Novel Kraft Softwood Lignin-Derived Carbon Quantum Dots: Synthesis, Characterization, and In Vitro Cytocompatibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbon Quantum Dot Synthesis
2.2. Carbon Quantum Dot Characterization
2.2.1. Physical Characterization
2.2.2. Microscopy
2.2.3. In Vitro Testing of CQDs
3. Results
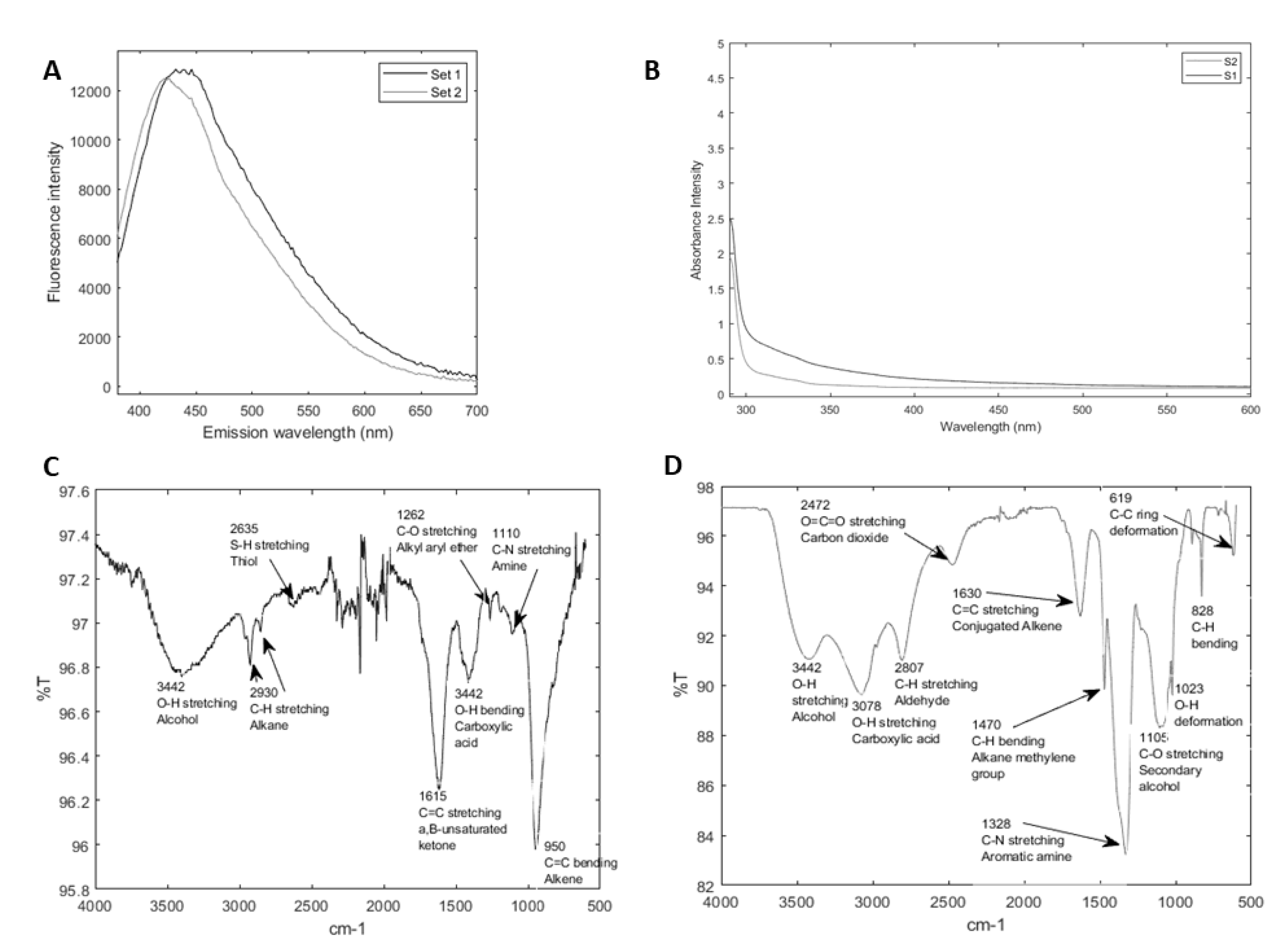
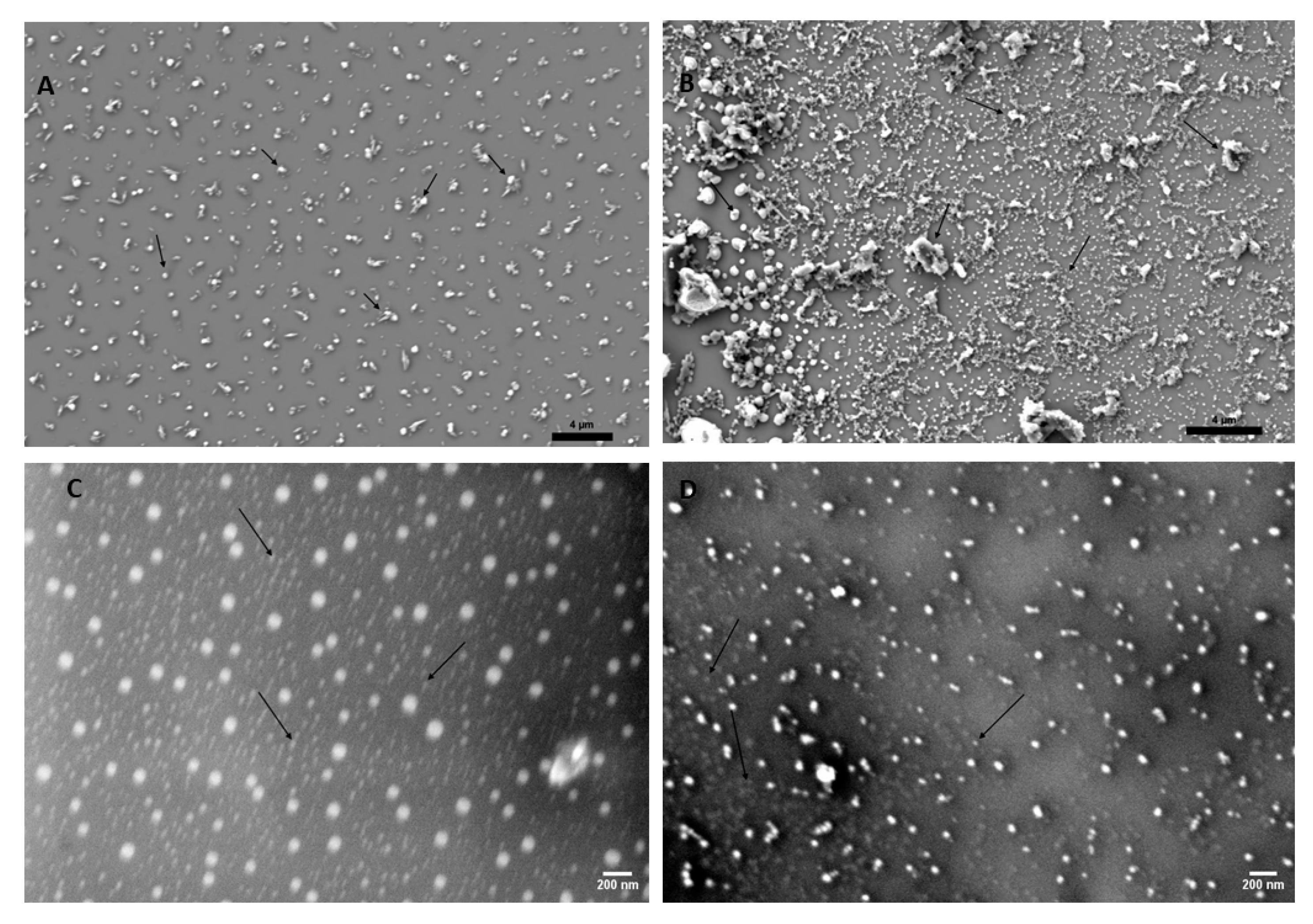
3.1. Lignin-Derived CQDs Exhibit Typical Features of Nanoparticles
3.2. S1 and S2 CQDs Support Cell Attachment
3.3. S1 and S2 CQDs Are Cytocompatible
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is Lignin from being a biomedical material? Bioact. Mater. 2021, 8, 71–94. [Google Scholar] [CrossRef]
- Hasan, M.S.; Sardar, M.R.; Shafin, A.A.; Rahman, M.S.; Mahmud, M.; Hossen, M.M. A Brief Review on Applications of Lignin. J. Chem. Rev. 2023, 5, 56–82. [Google Scholar]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crop Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Hosseinaei, O.; Harper, D.P.; Bozell, J.J.; Rials, T.G. Role of Physicochemical Structure of Organosolv Hardwood and Herbaceous Lignins on Carbon Fiber Performance. ACS Sustain. Chem. Eng. 2016, 4, 5785–5798. [Google Scholar] [CrossRef]
- Yu, L.; Liang, L.; Bajaj, I.; Seabright, K.; Keffer, D.J.; Ivanov, I.N.; Chen, H.; Dai, S.; Ragauskas, A.J.; Maravelias, C.T.; et al. Tailored Mesoporous Structures of Lignin-Derived Nano-Carbons for Multiple Applications. Carbon 2023, 213, 118285. [Google Scholar] [CrossRef]
- Kizzire, D.G.; García-Negrón, V.; Harper, D.P.; Keffer, D.J. Local Structure Analysis and Modelling of Lignin-Based Carbon Composites through the Hierarchical Decomposition of the Radial Distribution Function. ChemistryOpen 2022, 11, e202100220. [Google Scholar] [CrossRef]
- García-Negrón, V.; Kizzire, D.G.; Rios, O.; Keffer, D.J.; Harper, D.P. Elucidating nano and meso-structures of lignin carbon composites: A comprehensive study of feedstock and temperature dependence. Carbon 2020, 161, 856–869. [Google Scholar] [CrossRef]
