Synthesis of Ag-Doped Tetrahedral Amorphous Carbon Coatings and Their Antibiofilm Efficacy for Medical Implant Application
Abstract
:1. Introduction
2. Materials and Methods
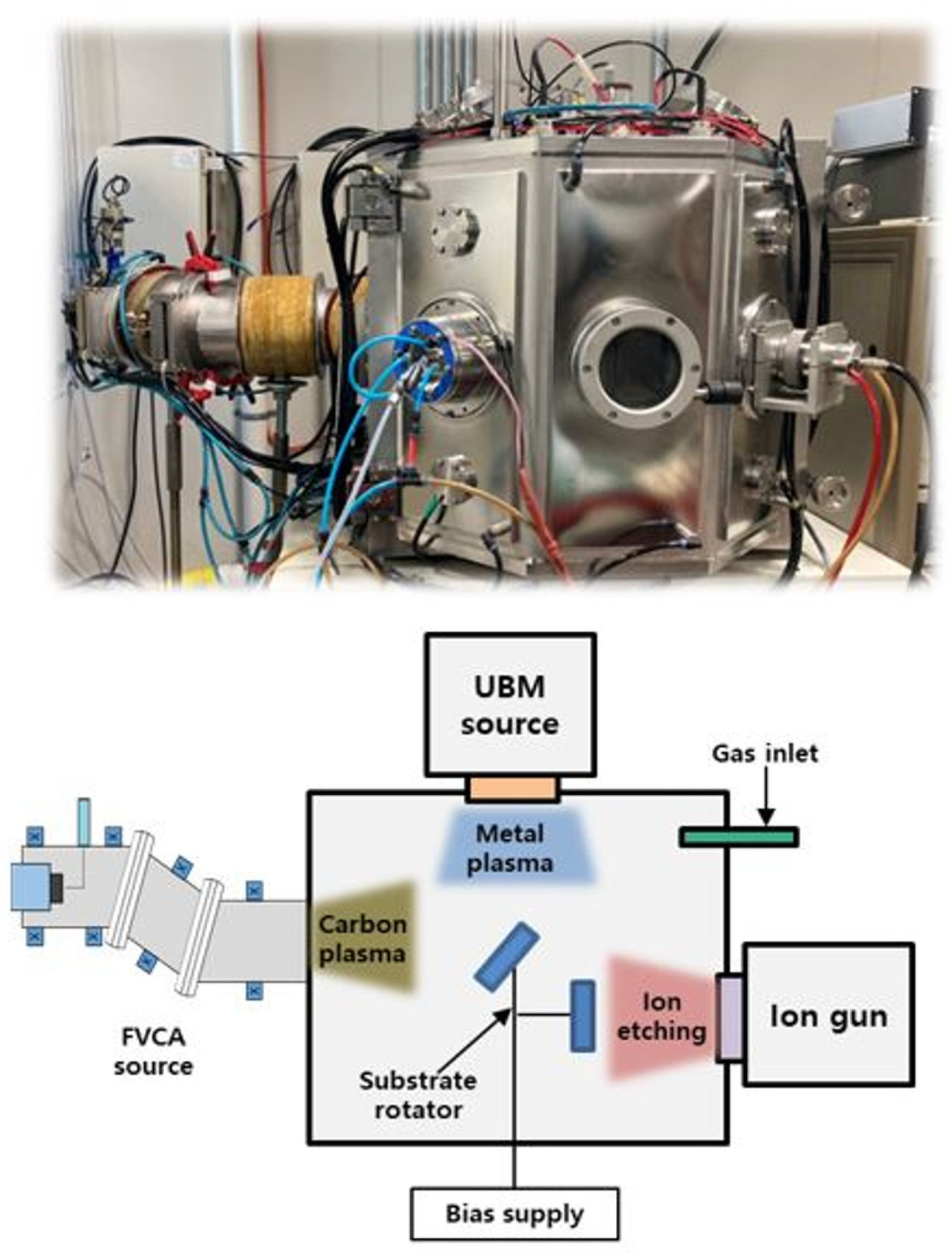
2.1. taC Coating Preparation
2.2. Surface Characterizations
2.3. Biofilm Formation on taC and Ag-taC
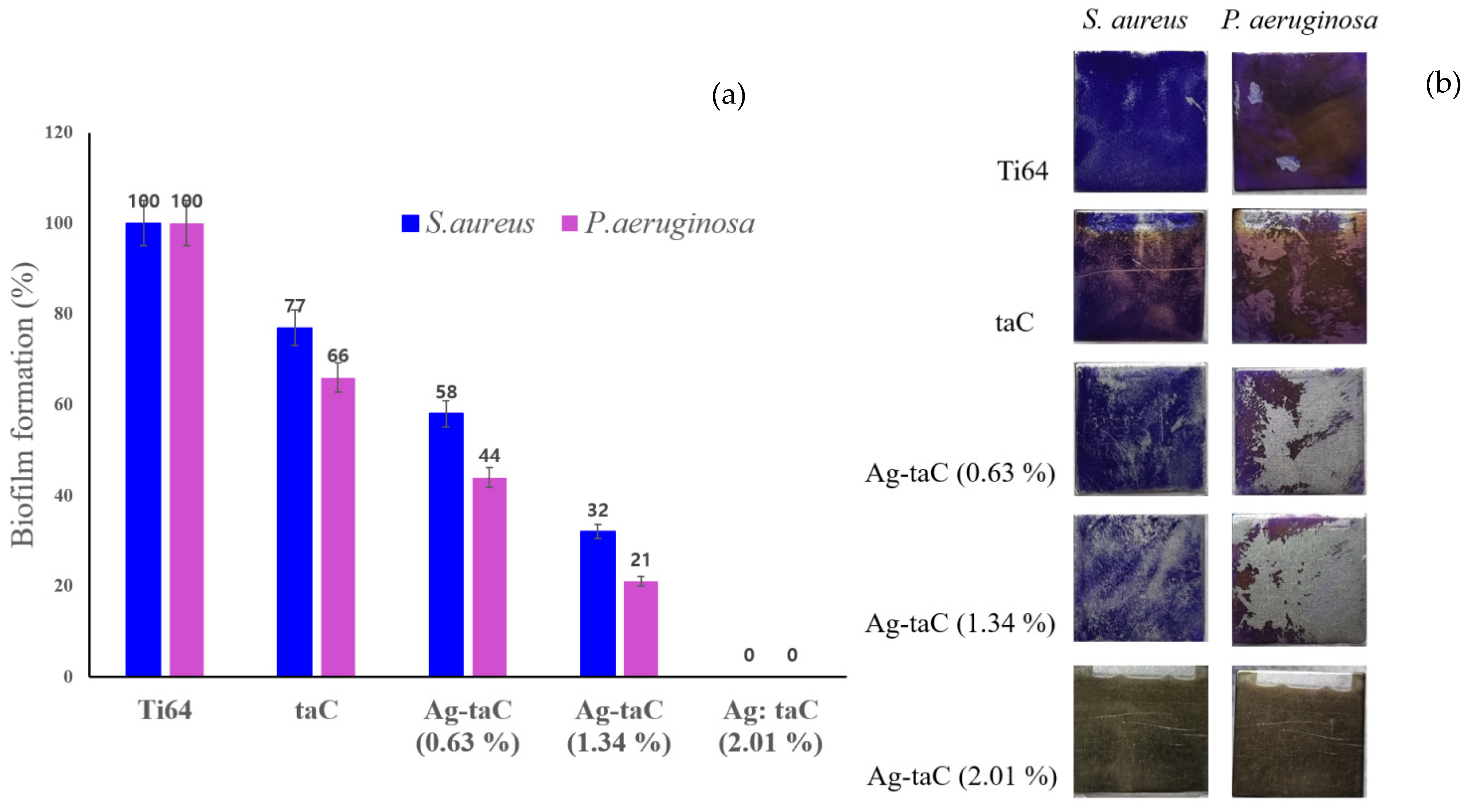
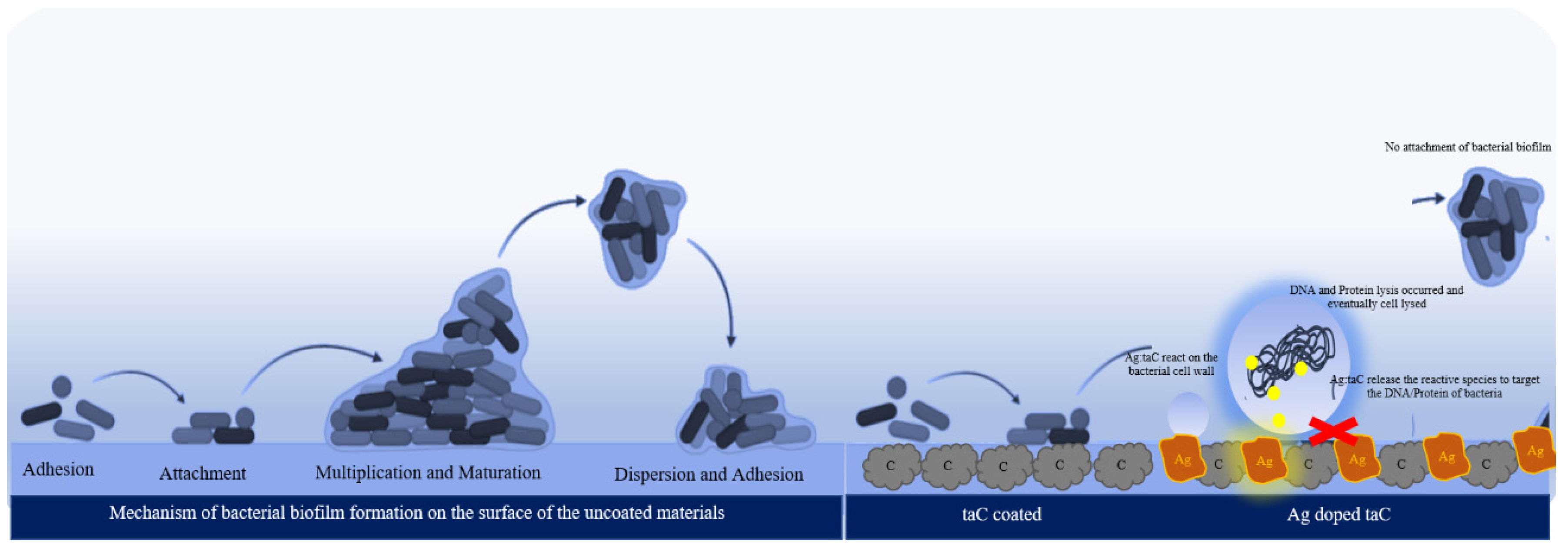
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Venkateswarlu, K.; Rameshbabu, N.; Bose, A.C.; Muthupandi, V.; Subramanian, S.; MubarakAli, D.; Thajuddin, N. Fabrication of corrosion resistant, bioactive and antibacterial silver substituted hydroxyapatite/titania composite coating on Cp Ti. Ceram. Int. 2012, 38, 731–740. [Google Scholar] [CrossRef]
- MubarakAli, D.; Arunkumar, J.; Pooja, P.; Subramanian, G.; Thajuddin, N.; Alharbi, N.S. Harbi, Synthesis and characterization of biocompatible tenorite nanoparticles for implant coatings with potential property against biofilm formation. Saudi Pharm. J. 2015, 23, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Huang, N.; Leng, Y.; Chen, J.; Fu, R.; Kwok, S.; Leng, Y.; Chu, P. Activation