Exploration and Optimization of the Polymer-Modified NiOx Hole Transport Layer for Fabricating Inverted Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
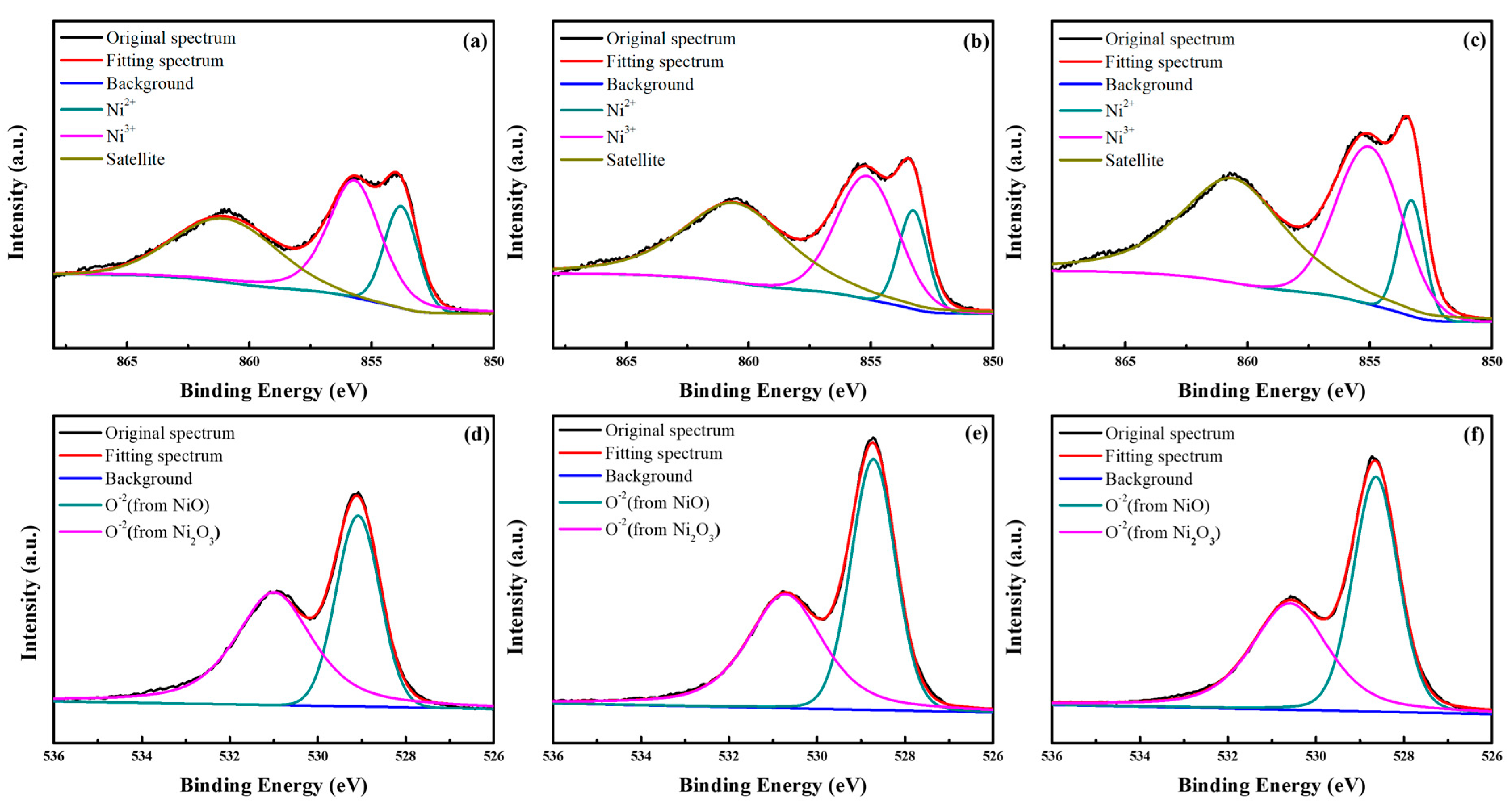
3.1. Characterization of the p-NiOx
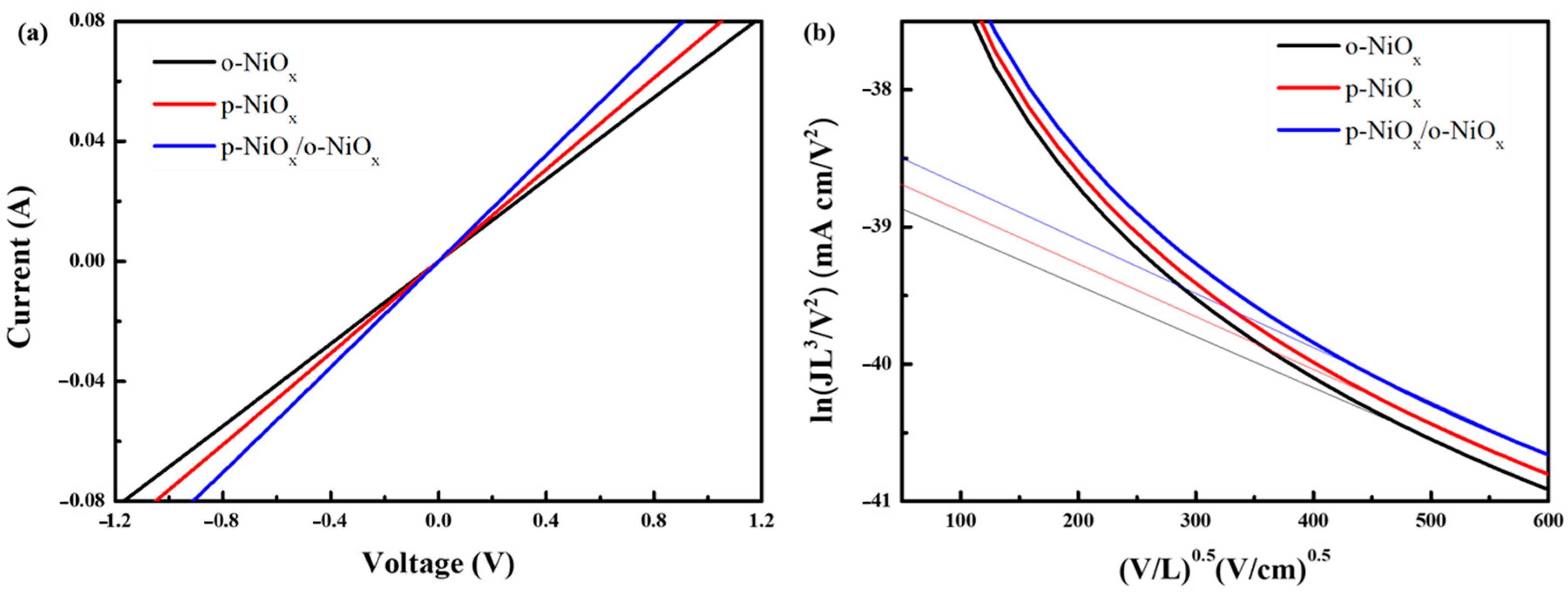
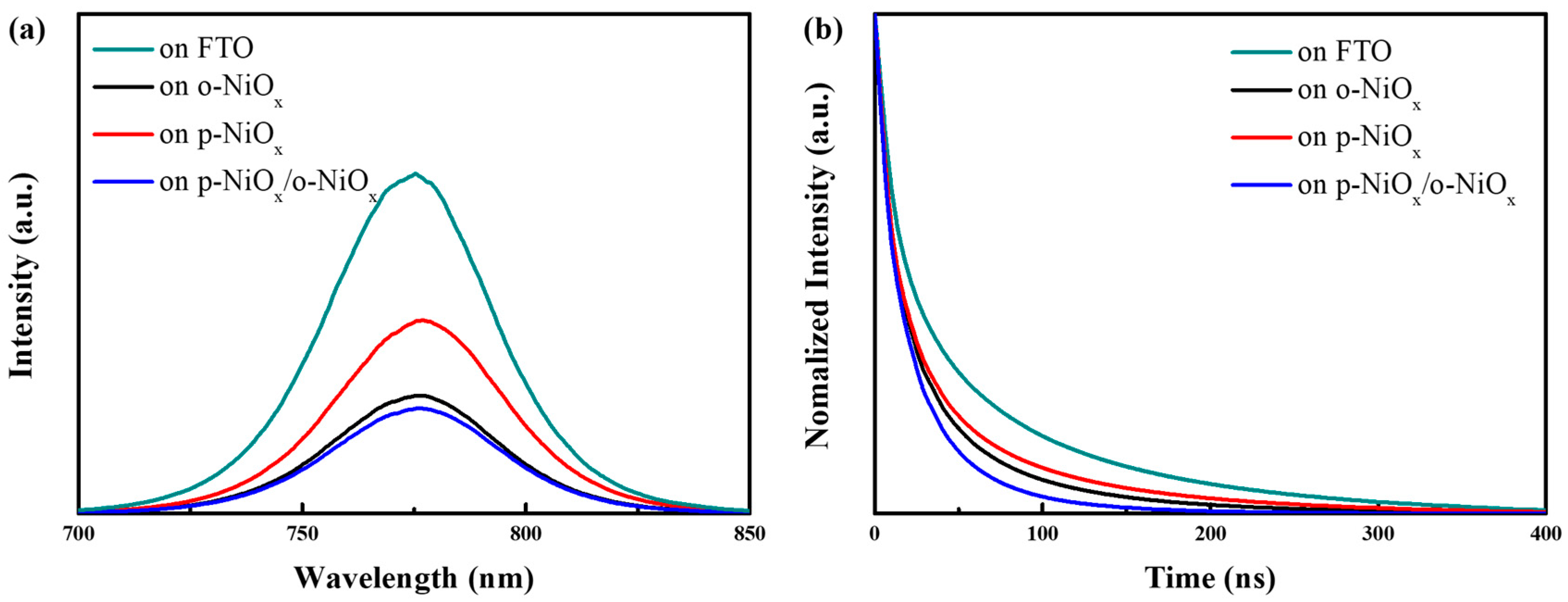
3.2. Characterization of Perovskite Layers on NiOx
3.3. Device Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, Q.; Armin, A.; Nagiri, R.C.R.; Burn, P.L.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photonics 2015, 9, 106–112. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Wu, Y.; Dong, B.; Song, H. Novel broad spectral response perovskite solar cells: A review of the current status and advanced strategies for breaking the theoretical limit efficiency. J. Mater. Sci. Technol. 2023, 140, 33–57. [Google Scholar] [CrossRef]
- Park, H.; Chaurasiya, R.; Jeong, B.H.; Sakthivel, P.; Park, H.J. Nickel Oxide for Perovskite Photovoltaic Cells. Adv. Photonics Res. 2021, 2, 2000178. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, X.; You, J. SnO2: A Wonderful Electron Transport Layer for Perovskite Solar Cells. Small 2018, 14, 1801154. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory (NREL). Efficiency Chart. 2024. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 2 May 2024).
- Hou, Y.; Aydin, E.; De Bastiani, M.; Xiao, C.; Isikgor, F.H.; Xue, D.-J.; Chen, B.; Chen, H.; Bahrami, B.; Chowdhury, A.H.; et al. Efficient tandem solar cells with solution-processed perovskite on textured crystalline silicon. Science 2020, 367, 1135–1140. [Google Scholar] [CrossRef]
- Pu, X.; Zhao, J.; Li, Y.; Zhang, Y.; Loi, H.-L.; Wang, T.; Chen, H.; He, X.; Yang, J.; Ma, X.; et al. Stable NiOx-based inverted perovskite solar cells achieved by passivation of multifunctional star polymer. Nano Energy 2023, 112, 108506. [Google Scholar] [CrossRef]
