Synthesis and Optical Properties of CdSeTe/CdZnS/ZnS Core/Shell Nanorods
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Precursors
2.3. Synthesis of CdSeTe QDs
2.4. Synthesis of CdSeTe/CdS Core/Shell NRs
2.5. Synthesis of CdSeTe/CdZnS Core/Shell NRs
2.6. Synthesis of CdSeTe/CdZnS/ZnS Core/Shell NRs
2.7. Characterizations
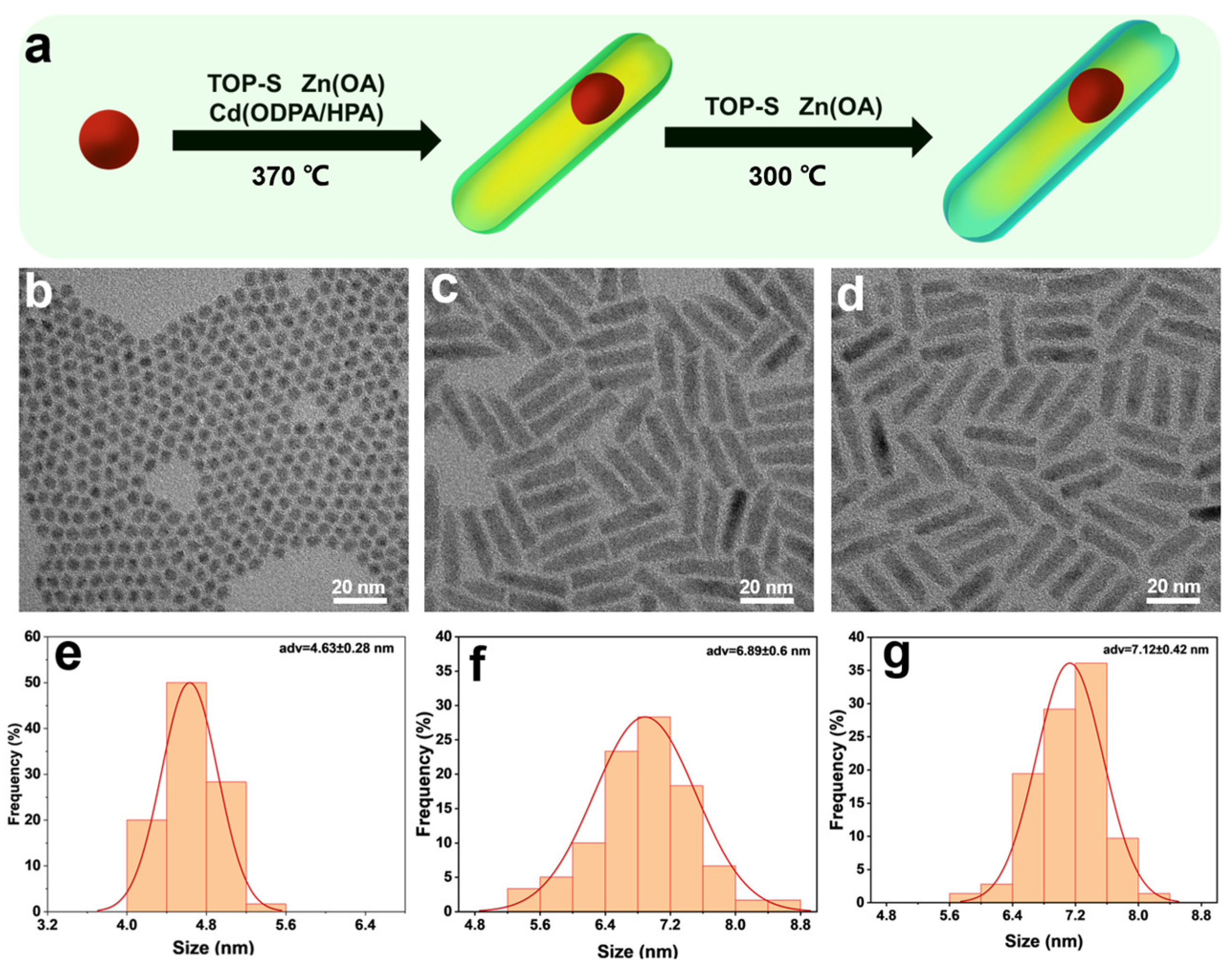
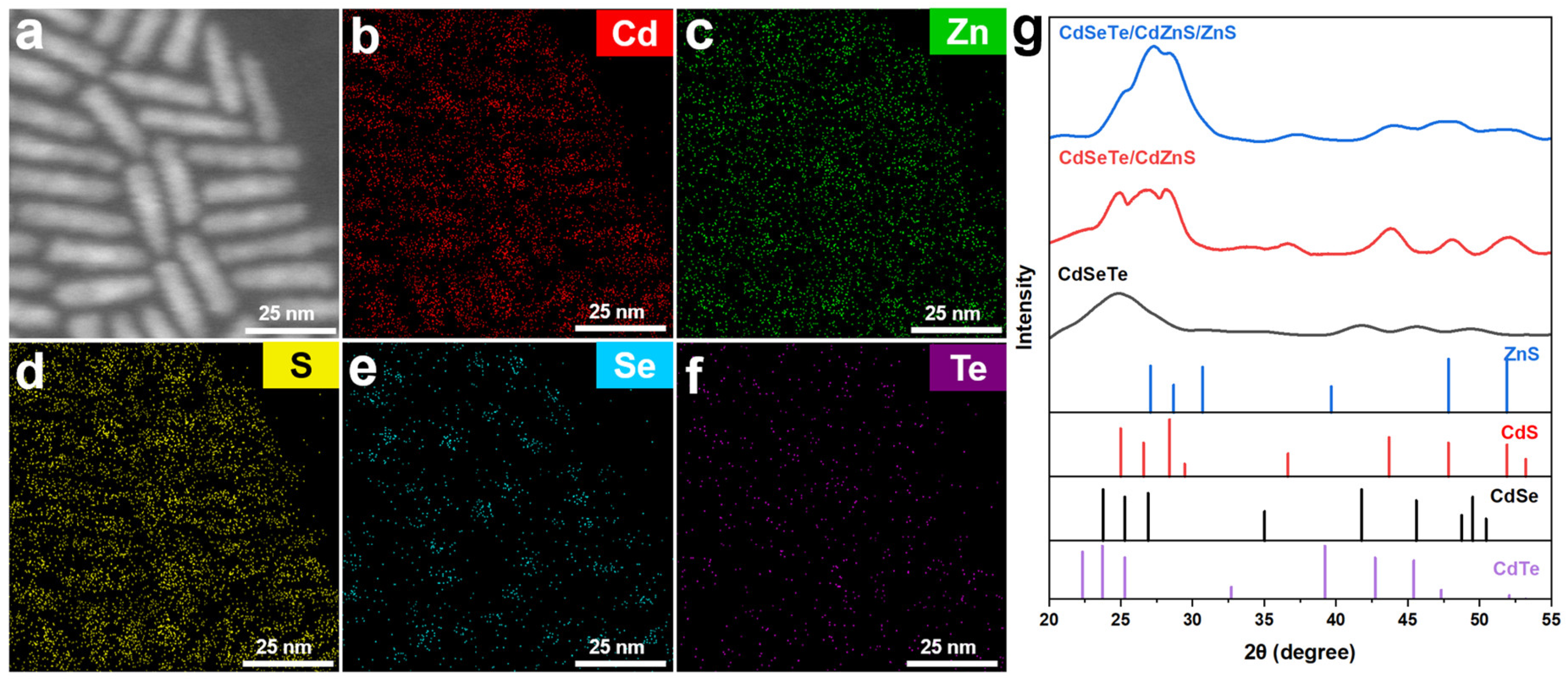
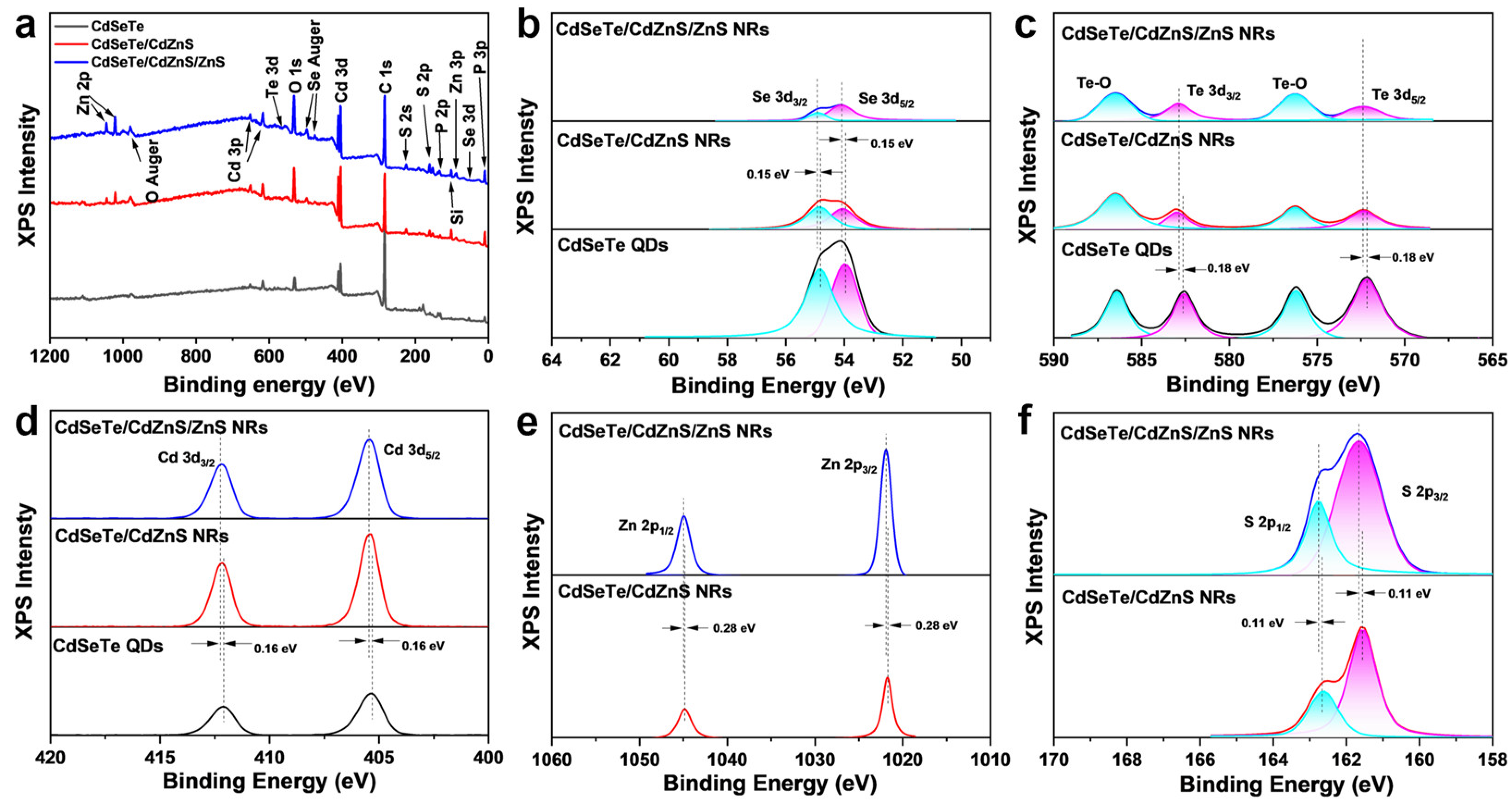
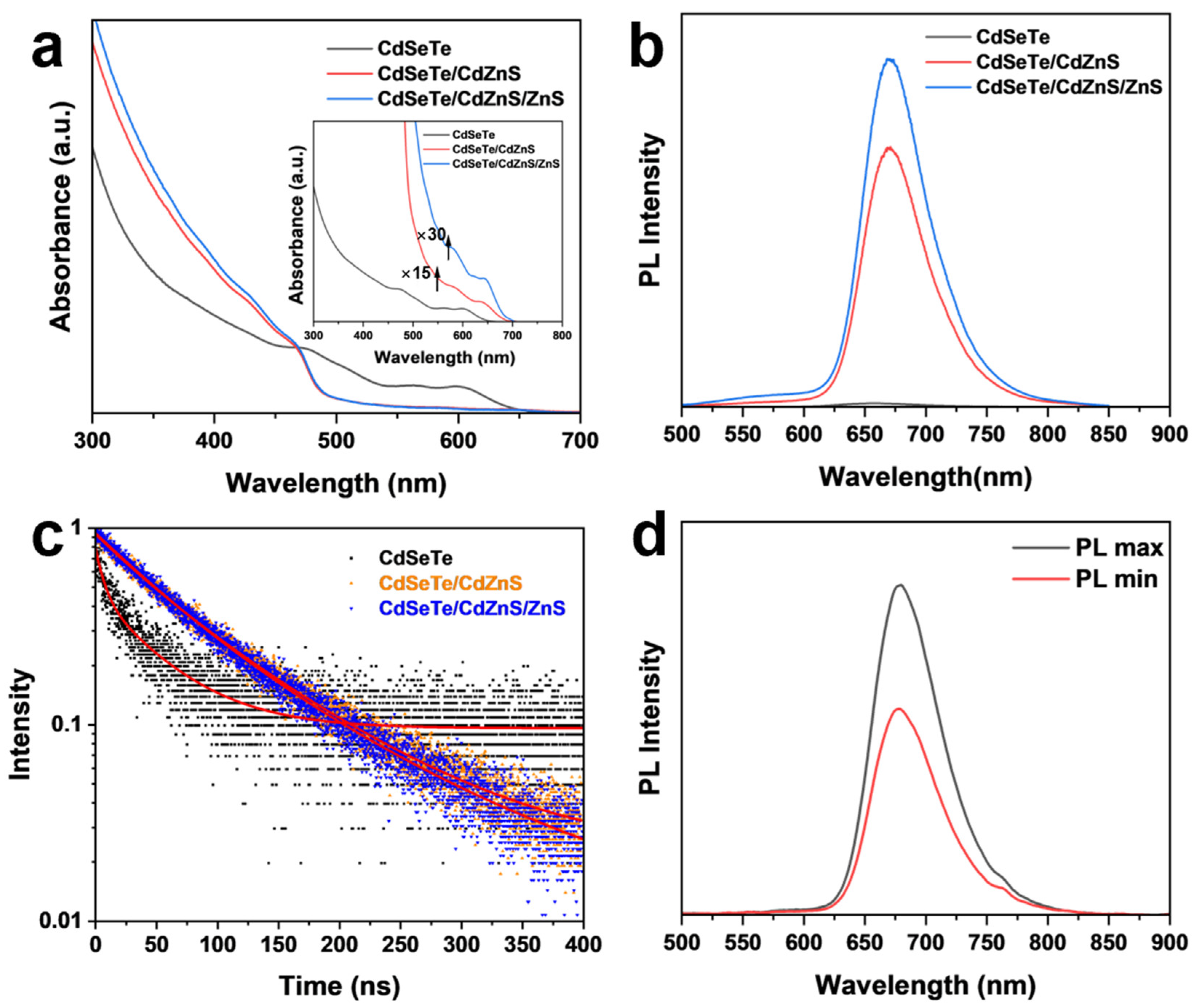
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulović, V. Emergence of Colloidal Quantum-Dot Light-Emitting Technologies. Nat. Photonics 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, Y.; Cao, W.; Titov, A.; Hyvonen, J.; Manders, J.R.; Xue, J.; Holloway, P.H.; Qian, L. High-Efficiency Light-Emitting Devices Based on Quantum Dots with Tailored Nanostructures. Nat. Photonics 2015, 9, 259–266. [Google Scholar] [CrossRef]
- Kim, J.S.; Heo, J.-M.; Park, G.-S.; Woo, S.-J.; Cho, C.; Yun, H.J.; Kim, D.-H.; Park, J.; Lee, S.-C.; Park, S.-H.; et al. Ultra-Bright, Efficient and Stable Perovskite Light-Emitting Diodes. Nature 2022, 611, 688–694. [Google Scholar] [CrossRef]
- Pattantyus-Abraham, A.G.; Kramer, I.J.; Barkhouse, A.R.; Wang, X.; Konstantatos, G.; Debnath, R.; Levina, L.; Raabe, I.; Nazeeruddin, M.K.; Grätzel, M.; et al. Depleted-Heterojunction Colloidal Quantum Dot Solar Cells. ACS Nano 2010, 4, 3374–3380. [Google Scholar] [CrossRef] [PubMed]
- Semonin, O.E.; Luther, J.M.; Choi, S.; Chen, H.-Y.; Gao, J.; Nozik, A.J.; Beard, M.C. Peak External Photocurrent Quantum Efficiency Exceeding 100% via MEG in a Quantum Dot Solar Cell. Science 2011, 334, 1530–1533. [Google Scholar] [CrossRef]
- Snee, P.T.; Somers, R.C.; Nair, G.; Zimmer, J.P.; Bawendi, M.G.; Nocera, D.G. A Ratiometric CdSe/ZnS Nanocrystal pH Sensor. J. Am. Chem. Soc. 2006, 128, 13320–13321. [Google Scholar] [CrossRef] [PubMed]
