Phosphorus-Modified Palladium and Tungsten Carbide/Mesoporous Carbon Composite for Hydrogen Oxidation Reaction of Proton Exchange Membrane Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
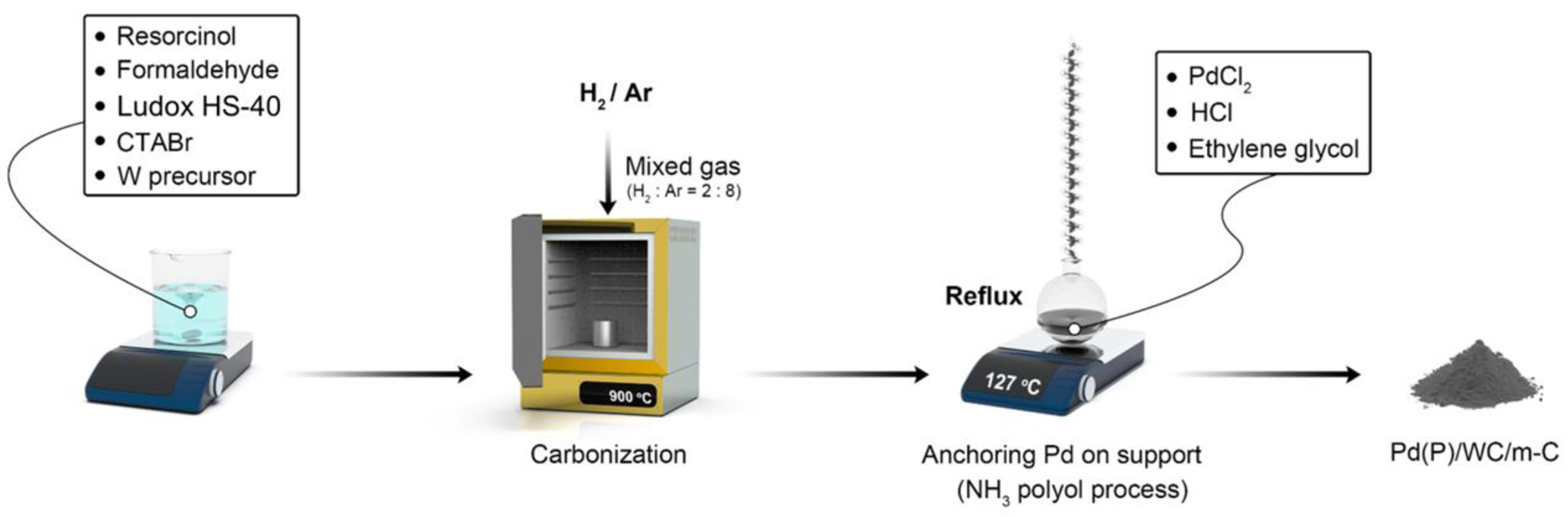
2.1. Synthesis of m-C
2.2. Synthesis of WC/m-C
2.3. Pd or Pd(P) Nanoparticle Loading onto Support Materials
2.4. Catalyst Characterization
2.5. Electrochemical Tests
3. Results
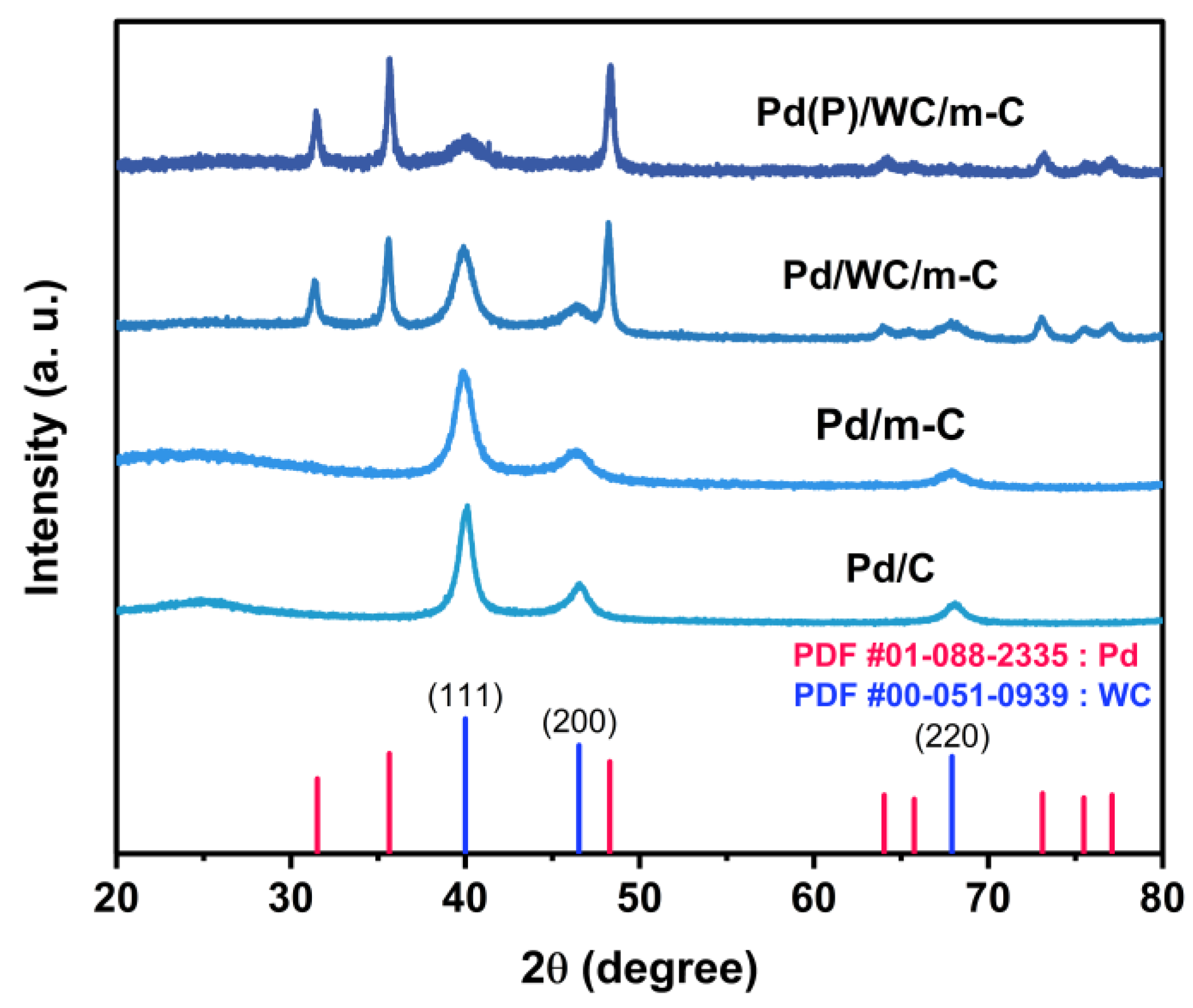
3.1. Physical and Electrochemical Properties of m-C and WC/m-C Supports
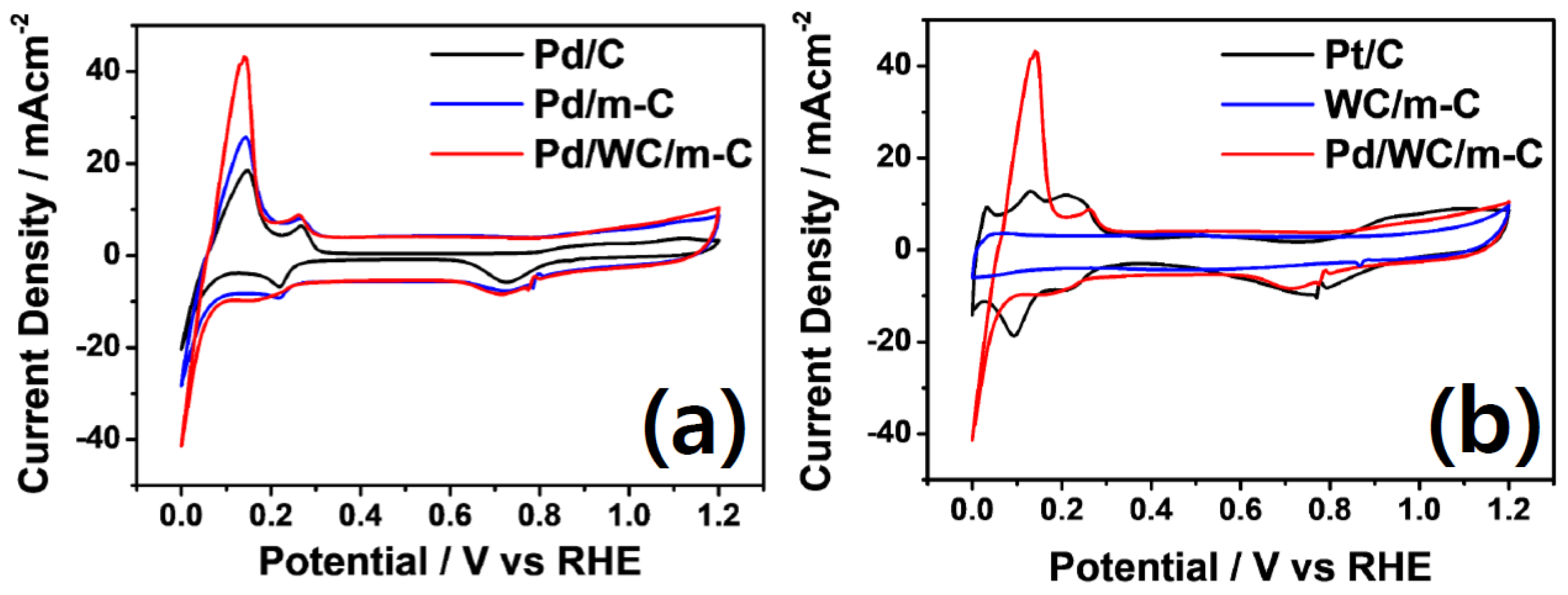
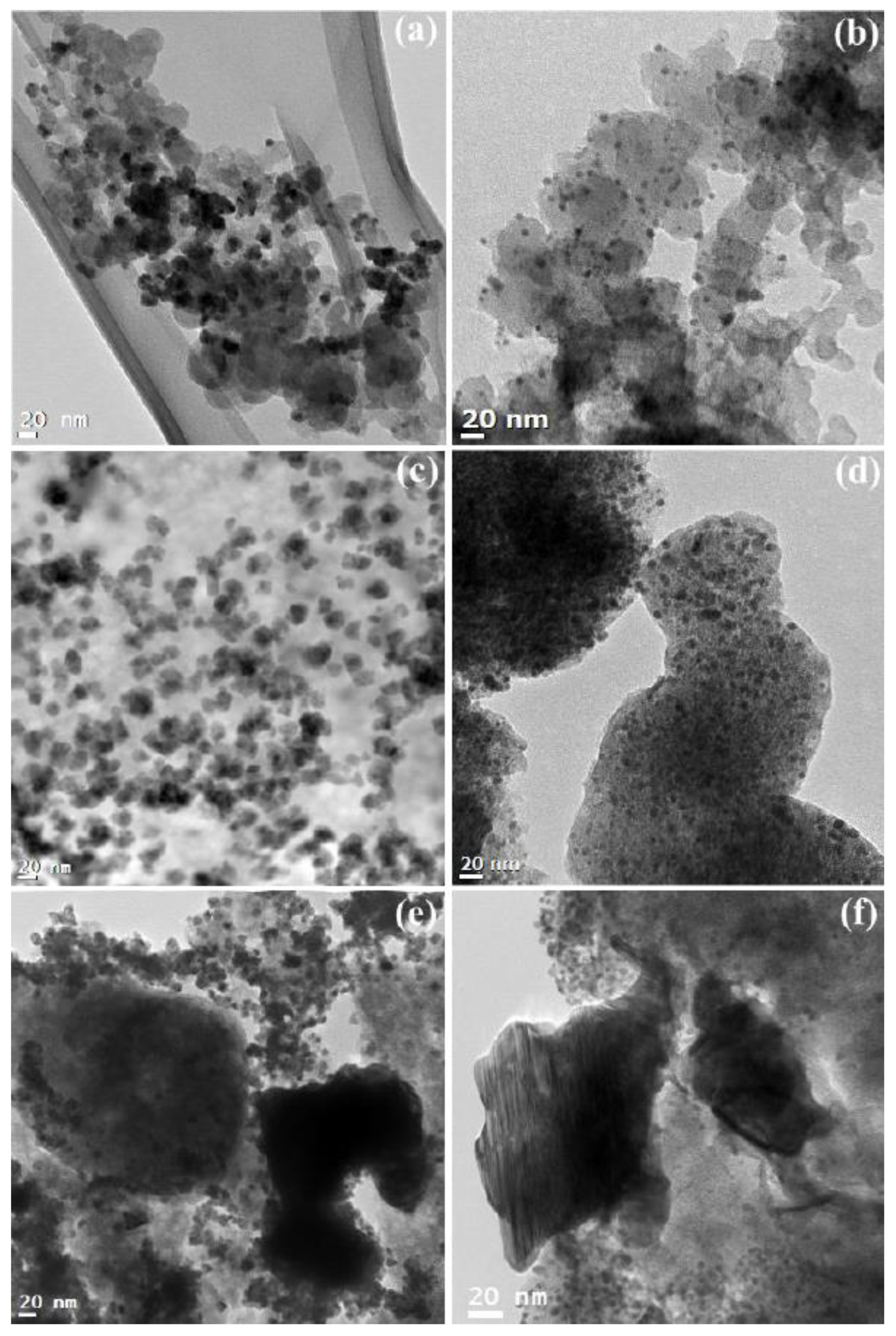
3.2. Physical and Electrochemical Properties of Pd Nanoparticles Loaded on the Supports
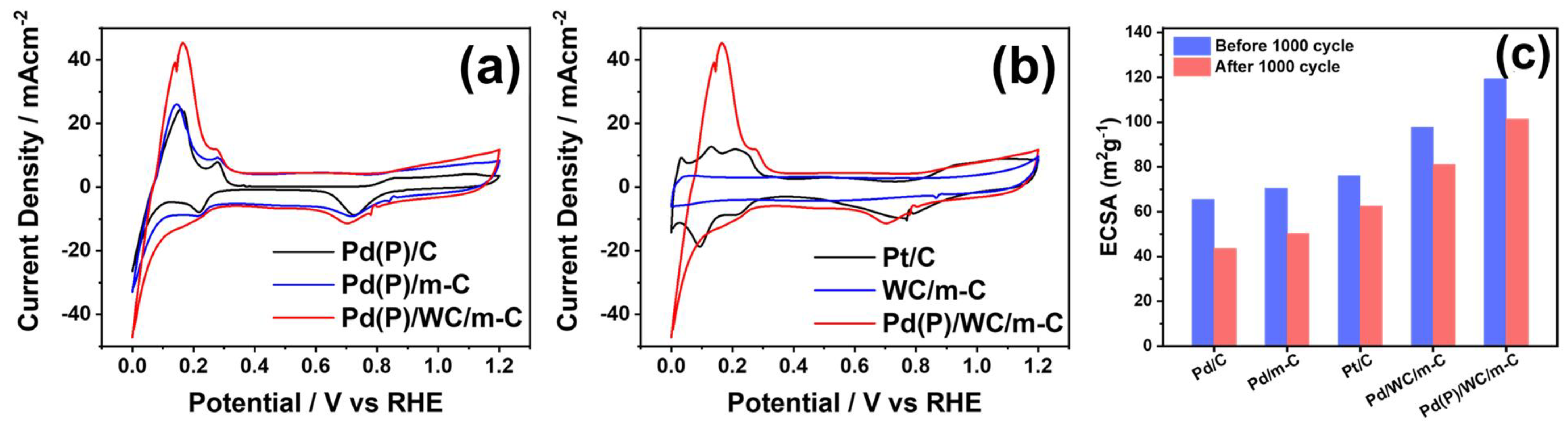
3.3. Physical and Electrochemical Properties of Phosphorus-Modified Pd Nanoparticles Loaded on the Supports
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prats, H.; Stamatakis, M. Transition Metal Carbides as Supports for Catalytic Metal Particles: Recent Progress and Opportunities. J. Phys. Chem. Lett. 2024, 15, 3450–3460. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G. Carbide and Nitride Overlayers on Early Transition Metal Surfaces: Preparation, Characterization, and Reactivities. Chem. Rev. 1996, 96, 1477–1498. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Denny, S.R.; Chen, J.G. Transition Metal Carbides and Nitrides as Catalysts for Thermochemical Reactions. J. Catal. 2021, 404, 929–942. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Kim, J.Y.; Youn, D.H. In-situ Construction of Mo2C–MoC Heterostructure on Ni Foam for Efficient Hydrogen Evolution in Alkaline Media. Int. J. Hydrogen Energy 2024, 49, 58–66. [Google Scholar] [CrossRef]