- García-Negrón, V.; Chmely, S.C.; Ilavsky, J.; Keffer, D.J.; Harper, D.P. Development of Nanocrystalline Graphite from Lignin Sources. ACS Sustain. Chem. Eng. 2022, 10, 1786–1794. [Google Scholar] [CrossRef]
- Ealias, S.A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Parak, W.J.; Pellegrino, T.; Plank, C. Labelling of cells with quantum dots. Nanotechnology 2005, 16, R9–R25. [Google Scholar] [CrossRef]
- Ding, H.; Yu, S.-B.; Wei, J.-S.; Xiong, H.-M. Full-Color Light-Emitting Carbon Dots with a Surface-State-Controlled Luminescence Mechanism. ACS Nano 2016, 10, 484–491. [Google Scholar] [CrossRef]
- Elkhenany, H.; Amelse, L.; Lafont, A.; Bourdo, S.; Caldwell, M.; Neilsen, N.; Dervishi, E.; Derek, O.; Biris, A.S.; Anderson, D.; et al. Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells: Potential for bone tissue engineering. J. Appl. Toxicol. 2015, 35, 367–374. [Google Scholar] [CrossRef]
- Shang, W.H.; Zhang, X.; Zhang, M.; Fan, Z.; Sun, Y.; Han, M.; Fan, L. The uptake mechanism and biocompatibility of graphene quantum dots with human neural stem cells. Nanoscale 2014, 6, 5799–5806. [Google Scholar] [CrossRef]
- Chung, T.-H.; Wu, S.-H.; Yao, M.; Lu, C.-W.; Lin, Y.-S.; Hung, Y.; Mou, C.-Y.; Chen, Y.-C.; Huang, D.-M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials 2007, 28, 2959–2966. [Google Scholar] [CrossRef]
- Yan, J.Y.; Hou, S.; Yu, Y.; Qiao, Y.; Xiao, T.; Mei, Y.; Zhang, Z.; Wang, B.; Huang, C.-C.; Lin, C.-H.; et al. The effect of surface charge on the cytotoxicity and uptake of carbon quantum dots in human umbilical cord derived mesenchymal stem cells. Colloids Surf. B Biointerfaces 2018, 171, 241–249. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, H.; Guo, Y.; Liu, C.; Song, H.; Zhou, P.; Zhang, H.; Zhou, K.; Guo, Y.; Chen, H. Developing electropositive citric acid–polyethylenimine carbon quantum dots with high biocompatibility and labeling performance for mesenchymal stem cells in vitro and in vivo. New J. Chem. 2022, 46, 2508–2517. [Google Scholar] [CrossRef]
