Enhancing Efficiency of Dye Sensitized Solar Cells by Coinage Metal Doping of Cyanidin-Silver Trimer Hybrids at TiO2 Support Based on Theoretical Study
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Structural and Electronic Properties of Cyanidin-Trimer Hybrids
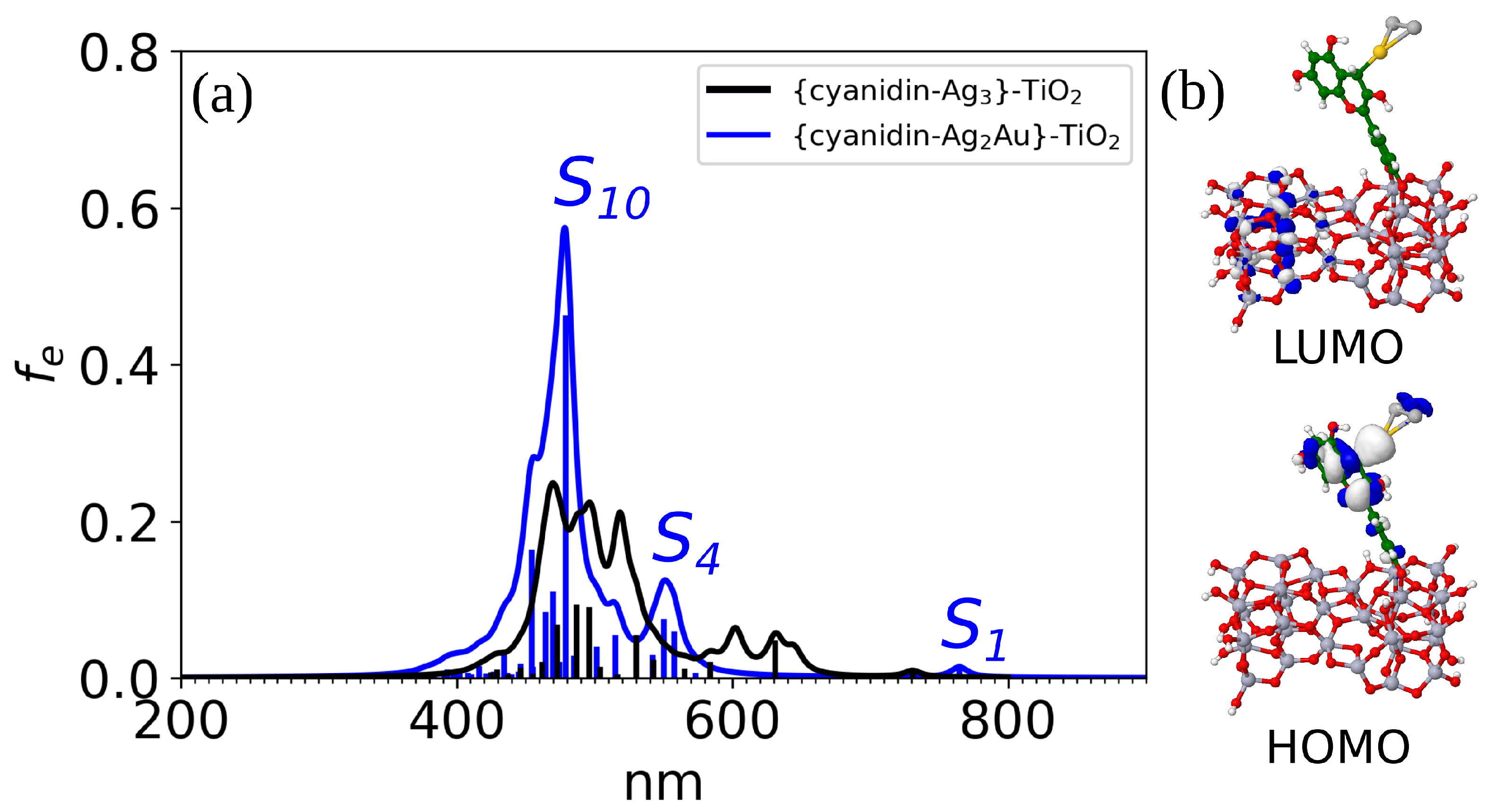
3.2. Linear Optical and Photovoltaic Properties of Cyanidin-Trimer Hybrids
3.3. Nonlinear Optical Properties of Cyanidin-Trimers Hybrids
3.4. Cyanidin-Trimer Sensitizer Anchored on a TiO2 Model Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kılkış, S.; Krajačić, G.; Duić, N.; Montorsi, L.; Wang, Q.; Rosen, M.A.; Ahmad Al-Nimr, M. Research frontiers in sustainable development of energy, water and environment systems in a time of climate crisis. Energy Convers. Manag. 2019, 199, 111938. [Google Scholar] [CrossRef]
- Pulli, E.; Rozzi, E.; Bella, F. Transparent photovoltaic technologies: Current trends towards upscaling. Energy Convers. Manag. 2020, 219, 112982. [Google Scholar] [CrossRef]
- Rennuit-Mortensen, A.W.; Dalgas Rasmussen, K.; Grahn, M. How replacing fossil fuels with electrofuels could influence the demand for renewable energy and land area. Smart Energy 2023, 10, 100107. [Google Scholar] [CrossRef]
- SolarPower Europe. EU Market Outlook for Solar Power 2023–2027; SolarPower Europe: Brussels, Belgium, 2023. [Google Scholar]
- Traverse, C.; Pandey, R.; Barr, M.; Lunt, R. Emergence of highly transparent photovoltaics for distributed applications. Nat. Energy 2017, 2, 849–860. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Bagher, A.M.; Vahid, M.M.A.; Mohsen, M. Types of Solar Cells and Application. Am. J. Opt. Photonics 2015, 3, 94–113. [Google Scholar] [CrossRef]
- Fan, W.; Tan, D.; Deng, W.Q. Acene-modified triphenylamine dyes for dye-sensitized solar cells: A computational study. ChemPhysChem 2012, 13, 2051–2060. [Google Scholar] [CrossRef]
- Carella, A.; Borbone, F.; Centore, R. Research Progress on Photosensitizers for DSSC. Front. Chem. 2018, 6, 481. [Google Scholar] [CrossRef]
- Chauke, N.M.; Mohlala, R.L.; Ngqoloda, S.; Raphulu, M.C. Harnessing visible light: Enhancing TiO2 photocatalysis with photosensitizers for sustainable and efficient environmental solutions. Front. Chem. Eng. 2024, 6, 1356021. [Google Scholar] [CrossRef]
- Birel, Ö.; Nadeem, S.; Duman, H. Porphyrin-Based Dye-Sensitized Solar Cells (DSSCs). J. Fluoresc. 2017, 27, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Tomar, N.; Agrawal, A.; Dhaka, V.S.; Surolia, P.K. Ruthenium complexes based dye sensitized solar cells: Fundamentals and research trends. Sol. Energy 2020, 207, 59–76. [Google Scholar] [CrossRef]
- Alizadeh, A.; Roudgar-Amoli, M.; Bonyad-Shekalgourabi, S.M.; Shariatinia, Z.; Mahmoudi, M.; Saadat, F. Dye sensitized solar cells go beyond using perovskite and spinel inorganic materials: A review. Renew. Sustain. Energy Rev. 2022, 157, 112047. [Google Scholar] [CrossRef]