- Paek, K.; Yang, H.; Lee, J.; Park, J.; Kim, B.J. Efficient Colorimetric pH Sensor Based on Responsive Polymer–Quantum Dot Integrated Graphene Oxide. ACS Nano 2014, 8, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Thibert, A.; Frame, F.A.; Busby, E.; Holmes, M.A.; Osterloh, F.E.; Larsen, D.S. Sequestering High-Energy Electrons to Facilitate Photocatalytic Hydrogen Generation in CdSe/CdS Nanocrystals. J. Phys. Chem. Lett. 2011, 2, 2688–2694. [Google Scholar] [CrossRef]
- Liu, Y.; Guo Sun, C.; Feng, J.; Zhu, J.; Shen, Y. CdSe Nanoplatelets as Photocatalyst for Organic Photochemistry: Synthesis of Amino Acid Derivatives by Decarboxylative Alkylation of Aldimines. Tetrahedron Lett. 2023, 123, 154551. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Dutta, A.; Medda, A.; Bera, R.; Sarkar, K.; Sain, S.; Kumar, P.; Patra, A. Hybrid Nanostructures of 2D CdSe Nanoplatelets for High-Performance Photodetector Using Charge Transfer Process. ACS Appl. Nano Mater. 2020, 3, 4717–4727. [Google Scholar] [CrossRef]
- Kwak, D.-H.; Ramasamy, P.; Lee, Y.-S.; Jeong, M.-H.; Lee, J.-S. High-Performance Hybrid InP QDs/Black Phosphorus Photodetector. ACS Appl. Mater. Interfaces 2019, 11, 29041–29046. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- García De Arquer, F.P.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor Quantum Dots: Technological Progress and Future Challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Efros, A.L.; Brus, L.E. Nanocrystal Quantum Dots: From Discovery to Modern Development. ACS Nano 2021, 15, 6192–6210. [Google Scholar] [CrossRef] [PubMed]