- Newby, S.D.; Masi, T.; Griffin, C.D.; King, W.J.; Chipman, A.; Stephenson, S.; E Anderson, D.; Biris, A.S.; E Bourdo, S.; Dhar, M. Functionalized Graphene Nanoparticles Induce Human Mesenchymal Stem Cells to Express Distinct Extracellular Matrix Proteins Mediating Osteogenesis. Int. J. Nanomed. 2020, 15, 2501–2513. [Google Scholar] [CrossRef]
- Limbach, L.K.; Li, Y.; Grass, R.N.; Brunner, T.J.; Hintermann, M.A.; Muller, M.; Gunther, D.; Stark, W.J. Oxide nanoparticle uptake in human lung fibroblasts: Effects of particle size, agglomeration, and diffusion at low concentrations. Environ. Sci. Technol. 2005, 39, 9370–9376. [Google Scholar] [CrossRef]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar] [CrossRef]
- Mahmoud, N.S.; Ahmed, H.H.; Mohamed, M.R.; Amr, K.S.; Aglan, H.A.; Ali, M.A.M.; Tantawy, M.A. Role of nanoparticles in osteogenic differentiation of bone marrow mesenchymal stem cells. Cytotechnology 2020, 72, 1–22. [Google Scholar] [CrossRef]
- Chu, K.-W.; Lee, S.L.; Chang, C.-J.; Liu, L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef]
- Yu, L.; Hsieh, C.-T.; Keffer, D.J.; Chen, H.; Goenaga, G.A.; Dai, S.; Zawodzinski, T.A.; Harper, D.P. Hierarchical Lignin-Based Carbon Matrix and Carbon Dot Composite Electrodes for High-Performance Supercapacitors. ACS Omega 2021, 6, 7851–7861. [Google Scholar] [CrossRef]
- Kasouni, A.I.; Chatzimitakos, T.G.; Troganis, A.N.; Stalikas, C.D. Citric acid-based carbon dots: From revealing new insights into their biological properties to demonstrating their enhanced wound healing potential by in vitro and in vivo experiments. Mater. Today Commun. 2021, 26, 102019. [Google Scholar] [CrossRef]
- Praseetha, P.; Vibala, B.; Sreedevy, K.; Vijayakumar, S. Aloe-vera conjugated natural Carbon Quantum dots as Bio-enhancers to accelerate the repair of chronic wounds. Ind. Crops Prod. 2021, 174, 114152. [Google Scholar]
- Han, Y.; Zhang, F.; Zhang, J.; Shao, D.; Wang, Y.; Li, S.; Lv, S.; Chi, G.; Zhang, M.; Chen, L.; et al. Bioactive carbon dots direct the osteogenic differentiation of human bone marrow mesenchymal stem cells. Colloids Surf. B Biointerfaces 2019, 179, 1–8. [Google Scholar] [CrossRef]