- Youn, D.H.; Han, S.; Kim, J.Y.; Kim, J.Y.; Park, H.; Choi, S.H.; Lee, J.S. Highly Active and Stable Hydrogen Evolution Electrocatalysts Based on Molybdenum Compounds on Carbon Nanotube–Graphene Hybrid Support. ACS Nano 2014, 8, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Abdelkareem, M.A.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Abdelghani, E.M.A.; Olabi, A.G. Transition Metal Carbides and Nitrides as Oxygen Reduction Reaction Catalyst or Catalyst Support in Proton Exchange Membrane Fuel Cells (PEMFCs). Int. J. Hydrogen Energy 2021, 46, 23529–23547. [Google Scholar] [CrossRef]
- Saha, S.; Rajbongshi, B.M.; Ramani, V.; Verma, A. Titanium Carbide: An Emerging Electrocatalyst for Fuel Cell and Electrolyser. Int. J. Hydrogen Energy 2021, 46, 12801–12821. [Google Scholar] [CrossRef]
- Li, G.-R.; Wang, F.; Jiang, Q.-W.; Gao, X.-P.; Shen, P.-W. Carbon Nanotubes with Titanium Nitride as a Low-Cost Counter-Electrode Material for Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2010, 49, 3653–3656. [Google Scholar] [CrossRef]
- Seol, M.; Youn, D.H.; Kim, J.Y.; Jang, J.-W.; Lee, J.S.; Yong, K. Mo-Compound/CNT-Graphene Composites as Efficient Catalytic Electrodes for Quantum-Dot-Sensitized Solar Cells. Adv. Energy Mater. 2014, 4, 1300775. [Google Scholar] [CrossRef]
- Wang, C.-H.; Kurra, N.; Alhabeb, M.; Chang, J.-K.; Alshareef, H.N.; Gogotsi, Y. Titanium Carbide (MXene) as a Current Collector for Lithium-Ion Batteries. ACS Omega 2018, 3, 12489–12494. [Google Scholar] [CrossRef]
- Syamsai, R.; Rodriguez, J.R.; Pol, V.G.; Van Le, Q.; Batoo, K.M.; Farooq Adil, S.; Pandiaraj, S.; Muthumareeswaran, M.R.; Raslan, E.H.; Grace, A.N. Double Transition Metal MXene (TixTa4−xC3) 2D Materials as Anodes for Li-Ion Batteries. Sci. Rep. 2021, 11, 688. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Fu, X.; Shang, Z.; Liu, J.; Luo, H. A BiVO4 Film Photoanode with Re-Annealing Treatment and 2D Thin Ti3C2Tx Flakes Decoration for Enhanced Photoelectrochemical Water Oxidation. Chem. Eng. J. 2019, 361, 853–861. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sivasankaran, R.; Arunachalam, M.; Lee, G.H.; Youn, D.H.; Kang, S.H. Mo2C Cocatalysts Supported Si Nanowire Photoanode for Solar Water Oxidation. J. Electrochem. Soc. 2021, 168, 066519. [Google Scholar] [CrossRef]
- Lori, O.; Gonen, S.; Kapon, O.; Elbaz, L. Durable Tungsten Carbide Support for Pt-Based Fuel Cells Cathodes. ACS Appl. Mater. Interfaces 2021, 13, 8315–8323. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; You, S.; Liu, M.; Zhang, P.; Dai, Y.; Yu, Y.; Ren, N.; Zou, J. ZIF-8-Derived Carbon-Thin-Layer Protected WC/W24O68 Micro-Sized Rods with Enriched Oxygen Vacancies as Efficient Pt Co-Catalysts for Methanol Oxidation and Oxygen Reduction. Appl. Catal. B Environ. 2020, 265, 118574. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Yu, C.; Hu, X.; Yin, Y.; Guo, S.; Zhong, S. Synergistic and Durable Pt-WC Catalyst for Methanol Electro-Oxidation in Ionic Liquid Aqueous Solution. ACS Appl. Energy Mater. 2019, 2, 8459–8463. [Google Scholar] [CrossRef]
- Ganesan, R.; Lee, J.S. Tungsten Carbide Microspheres as a Noble-Metal-Economic Electrocatalyst for Methanol Oxidation. Angew. Chem. Int. Ed. 2005, 44, 65576560. [Google Scholar] [CrossRef]
- Polini, R.; Marcucci, A.; D’Ottavi, C.; Nunziante, P.; De Filippis, P.; Marcheselli, G. Toward Greener Synthesis of WC Powders for Cemented Tungsten Carbides Manufacturing. ACS Sustain. Chem. Eng. 2021, 9, 8458–8466. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, L.; Qi, X.; Song, H.; Wang, J.