- Badawy, S.A.; Salem, K.E.; Fadda, A.A.; Abdel-Latif, E.; Elmorsy, M.R. Advancements in metal-free organic dyes: Achieving over 10% efficiency in DSSCs. Dyes Pigment. 2024, 225, 112096. [Google Scholar] [CrossRef]
- Muniz, C.N.; Archer, C.A.; Applebaum, J.S.; Alagaratnam, A.; Schaab, J.; Djurovich, P.I.; Thompson, M.E. Two-Coordinate Coinage Metal Complexes as Solar Photosensitizers. J. Am. Chem. Soc. 2023, 145, 13846–13857. [Google Scholar] [CrossRef] [PubMed]
- Yazie, A.N.; Worku, A.D.; Tsigie, Y.A. Recent advances in anthocyanin dyes extracted from plants for dye sensitized solar cell. Mater. Renew. Sustain. Energy 2020, 9, 23. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Klein, C.; Liska, P.; Grätzel, M. Synthesis of novel ruthenium sensitizers and their application in dye-sensitized solar cells. Coord. Chem. Rev. 2005, 249, 1460–1467. [Google Scholar] [CrossRef]
- Yin, J.F.; Velayudham, M.; Bhattacharya, D.; Lin, H.C.; Lu, K.L. Structure optimization of ruthenium photosensitizers for efficient dye-sensitized solar cells–A goal toward a “bright” future. Coord. Chem. Rev. 2012, 256, 3008–3035. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Abualnaja, M.M.; El-Metwaly, N.M. Development of organic co-sensitizers based on piperonal for over 10% efficient ruthenium complex dye-sensitized solar cells. J. Mol. Liq. 2024, 398, 124337. [Google Scholar] [CrossRef]
- Hug, H.; Bader, M.; Mair, P.; Glatzel, T. Biophotovoltaics: Natural pigments in dye-sensitized solar cells. Appl. Energy 2014, 115, 216–225. [Google Scholar] [CrossRef]
- Pratiwi, D.D.; Nurosyid, F.; Supriyanto, A.; Suryana, R. Efficiency enhancement of dye-sensitized solar cells (DSSC) by addition of synthetic dye into natural dye (anthocyanin). IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 012012. [Google Scholar] [CrossRef]
- Prima, E.C.; Nuruddin, A.; Yuliarto, B.; Kawamura, G.; Matsuda, A. Combined spectroscopic and TDDFT study of single-double anthocyanins for application in dye-sensitized solar cells. New J. Chem. 2018, 42, 11616–11628. [Google Scholar] [CrossRef]
- Purwoko, A.; Setiawati, V.; Hadisaputra, S. Metal-pigment complex derived from natural dye of anthocyanin: A potential candidate for DSSC photosensitizer. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012130. [Google Scholar] [CrossRef]
- Shah, W.; Faraz, S.M.; Awan, Z.H. Photovoltaic properties and impedance spectroscopy of dye sensitized solar cells co-sensitized by natural dyes. Phys. B Condens. Matter 2023, 654, 414716. [Google Scholar] [CrossRef]
- Pramananda, V.; Fityay, T.A.H.; Misran, E. Anthocyanin as natural dye in DSSC fabrication: A review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1122, 012104. [Google Scholar] [CrossRef]