of platelets adhered on amorphous hydrogenated carbon (a-C:H) films synthesized by plasma immersion ion implantation-deposition (PIII-D). Biomaterials 2003, 24, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.I.; McColl, I.R.; Grant, D.M.; Parker, K.G.; Parker, T.L. Protein adsorption and platelet attachment and activation, on TiN, TiC, and DLC coatings on titanium for cardiovascular applications. J. Biomed. Mater. Res. 2000, 52, 413–421. [Google Scholar] [PubMed]
- Casiraghi, C.; Robertson, J.; Ferrari, A.C. Diamond-like carbon for data and beer storage. Mater. Today 2007, 10, 44–53. [Google Scholar] [CrossRef]
- Moseler, M.; Gumbsch, P.; Casiraghi, C.; Ferrari, A.C.; Robertson, J. The ultrasmoothness of diamond-like carbon surfaces. Science 2005, 309, 1545–1548. [Google Scholar] [CrossRef] [PubMed]
- Laurila, T.; Sainio, S.; Caro, M.A. Hybrid carbon based nanomaterials for electrochemical detection of biomolecules. Prog. Mater. Sci. 2017, 88, 499–594. [Google Scholar] [CrossRef]
- Etula, J.; Wester, N.; Sainio, S.; Laurila, T.; Koskinen, J. Characterization and electrochemical properties of iron-doped tetrahedral amorphous carbon (ta-C) thin films. RSC Adv. 2018, 8, 26356–26363. [Google Scholar] [CrossRef] [PubMed]
- Al Mahmud, K.A.H.; Kalam, M.A.; Masjuki, H.H.; Mobarak, H.M.; Zulkifli, N.M. An updated overview of diamond-like carbon coating in tribology. Crit. Rev. Solid State Mater. Sci. 2014, 40, 90–118. [Google Scholar] [CrossRef]
- Kim, J.-I.; Jang, Y.-J.; Kim, J.; Kim, J. Effects of silicon doping on low-friction and high-hardness diamond-like carbon coating via filtered cathodic vacuum arc deposition. Sci. Rep. 2021, 11, 3529. [Google Scholar] [CrossRef]
- Tujunen, N.; Kaivosoja, E.; Protopopova, V.; Valle-Delgado, J.J.; Österberg, M.; Koskinen, J.; Laurila, T. Electrochemical detection of hydrogen peroxide on platinum-containing tetrahedral amorphous carbon sensors and evaluation of their biofouling properties. Mater. Sci. Eng. C 2015, 55, 70–78. [Google Scholar] [CrossRef] [PubMed]
- ISO 20523; International Standardization and Organization, Carbon Based Films—Classification and Designations. ISO: Geneva, Switzerland, 2017.