-Y.; Zhang, H.; Wang, X. Template-Free Pseudomorphic Synthesis of Tungsten Carbide Nanorods. Small 2012, 8, 3350–3356. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Cai, M.; Shen, P.K. Nanosized tungsten carbide synthesized by a novel route at low temperature for high performance electrocatalysis. Sci. Rep. 2013, 3, 1646. [Google Scholar] [CrossRef]
- Wang, Y.; He, C.; Brouzgou, A.; Liang, Y.; Fu, R.; Wu, D.; Tsiakaras, P.; Song, S. A facile soft-template synthesis of ordered mesoporous carbon/tungsten carbide composites with high surface area for methanol electrooxidation. J. Power Sources 2012, 200, 8–13. [Google Scholar] [CrossRef]
- Ma, C.; Kang, L.; Shi, M.; Lang, X.; Jiang, Y. Preparation of Pt-mesoporous tungsten carbide/carbon composites via a soft-template method for electrochemical methanol oxidation. J. Alloys Compd. 2014, 588, 481–487. [Google Scholar] [CrossRef]
- Hong, S.; Chung, S.; Park, J.; Hwang, J.P.; Lee, C.H.; Uhm, S.; Bong, S.; Lee, J. Contribution of Interstitial Boron in a Boron-Incorporated Palladium Catalyst Toward Formate Oxidation in an Alkaline Direct Formate Fuel Cell. ACS Catal. 2021, 11, 4722–4729. [Google Scholar] [CrossRef]
- Kakaei, K.; Rahnavardi, M. Synthesis of Nitrogen-Doped Reduced Graphene Oxide and Its Decoration with High Efficiency Palladium Nanoparticles for Direct Ethanol Fuel Cell. Renew. Energy 2021, 163, 1277–1286. [Google Scholar] [CrossRef]
- Sankar, S.; Anilkumar, G.M.; Tamaki, T.; Yamaguchi, T. Cobalt-Modified Palladium Bimetallic Catalyst: A Multifunctional Electrocatalyst with Enhanced Efficiency and Stability toward the Oxidation of Ethanol and Formate in Alkaline Medium. ACS Appl. Energy Mater. 2018, 1, 4140–4149. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuelcell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, Z.; Niu, W.; Xu, G.; Zhu, L. Carbon-supported Pd nanocatalyst modified by non-metal phosphorus for the oxygen reduction reaction. J. Power Sources 2008, 182, 91–94. [Google Scholar] [CrossRef]