- Balayeva, N.O.; Mamiyev, Z.Q. Synthesis and Studies of CdS and ZnS-PE/NBR Modified Thermoplastic Elastomeric Copolymer Nanocomposite Films. Mater. Lett. 2016, 162, 121–125. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Chen, L.; Wang, J.; Peng, X. Solution-Processed, High-Performance Light-Emitting Diodes Based on Quantum Dots. Nature 2014, 515, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, O.; Shen, H.; Lin, Q.; Li, Z.; Wang, L.; Zhang, X.; Li, L.S. Over 30% External Quantum Efficiency Light-Emitting Diodes by Engineering Quantum Dot-Assisted Energy Level Match for Hole Transport Layer. Adv. Funct. Mater. 2019, 29, 1808377. [Google Scholar] [CrossRef]
- Song, Y.; Liu, R.; Wang, Z.; Xu, H.; Ma, Y.; Fan, F.; Voznyy, O.; Du, J. Enhanced Emission Directivity from Asymmetrically Strained Colloidal Quantum Dots. Sci. Adv. 2022, 8, eabl8219. [Google Scholar] [CrossRef]
- Jiang, Y.; Cho, S.-Y.; Shim, M. Light-Emitting Diodes of Colloidal Quantum Dots and Nanorod Heterostructures for Future Emissive Displays. J. Mater. Chem. C 2018, 6, 2618–2634. [Google Scholar] [CrossRef]
- Kim, W.D.; Kim, D.; Yoon, D.-E.; Lee, H.; Lim, J.; Bae, W.K.; Lee, D.C. Pushing the Efficiency Envelope for Semiconductor Nanocrystal-Based Electroluminescence Devices Using Anisotropic Nanocrystals. Chem. Mater. 2019, 31, 3066–3082. [Google Scholar] [CrossRef]
- Galogahi, F.M.; Zhu, Y.; An, H.; Nguyen, N.-T. Core-Shell Microparticles: Generation Approaches and Applications. J. Sci. Adv. Mater. Devices 2020, 5, 417–435. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Guo, X.; Luo, Y.; Li, Q.; Zhu, R.; Pang, H. Core-Shell Materials for Advanced Batteries. Chem. Eng. J. 2019, 355, 208–237. [Google Scholar] [CrossRef]
- Gueorguiev, G.K.; Stafström, S.; Hultman, L. Nano-Wire Formation by Self-Assembly of Silicon–Metal Cage-like Molecules. Chem. Phys. Lett. 2008, 458, 170–174. [Google Scholar] [CrossRef]