- Mali, S.S.; Kim, H.; Kim, H.H.; Shim, S.E.; Hong, C.K. Nanoporous p-type NiOx electrode for p-i-n inverted perovskite solar cell toward air stability. Mater. Today 2018, 21, 483–500. [Google Scholar] [CrossRef]
- Li, L.; Shen, W.; Yang, C.; Dou, Y.; Zhu, X.; Dong, Y.; Zhao, J.; Xiao, J.; Huang, F.; Cheng, Y.-B.; et al. In-situ monitored chemical bath deposition of planar NiOx layer for inverted perovskite solar cell with enhanced efficiency. J. Mater. Sci. Technol. 2023, 133, 145–153. [Google Scholar] [CrossRef]
- Gidey, A.T.; Kuo, D.-W.; Fenta, A.D.; Chen, C.-T.; Chen, C.-T. First Conventional Solution Sol–Gel-Prepared Nanoporous Materials of Nickel Oxide for Efficiency Enhancing and Stability Extending MAPbI3 Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2021, 4, 6486–6499. [Google Scholar] [CrossRef]
- Koushik, D.; Jošt, M.; Dučinskas, A.; Burgess, C.; Zardetto, V.; Weijtens, C.; Verheijen, M.A.; Kessels, W.M.M.; Albrecht, S.; Creatore, M. Plasma-assisted atomic layer deposition of nickel oxide as hole transport layer for hybrid perovskite solar cells. J. Mater. Chem. C 2019, 7, 12532–12543. [Google Scholar] [CrossRef]
- Ukoba, K.O.; Eloka-Eboka, A.C.; Inambao, F.L. Review of nanostructured NiO thin film deposition using the spray pyrolysis technique. Renew. Sustain. Energy Rev. 2018, 82, 2900–2915. [Google Scholar] [CrossRef]
- Cai, C.; Zhou, K.; Guo, H.; Pei, Y.; Hu, Z.; Zhang, J.; Zhu, Y. Enhanced hole extraction by NiO nanoparticles in carbon-based perovskite solar cells. Electrochim. Acta 2019, 312, 100–108. [Google Scholar] [CrossRef]
- Yin, X.; Guo, Y.; Xie, H.; Que, W.; Kong, L.B. Nickel Oxide as Efficient Hole Transport Materials for Perovskite Solar Cells. Sol. RRL 2019, 3, 1900001. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Feng, X.; Guo, X.; Lu, C.; Li, X.; Fang, J. Photoconductive NiOx hole transport layer for efficient perovskite solar cells. Chem. Eng. J. 2022, 435, 135140. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Li, Y.; Pu, X.; Chen, H.; Li, Y.; Yang, B.; Zhang, Y.; Zhao, J.; Cao, Q.; et al. Overcome Low Intrinsic Conductivity of NiOx Through Triazinyl Modification for Highly Efficient and Stable Inverted Perovskite Solar Cells. Sol. RRL 2022, 6, 2200422. [Google Scholar] [CrossRef]