- Song, X.; Zhao, S.; Xu, Y.; Chen, X.; Wang, S.; Zhao, P.; Pu, Y.; Ragauskas, A.J. Preparation, Properties, and Application of Lignocellulosic-Based Fluorescent Carbon Dots. ChemSusChem 2022, 15, e202102486. [Google Scholar] [CrossRef]
- Ding, Z.; Li, F.; Wen, J.; Wang, X.; Sun, R. Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green Chem. 2018, 20, 1383–1390. [Google Scholar] [CrossRef]
- Ambriz, X.; de Lanerolle, P.; Ambrosio, J.R. The Mechanobiology of the Actin Cytoskeleton in Stem Cells during Differentiation and Interaction with Biomaterials. Stem Cells Int. 2018, 2018, 2891957. [Google Scholar] [CrossRef]
- Tandale, P.; Choudhary, N.; Singh, J.; Sharma, A.; Shukla, A.; Sriram, P.; Soni, U.; Singla, N.; Barnwal, R.P.; Singh, G.; et al. Fluorescent quantum dots: An insight on synthesis and potential biological application as drug carrier in cancer. Biochem. Biophys. Rep. 2021, 26, 100962. [Google Scholar] [CrossRef]
- Mezhevikina, L.M.; Reshetnikov, D.A.; Fomkina, M.G.; Fesenko, E.E. Ultrathin polyethyleneimine (PEI) films for culturing of the human mesenchymal stromal cells (hMSCs). J. Cardiovasc. Med. Cardiol. 2020, 7, 255–261. [Google Scholar]
- Newby, S.D.; Forsynth, C.; Bow, A.J.; Bourdo, S.E.; Hung, M.; Cheever, J.; Moffat, R.; Gross, A.J.; Licari, F.W.; Dhar, M.S. Xenogenic Implantation of Human Mesenchymal Stromal Cells Using a Novel 3D-Printed Scaffold of PLGA and Graphene Leads to a Significant Increase in Bone Mineralization in a Rat Segmental Femoral Bone Defect. Nanomaterials 2023, 13, 1149. [Google Scholar] [CrossRef]
- Balkus, K.J. Chapter 9—Metal Oxide Nanotube, Nanorod, and Quantum Dot Photocatalysis. In New and Future Developments in Catalysis; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 213–244. [Google Scholar]
- Maxwell, T.; Campos, M.G.N.; Smith, S.; Doomra, M.; Thwin, Z.; Santra, S. Chapter 15—Quantum Dots. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–265. [Google Scholar]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Pisanic, T.R.; Zhang, Y.; Wang, T.H. Quantum dots in diagnostics and detection: Principles and paradigms. Analyst 2014, 139, 2968–2981. [Google Scholar] [CrossRef]
