.Y.; Yeritsyan, G.S.; Shaman, Y.P.; Kitsyuk, E.P.; Tarasov, A.; Shtyka, O.; Ciesielski, R.; Gromov, D.G. SERS in red spectrum region through array of Ag–Cu composite nanoparticles formed by vacuum-thermal evaporation. Opt. Mater. X 2020, 7, 100055. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, Z.Y.; Wu, L.; Pei, Y.W.; Chen, P.; Cui, Y.P. Rapid simultaneous detection of multi-pesticide residues on apple using SERS technique. Analyst 2014, 139, 5148–5154. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.Z.; Jiang, L.; Liu, R.Y.; Zhao, W.D.; Yang, Z.H.; Zhang, J.Y.; Jin, S.Z. Versatile self-assembled MXene-Au nanocomposites for SERS detection of bacteria, antibacterial and photothermal sterilization. Chem. Eng. J. 2021, 426, 12. [Google Scholar] [CrossRef]
- Liu, X.; Dang, A.L.; Li, T.H.; Sun, Y.T.; Lee, T.C.; Deng, W.B.; Wu, S.H.; Zada, A.; Zhao, T.K.; Li, H. Plasmonic Coupling of Au Nanoclusters on a Flexible MXene/ Graphene Oxide Fiber for Ultrasensitive SERS Sensing. ACS Sens. 2023, 8, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Tao, X.; Zhang, J.; Xia, Y.; Huang, H.; Zhang, L.; Gan, Y.; Liang, C.; Zhang, W. Sn4+ Ion Decorated Highly Conductive Ti3C2 MXene: Promising Lithium-Ion Anodes with Enhanced Volumetric Capacity and Cyclic Performance. ACS Nano 2016, 10, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Sarycheva, A.; Gogotsi, Y. Raman Spectroscopy Analysis of the Structure and Surface Chemistry of Ti3C2Tx MXene. Chem. Mat. 2020, 32, 3480–3488. [Google Scholar] [CrossRef]
- Liu, R.Y.; Jiang, L.; Lu, C.X.; Yu, Z.Z.; Li, F.H.; Jing, X.F.; Xu, R.; Zhou, W.; Jin, S.Z. Large-scale two-dimensional titanium carbide MXene as SERS-active substrate for reliable and sensitive detection of organic pollutants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 236, 8. [Google Scholar] [CrossRef]
- Sarycheva, A.; Makaryan, T.; Maleski, K.; Satheeshkumar, E.; Melikyan, A.; Minassian, H.; Yoshimura, M.; Gogotsi, Y. Two-Dimensional Titanium Carbide (MXene) as Surface-Enhanced Raman Scattering Substrate. J. Phys. Chem. C 2017, 121, 19983–19988. [Google Scholar] [CrossRef]
- Wang, D.; Xu, G.C.; Zhang, X.S.; Gong, H.Y.; Jiang, L.; Sun, G.L.; Li, Y.; Liu, G.R.; Li, Y.; Yang, S.K.; et al. Dual-functional ultrathin wearable 3D particle-in-cavity SF-AAO-Au SERS sensors for effective sweat glucose and lab-on-glove pesticide detection. Sens. Actuator B Chem. 2022, 359, 11. [Google Scholar] [CrossRef]
- Zhu, A.F.; Ali, S.; Xu, Y.; Ouyang, Q.; Chen, Q.S. A SERS aptasensor based on AuNPs functionalized PDMS film for selective and sensitive detection of Staphylococcus aureus. Biosens. Bioelectron. 2021, 172, 8. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Zhang, C.; Lia, C.H.; Yu, J.; Jiang, S.Z.; Xu, S.C.; Yang, C.; Liu, Y.J.; Gao, X.G.; Liu, A.H.; Man, B.Y. SERS activated platform with three-dimensional hot spots and tunable nanometer gap. Sens. Actuator B Chem. 2018, 258, 163–171. [Google Scholar] [CrossRef]
- Bai, Y.; Otitoju, T.A.; Wang, Y.; Chen, Q.; Sun, T. Highly sensitive in situ SERS monitoring of Fenton-like reaction by a PDDA-MXene@AuNP composite. New J. Chem. 2023, 47, 5174–5178. [Google Scholar] [CrossRef]
- Chen, M.; Luo, W.; Liu, Q.; Hao, N.Y.; Zhu, Y.Q.; Liu, M.Z.; Wang, L.M.; Yang, H.; Chen, X.Q. Simultaneous In Situ Extraction and Fabrication of Surface-Enhanced Raman Scattering Substrate for Reliable Detection of Thiram Residue. Anal. Chem. 2018, 90, 13647–13654. [Google Scholar] [CrossRef]
- Xiao, L.; Feng, S.L.; Hua, M.Z.; Lu, X.A. Rapid determination of thiram on apple using a flexible bacterial cellulose-based SERS substrate. Talanta 2023, 254, 7. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, B.; Kan, L.; Zhao, Y.; Jin, S.; Jiang, L. Simple Synthesis of Cellulose-Based Nanocomposites as SERS Substrates for In Situ Detection of Thiram. Nanomaterials 2024, 14, 987. https://doi.org/10.3390/nano14110987
Shi B, Kan L, Zhao Y, Jin S, Jiang L. Simple Synthesis of Cellulose-Based Nanocomposites as SERS Substrates for In Situ Detection of Thiram. Nanomaterials. 2024; 14(11):987. https://doi.org/10.3390/nano14110987
Chicago/Turabian StyleShi, Boya, Lian Kan, Yuliang Zhao, Shangzhong Jin, and Li Jiang. 2024. "Simple Synthesis of Cellulose-Based Nanocomposites as SERS Substrates for In Situ Detection of Thiram" Nanomaterials 14, no. 11: 987. https://doi.org/10.3390/nano14110987