- Huang, A.; Lei, L.; Chen, Y.; Yu, Y.; Zhou, Y.; Liu, Y.; Yang, S.; Bao, S.; Li, R.; Jin, P. Minimizing the energy loss of perovskite solar cells with Cu+ doped NiOx processed at room temperature. Sol. Energy Mater. Sol. Cells 2018, 182, 128–135. [Google Scholar] [CrossRef]
- Kim, J.H.; Liang, P.-W.; Williams, S.T.; Cho, N.; Chueh, C.-C.; Glaz, M.S.; Ginger, D.S.; Jen, A.K.-Y. High-Performance and Environmentally Stable Planar Heterojunction Perovskite Solar Cells Based on a Solution-Processed Copper-Doped Nickel Oxide Hole-Transporting Layer. Adv. Mater. 2015, 27, 695–701. [Google Scholar] [CrossRef]
- Wei, Y.; Yao, K.; Wang, X.; Jiang, Y.; Liu, X.; Zhou, N.; Li, F. Improving the efficiency and environmental stability of inverted planar perovskite solar cells via silver-doped nickel oxide hole-transporting layer. Appl. Surf. Sci. 2018, 427, 782–790. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, K.; Duan, J.; Jiang, Y.; Hu, L.; Liu, T.; Zhou, Y.; Hu, B. Enhancing Photovoltaic Performance of Inverted Planar Perovskite Solar Cells by Cobalt-Doped Nickel Oxide Hole Transport Layer. ACS Appl. Mater. Interfaces 2018, 10, 14153–14159. [Google Scholar] [CrossRef]
- Purushothaman, K.K.; Muralidharan, G. Electrochromic properties of nickel oxide and mixed Co/Ni oxide films prepared via sol–gel route. J. Non-Cryst. Solids 2012, 358, 354–359. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Chang, C.-Y.; Chiu, F.-B.; Yang, S.-H. Efficient and stable perovskite solar cells using manganese-doped nickel oxide as the hole transport layer. RSC Adv. 2022, 12, 22984–22995. [Google Scholar] [CrossRef]