- Joseph, E.; Singhvi, G. Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–116. [Google Scholar]
- Zhang, J.; Tang, H.; Liu, Z.; Chen, B. Effects of major parameters of nanoparticles on their physical and chemical properties and recent application of nanodrug delivery system in targeted chemotherapy. Int. J. Nanomed. 2017, 12, 8483–8493. [Google Scholar] [CrossRef]
- Carone, A.; Emilsson, S.; Mariani, P.; Désert, A.; Parola, S. Gold nanoparticle shape dependence of colloidal stability domains. Nanoscale Adv. 2023, 5, 2017–2026. [Google Scholar] [CrossRef]
- Bao, L.; Liu, C.; Zhang, Z.-L.; Pang, D.-W. Photoluminescence-Tunable Carbon Nanodots: Surface-State Energy-Gap Tuning. Adv. Mater. 2015, 27, 1663–1667. [Google Scholar] [CrossRef]
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.; Chen, A.; et al. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 2018, 9, 2249. [Google Scholar] [CrossRef]
- Rangamani, P.; Lipshtat, A.; Azeloglu, E.U.; Calizo, R.C.; Hu, M.; Ghassemi, S.; Hone, J.; Scarlata, S.; Neves, S.R.; Iyengar, R. Decoding information in cell shape. Cell 2013, 154, 1356–1369. [Google Scholar] [CrossRef]
- Sikavitsas, V.I.; Temenoff, J.S.; Mikos, A.G. Biomaterials and bone mechanotransduction. Biomaterials 2001, 22, 2581–2593. [Google Scholar] [CrossRef]
- Pockwinse, S.M.; Stein, J.L.; Lian, J.B.; Stein, G.S. Developmental Stage-Specific Cellular Responses to Vitamin D and Glucocorticoids during Differentiation of the Osteoblast Phenotype: Interrelationship of Morphology and Gene Expression by in situ Hybridization. Exp. Cell Res. 1995, 216, 244–260. [Google Scholar] [CrossRef]
- López-de-Uralde, J.; Ruiz, I.; Santos, I.; Zubillaga, A.; Bringas, P.G.; Okariz, A.; Guraya, T. Automatic Morphological Categorisation of Carbon Black Nano-aggregates. In Database and Expert Systems Applications, Proceedings of the 21st International Conference, DEXA 2010, Bilbao, Spain, 30 August–3 September 2010; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6262, pp. 185–193. Available online: https://www.webofscience.com/wos/woscc/full-record/WOS:000285525100015?SID=USW2EC0C6BToQS8BAv6GqctzDucDc (accessed on 1 August 2023).