- Bužančić Milosavljević, M.; Mravak, A.; Perić Bakulić, M.; Bonačić-Koutecký, V. Model systems for dye-sensitized solar cells: Cyanidin-silver nanocluster hybrids at TiO2 support. RSC Adv. 2023, 13, 6010–6016. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S. Dye-sensitized solar cells: Fundamentals and current status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Bonačić-Kouteckỳ, V.; Kulesza, A.; Gell, L.; Mitrić, R.; Antoine, R.; Bertorelle, F.; Hamouda, R.; Rayane, D.; Broyer, M.; Tabarin, T.; et al. Silver cluster–biomolecule hybrids: From basics towards sensors. Phys. Chem. Chem. Phys. 2012, 14, 9282–9290. [Google Scholar] [CrossRef]
- Ko, K.H.; Lee, Y.C.; Jung, Y.J. Enhanced efficiency of dye-sensitized TiO2 solar cells (DSSC) by doping of metal ions. J. Colloid Interface Sci. 2005, 283, 482–487. [Google Scholar] [CrossRef]
- Yang, J.; Pang, R.; Song, D.; Li, M.B. Tailoring silver nanoclusters via doping: Advances and opportunities. Nanoscale Adv. 2021, 3, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.; Gazi, T.R.; Roy, S.; Chakraborty, I. Nanohybrids of atomically precise metal nanoclusters. Commun. Chem. 2023, 6, 157. [Google Scholar] [CrossRef] [PubMed]
- Bonačić-Kouteckỳ, V.; Burda, J.; Mitrić, R.; Ge, M.; Zampella, G.; Fantucci, P. Density functional study of structural and electronic properties of bimetallic silver–gold clusters: Comparison with pure gold and silver clusters. J. Chem. Phys. 2002, 117, 3120–3131. [Google Scholar] [CrossRef]
- Kulesza, A.; Mitrić, R.; Bonačić-Kouteckỳ, V. Unique optical properties of silver cluster-biochromophore hybrids: Comparison with copper and gold. Chem. Phys. Lett. 2011, 501, 211–214. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Roberto, D.; Fagnani, F. Copper complexes as alternative redox mediators in dye-sensitized solar cells. Molecules 2021, 26, 194. [Google Scholar] [CrossRef]
- Pniakowska, A.; Kumaranchira Ramankutty, K.; Obstarczyk, P.; Perić Bakulić, M.; Sanader Maršić, Ž.; Bonačić-Kouteckỳ, V.; Bürgi, T.; Olesiak-Bańska, J. Gold-Doping Effect on Two-Photon Absorption and Luminescence of Atomically Precise Silver Ligated Nanoclusters. Angew. Chem. Int. Ed. 2022, 61, e202209645. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868, Erratum in Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Andrae, D.; Haeussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Aidas, K.; Angeli, C.; Bak, K.L.; Bakken, V.; Bast, R.; Boman, L.; Christiansen, O.; Cimiraglia, R.; Coriani, S.; Dahle, P.; et al. The Dalton quantum chemistry program system. WIREs Comput. Mol. Sci. 2014, 4, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Dalton, a Molecular Electronic Structure Program, Release Dalton2020.alpha. 2020. Available online: http://daltonprogram.org (accessed on 14 May 2024).