- Chen, J.; Lau, S.; Sun, Z.; Chen, G.; Li, Y.; Tay, B.; Chai, J. Metal-containing amorphous carbon films for hydrophobic application. Thin Solid Films 2001, 398–399, 110–115. [Google Scholar] [CrossRef]
- Konicek, A.R.; Grierson, D.S.; Sumant, A.V.; Friedmann, T.A.; Sullivan, J.P.; Gilbert, P.U.P.A.; Sawyer, W.G.; Carpick, R.W. Influence of surface passivation on the friction and wear behavior of ultrananocrystalline diamond and tetrahedral amorphous carbon thin films. Phys. Rev. B 2012, 85, 155448. [Google Scholar] [CrossRef]
- Wester, N.; Etula, J.; Lilius, T.; Sainio, S.; Laurila, T.; Koskinen, J. Selective detection of morphine in the presence of paracetamol with anodically pretreated dual layer Ti/tetrahedral amorphous carbon electrodes. Electrochem. Commun. 2018, 86, 166–170. [Google Scholar] [CrossRef]
- Sainio, S.; Nordlund, D.; Caro, M.A.; Gandhiraman, R.P.; Koehne, J.E.; Wester, N.; Koskinen, J.; Meyyappan, M.; Laurila, T. Correlation between sp3-to-sp2 ratio and surface oxygen functionalities in tetrahedral amorphous carbon (ta-C) thin film electrodes and implications of their electrochemical properties. J. Phys. Chem. C 2016, 120, 8298–8304. [Google Scholar] [CrossRef]
- Cortese, B.; Caschera, D.; Federici, F.; Ingo, G.M.; Gigli, G. Superhydrophobic fabrics for oil–water separation through a diamond like carbon (DLC) coating. J. Mater. Chem. A 2014, 2, 6781–6789. [Google Scholar] [CrossRef]
- Kaivosoja, E.; Berg, E.; Rautiainen, A.; Palomaki, T.; Koskinen, J.; Paulasto-Krockel, M.; Laurila, T. Improving the function of dopamine electrodes with novel carbon materials. In Proceedings of the International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 632–634. [Google Scholar] [CrossRef]
- Matsuura, Y.S.; Tuoya, M.; Tokutaka, H.; Ohkita, M. The identification of a cancer cell gene by using SOM. In Proceedings of the 16th International Conference on Genome Informatics, Yokohama Pacifico, Yokohama, Japan, 19–21 December 2005; pp. 19–21. [Google Scholar]
- Müller, I.C.; Sharp, J.; Rainforth, W.M.; Hovsepian, P.; Ehiasarian, A. Tribological response and characterization of Mo–W doped DLC coating. Wear 2017, 376–377, 1622–1629. [Google Scholar] [CrossRef]
- Koutsokeras, L.; Constantinou, M.; Nikolaou, P.; Constantinides, G.; Kelires, P. Synthesis and characterization of hydrogenated diamond-like carbon (HDLC) nanocomposite films with metal (Ag, Cu) nanoparticles. Materials 2020, 13, 1753. [Google Scholar] [CrossRef] [PubMed]