- Lamy, C. Electrocatalytic oxidation of organic compounds on noble metals in aqueous solution. Electrochim. Acta 1984, 29, 1581–1588. [Google Scholar] [CrossRef]
- Capon, A.; Parsons, R. The oxidation of formic acid on noble metal electrodes: II. A comparison of the behaviour of pure electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1973, 44, 239–254. [Google Scholar] [CrossRef]
- Uhm, S.; Lee, H.J.; Kwon, Y.; Lee, J. A Stable and Cost-Effective Anode Catalyst Structure for Formic Acid Fuel Cells. Angew. Chem. Int. Ed. 2008, 47, 10163–10166. [Google Scholar] [CrossRef]
- Kim, S.-W.; Park, J.; Jang, Y.; Chung, Y.; Hwang, S.; Hyeon, T.; Kim, Y.W. Synthesis of Monodisperse Palladium Nanoparticles. Nano Lett. 2003, 3, 1289–1291. [Google Scholar] [CrossRef]
- Xiong, Y.; McLellan, J.M.; Chen, J.; Yin, Y.; Li, Z.-Y.; Xia, Y. Kinetically controlled synthesis of triangular and hexagonal nanoplates of palladium and their SPR/SERS properties. J. Am. Chem. Soc. 2005, 127, 17118–17127. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kim, S.; Tracy, J.B.; Jasanoff, A.; Bawendi, M.G. Phosphine Oxide Polymer for Water-Soluble Nanoparticles. J. Am. Chem. Soc. 2005, 127, 4556–4557. [Google Scholar] [CrossRef]
- Yu, X.; Liu, J.; Li, J.; Luo, Z.; Zuo, Y.; Xing, C.; Llorca, J.; Nasiou, D.; Arbiol, J.; Pan, K.; et al. Phosphorous Incorporation in Pd2Sn Alloys for Electrocatalytic Ethanol Oxidation. Nano Energy 2020, 77, 105116. [Google Scholar] [CrossRef]