- Frediani, L.; Rinkevicius, Z.; Ågren, H. Two-photon absorption in solution by means of time-dependent density-functional theory and the polarizable continuum model. J. Chem. Phys. 2005, 122, 244104. [Google Scholar] [CrossRef] [PubMed]
- Norman, P. A perspective on nonresonant and resonant electronic response theory for time-dependent molecular properties. Phys. Chem. Chem. Phys. 2011, 13, 20519–20535. [Google Scholar] [CrossRef] [PubMed]
- List, N.H.; Zalesny, R.; Murugan, N.A.; Kongsted, J.; Bartkowiak, W.; Ågren, H. Relation between nonlinear optical properties of push–pull molecules and metric of charge transfer excitations. J. Chem. Theory Comput. 2015, 11, 4182–4188. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, J.; Eda, T.; Hanaya, M. Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem. Phys. Lett. 2017, 685, 23–26. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, C.; Mosconi, E.; Pastore, M.; Ronca, E.; De Angelis, F. Adsorption of organic dyes on TiO2 surfaces in dye-sensitized solar cells: Interplay of theory and experiment. Phys. Chem. Chem. Phys. 2012, 14, 15963–15974. [Google Scholar] [CrossRef]
- Adhikari, S.G.; Gascooke, J.R.; Alotabi, A.S.; Andersson, G.G. Anchoring Modes of Ru-Based N719 Dye onto Titania Substrates. J. Phys. Chem. C 2024, 128, 3136–3147. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, Y.; Li, R.; Shi, D.; Wenger, S.; Zakeeruddin, S.M.; Grätzel, M.; Wang, P. Employ a bisthienothiophene linker to construct an organic chromophore for efficient and stable dye-sensitized solar cells. Energy Environ. Sci. 2009, 2, 92–95. [Google Scholar] [CrossRef]
- Ørnsø, K.B.; Garcia-Lastra, J.M.; De La Torre, G.; Himpsel, F.J.; Rubio, A.; Thygesen, K.S. Design of two-photon molecular tandem architectures for solar cells by ab initio theory. Chem. Sci. 2015, 6, 3018–3025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guillén-López, A.; Delesma, C.; Amador-Bedolla, C.; Robles, M.; Muñiz, J. Electronic structure and nonlinear optical properties of organic photovoltaic systems with potential applications on solar cell devices: A DFT approach. Theor. Chem. Acc. 2018, 137, 85. [Google Scholar] [CrossRef]
