Investigating UV-Irradiation Parameters in the Green Synthesis of Silver Nanoparticles from Water Hyacinth Leaf Extract: Optimization for Future Sensor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Green Synthesis of AgNPs
2.2. Optimization of Conditions for Synthesized AgNPs
2.3. Characterization of AgNPs
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of UV-Irradiation Parameters on AgNP Synthesis
3.2. Effect of Reaction Time on Different Types of UV Rays
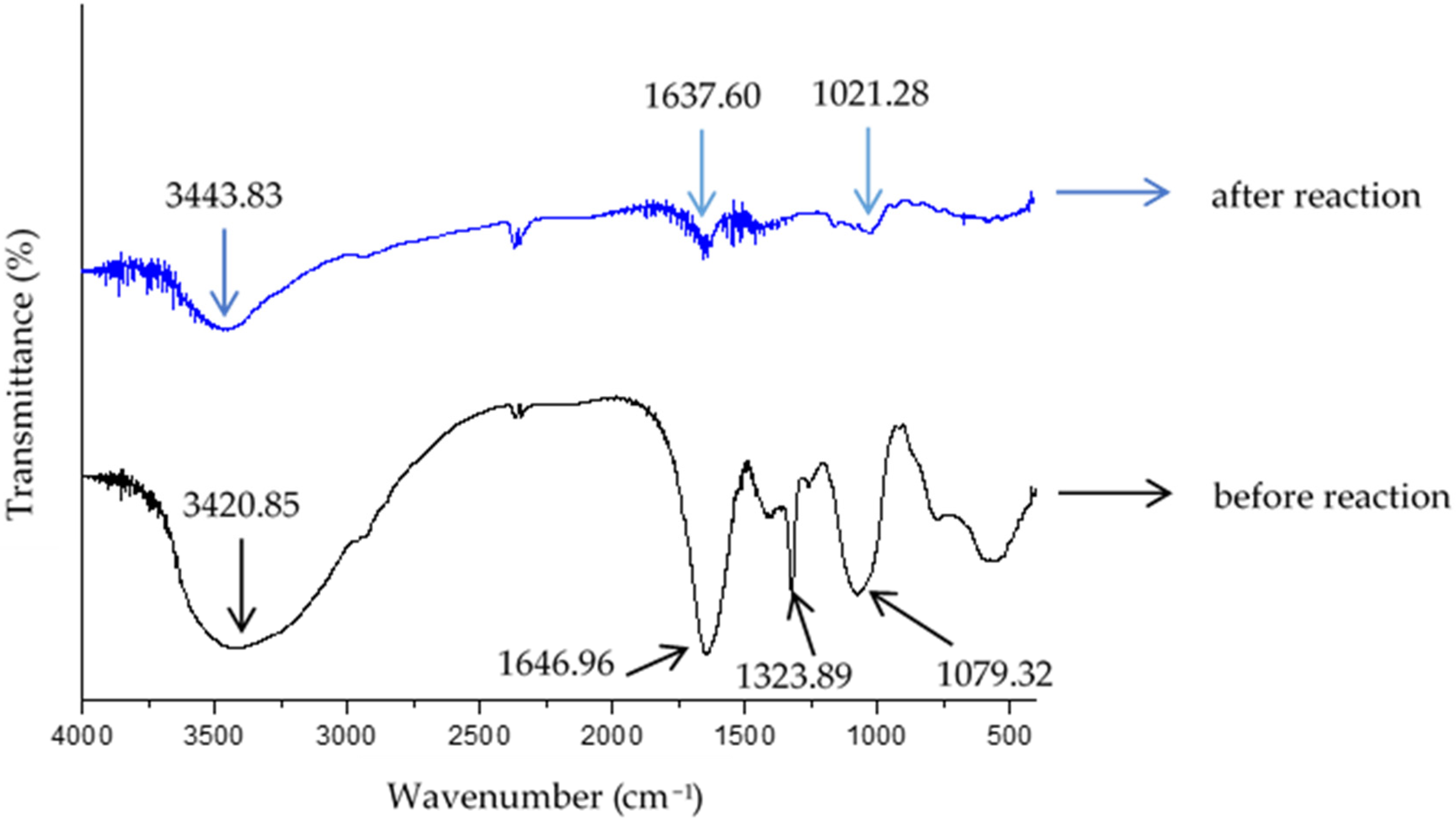
3.3. Characterization of Green Synthesized AgNPs
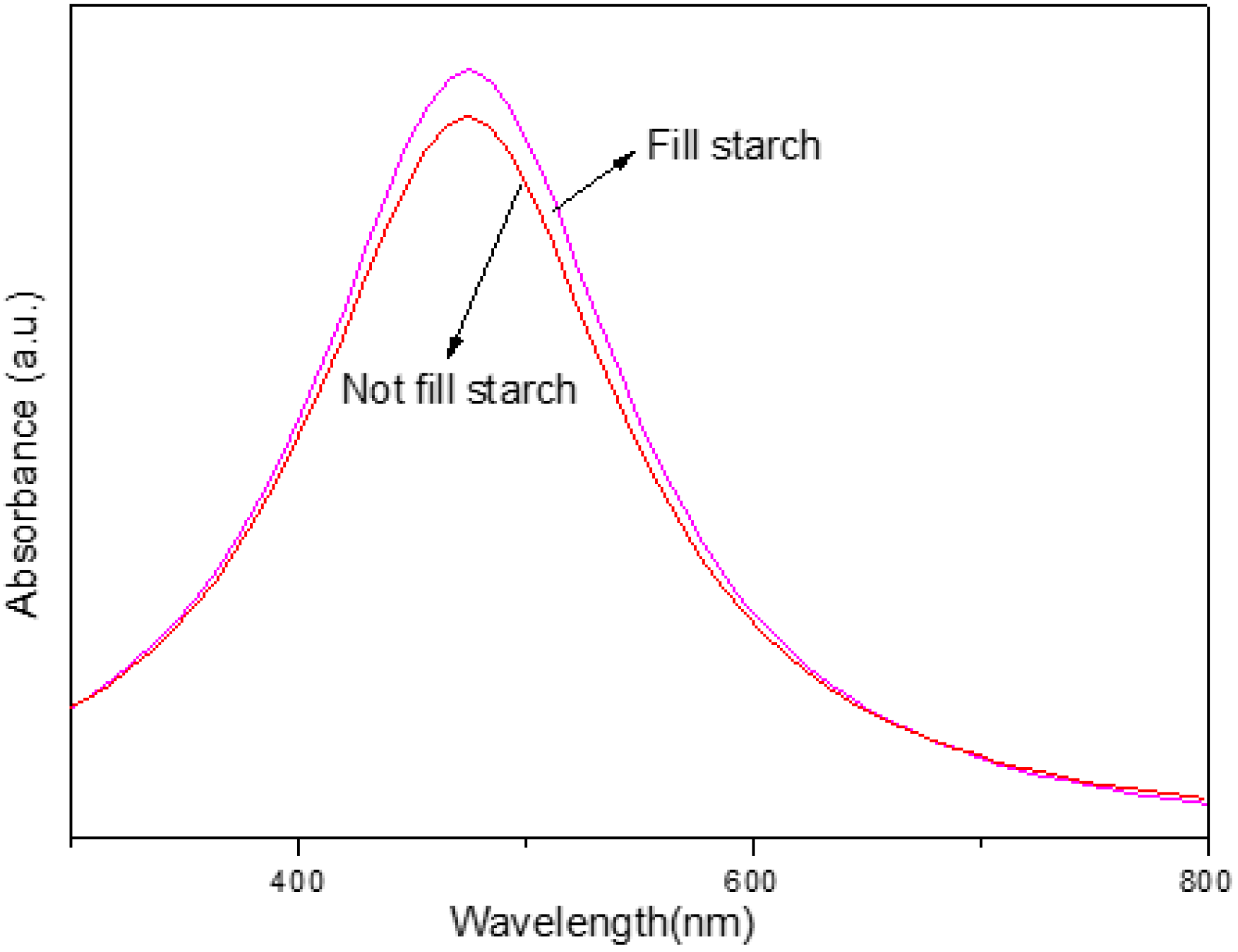
3.4. Effect of pH Levels
4. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129–141. [Google Scholar]
- Sharma, A.; Agarwal, P.; Sebghatollahi, Z.; Mahato, N. Functional Nanostructured Materials in the Cosmetics Industry: A Review. ChemEngineering 2023, 7, 66. [Google Scholar] [CrossRef]
- Wong, Y.W.H.; Yuen, C.W.M.; Leung, M.Y.S.; Ku, S.K.A.; Lam, H.L.I. Selected applications of nanotechnology in textiles. Autex Res. J. 2006, 6, 1–8. [Google Scholar] [CrossRef]
- Villalba-Rodríguez, A.M.; Martínez-Zamudio, L.Y.; Hernández Martínez, S.A.; Rodríguez-Hernández, J.A.; Melchor-Martínez, E.M.; Flores-Contreras, E.A.; González-González, R.B.; Parra-Saldívar, R. Nanomaterial Constructs for Catalytic Applications in Biomedicine: Nanobiocatalysts and Nanozymes. Top. Catal. 2023, 66, 707–722. [Google Scholar] [CrossRef]
- Asghar, N.; Hussain, A.; Nguyen, D.A.; Ali, S.; Hussain, I.; Junejo, A.; Ali, A. Advancement in nanomaterials for environmental pollutants remediation: A systematic review on bibliometrics analysis, material types, synthesis pathways, and related mechanisms. J. Nanobiotechnol. 2024, 22, 26. [Google Scholar] [CrossRef]