- Chen, L.; Lu, L.; Zhu, H.; Chen, Y.; Huang, Y.; Li, Y.; Wang, L. Improved Ethanol Electrooxidation Performance by Shortening Pd–Ni Active Site Distance in Pd–Ni–P Nanocatalysts. Nat. Commun. 2017, 8, 14136. [Google Scholar] [CrossRef] [PubMed]
- Bampos, G.; Bebelis, S.; Kondarides, D.I.; Verykios, X. Comparison of the activity of Pd–M (M: Ag, Co, Cu, Fe, Ni, Zn) bimetallic electrocatalysts for oxygen reduction reaction. Top. Catal. 2017, 60, 1260–1273. [Google Scholar] [CrossRef]
- Zhao, F.; Li, C.; Yuan, Q.; Yang, F.; Luo, B.; Xie, Z.; Yang, X.; Zhou, Z.; Wang, X. Trimetallic Palladium–Copper–Cobalt Alloy Wavy Nanowires Improve Ethanol Electrooxidation in Alkaline Medium. Nanoscale 2019, 11, 19448–19454. [Google Scholar] [CrossRef]
- Larcher, D.; Patrice, R. Preparation of Metallic Powders and Alloys in Polyol Media: A Thermodynamic Approach. J. Solid State Chem. 2000, 154, 405–411. [Google Scholar] [CrossRef]
- Ham, D.J.; Pak, C.; Bae, G.H.; Han, S.; Kwon, K.; Jin, S.-A.; Chang, H.; Choi, S.H.; Lee, J.S. Palladium–nickel alloys loaded on tungsten carbide as platinum-free anode electrocatalysts for polymer electrolyte membrane fuel cells. Chem. Commun. 2011, 47, 5792–5794. [Google Scholar] [CrossRef]
- Yin, S.; Cai, M.; Wang, C.; Shen, P.K. Tungsten carbide promoted Pd–Fe as alcohol-tolerant electrocatalysts for oxygen reduction reactions. Energy Environ. Sci. 2011, 4, 558–563. [Google Scholar] [CrossRef]
- Mellinger, Z.J.; Kelly, T.G.; Chen, J.G. Pd-Modified Tungsten Carbide for Methanol Electro-oxidation: From Surface Science Studies to Electrochemical Evaluation. ACS Catal. 2012, 2, 751–758. [Google Scholar] [CrossRef]
- Ham, D.J.; Han, S.; Pak, C.; Ji, S.M.; Jin, S.A.; Chang, H.; Lee, J.S. High electrochemical performance and stability of co-deposited Pd–Au on phase-pure tungsten carbide for hydrogen oxidation. Top. Catal. 2012, 55, 922–930. [Google Scholar] [CrossRef]