- Alves Machado Filho, M.; Hsiao, C.-L.; dos Santos, R.B.; Hultman, L.; Birch, J.; Gueorguiev, G.K. Self-Induced Core–Shell InAlN Nanorods: Formation and Stability Unraveled by Ab Initio Simulations. ACS Nanosci. Au 2023, 3, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kim, B.J.; Lee, H.; Hahm, D.; Bae, W.K.; Lim, J.; Kwak, J. Bright and Stable Quantum Dot Light-Emitting Diodes. Adv. Mater. 2022, 34, 2106276. [Google Scholar] [CrossRef]
- Shen, H.; Gao, Q.; Zhang, Y.; Lin, Y.; Lin, Q.; Li, Z.; Chen, L.; Zeng, Z.; Li, X.; Jia, Y.; et al. Visible Quantum Dot Light-Emitting Diodes with Simultaneous High Brightness and Efficiency. Nat. Photonics 2019, 13, 192–197. [Google Scholar] [CrossRef]
- Mallem, K.; Prodanov, M.F.; Dezhang, C.; Marus, M.; Kang, C.; Shivarudraiah, S.B.; Vashchenko, V.V.; Halpert, J.E.; Srivastava, A.K. Solution-Processed Red, Green, and Blue Quantum Rod Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2022, 14, 18723–18735. [Google Scholar] [CrossRef]
- Kang, C.; Prodanov, M.F.; Gao, Y.; Mallem, K.; Yuan, Z.; Vashchenko, V.V.; Srivastava, A.K. Quantum-Rod On-Chip LEDs for Display Backlights with Efficacy of 149 Lm W−1: A Step toward 200 Lm W−1. Adv. Mater. 2021, 33, 2104685. [Google Scholar] [CrossRef]
- Zeng, Y.; Su, H.; Liu, Y.; Chen, W.; Liu, F.; Gao, H.; Wu, Y.; Manna, L.; Li, H. Synthesis and Assembly of Core–Shell Nanorods with High Quantum Yield and Linear Polarization. Adv. Funct. Mater. 2023, 33, 2306091. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, X.; Liu, Y.; Chen, W.; Liu, F.; Li, H. 22% Record Efficiency in Nanorod Light-Emitting Diodes Achieved by Gradient Shells. Adv. Mater. 2024, 36, 2310705. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, S.; Cao, F.; Chen, W.; Wang, Q.; Jin, G.; Wei, J.; Liu, F.; Manna, L.; Yang, X.; et al. High-Efficiency and Stable Colloidal One-Dimensional Core/Shell Nanorod Light-Emitting Diodes. Nano Lett. 2024, 24, 5647–5655. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Brescia, R.; Krahne, R.; Bertoni, G.; Alcocer, M.J.P.; D’Andrea, C.; Scotognella, F.; Tassone, F.; Zanella, M.; De Giorgi, M.; et al. Blue-UV-Emitting ZnSe(Dot)/ZnS(Rod) Core/Shell Nanocrystals Prepared from CdSe/CdS Nanocrystals by Sequential Cation Exchange. ACS Nano 2012, 6, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Sun, X.; Liu, Y.; Jin, W.; He, S.; Zhu, X.; Niu, K.; Sun, G.; Li, J.; He, H.; et al. Switchable Interfacial Reaction Enables Bright and Stable Deep-Red Perovskite Light-Emitting Diodes. Nat. Photonics 2024, 18, 325–333. [Google Scholar] [CrossRef]
- Shinhmar, H.; Hogg, C.; Neveu, M.; Jeffery, G. Weeklong Improved Colour Contrasts Sensitivity after Single 670 Nm Exposures Associated with Enhanced Mitochondrial Function. Sci. Rep. 2021, 11, 22872. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, L.; Han, Y.; Li, H.; Ji, Z.; Zhang, Z. Luminescent Properties of Zn2+-Doped CaAl12O19:Mn4+ Deep-Red Phosphor for Indoor Plant Cultivation. Ceram. Int. 2019, 45, 8265–8270. [Google Scholar] [CrossRef]
- Zhu, R.; Luo, Z.; Chen, H.; Dong, Y.; Wu, S.-T. Realizing Rec. 2020 Color Gamut with Quantum Dot Displays. Opt. Express 2015, 23, 23680–23693. [Google Scholar] [CrossRef]
- Polovitsyn, A.; Khan, A.H.; Angeloni, I.; Grim, J.Q.; Planelles, J.; Climente, J.I.; Moreels, I. Synthesis of Anisotropic CdSe/CdS Dot-in-Giant-Rod Nanocrystals with Persistent Blue-Shifted Biexciton Emission. ACS Photonics 2018, 5, 4561–4568. [Google Scholar] [CrossRef]
- Huang, P.; Liu, X.; Jin, G.; Liu, F.; Shen, H.; Li, H. Deep-Red InP Core-Multishell Quantum Dots for Highly Bright and Efficient Light-Emitting Diodes. Adv. Opt. Mater. 2023, 11, 2300612. [Google Scholar] [CrossRef]
- Leach, A.D.P.; Macdonald, J.E. Optoelectronic Properties of CuInS2 Nanocrystals and Their Origin. J. Phys. Chem. Lett. 2016, 7, 572–583. [Google Scholar] [CrossRef]