- Gupta, D.; Boora, A.; Thakur, A.; Gupta, T.K. Green and sustainable synthesis of nanomaterials: Recent advancements and limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Alshehri, A.H.; Jakubowska, M.; Młożniak, A.; Horaczek, M.; Rudka, D.; Free, C.; Carey, J.D. Enhanced Electrical Conductivity of Silver Nanoparticles for High Frequency Electronic Applications. ACS Appl. Mater. Interfaces 2012, 4, 7007–7010. [Google Scholar] [CrossRef]
- Nguyen, N.P.U.; Dang, N.T.; Doan, L.; Nguyen, T.T.H. Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review. Processes 2023, 11, 2617. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Silver Nanoparticles: Synthesis and Therapeutic Applications. J. Biomed. Nanotechnol. 2007, 3, 301–316. [Google Scholar] [CrossRef]
- Vishwanath, R.; Negi, B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. Curr. Res. Green Sustain. Chem. 2021, 4, 100205. [Google Scholar] [CrossRef]
- Rahman, H.; Rauf, A.; Khan, S.A.; Ahmad, Z.; Alshammari, A.; Alharbi, M.; Alam, A.; Suleria, H.A.R. Green Synthesis of Silver Nanoparticles Using Rhazya stricta Decne Extracts and Their Anti-Microbial and Anti-Oxidant Activities. Crystals 2023, 13, 398. [Google Scholar] [CrossRef]
- Jorge de Souza, T.A.; Rosa Souza, L.R.; Franchi, L.P. Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol. Environ. Saf. 2019, 171, 691–700. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–General Aspects: A Chemical Reduction Approach to the Synthesis of Nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef]
- Dhaka, A.; Mali, S.C.; Sharma, S.; Trivedi, R. A review on biological synthesis of silver nanoparticles and their potential applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Khan, F.; Shahid, A.; Zhu, H.; Wang, N.; Javed, M.R.; Ahmad, N.; Xu, J.; Alam, M.A.; Mehmood, M.A. Prospects of algae-based green synthesis of nanoparticles for environmental applications. Chemosphere 2022, 293, 133571. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Morsi, H.H.; Hassan, L.H.S.; Ali, S.S. The efficient role of algae as green factories for nanotechnology and their vital applications. Microbiol. Res. 2022, 263, 127111. [Google Scholar] [CrossRef]
- Filho, S.A.; dos Santos, M.S.; dos Santos, O.A.L.; Backx, B.P.; Soran, M.L.; Opriş, O.; Lung, I.; Stegarescu, A.; Bououdina, M. Biosynthesis of Nanoparticles Using Plant Extracts and Essential Oils. Molecules 2023, 28, 3060. [Google Scholar] [CrossRef]
- Ansari, M.; Ahmed, S.; Abbasi, A.; Khan, M.T.; Subhan, M.; Bukhari, N.A.; Hatamleh, A.A.; Abdelsalam, N.R. Plant mediated fabrication of silver nanoparticles, process optimization, and impact on tomato plant. Sci. Rep. 2023, 13, 18048. [Google Scholar] [CrossRef]
- Soni, V.; Raizada, P.; Singh, P.; Cuong, H.N.; Selvasembian, R.; Saini, A.; Saini, R.V.; Le, Q.V.; Nadda, A.K.; Le, T.T.; et al. Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: A review. Environ. Res. 2021, 202, 111622. [Google Scholar] [CrossRef]
- Bao, Y.; He, J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Plant-Extract-Mediated Synthesis of Metal Nanoparticles. J. Chem. 2021, 2021, 6562687. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Islam Masum, M.M.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnology 2021, 19, 86. [Google Scholar] [CrossRef]
- Castañeda-Aude, J.E.; Morones-Ramírez, J.R.; De Haro-Del Río, D.A.; León-Buitimea, A.; Barriga-Castro, E.D.; Escárcega-González, C.E. Ultra-Small Silver Nanoparticles: A Sustainable Green Synthesis Approach for Antibacterial Activity. Antibiotics 2023, 12, 574. [Google Scholar] [CrossRef]