- Lee, J.H.; Noh, Y.W.; Jin, I.S.; Jung, J.W. Efficient planar heterojunction perovskite solar cells employing a solution-processed Zn-doped NiOX hole transport layer. Electrochim. Acta 2018, 284, 253–259. [Google Scholar] [CrossRef]
- Wan, X.; Jiang, Y.; Qiu, Z.; Zhang, H.; Zhu, X.; Sikandar, I.; Liu, X.; Chen, X.; Cao, B. Zinc as a New Dopant for NiOx-Based Planar Perovskite Solar Cells with Stable Efficiency near 20%. ACS Appl. Energy Mater. 2018, 1, 3947–3954. [Google Scholar] [CrossRef]
- Wang, K.-C.; Jeng, J.-Y.; Shen, P.-S.; Chang, Y.-C.; Diau, E.W.-G.; Tsai, C.-H.; Chao, T.-Y.; Hsu, H.-C.; Lin, P.-Y.; Chen, P.; et al. p-type Mesoscopic Nickel Oxide/Organometallic Perovskite Heterojunction Solar Cells. Sci. Rep. 2014, 4, 4756. [Google Scholar] [CrossRef]
- Wang, T.; Ding, D.; Wang, X.; Zeng, R.; Liu, H.; Shen, W. High-Performance Inverted Perovskite Solar Cells with Mesoporous NiOx Hole Transport Layer by Electrochemical Deposition. ACS Omega 2018, 3, 18434–18443. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Wang, S.; Zheng, X.; Wu, Y.; Yuan, N.; Zhang, W.-H.; Liu, S.F. Design of an Inorganic Mesoporous Hole-Transporting Layer for Highly Efficient and Stable Inverted Perovskite Solar Cells. Adv. Mater. 2018, 30, 1805660. [Google Scholar] [CrossRef]
- El-Din, N.M.S.; Sabaa, M.W. Thermal degradation of poly(viny1 butyral) laminated safety glass. Polym. Degrad. Stabil. 1995, 41, 283–288. [Google Scholar] [CrossRef]
- Kim, K.H.; Takahashi, C.; Abe, Y.; Kawamura, M. Effects of Cu doping on nickel oxide thin film prepared by sol–gel solution process. Optik 2014, 125, 2899–2901. [Google Scholar] [CrossRef]
- Yadav, M.S.; Tripathi, S.K. Synthesis and characterization of nanocomposite NiO/activated charcoal electrodes for supercapacitor application. Ionics 2017, 23, 2919–2930. [Google Scholar] [CrossRef]
- Meybodi, S.M.; Hosseini, S.A.; Rezaee, M.; Sadrnezhaad, S.K.; Mohammadyani, D. Synthesis of wide band gap nanocrystalline NiO powder via a sonochemical method. Ultrason. Sonochem. 2012, 19, 841–845. [Google Scholar] [CrossRef]