- Erwin, S.C.; Zu, L.; Haftel, M.I.; Efros, A.L.; Kennedy, T.A.; Norris, D.J. Doping Semiconductor Nanocrystals. Nature 2005, 436, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Dufour, M.; Izquierdo, E.; Livache, C.; Martinez, B.; Silly, M.G.; Pons, T.; Lhuillier, E.; Delerue, C.; Ithurria, S. Doping as a Strategy to Tune Color of 2D Colloidal Nanoplatelets. ACS Appl. Mater. Interfaces 2019, 11, 10128–10134. [Google Scholar] [CrossRef]
- Freyer, A.R.; Sercel, P.C.; Hou, Z.; Savitzky, B.H.; Kourkoutis, L.F.; Efros, A.L.; Krauss, T.D. Explaining the Unusual Photoluminescence of Semiconductor Nanocrystals Doped via Cation Exchange. Nano Lett. 2019, 19, 4797–4803. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Sharma, M.; Delikanli, S.; Bhattacharya, A.; Murphy, J.R.; Pientka, J.; Sharma, A.; Quinn, A.P.; Erdem, O.; Kattel, S.; et al. Light-Induced Paramagnetism in Colloidal Ag+-Doped CdSe Nanoplatelets. J. Phys. Chem. Lett. 2021, 12, 2892–2899. [Google Scholar] [CrossRef]
- Mondal, P.; Viswanatha, R. Insights into the Oxidation State of Cu Dopants in II–VI Semiconductor Nanocrystals. J. Phys. Chem. Lett. 2022, 13, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, Z.; Luo, J.-W.; Franceschetti, A. The Birth of a Type-II Nanostructure: Carrier Localization and Optical Properties of Isoelectronically Doped CdSe:Te Nanocrystals. ACS Nano 2012, 6, 8325–8334. [Google Scholar] [CrossRef]
- Franzl, T.; Müller, J.; Klar, T.A.; Rogach, A.L.; Feldmann, J.; Talapin, D.V.; Weller, H. CdSe:Te Nanocrystals: Band-Edge versus Te-Related Emission. J. Phys. Chem. C 2007, 111, 2974–2979. [Google Scholar] [CrossRef]
- Zou, H.; Liu, M.; Zhou, D.; Zhang, X.; Liu, Y.; Yang, B.; Zhang, H. Employing CdSexTe1−x Alloyed Quantum Dots to Avoid the Temperature-Dependent Emission Shift of Light-Emitting Diodes. J. Phys. Chem. C 2017, 121, 5313–5323. [Google Scholar] [CrossRef]