- Asif, M.; Yasmin, R.; Asif, R.; Ambreen, A.; Mustafa, M.; Umbreen, S. Green Synthesis of Silver Nanoparticles (AgNPs), Structural Characterization, and their Antibacterial Potential. Dose-Response 2022, 20, 15593258221088709. [Google Scholar] [CrossRef]
- Goyal, G.; Hwang, J.; Aviral, J.; Seo, Y.; Jo, Y.; Son, J.; Choi, J. Green synthesis of silver nanoparticles using β-glucan, and their incorporation into doxorubicin-loaded water-in-oil nanoemulsions for antitumor and antibacterial applications. J. Ind. Eng. Chem. 2016, 47, 179–186. [Google Scholar] [CrossRef]
- Melkamu, W.W.; Bitew, L.T. Green synthesis of silver nanoparticles using Hagenia abyssinica (Bruce) J.F. Gmel plant leaf extract and their antibacterial and anti-oxidant activities. Heliyon 2021, 7, e08459. [Google Scholar] [CrossRef]
- Arshad, F.; Naikoo, G.A.; Hassan, I.U.; Chava, S.R.; El-Tanani, M.; Aljabali, A.A.; Tambuwala, M.M. Bioinspired and Green Synthesis of Silver Nanoparticles for Medical Applications: A Green Perspective. Appl. Biochem. Biotechnol. 2023, 196, 3636–3669. [Google Scholar] [CrossRef]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: Synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ulbrich, P.; Ruml, T. Effect of the physicochemical changes in the antimicrobial durability of green synthesized silver nanoparticles during their long-term storage. RSC Adv. 2022, 12, 30386–30403. [Google Scholar] [CrossRef]
- Bhat, R.S.; Almusallam, J.; Daihan, S.A.; Dbass, A.A. Biosynthesis of silver nanoparticles using Azadirachta indica leaves: Characterisation and impact on Staphylococcus aureus growth and glutathione-S-transferase activity. IET Nanobiotechnol. 2019, 13, 42–46. [Google Scholar] [CrossRef]