- Sasi, B.; Gopchandran, K.G. Preparation and characterization of nanostructured NiO thin films by reactive-pulsed laser ablation technique. Sol. Energy Mater. Sol. Cells 2007, 91, 1505–1509. [Google Scholar] [CrossRef]
- Chen, S.C.; Kuo, T.Y.; Lin, Y.C.; Lin, H.C. Preparation and properties of p-type transparent conductive Cu-doped NiO films. Thin Solid Film. 2011, 519, 4944–4947. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.-Z.; Feng, X.-Y.; Djurišić, A.B.; Chan, W.K.; He, Z.-B. Cesium Doped NiOx as an Efficient Hole Extraction Layer for Inverted Planar Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1700722. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, J.; Wang, H.; Ye, F.; Xiong, L.; Lei, H.; Tan, Z. Overcoming Ni3+-Induced Non-Radiative Recombination at Perovskite-Nickel Oxide Interfaces to Boost Voltages in Perovskite Solar Cells. Adv. Mater. Interfaces 2021, 8, 2100920. [Google Scholar] [CrossRef]
- Dubey, P.; Kaurav, N.; Devan, R.S.; Okram, G.S.; Kuo, Y.K. The effect of stoichiometry on the structural thermal and electronic properties of thermally decomposed nickel oxide. RSC Adv. 2018, 8, 5882–5890. [Google Scholar] [CrossRef]
- Xie, L.; Cao, Z.; Wang, J.; Wang, A.; Wang, S.; Cui, Y.; Xiang, Y.; Niu, X.; Hao, F.; Ding, L. Improving energy level alignment by adenine for efficient and stable perovskite solar cells. Nano Energy 2020, 74, 104846. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, M.; Chen, C.; Zhu, Z.; Zheng, X.; Chen, Z.; Guo, Y.; Liu, C.; Yan, Y.; Fang, G. Efficient and Stable Nonfullerene-Graded Heterojunction Inverted Perovskite Solar Cells with Inorganic Ga2O3 Tunneling Protective Nanolayer. Adv. Funct. Mater. 2018, 28, 1804128. [Google Scholar] [CrossRef]
- Xiong, S.; Yuan, M.; Yang, J.; Song, J.; Guo, X.; Li, X.; Li, B.; Liu, X.; Duan, C.; Liu, F.; et al. Engineering of the Back Contact between PCBM and Metal Electrode for Planar Perovskite Solar Cells with Enhanced Efficiency and Stability. Adv. Opt. Mater. 2019, 7, 1900542. [Google Scholar] [CrossRef]