- Hu, Z.; Khadka, V.; Wang, W.; Galipeau, D.; Yan, X. Theoretical study of two-photon absorption properties and up-conversion efficiency of new symmetric organic π-conjugated molecules for photovoltaic devices. J. Mol. Model. 2012, 18, 3657–3667. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Suemoto, T.; Yamanaka, K.; Sugimoto, N.; Kobayashi, Y.; Otsu, T.; Tani, S.; Koyama, T. Relaxation dynamics of hot electrons in the transition metals Au, Ag, Cu, Pt, Pd, and Ni studied by ultrafast luminescence spectroscopy. J. Appl. Phys. 2021, 130, 025101. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, B.; Rogach, A.L. Synthesis, optical properties and applications of light-emitting copper nanoclusters. Nanoscale Horiz. 2017, 2, 135–146. [Google Scholar] [CrossRef]
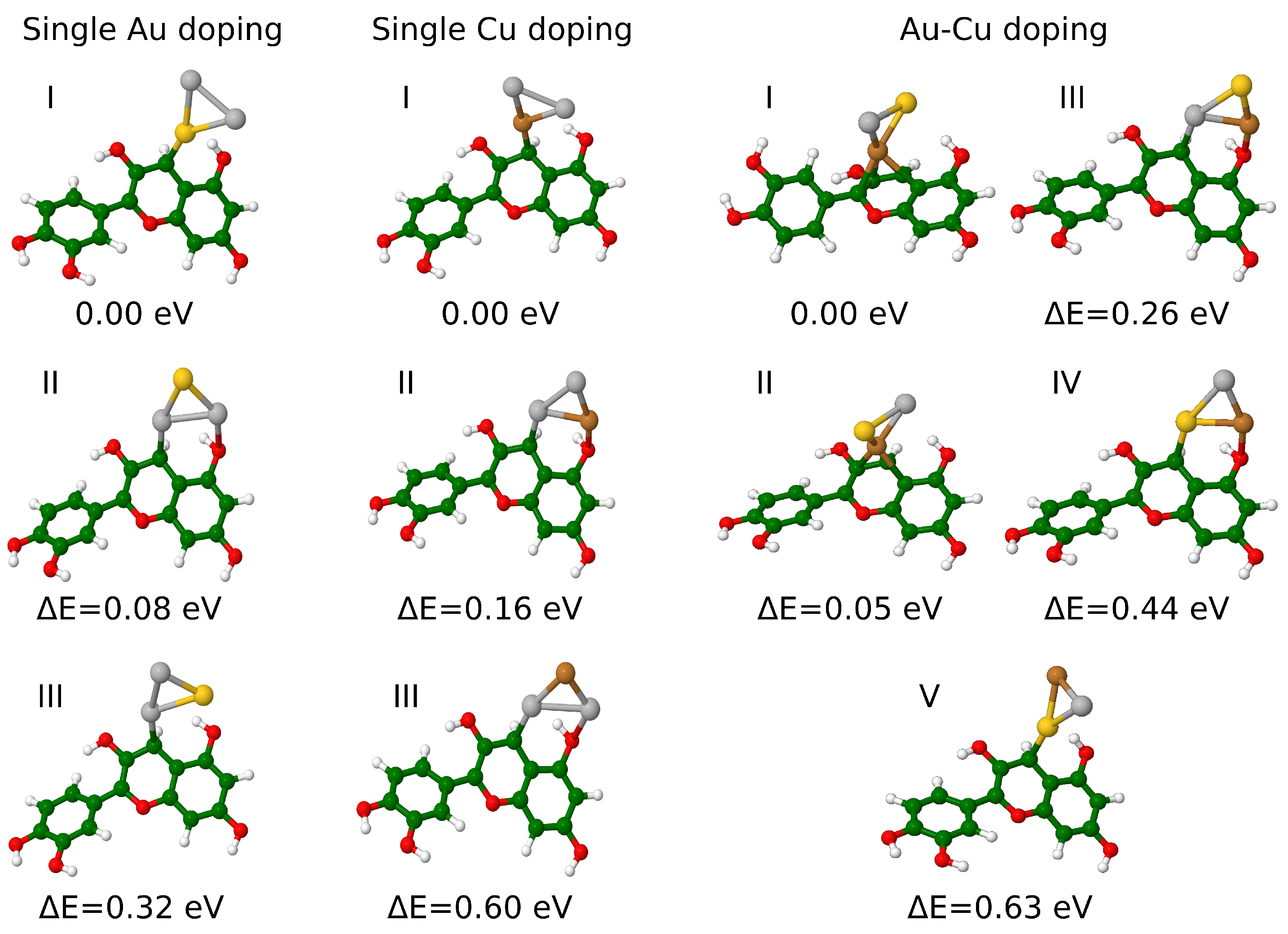

| Au Doping | |||||
| [eV] | Net Trimer Charge | Au Charge | Ag1 Charge | Ag2 Charge | |
| Isomer I | −12.95 | 0.02 | −0.20 | 0.09 | 0.13 |
| Isomer II | −12.87 | 0.07 | −0.07 | 0.15 | −0.01 |
| Isomer III | −12.63 | −0.19 | −0.28 | 0.05 | 0.04 |
| Cu Doping | |||||
| [eV] | Net Trimer Charge | Cu Charge | Ag1 Charge | Ag2 Charge | |
| Isomer I | −12.77 | 0.20 | 0.17 | 0.01 | 0.02 |
| Isomer II | −12.61 | 0.11 | 0.20 | −0.03 | −0.06 |
| Isomer III | −12.17 | 0.12 | 0.09 | 0.09 | −0.06 |
| Au-Cu Doping | |||||
| [eV] | Net Trimer Charge | Au Charge | Cu Charge | Ag Charge | |
| Isomer I | −13.56 | −0.24 | −0.22 | −0.07 | 0.05 |
| Isomer II | −13.50 | −0.06 | −0.21 | 0.15 | −0.001 |
| Isomer III | −13.29 | 0.08 | −0.13 | 0.22 | −0.01 |
| Isomer IV | −13.12 | 0.02 | −0.10 | 0.20 | −0.08 |
| Isomer V | −12.92 | 0.02 | −0.17 | 0.21 | −0.02 |
| HOMO [eV] | LUMO [eV] | [nm] | ΔGinject [eV] | HOMO-LUMO | |||
|---|---|---|---|---|---|---|---|
| cyanidin-Ag3 * | −5.35 | −1.65 | 467 | 0.57 | 0.73 | −1.30 | 3.70 |
| cyanidin-Ag9 * | −5.28 | −1.68 | 311 | 0.71 | 0.80 | −2.71 | 3.60 |
| cyanidin-Ag21 * | −4.82 | −2.52 | 332 | 0.68 | 0.79 | −2.91 | 2.30 |
| cyanidin-Ag2Au I | −5.58 | −2.08 | 463 | 0.81 | 0.85 | −1.10 | 3.50 |