- Meir, N.; Martín-García, B.; Moreels, I.; Oron, D. Revisiting the Anion Framework Conservation in Cation Exchange Processes. Chem. Mater. 2016, 28, 7872–7877. [Google Scholar] [CrossRef]
- Lahad, O.; Meir, N.; Pinkas, I.; Oron, D. Long-Lived Population Inversion in Isovalently Doped Quantum Dots. ACS Nano 2015, 9, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Nobile, C.; De Giorgi, M.; Sala, F.D.; Morello, G.; Pompa, P.; Hytch, M.; Snoeck, E.; Fiore, A.; Franchini, I.R.; et al. Synthesis and Micrometer-Scale Assembly of Colloidal CdSe/CdS Nanorods Prepared by a Seeded Growth Approach. Nano Lett. 2007, 7, 2942–2950. [Google Scholar] [CrossRef]
- Deka, S.; Quarta, A.; Lupo, M.G.; Falqui, A.; Boninelli, S.; Giannini, C.; Morello, G.; De Giorgi, M.; Lanzani, G.; Spinella, C.; et al. CdSe/CdS/ZnS Double Shell Nanorods with High Photoluminescence Efficiency and Their Exploitation As Biolabeling Probes. J. Am. Chem. Soc. 2009, 131, 2948–2958. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.A.; Peng, X. Mechanisms of the Shape Evolution of CdSe Nanocrystals. J. Am. Chem. Soc. 2001, 123, 1389–1395. [Google Scholar] [CrossRef]
- Whitehead, C.B.; Özkar, S.; Finke, R.G. LaMer’s 1950 Model for Particle Formation of Instantaneous Nucleation and Diffusion-Controlled Growth: A Historical Look at the Model’s Origins, Assumptions, Equations, and Underlying Sulfur Sol Formation Kinetics Data. Chem. Mater. 2019, 31, 7116–7132. [Google Scholar] [CrossRef]