- Juknius, T.; Ružauskas, M.; Tamulevičius, T.; Šiugždinienė, R.; Juknienė, I.; Vasiliauskas, A.; Jurkevičiūtė, A.; Tamulevičius, S. Antimicrobial properties of diamond-like carbon/silver nanocomposite thin films deposited on textiles: Towards smart bandages. Materials 2016, 9, 371. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Moon, M.-W.; Lee, K.-R. Enhancement of electron field emission property with silver incorporation into diamondlike carbon matrix. Appl. Phys. Lett. 2008, 92, 193502. [Google Scholar] [CrossRef]
- Wai, K.P.; Koo, C.H.; Chong, W.C.; Lai, S.O.; Pang, Y.L. Improving Hydrophilicity of Polyethersulfone Membrane Using Silver Nanoparticles for Humic Substances Removal. Int. J. Eng. Trans. B Appl. 2018, 31, 1364–1372. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Y.; Pu, X.; Jiang, Y.; Cheng, L.; Jiao, Z. One-step synthesis of high conductivity silver nanoparticle-reduced graphene oxide composite films by electron beam irradiation. Appl. Surf. Sci. 2015, 349, 570–575. [Google Scholar] [CrossRef]
- Tian, P.; Zhang, X.; Xue, Q. Enhanced room-temperature positive magnetoresistance of a-C:Fe film. Carbon 2007, 45, 1764–1768. [Google Scholar] [CrossRef]
- Morrison, N.; Rodil, S.; Ferrari, A.; Robertson, J.; Milne, W. High rate deposition of ta-C:H using an electron cyclotron wave resonance plasma source. Thin Solid Films 1999, 337, 71–73. [Google Scholar] [CrossRef]
- Panwar, O.; Khan, M.A.; Kumar, S.; Basu, A.; Mehta, B.; Kumar, S.; Ishpal, I. Effect of high substrate bias and hydrogen and nitrogen incorporation on spectroscopic ellipsometric and atomic force microscopic studies of tetrahedral amorphous carbon films. Surf. Coat. Technol. 2010, 205, 2126–2133. [Google Scholar] [CrossRef]
- Ulrich, S.; Bauer, C.; Holleck, H.; Leiste, H.; Stüber, M.; Ye, J.; Schwan, J. Influence of the energy of sputtered carbon atoms on the constitution of diamond-like carbon thin films. Diam. Relat. Mater. 2002, 11, 1010–1014. [Google Scholar] [CrossRef]
- Martin, P.; Bendavid, A. Review of the filtered vacuum arc process and materials deposition. Thin Solid Films 2001, 394, 1–14. [Google Scholar] [CrossRef]