- Hung, C.-M.; Lin, J.-T.; Yang, Y.-H.; Liu, Y.-C.; Gu, M.-W.; Chou, T.-C.; Wang, S.-F.; Chen, Z.-Q.; Wu, C.-C.; Chen, L.-C.; et al. Modulation of Perovskite Grain Boundaries by Electron Donor–Acceptor Zwitterions R,R-Diphenylamino-phenyl-pyridinium-(CH2)n-sulfonates: All-Round Improvement on the Solar Cell Performance. JACS Au 2022, 2, 1189–1199. [Google Scholar] [CrossRef]
- Ye, L.-H.; Luo, N.; Peng, L.-M. Dielectric constant of NiO and LDA+U. Phys. Rev. B 2013, 87, 075115. [Google Scholar]
- Schlaf, R.; Parkinson, B.A.; Lee, P.A.; Nebesny, K.W.; Jabbour, G.; Kippelen, B.; Peyghambarian, N.; Armstrong, N.R. Photoemission spectroscopy of LiF coated Al and Pt electrodes. J. Appl. Phys. 1998, 84, 6729–6736. [Google Scholar] [CrossRef]
- Schroeder, P.G.; France, C.B.; Parkinson, B.A.; Schlaf, R. Orbital alignment at p-sexiphenyl and coronene/layered materials interfaces measured with photoemission spectroscopy. J. Appl. Phys. 2002, 91, 9095–9107. [Google Scholar] [CrossRef]
- Shao, G. Work Function and Electron Affinity of Semiconductors: Doping Effect and Complication due to Fermi Level Pinning. Energy Environ. Mater. 2021, 4, 273–276. [Google Scholar] [CrossRef]
- Kashiwaya, S.; Morasch, J.; Streibel, V.; Toupance, T.; Jaegermann, W.; Klein, A. The Work Function of TiO2. Surfaces 2018, 1, 73–89. [Google Scholar] [CrossRef]
- Klein, A.; Albe, K.; Bein, N.; Clemens, O.; Creutz, K.A.; Erhart, P.; Frericks, M.; Ghorbani, E.; Hofmann, J.P.; Huang, B.; et al. The Fermi energy as common parameter to describe charge compensation mechanisms: A path to Fermi level engineering of oxide electroceramic. J. Electroceram 2023, 51, 147–177. [Google Scholar] [CrossRef]