- Sundeep, D.; Vijaya Kumar, T.; Rao, P.S.S.; Ravikumar, R.V.S.S.N.; Gopala Krishna, A. Green synthesis and characterization of Ag nanoparticles from Mangifera indica leaves for dental restoration and antibacterial applications. Prog. Biomater. 2017, 6, 57–66. [Google Scholar] [CrossRef]
- Parameshwaran, R.; Kalaiselvam, S.; Jayavel, R. Green synthesis of silver nanoparticles using Beta vulgaris: Role of process conditions on size distribution and surface structure. Mater. Chem. Phys. 2013, 140, 135–147. [Google Scholar] [CrossRef]
- Ibrahim, H.M.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Degaga, A.H. Water Hyacinth (Eichhornia crassipes) Biology and its Impacts on Ecosystem, Biodiversity, Economy and Human Well-being. J. Life Sci. Biomed. 2018, 8, 94–100. [Google Scholar]
- Harun, I.; Pushiri, H.; Amirul-Aiman, A.J.; Zulkeflee, Z. Invasive Water Hyacinth: Ecology, Impacts and Prospects for the Rural Economy. Plants 2021, 10, 1613. [Google Scholar] [CrossRef]
- Mochochoko, T.; Oluwafemi, O.S.; Jumbam, D.N.; Songca, S.P. Green synthesis of silver nanoparticles using cellulose extracted from an aquatic weed; water hyacinth. Carbohydr. Polym. 2013, 98, 290–294. [Google Scholar] [CrossRef]
- Hublikar, L.V.; Ganachari, S.V.; Raghavendra, N.; Patil, V.B.; Banapurmath, N.R. Green synthesis silver nanoparticles via Eichhornia Crassipes leaves extract and their applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100212. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Ragadhita, R.; Hoffah, S.N.; Husaeni, D.F.A.; Husaeni, D.N.A.; Fiandini, M.; Luckiardi, S.; Soegoto, E.S.; Darmawan, A.; Aziz, M. Progress in the utilization of water hyacinth as effective biomass material. Environ. Dev. Sustain. 2023, 1–48. [Google Scholar] [CrossRef]
- Su, W.; Sun, Q.; Xia, M.; Wen, Z.; Yao, Z. The Resource Utilization of Water Hyacinth (Eichhornia crassipes [Mart.] Solms) and Its Challenges. Resources 2018, 7, 46. [Google Scholar] [CrossRef]