- Serov, A.A.; Cho, S.-Y.; Han, S.; Min, M.; Chai, G.; Nam, K.H.; Kwak, C. Modification of palladium-based catalysts by chalcogenes for direct methanol fuel cells. Electrochem. Commun. 2007, 9, 2041–2044. [Google Scholar] [CrossRef]
- Abdellah, A.M.; Ismail, F.; Siig, O.W.; Yang, J.; Andrei, C.M.; DiCecco, L.-A.; Rakhsha, A.; Salem, K.E.; Grandfield, K.; Bassim, N.; et al. Impact of palladium/palladium hydride conversion on electrochemical CO2 reduction via in-situ transmission electron microscopy and diffraction. Nat. Commun. 2024, 15, 938. [Google Scholar] [CrossRef] [PubMed]
- Bampos, G.; Tsatsos, S.; Kyriakou, G.; Bebelis, S. Pd-based bimetallic electrocatalysts for hydrogen oxidation reaction in acidic medium. J. Electroanal. Chem. 2023, 928, 117008. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.-H.; Sasaki, K.; Adzic, R.R. Pd–Fe Nanoparticles as Electrocatalysts for Oxygen Reduction. J. Am. Chem. Soc. 2006, 128, 3526–3527. [Google Scholar] [CrossRef]
- Lee, S.-P.; Chen, Y.-W. Selective Hydrogenation of Furfural on Ni–P, Ni–B, and Ni–P–B Ultrafine Materials. Ind. Eng. Chem. Res. 1999, 38, 2548–2556. [Google Scholar] [CrossRef]
- Jørgensen, S.; Horst, J.A.; Dyrlie, O.; Larring, Y.; Ræder, H.; Norby, T. XPS surface analyses of LaPO4 ceramics prepared by precipitation with or without excess of PO43−. Surf. Interface Anal. 2002, 34, 306–310. [Google Scholar] [CrossRef]
- Lo, P.H.; Tsai, W.T.; Lee, J.T.; Hung, M.P. The Electrochemical Behavior of Electroless Plated Ni-P Alloys in Concentrated NaOH Solution. J. Electrochem. Soc. 1995, 142, 91. [Google Scholar] [CrossRef]
- Rodríguez-reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159. [Google Scholar] [CrossRef]
| XRD [nm] | TEM [nm] | XRD [nm] | TEM [nm] | ||