- Mezhevikina, L.M.; Reshetnikov, D.A.; Fomkina, M.G.; Appazov, N.O.; Ibadullayeva, S.Z.; Fesenko, E.E. Growth characteristics of human bone marrow mesenchymal stromal cells at cultivation on synthetic polyelectrolyte nanofilms. Heliyon 2021, 7, e06517. [Google Scholar] [CrossRef]
- Ma, J.; Liang, Q.; Qin, W.; Lartey, P.O.; Li, Y.; Feng, X. Bioactivity of nitric acid and calcium chloride treated carbon-fibers reinforced polyetheretherketone for dental implant. J. Mech. Behav. Biomed. Mater. 2020, 102, 103497. [Google Scholar] [CrossRef]
- Martocq, L.; Douglas, T.E.L. Amine-Rich Coatings to Potentially Promote Cell Adhesion, Proliferation and Differentiation, and Reduce Microbial Colonization: Strategies for Generation and Characterization. Coatings 2021, 11, 983. [Google Scholar] [CrossRef]
- Sen, B.; Uzer, G.; Samsonraj, R.M.; Xie, Z.; McGrath, C.; Styner, M.; Dudakovic, A.; van Wijnen, A.J.; Rubin, J. Intranuclear Actin Structure Modulates Mesenchymal Stem Cell Differentiation. Stem Cells 2017, 35, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Pullikuth, A.K.; Catling, A.D. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: A perspective. Cell. Signal. 2007, 19, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Langenbach, A.; Kaminski, A.; Rychly, J. Modulating the actin cytoskeleton affects mechanically induced signal transduction and differentiation in mesenchymal stem cells. PLoS ONE 2013, 8, e71283. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christoph, E.; Yu, L.; Newby, S.D.; Rivera Orsini, M.A.; Scroggins, J.; Keffer, D.J.; Harper, D.P.; Dhar, M. Novel Kraft Softwood Lignin-Derived Carbon Quantum Dots: Synthesis, Characterization, and In Vitro Cytocompatibility. Nanomaterials 2024, 14, 1029. https://doi.org/10.3390/nano14121029
Christoph E, Yu L, Newby SD, Rivera Orsini MA, Scroggins J, Keffer DJ, Harper DP, Dhar M. Novel Kraft Softwood Lignin-Derived Carbon Quantum Dots: Synthesis, Characterization, and In Vitro Cytocompatibility. Nanomaterials. 2024; 14(12):1029. https://doi.org/10.3390/nano14121029
Chicago/Turabian StyleChristoph, Eli, Lu Yu, Steven D. Newby, Michael A. Rivera Orsini, Jakob Scroggins, David J. Keffer, David P. Harper, and Madhu Dhar. 2024. "Novel Kraft Softwood Lignin-Derived Carbon Quantum Dots: Synthesis, Characterization, and In Vitro Cytocompatibility" Nanomaterials 14, no. 12: 1029. https://doi.org/10.3390/nano14121029
APA StyleChristoph, E., Yu, L., Newby, S. D., Rivera Orsini, M. A., Scroggins, J., Keffer, D. J., Harper, D. P., & Dhar, M. (2024). Novel Kraft Softwood Lignin-Derived Carbon Quantum Dots: Synthesis, Characterization, and In Vitro Cytocompatibility. Nanomaterials, 14(12), 1029. https://doi.org/10.3390/nano14121029