- Oluwafemi, O.S.; Anyik, J.L.; Zikalala, N.E.; Sakho, E.H.M. Biosynthesis of silver nanoparticles from water hyacinth plant leaves extract for colourimetric sensing of heavy metals. Nano-Struct. Nano-Objects 2019, 20, 100387. [Google Scholar] [CrossRef]
- Munive-Olarte, A.; Rosano-Ortega, G.; Schabes-Retchkiman, P.; Martínez-Gallegos, M.S.M.; Kassis, E.E.; González-Pérez, M.; Pacheco-García, F. Assessment of Biomass of Leaves of Water Hyacinth (Eichhornia crassipes) as Reducing Agents for the Synthesis of Nanoparticles of Gold and Silver. Int. J. Adv. Eng. Manag. Sci. 2017, 3, 364–370. [Google Scholar] [CrossRef]
- Kiruba Daniel, S.C.G.; Nehru, K.; Sivakumar, M. Rapid Biosynthesis of Silver Nanoparticles using Eichornia crassipes and its Antibacterial Activity. Curr. Nanosci. 2012, 8, 125–129. [Google Scholar] [CrossRef]
- Martínez-Espinosa, J.C.; Ramírez-Morales, M.A.; Carrera-Cerritos, R. Silver Nanoparticles Synthesized Using Eichhornia crassipes Extract from Yuriria Lagoon, and the Perspective for Application as Antimicrobial Agent. Crystals 2022, 12, 814. [Google Scholar] [CrossRef]
- Thombre, R.; Chitnis, A.; Kadam, V.; Bogawat, Y.; Colaco, R.; Kale, A. A facile method for synthesis of biostabilized silver nanoparticles using Eichhornia crassipes (Mart.) Solms (water hyacinth). Indian J. Biotechnol. 2014, 13, 337–341. [Google Scholar]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Chitra, K.; Annadurai, G. Antibacterial Activity of pH-Dependent Biosynthesized Silver Nanoparticles against Clinical Pathogen. BioMed Res. Int. 2014, 2014, 725165. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Qu, R.; Chen, H.; Ji, C.; Wang, C.; Sun, Y.; Wang, B. Degradation behavior of chitosan chains in the ‘green’ synthesis of gold nanoparticles. Carbohydr. Res. 2008, 343, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Song, L.; Liu, Y.; Fang, Y. Synthesis of silver nanoparticles by γ-ray irradiation in acetic water solution containing chitosan. Radiat. Phys. Chem. 2007, 76, 1165–1168. [Google Scholar] [CrossRef]
- Long, D.; Wu, G.; Chen, S. Preparation of oligochitosan stabilized silver nanoparticles by gamma irradiation. Radiat. Phys. Chem. 2007, 76, 1126–1131. [Google Scholar] [CrossRef]
- Yoksan, R.; Chirachanchai, S. Silver nanoparticles dispersing in chitosan solution: Preparation by γ-ray irradiation and their antimicrobial activities. Mater. Chem. Phys. 2009, 115, 296–302. [Google Scholar] [CrossRef]
- Huang, L.; Zhai, M.L.; Long, D.W.; Peng, J.; Xu, L.; Wu, G.Z.; Li, J.Q.; Wei, G.S. UV-induced synthesis, characterization and formation mechanism of silver nanoparticles in alkalic carboxymethylated chitosan solution. J. Nanopart. Res. 2008, 10, 1193–1202. [Google Scholar] [CrossRef]
- Ramnani, S.P.; Biswal, J.; Sabharwal, S. Synthesis of silver nanoparticles supported on silica aerogel using gamma radiolysis. Radiat. Phys. Chem. 2007, 76, 1290–1294. [Google Scholar] [CrossRef]
- Huang, Q.; Guo, Y.; Chen, D.; Zhang, L.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. Rational construction of ultrafine noble metals onto carbon nanoribbons with efficient oxygen reduction in practical alkaline fuel cell. Chem. Eng. J. 2021, 424, 130336. [Google Scholar] [CrossRef]
- Chen, D.; Han, C.; Sun, Q.; Ding, J.; Huang, Q.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. Bimetallic AgNi nanoparticles anchored onto MOF-derived nitrogen-doped carbon nanostrips for efficient hydrogen evolution. Green Energy Environ. 2023, 8, 258–266. [Google Scholar] [CrossRef]