- Jang, Y.-J.; Kim, J.-I.; Lee, W.-Y.; Kim, J. Friction properties of thick tetrahedral amorphous carbon coating with different surface defects under dry contact conditions. Appl. Surf. Sci. 2021, 550, 149332. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Robertson, Raman spectroscopy of amorphous, nanostructured, diamond–like carbon, and nanodiamond. Philosophical Transactions of the Royal Society of London. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Mohagheghpour, E.; Larijani, M.M.; Rajabi, M.; Gholamipour, R. Effect of Silver Clusters Deposition on Wettability and Optical Properties of Diamond-like Carbon Films. Int. J. Eng. 2021, 34, 706–713. [Google Scholar] [CrossRef]
- Neuville, S.; Matthews, A. A perspective on the optimisation of hard carbon and related coatings for engineering applications. Thin Solid Films 2007, 515, 6619–6653. [Google Scholar] [CrossRef]
- Neuville, S. Quantum electronic mechanisms of atomic rearrangements during growth of hard carbon films. Surf. Coat. Technol. 2011, 206, 703–726. [Google Scholar] [CrossRef]
- Ding, J.C.; Mei, H.; Jeong, S.; Zheng, J.; Wang, Q.M.; Kim, K.H. Effect of bias voltage on the microstructure and properties of Nb-DLC films prepared by a hybrid sputtering system. J. Alloy Compd. 2020, 861, 158505. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Ismail, R.A.; Mousa, A.M.; Hassan, M.A. Synthesis and characterization of diamond-like carbon film on silicon by electrodeposition from solution of ethanol and methanol. Mater. Sci. Semicond. Process. 2014, 27, 461–467. [Google Scholar] [CrossRef]
- Dai, W.; Ke, P.; Wang, A. Influence of bias voltage on microstructure and properties of Al-containing diamond-like carbon films deposited by a hybrid ion beam system. Surf. Coat. Technol. 2013, 229, 217–221. [Google Scholar] [CrossRef]
- Haerle, R.; Riedo, E.; Pasquarello, A.; Baldereschi, A. sp2/sp3hybridization ratio in amorphous carbon from C1score-level shifts: X-ray photoelectron spectroscopy and first-principles calculation. Phys. Rev. B 2001, 65, 045101. [Google Scholar] [CrossRef]
- Kung, C.H.; Sow, P.K.; Zahiri, B.; Mérida, W. Assessment and Interpretation of Surface Wettability Based on Sessile Droplet Contact Angle Measurement: Challenges and Opportunities. Adv. Mater. Interfaces 2019, 6, 1900839. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Salunkhe, P.; A.V, M.A.; Kekuda, D. Structural, spectroscopic and electrical properties of dc magnetron sputtered NiO thin films and an insight into different defect states. Appl. Phys. A 2021, 127, 390. [Google Scholar] [CrossRef]