|---|---|---|---|---|---|
| Pd/C | 13.9 | 14.5 | Pd(P)/C | 5.7 | 6.3 |
| Pd/m-C | 9.8 | 10.1 | Pd(P)/m-C | 4.4 | 5.1 |
| Pd/WC/m-C | 9.6 | 9.8 | Pd(P)/WC/m-C | 3.6 | 4.2 |
| ECSA [m2g−1] * | ECSA [m2g−1] | ||||
|---|---|---|---|---|---|
| HDR | HAR | HDR | HAR | ||
| Pt/C (ETEK) | 68.93 | 74.00 | |||
| Pd/C | 65.16 | 43.05 | Pd(P)/C | 92.67 | 63.78 |
| Pd/m-C | 65.62 | 46.47 | Pd(P)/m-C | 78.50 | 60.87 |
| Pd/WC/m-C | 99.07 | 61.37 | Pd(P)/WC/m-C | 155.44 | 96.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, G.; Byun, W.J.; Lee, J.H.; Lee, M.H.; Choi, Y.; Kim, J.Y.; Youn, D.H. Phosphorus-Modified Palladium and Tungsten Carbide/Mesoporous Carbon Composite for Hydrogen Oxidation Reaction of Proton Exchange Membrane Fuel Cells. Nanomaterials 2024, 14, 1024. https://doi.org/10.3390/nano14121024
Bae G, Byun WJ, Lee JH, Lee MH, Choi Y, Kim JY, Youn DH. Phosphorus-Modified Palladium and Tungsten Carbide/Mesoporous Carbon Composite for Hydrogen Oxidation Reaction of Proton Exchange Membrane Fuel Cells. Nanomaterials. 2024; 14(12):1024. https://doi.org/10.3390/nano14121024
Chicago/Turabian StyleBae, Ganghong, Woo Jin Byun, Jin Ho Lee, Min Hee Lee, Yeji Choi, Jae Young Kim, and Duck Hyun Youn. 2024. "Phosphorus-Modified Palladium and Tungsten Carbide/Mesoporous Carbon Composite for Hydrogen Oxidation Reaction of Proton Exchange Membrane Fuel Cells" Nanomaterials 14, no. 12: 1024. https://doi.org/10.3390/nano14121024
APA StyleBae, G., Byun, W. J., Lee, J. H., Lee, M. H., Choi, Y., Kim, J. Y., & Youn, D. H. (2024). Phosphorus-Modified Palladium and Tungsten Carbide/Mesoporous Carbon Composite for Hydrogen Oxidation Reaction of Proton Exchange Membrane Fuel Cells. Nanomaterials, 14(12), 1024. https://doi.org/10.3390/nano14121024