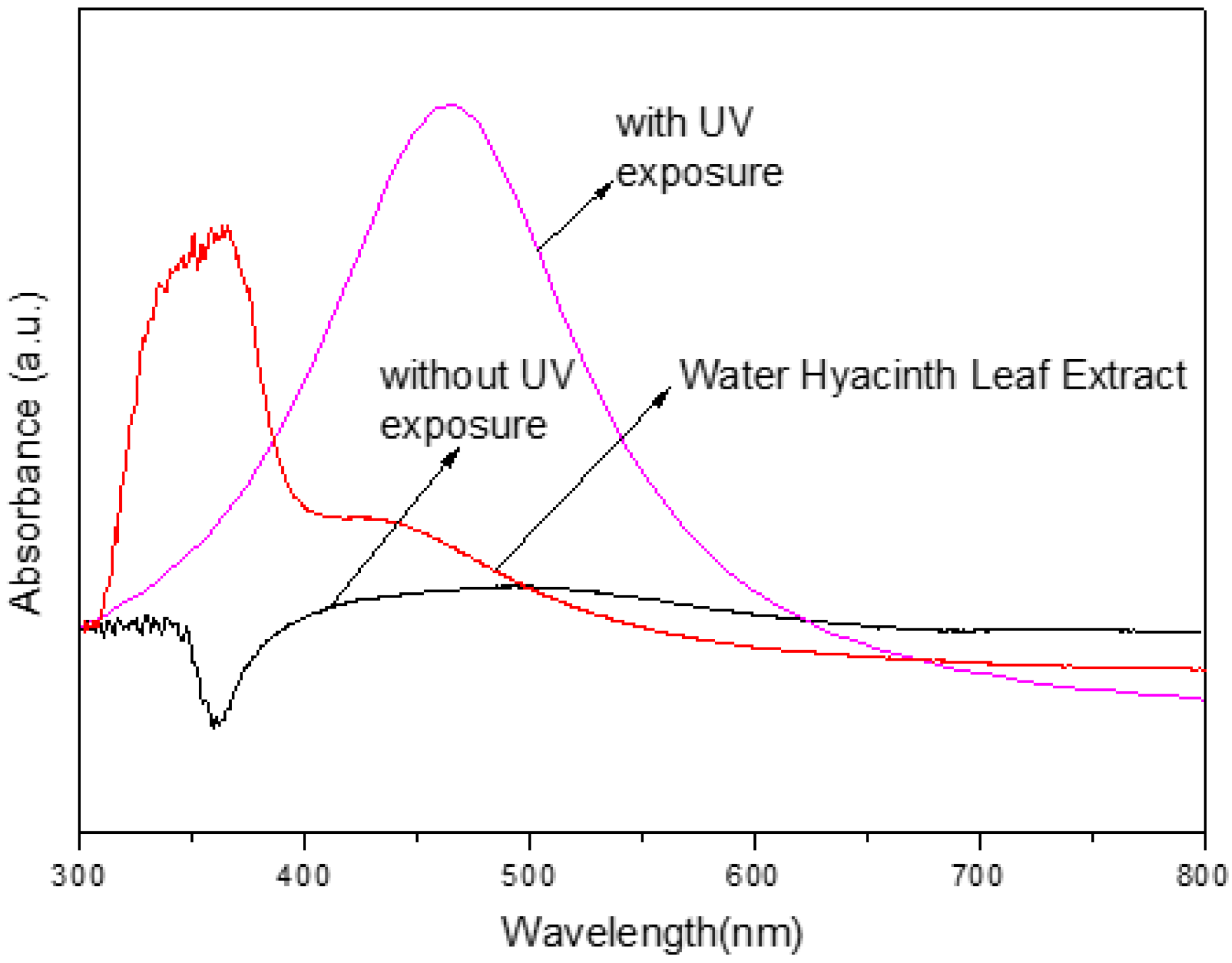


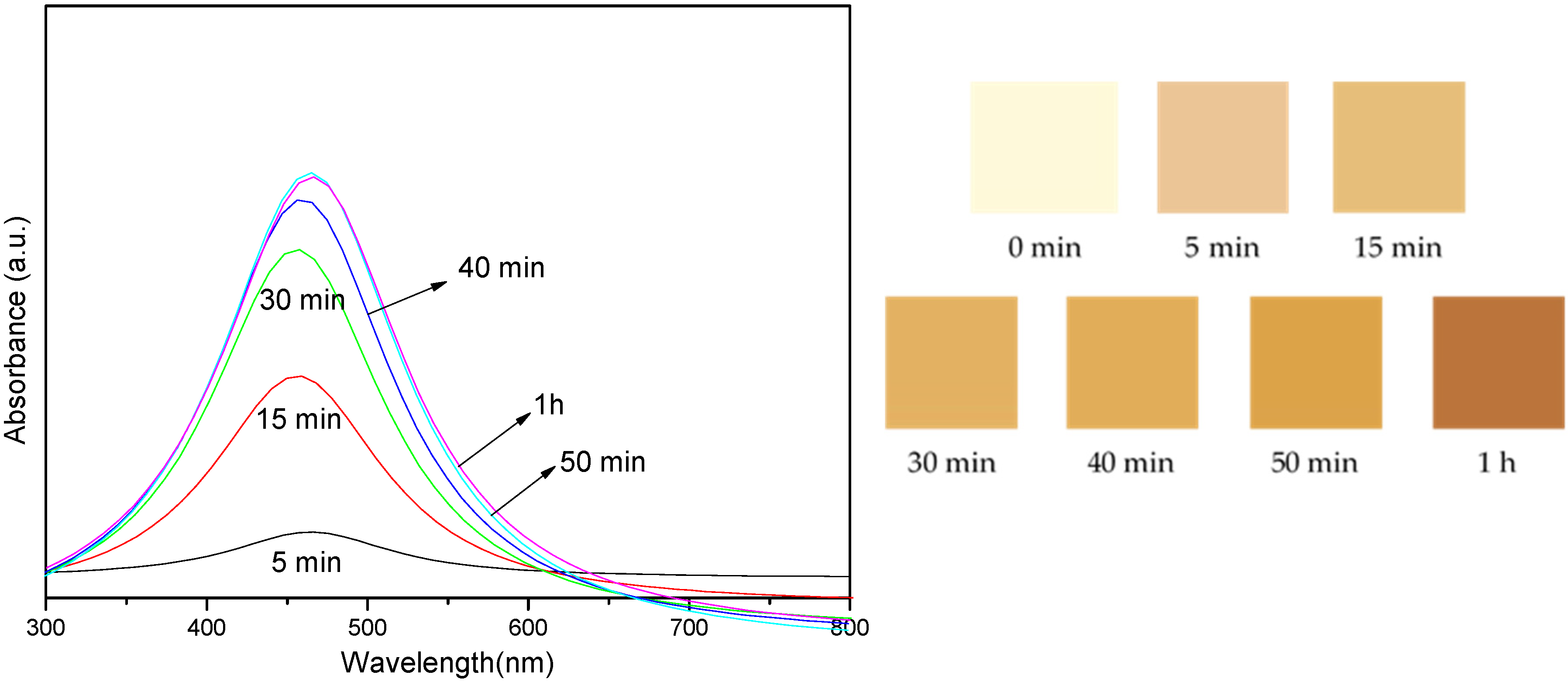
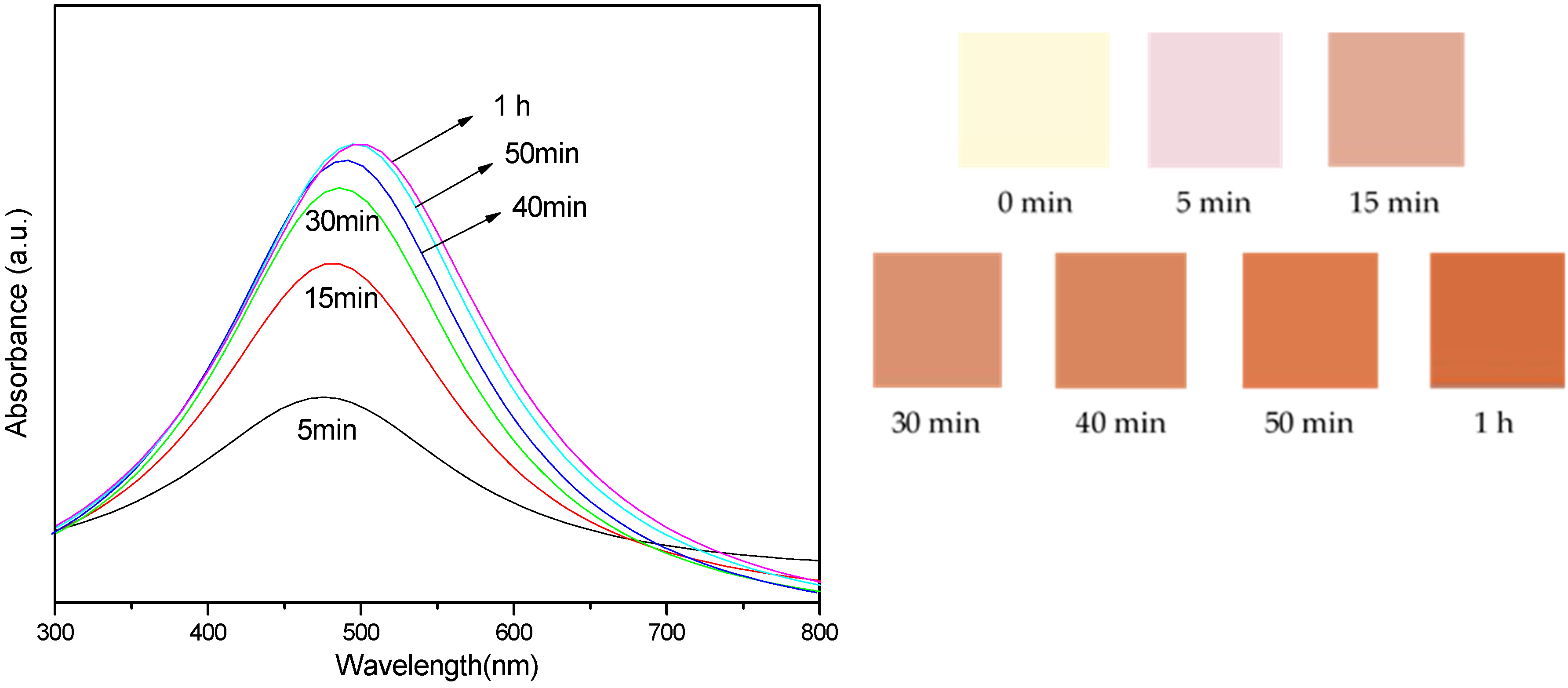

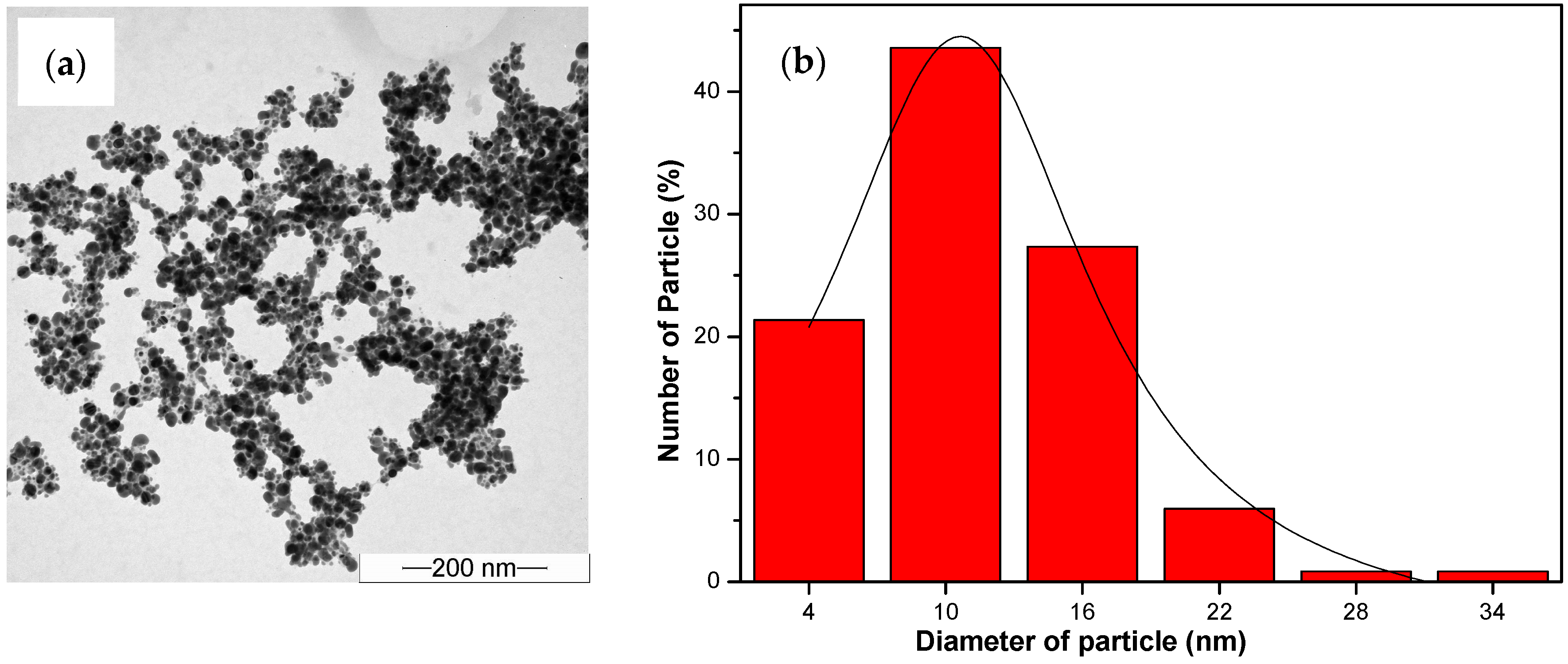
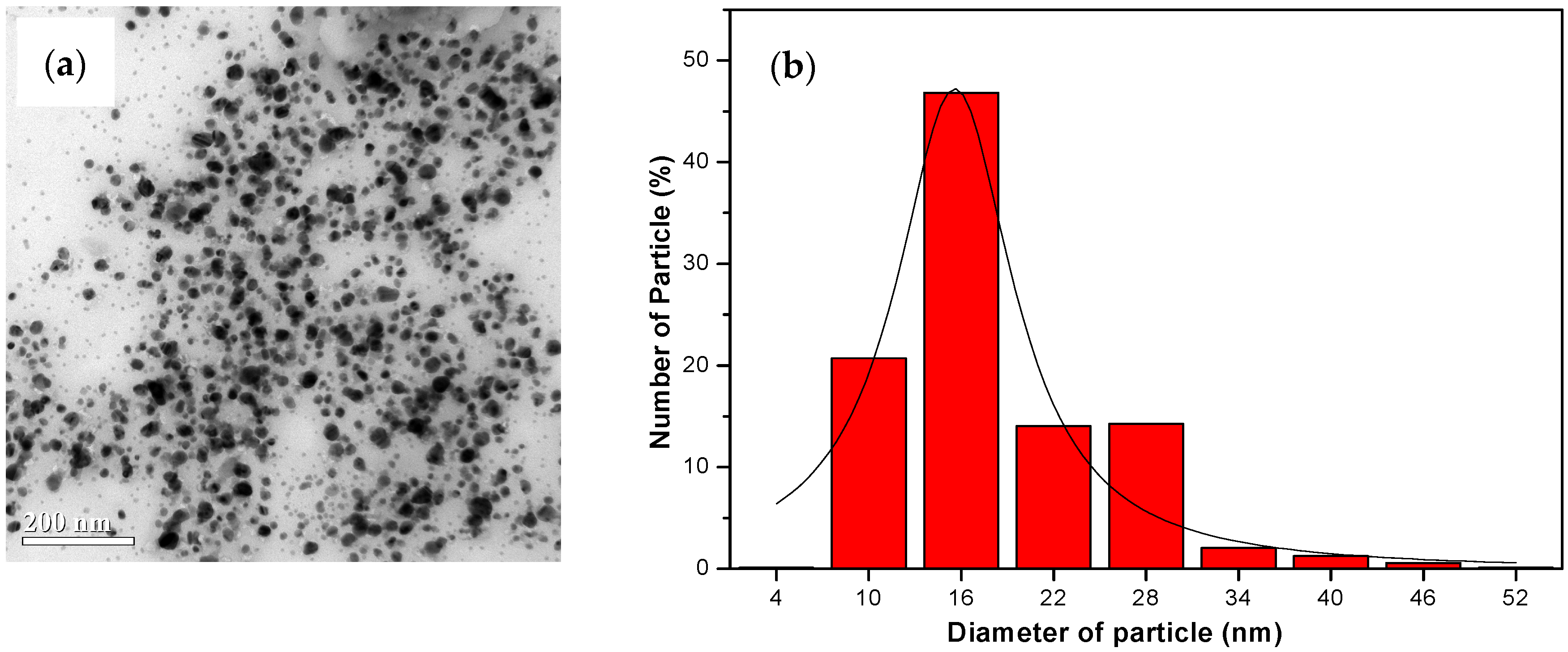


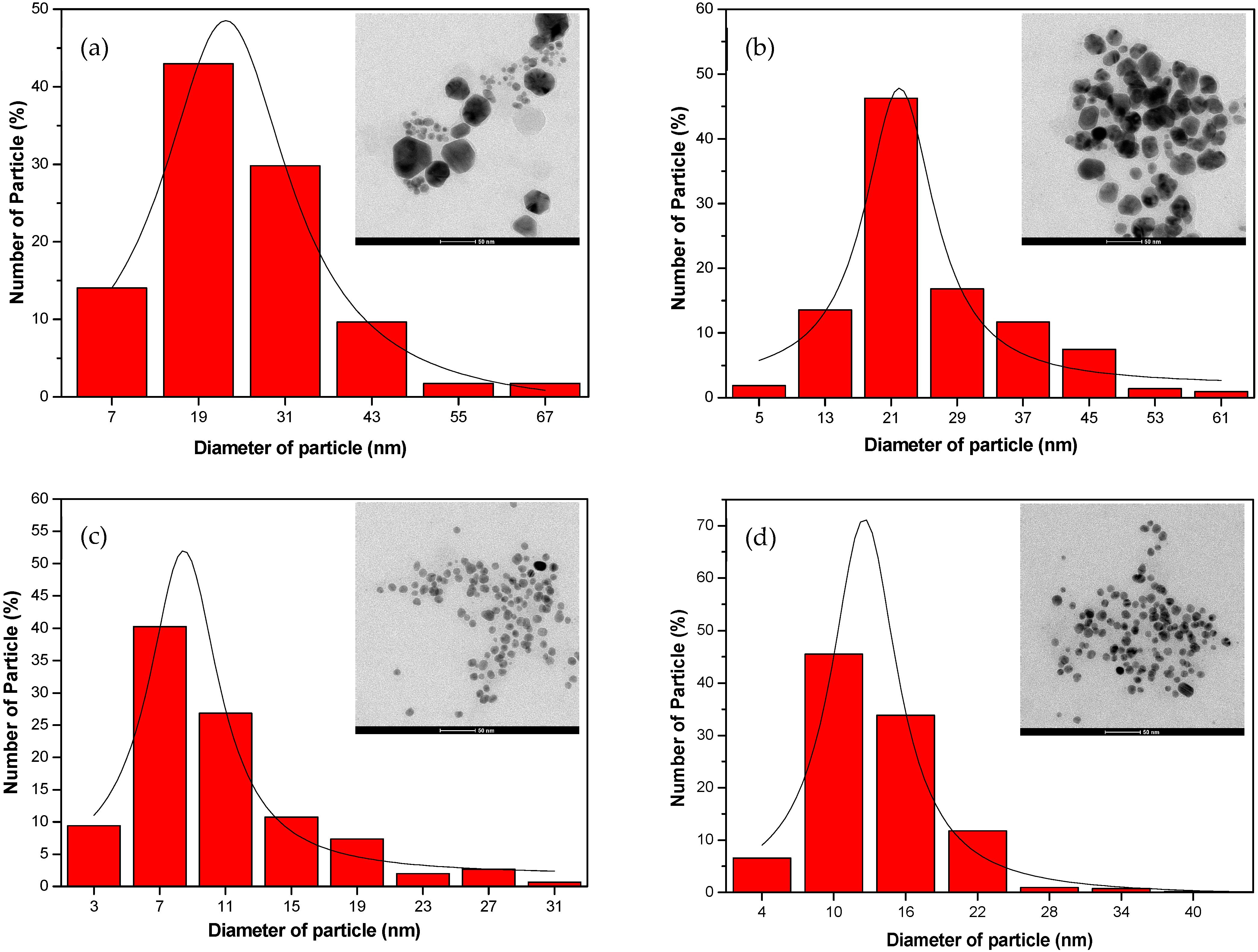
| UV | λmax (nm) | FWHM (nm) | Average Size (nm) |
|---|---|---|---|
| UV-A | 430 | 137.73 | 12.54 ± 0.19 |
| UV-B | 440 | 143.06 | 13.14 ± 0.23 |
| UV-C | 500 | 173.54 | 18.39 ± 0.48 |
| Sample | λmax (nm) | Average Size (nm) | Intensity (a.u.) | Yield (%) |
|---|---|---|---|---|
| UV-A pH 4.5 (starch) | 456 | 25.25 ± 0.70 | 1.01 | 86.92 |
| UV-A pH 5.4 (starch) | 441 | 24.74 ± 0.71 | 1.72 | 95.61 |
| UV-A pH 8.5 (starch) | 420 | 10.60 ± 0.19 | 2.66 | 99.87 |
| UV-A pH 12 (starch) | 430 | 12.56 ± 0.42 | 2.17 | 96.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chutrakulwong, F.; Thamaphat, K.; Intarasawang, M. Investigating UV-Irradiation Parameters in the Green Synthesis of Silver Nanoparticles from Water Hyacinth Leaf Extract: Optimization for Future Sensor Applications. Nanomaterials 2024, 14, 1018. https://doi.org/10.3390/nano14121018
Chutrakulwong F, Thamaphat K, Intarasawang M. Investigating UV-Irradiation Parameters in the Green Synthesis of Silver Nanoparticles from Water Hyacinth Leaf Extract: Optimization for Future Sensor Applications. Nanomaterials. 2024; 14(12):1018. https://doi.org/10.3390/nano14121018
Chicago/Turabian StyleChutrakulwong, Fueangfakan, Kheamrutai Thamaphat, and Mana Intarasawang. 2024. "Investigating UV-Irradiation Parameters in the Green Synthesis of Silver Nanoparticles from Water Hyacinth Leaf Extract: Optimization for Future Sensor Applications" Nanomaterials 14, no. 12: 1018. https://doi.org/10.3390/nano14121018