- Zhumekenov, A.A.; Saidaminov, M.I.; Haque, M.A.; Alarousu, E.; Sarmah, S.P.; Murali, B.; Dursun, I.; Miao, X.-H.; Abdelhady, A.L.; Wu, T.; et al. Formamidinium Lead Halide Perovskite Crystals with Unprecedented Long Carrier Dynamics and Diffusion Length. ACS Energy Lett. 2016, 1, 32–37. [Google Scholar] [CrossRef]
- She, Z.-X.; Yang, S.-H. Interfacial modification between NiOx and perovskite layers with hexafluorophosphate salts for enhancing device efficiency and stability of perovskite solar cells. RSC Appl. Interfaces 2024, 1, 443–454. [Google Scholar] [CrossRef]
- Zheng, G.; Zhu, C.; Ma, J.; Zhang, X.; Tang, G.; Li, R.; Chen, Y.; Li, L.; Hu, J.; Hong, J.; et al. Manipulation of facet orientation in hybrid perovskite polycrystalline films by cation cascade. Nat. Commun. 2018, 9, 2793. [Google Scholar] [CrossRef]
- Dong, P.; Yuan, S.; Zhu, D.; Du, Y.; Mu, C.; Ai, X.-C. Electron transport layer assisted by nickel chloride hexahydrate for open-circuit voltage improvement in MAPbI3 perovskite solar cells. RSC Adv. 2022, 12, 13820–13825. [Google Scholar] [CrossRef]
- Chen, P.; Bai, Y.; Wang, S.; Lyu, M.; Yun, J.-H.; Wang, L. In Situ Growth of 2D Perovskite Capping Layer for Stable and Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1706923. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, L.; Yun, J.S.; Zhang, M.; Lau, C.F.J.; Bing, J.; Deng, X.; Ma, Q.; Cho, Y.; Fu, W.; et al. Solution Processed, Silver Doped NiOx as Hole Transporting Layer for High-Efficiency Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 561–570. [Google Scholar] [CrossRef]
- Abate, S.Y.; Yang, Z.; Jha, S.; Emodogo, J.; Ma, G.; Ouyang, Z.; Muhammad, S.; Pradhan, N.; Gu, X.; Patton, D.; et al. Promoting Large-Area Slot-Die-Coated Perovskite Solar Cell Performance and Reproducibility by Acid-Based Sulfono-γ-AApeptide. ACS Appl. Mater. Interfaces 2023, 15, 25495–25505. [Google Scholar] [CrossRef]
- Lee, D.G.; Kim, M.-C.; Kim, B.J.; Kim, D.H.; Lee, S.M.; Choi, M.; Lee, S.; Jung, H.S. Effect of TiO2 particle size and layer thickness on mesoscopic perovskite solar cells. Appl. Surf. Sci. 2019, 477, 131–136. [Google Scholar] [CrossRef]