- Philipp, P.; Bischoff, L. Investigation of conducting nano-structures on ta-C films made by FIB lithography. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2012, 282, 121–124. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Z.; Liao, B.; Wu, X.; Zhang, X. Comparative study on effects of Ni ion implantation on amorphous carbon (a-C) coating and tetrahedral amorphous carbon (ta-C) coating. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 467, 1–8. [Google Scholar] [CrossRef]
- Arunachalam, K.; Pandurangan, P.; Shi, C.; Lagoa, R. Regulation of Staphylococcus aureus virulence and application of nanotherapeutics to eradicate S. aureus infection. Pharmaceutics 2023, 15, 310. [Google Scholar] [CrossRef]
- MubarakAli, D.; Arunachalam, K.; Lakshmanan, M.; Badar, B.; Kim, J.-W.; Lee, S.-Y. Unveiling the Anti-Biofilm Property of Hydroxyapatite on Pseudomonas aeruginosa: Synthesis and Strategy. Pharmaceutics 2023, 15, 463. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Krishnan, G.P.; Sethuraman, S.; Abraham, S.V.P.I.; Krishnan, S.T.; Venkateswar, A.; Arunkumar, J.; Shi, C.; MubarakAli, D. Exploring Possible Ways to Enhance the Potential and Use of Natural Products through Nanotechnology in the Battle against Biofilms of Foodborne Bacterial Pathogens. Pathogens 2023, 12, 270. [Google Scholar] [CrossRef]
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef] [PubMed]
- LewisOscar, F.; MubarakAli, D.; Nithya, C.; Priyanka, R.; Gopinath, V.; Alharbi, N.S.; Thajuddin, N. One pot synthesis and anti-biofilm potential of copper nanoparticles (CuNPs) against clinical strains of Pseudomonas aeruginosa. Biofouling 2015, 31, 379–391. [Google Scholar] [CrossRef] [PubMed]
- MubarakAli, D.; Manzoor, M.A.; Sabarinathan, A.; Devi, C.A.; Rekha, P.; Thajuddin, N.; Lee, S.-Y. An investigation of antibiofilm and cytotoxic property of MgO nanoparticles. Biocatal. Agric. Biotechnol. 2019, 18, 101069. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Balabanyan, V.Y.; Tsiskarashvili, A.V. Antibacterial properties of modified a-C and ta-C coatings: The effects of the sp2/sp3 ratio, oxidation, nitridation, and silver incorporation. Appl. Phys. A 2022, 128, 929. [Google Scholar] [CrossRef]
- Oliveira, S.E.O.; Costa, F.A.D.; Gibson, T.J.; Costa, O.A.D.; Coldebella, A.; Gregory, N.G. Evaluation of brain damage resulting from penetrating and non-penetrating stunning in Nelore Cattle using pneumatically powered captive bolt guns. Meat Sci. 2018, 145, 347–351. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.; Fong, A.; Lai, K.; Shum, P.; Zhou, Z.; Gao, Z.; Fu, T. Effects of silver segregation on sputter deposited antibacterial silver-containing diamond-like carbon films. Thin Solid Films 2018, 650, 58–64. [Google Scholar] [CrossRef]