- Yin, Y.; Alivisatos, A.P. Colloidal Nanocrystal Synthesis and the Organic–Inorganic Interface. Nature 2005, 437, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Tenne, R.; Pedetti, S.; Kazes, M.; Ithurria, S.; Houben, L.; Nadal, B.; Oron, D.; Dubertret, B. From Dilute Isovalent Substitution to Alloying in CdSeTe Nanoplatelets. Phys. Chem. Chem. Phys. 2016, 18, 15295–15303. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ye, H.; Ruan, X. Luminescence Dynamics of Te Doped CdS Quantum Dots at Different Doping Levels. Nanotechnology 2010, 21, 265704. [Google Scholar] [CrossRef]
- Ning, J.; Xiong, Y.; Kershaw, S.V.; Rogach, A.L. Phase-Dependent Shell Growth and Optical Properties of ZnSe/ZnS Core/Shell Nanorods. Chem. Mater. 2021, 33, 3413–3427. [Google Scholar] [CrossRef]
- De Mello Donegá, C. Formation of Nanoscale Spatially Indirect Excitons: Evolution of the Type-II Optical Character of CdTe/CdSe Heteronanocrystals. Phys. Rev. B 2010, 81, 165303. [Google Scholar] [CrossRef]
- Sitt, A.; Salant, A.; Menagen, G.; Banin, U. Highly Emissive Nano Rod-in-Rod Heterostructures with Strong Linear Polarization. Nano Lett. 2011, 11, 2054–2060. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, G.; Zeng, Y.; Liu, X.; Wang, Q.; Wei, J.; Liu, F.; Li, H. Synthesis and Optical Properties of CdSeTe/CdZnS/ZnS Core/Shell Nanorods. Nanomaterials 2024, 14, 989. https://doi.org/10.3390/nano14110989
Jin G, Zeng Y, Liu X, Wang Q, Wei J, Liu F, Li H. Synthesis and Optical Properties of CdSeTe/CdZnS/ZnS Core/Shell Nanorods. Nanomaterials. 2024; 14(11):989. https://doi.org/10.3390/nano14110989
Chicago/Turabian StyleJin, Geyu, Yicheng Zeng, Xiao Liu, Qingya Wang, Jing Wei, Fangze Liu, and Hongbo Li. 2024. "Synthesis and Optical Properties of CdSeTe/CdZnS/ZnS Core/Shell Nanorods" Nanomaterials 14, no. 11: 989. https://doi.org/10.3390/nano14110989
APA StyleJin, G., Zeng, Y., Liu, X., Wang, Q., Wei, J., Liu, F., & Li, H. (2024). Synthesis and Optical Properties of CdSeTe/CdZnS/ZnS Core/Shell Nanorods. Nanomaterials, 14(11), 989. https://doi.org/10.3390/nano14110989