- Ke, W.; Stoumpos, C.C.; Spanopoulos, I.; Chen, M.; Wasielewski, M.R.; Kanatzidis, M.G. Diammonium Cations in the FASnI3 Perovskite Structure Lead to Lower Dark Currents and More Efficient Solar Cells. ACS Energy Lett. 2018, 3, 1470–1476. [Google Scholar] [CrossRef]
- Vollbrecht, J.; Brus, V.V.; Ko, S.-J.; Lee, J.; Karki, A.; Cao, D.X.; Cho, K.; Bazan, G.C.; Nguyen, T.-Q. Quantifying the Nongeminate Recombination Dynamics in Nonfullerene Bulk Heterojunction Organic Solar Cells. Adv. Energy Mater. 2019, 9, 1901438. [Google Scholar] [CrossRef]
- Brus, V.V.; Proctor, C.M.; Ran, N.A.; Nguyen, T.-Q. Capacitance Spectroscopy for Quantifying Recombination Losses in Nonfullerene Small-Molecule Bulk Heterojunction Solar Cells. Adv. Energy Mater. 2016, 6, 1502250. [Google Scholar] [CrossRef]


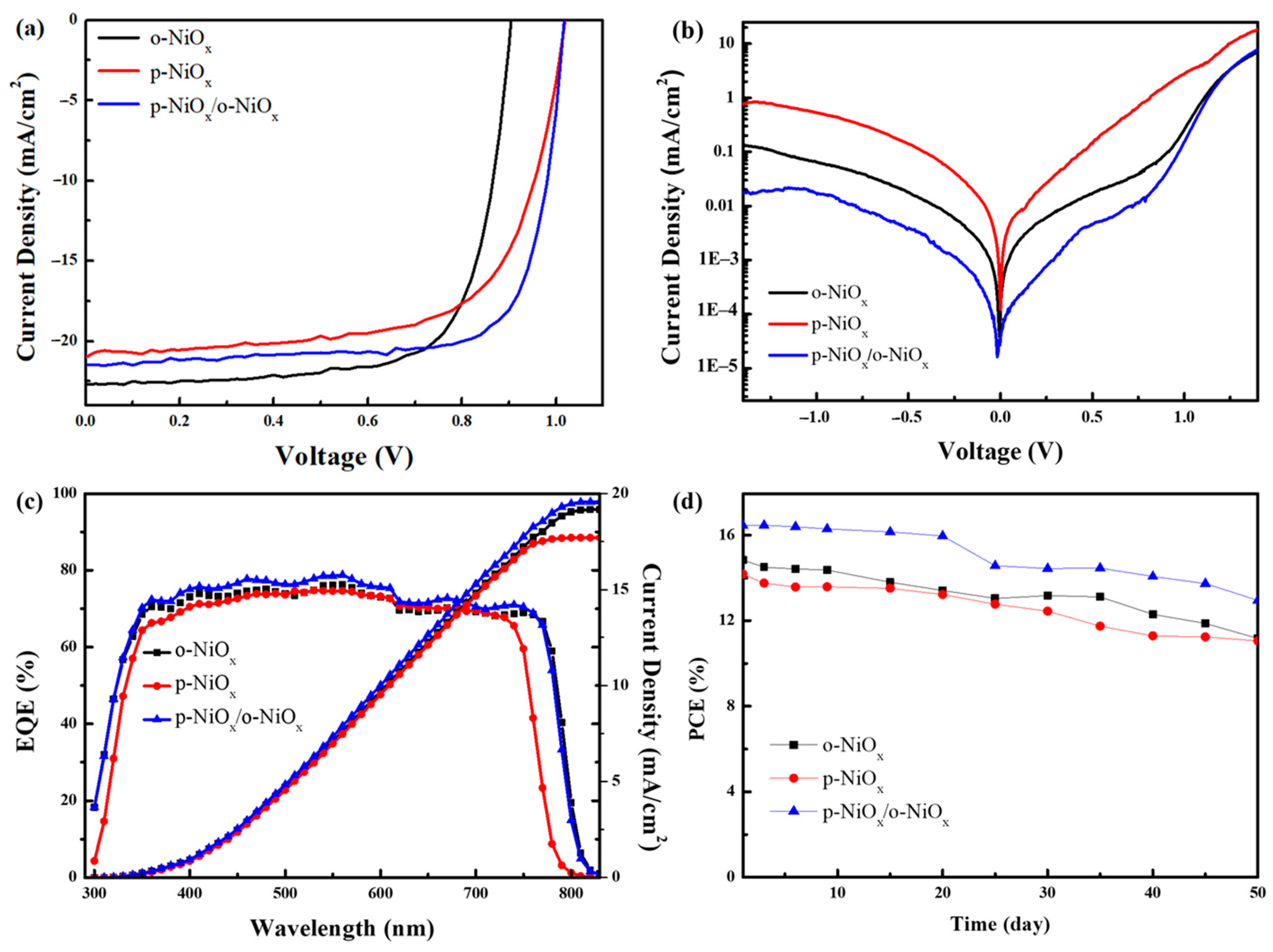
| HTL | JSC (mA/cm2) | JSC from EQE (mA/cm2) | VOC (V) | FF (%) | Best PCE (%) | Avg. PCE a (%) |
|---|---|---|---|---|---|---|
| o-NiOx | 22.7 | 19.2 | 0.90 | 72 | 14.8 | 14.1 |
| p-NiOx | 21.0 | 17.7 | 1.01 | 66 | 14.2 | 13.5 |
| p-NiOx/o-NiOx | 21.5 | 19.6 | 1.01 | 75 | 16.5 | 15.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-W.; Wang, C.-Y.; Yang, S.-H. Exploration and Optimization of the Polymer-Modified NiOx Hole Transport Layer for Fabricating Inverted Perovskite Solar Cells. Nanomaterials 2024, 14, 1054. https://doi.org/10.3390/nano14121054
Wu Y-W, Wang C-Y, Yang S-H. Exploration and Optimization of the Polymer-Modified NiOx Hole Transport Layer for Fabricating Inverted Perovskite Solar Cells. Nanomaterials. 2024; 14(12):1054. https://doi.org/10.3390/nano14121054
Chicago/Turabian StyleWu, You-Wei, Ching-Ying Wang, and Sheng-Hsiung Yang. 2024. "Exploration and Optimization of the Polymer-Modified NiOx Hole Transport Layer for Fabricating Inverted Perovskite Solar Cells" Nanomaterials 14, no. 12: 1054. https://doi.org/10.3390/nano14121054