- Kannappan, S.; Yang, H.; Kaliyappan, K.; Manian, R.K.; Pandian, A.S.; Lee, Y.S.; Jang, J.-H.; Lu, W. Thiolated-graphene-based supercapacitors with high energy density and stable cycling performance. Carbon 2018, 134, 326–333. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; MubarakAli, D.; Prabakar, D.; Muthukumar, H.; Thajuddin, N.; Kumar, S.S.; Pugazhendhi, A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: Green approach. Environ. Sci. Pollut. Res. 2017, 25, 10362–10370. [Google Scholar] [CrossRef] [PubMed]

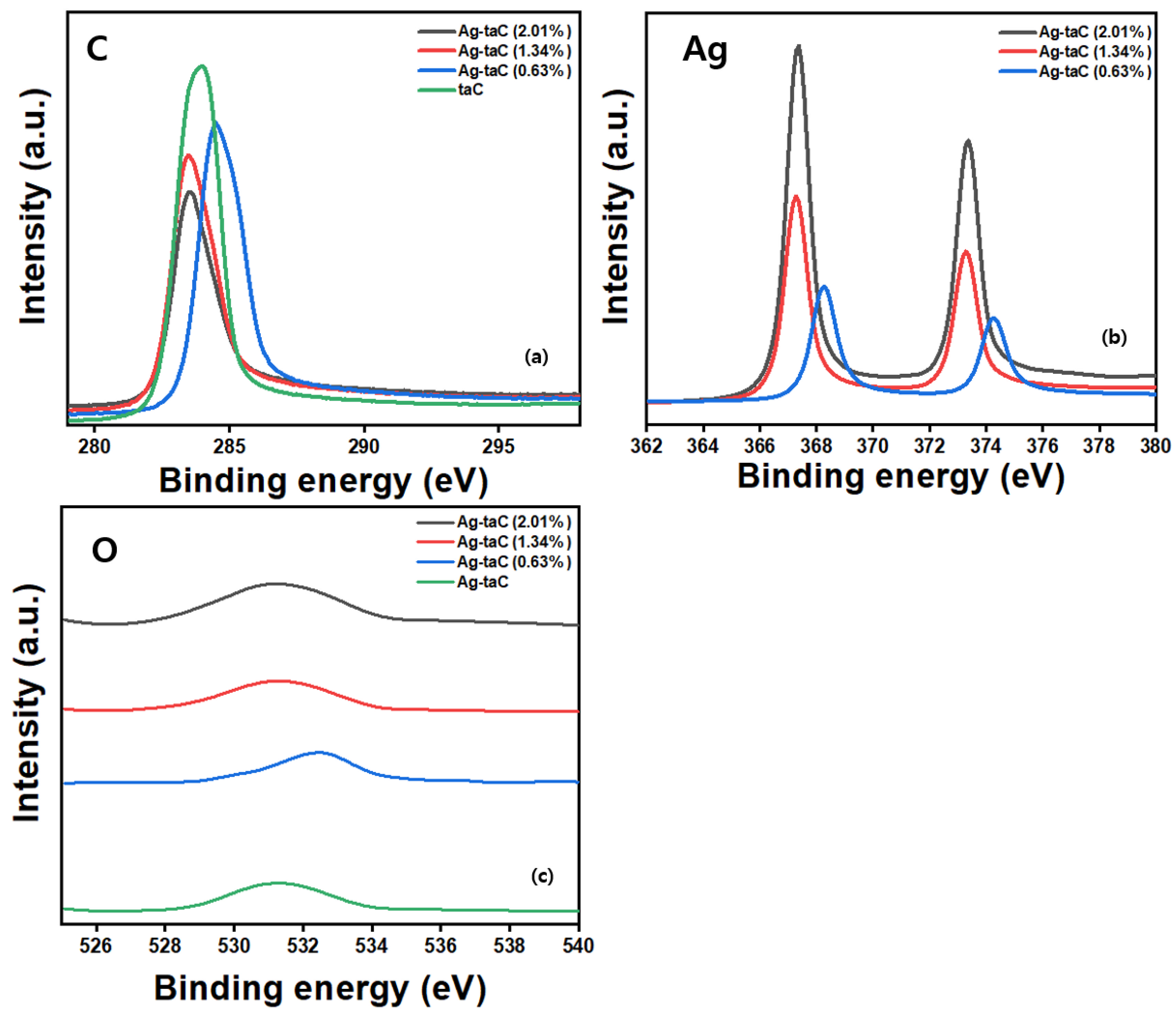

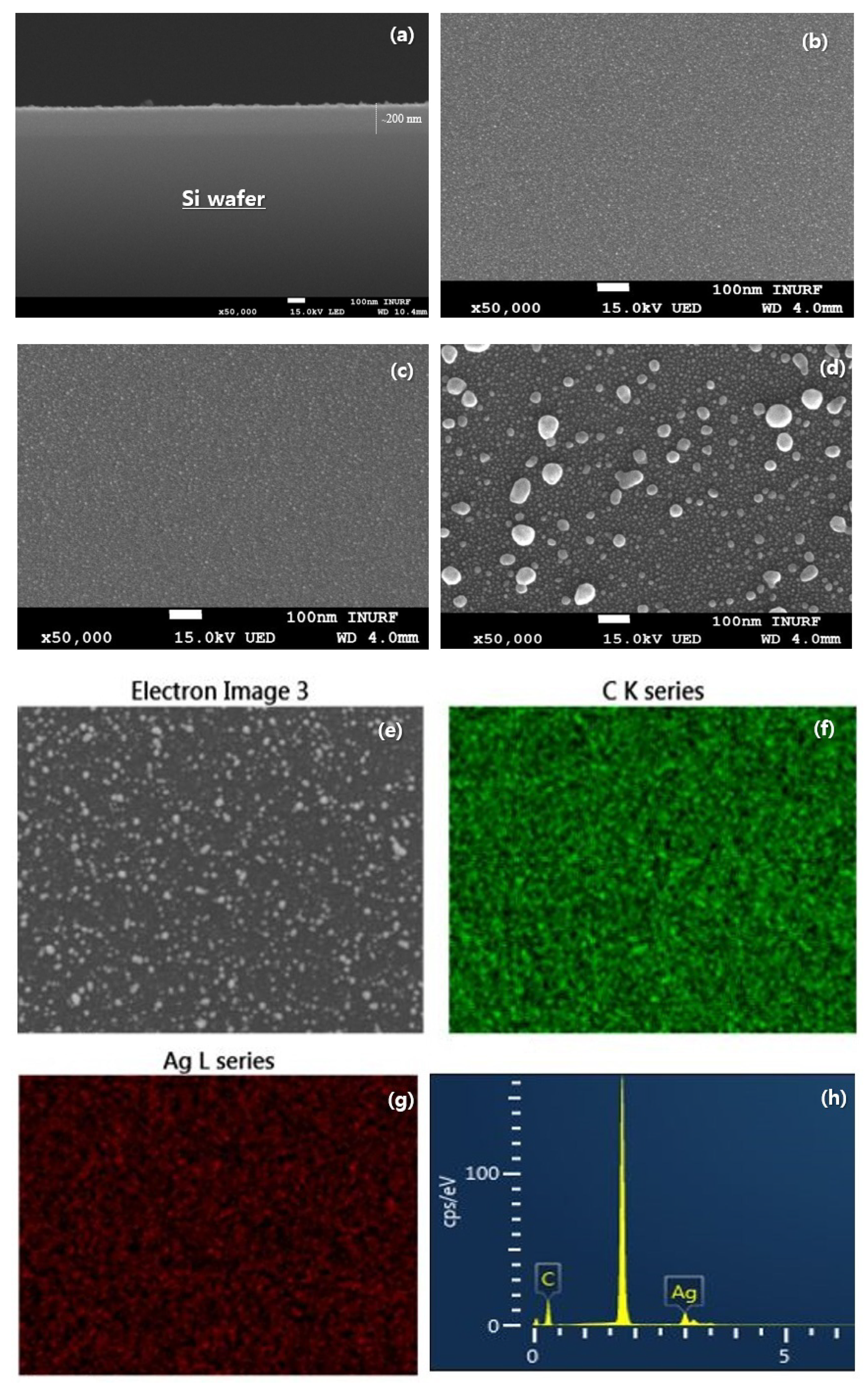
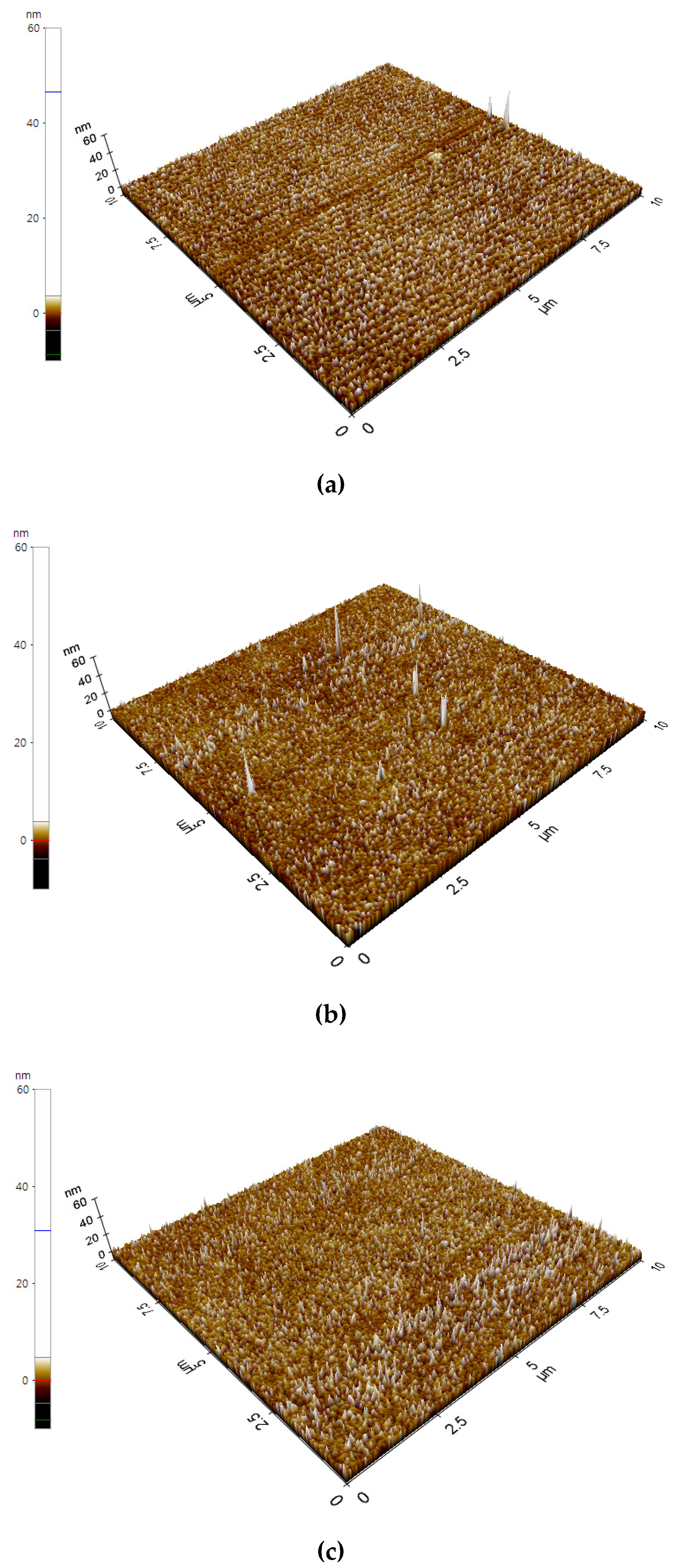

| Sample | C (at. %) | O (at. %) | Ag (at. %) | Binding Energy (eV) |
|---|---|---|---|---|
| taC | 98.7 | 1.3 | 0.0 | 283.86 (C); 531.38 (O) |
| Ag-taC (0.63, at %) | 95.41 | 1.47 | 3.12 | 284.58 (C); 532.43 (O); 368.26 (Ag) |
| Ag-taC (1.34, at %) | 92.56 | 1.96 | 5.48 | 283.55 (C); 531.45 (O); 363.28 (Ag) |
| Ag-taC (2.01, at %) | 86.66 | 3.08 | 10.26 | 283.57 (C); 531.55 (O); 367.35 (Ag) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MubarakAli, D.; Kim, S.-M.; Ko, Y.-B.; Kim, J.-W.; Jang, Y.-J.; Lee, S.-Y. Synthesis of Ag-Doped Tetrahedral Amorphous Carbon Coatings and Their Antibiofilm Efficacy for Medical Implant Application. Nanomaterials 2024, 14, 1017. https://doi.org/10.3390/nano14121017
MubarakAli D, Kim S-M, Ko Y-B, Kim J-W, Jang Y-J, Lee S-Y. Synthesis of Ag-Doped Tetrahedral Amorphous Carbon Coatings and Their Antibiofilm Efficacy for Medical Implant Application. Nanomaterials. 2024; 14(12):1017. https://doi.org/10.3390/nano14121017
Chicago/Turabian StyleMubarakAli, Davoodbasha, Sung-Min Kim, Yu-Been Ko, Jung-Wan Kim, Young-Jun Jang, and Sang-Yul Lee. 2024. "Synthesis of Ag-Doped Tetrahedral Amorphous Carbon Coatings and Their Antibiofilm Efficacy for Medical Implant Application" Nanomaterials 14, no. 12: 1017. https://doi.org/10.3390/nano14121017
APA StyleMubarakAli, D., Kim, S. -M., Ko, Y. -B., Kim, J. -W., Jang, Y. -J., & Lee, S. -Y. (2024). Synthesis of Ag-Doped Tetrahedral Amorphous Carbon Coatings and Their Antibiofilm Efficacy for Medical Implant Application. Nanomaterials, 14(12), 1017. https://doi.org/10.3390/nano14121017