| cyanidin-Ag2Au II | −5.52 | −1.71 | 435 | 0.52 | 0.70 | −1.33 | 3.81 |
| cyanidin-Ag2Au III | −5.29 | −2.53 | 518 | 0.46 | 0.65 | −1.10 | 2.77 |
| cyanidin-Ag2Cu I | −5.51 | −1.84 | 440 | 0.37 | 0.57 | −1.31 | 3.67 |
| cyanidin-Ag2Cu II | −5.30 | −0.98 | 444 | 0.52 | 0.70 | −1.49 | 4.33 |
| cyanidin-Ag2Cu III | −5.29 | −1.72 | 457 | 0.51 | 0.69 | −1.42 | 3.57 |
| cyanidin-AgAuCu I | −5.68 | −1.96 | 449 | 0.23 | 0.41 | −0.26 | 3.72 |
| cyanidin-AgAuCu II | −5.42 | −2.12 | 228 | 0.27 | 0.46 | −4.01 | 3.29 |
| cyanidin-AgAuCu III | −5.51 | −0.86 | 425 | 0.45 | 0.65 | −1.41 | 4.65 |
| cyanidin-AgAuCu IV | −5.53 | −1.06 | 419 | 0.62 | 0.76 | −1.43 | 4.47 |
| cyanidin-AgAuCu V | −5.54 | −1.89 | 468 | 0.72 | 0.81 | −1.11 | 3.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bužančić Milosavljević, M.; Perić Bakulić, M.; Sanader Maršić, Ž.; Mravak, A.; Bonačić-Koutecký, V. Enhancing Efficiency of Dye Sensitized Solar Cells by Coinage Metal Doping of Cyanidin-Silver Trimer Hybrids at TiO2 Support Based on Theoretical Study. Nanomaterials 2024, 14, 1034. https://doi.org/10.3390/nano14121034
Bužančić Milosavljević M, Perić Bakulić M, Sanader Maršić Ž, Mravak A, Bonačić-Koutecký V. Enhancing Efficiency of Dye Sensitized Solar Cells by Coinage Metal Doping of Cyanidin-Silver Trimer Hybrids at TiO2 Support Based on Theoretical Study. Nanomaterials. 2024; 14(12):1034. https://doi.org/10.3390/nano14121034
Chicago/Turabian StyleBužančić Milosavljević, Margarita, Martina Perić Bakulić, Željka Sanader Maršić, Antonija Mravak, and Vlasta Bonačić-Koutecký. 2024. "Enhancing Efficiency of Dye Sensitized Solar Cells by Coinage Metal Doping of Cyanidin-Silver Trimer Hybrids at TiO2 Support Based on Theoretical Study" Nanomaterials 14, no. 12: 1034. https://doi.org/10.3390/nano14121034
APA StyleBužančić Milosavljević, M., Perić Bakulić, M., Sanader Maršić, Ž., Mravak, A., & Bonačić-Koutecký, V. (2024). Enhancing Efficiency of Dye Sensitized Solar Cells by Coinage Metal Doping of Cyanidin-Silver Trimer Hybrids at TiO2 Support Based on Theoretical Study. Nanomaterials, 14(12), 1034. https://doi.org/10.3390/nano14121034






