Understanding of Lanthanide-Doped Core–Shell Structure at the Nanoscale Level
Abstract
:1. Introduction
2. Synthesis Techniques and Structural Characterization
2.1. Synthesis Techniques
2.1.1. Thermal Synthesis in Water (Solvent)
2.1.2. Co-Precipitation Method
2.1.3. Thermal Injection Method
2.2. Structural Characterization
3. Interfacial Ion Mixing
4. Interfacial Stress
5. Luminescence Modulation
5.1. Upconversion Luminescence Mechanism
5.1.1. Excited State Absorption (ESA)
5.1.2. Energy Transfer Upconversion (ETU)
5.1.3. Co-Operative Sensitization Upconversion (CSU)
5.1.4. Cross-Relaxation (CR)
5.1.5. Photon Avalanche (PA)
5.1.6. Energy Transport-Mediated Upconversion (EMU)
5.2. Emission Color Modulation
5.2.1. Adjustment of Optically Active ion Distribution in the Core–Shell Structure
Homogeneous Core–Shell Structure
Heterogeneous Core–Shell Structure
Active Core–Active Shell Structure
5.2.2. Adjustment of Shell Thickness
5.3. Luminescence Enhancement
5.3.1. Design of the Shell Structure
5.3.2. Regulation of the Properties of the Core–Shell Interface
5.3.3. Size Effect
5.4. Tuning Upconversion Luminescence Lifetime
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef]
- Bünzli, J.C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Qju, H.L.; Prasad, P.N.; Chen, X.Y. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.L.; Li, C.X.; Yang, P.P.; Lin, J. Recent Progress in Rare Earth Micro/Nanocrystals: Soft Chemical Synthesis, Luminescent Properties, and Biomedical Applications. Chem. Rev. 2014, 114, 2343–2389. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.W.; Zhou, J.; Yue, J.Y.; Wei, Y.; Gao, C.; Xie, X.J.; Huang, L. Recent Development in Sensitizers for Lanthanide-Doped Upconversion Luminescence. Chem. Rev. 2022, 122, 15998–16050. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.Z.; Fan, J.Y.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.R.; Liu, X.G. Rare-Earth Doping in Nanostructured Inorganic Materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Yan, L.; Huang, J.S.; Zhang, Q.Y.; Zhou, B. Controlling upconversion in emerging multilayer core-shell nanostructures: From fundamentals to frontier applications. Chem. Soc. Rev. 2022, 51, 1729–1765. [Google Scholar] [CrossRef]
- Tian, L.; Shang, Y.F.; Hao, S.W.; Han, Q.; Chen, T.; Lv, W.Q.; Yang, C.H. Constructing a “Native” Oxyfluoride Layer on Fluoride Particles for Enhanced Upconversion Luminescence. Adv. Funct. Mater. 2018, 28, 1803946. [Google Scholar] [CrossRef]
- Fu, H.H.; Ma, Y.H.; Liu, Y.S.; Hong, M.C. Local-structure-dependent luminescence in lanthanide-doped inorganic nanocrystals for biological applications. Chem. Commun. 2021, 57, 2970–2981. [Google Scholar] [CrossRef]
- Kömpe, K.; Borchert, H.; Storz, J.; Lobo, A.; Adam, S.; Möller, T.; Haase, M. Green-emitting CePO4: Th/LaPO4 core-shell nanoparticles with 70% photoluminescence quantum yield. Angew. Chem. Int. Ed. 2003, 42, 5513–5516. [Google Scholar] [CrossRef]
- Chen, G.Y.; Shen, J.; Ohulchanskyy, T.Y.; Patel, N.J.; Kutikov, A.; Li, Z.P.; Song, J.; Pandey, R.K.; Ågren, H.; Prasad, P.N.; et al. (α-NaYbF4:Tm3+)/CaF2 Core/Shell Nanoparticles with Efficient Near-Infrared to Near-Infrared Upconversion for High-Contrast Deep Tissue Bioimaging. ACS Nano 2012, 6, 8280–8287. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; Mahalingam, V.; Morgan, C.G.; Capobianco, J.A. The Active-Core/Active-Shell Approach: A Strategy to Enhance the Upconversion Luminescence in Lanthanide-Doped Nanoparticles. Adv. Funct. Mater. 2009, 19, 2924–2929. [Google Scholar] [CrossRef]
- Xie, X.J.; Gao, N.Y.; Deng, R.R.; Sun, Q.; Xu, Q.H.; Liu, X.G. Mechanistic Investigation of Photon Upconversion in Nd3+-Sensitized Core-Shell Nanoparticles. J. Am. Chem. Soc. 2013, 135, 12608–12611. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Patra, A. Impacts of core-shell structures on properties of lanthanide-based nanocrystals: Crystal phase, lattice strain, downconversion, upconversion and energy transfer. Nanoscale 2012, 4, 3608–3619. [Google Scholar] [CrossRef]
- Fischer, S.; Swabeck, J.K.; Alivisatos, A.P. Controlled Isotropic and Anisotropic Shell Growth in β-NaLnF4 Nanocrystals Induced by Precursor Injection Rate. J. Am. Chem. Soc. 2017, 139, 12325–12332. [Google Scholar] [CrossRef]
- Garfield, D.J.; Borys, N.J.; Hamed, S.M.; Torquato, N.A.; Tajon, C.A.; Tian, B.; Shevitski, B.; Barnard, E.S.; Suh, Y.D.; Aloni, S.; et al. Enrichment of molecular antenna triplets amplifies upconverting nanoparticle emission. Nat. Photonics 2018, 12, 402–407. [Google Scholar] [CrossRef]
- Liu, Z.; Yun, B.F.; Han, Y.B.; Jiang, Z.L.; Zhu, H.Q.; Ren, F.; Li, Z. Dye-Sensitized Rare Earth Nanoparticles with Up/Down Conversion Luminescence for On-Demand Gas Therapy of Glioblastoma Guided by NIR-II Fluorescence Imaging. Adv. Healthc. Mater. 2022, 11, 2102042. [Google Scholar] [CrossRef] [PubMed]
- Shuquan, L.I.; Jianming, L.I.N.; Wu, J.; Xiukun, Z.; Biao, L.I.; Bo, X.U. Applications of upconversion luminescence of Ho~ (3+) in dye sensitized solar cell. J. Funct. Mater. 2009, 40, 82–85. [Google Scholar]
- Zhao, F.; Hu, J.L.; Guan, D.M.; Liu, J.Y.; Zhang, X.B.; Ling, H.; Zhang, Y.X.; Liu, Q. Boosting Dye-Sensitized Luminescence by Enhanced Short-Range Triplet Energy Transfer. Adv. Mater. 2023, 35, 2304907. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y.W.; Takle, K.; Bilsel, O.; Li, Z.J.; Lee, H.; Zhang, Z.J.; Li, D.S.; Fan, W.; Duan, C.Y.; et al. Dye-Sensitized Core/Active Shell Upconversion Nanoparticles for Optogenetics and Bioimaging Applications. ACS Nano 2016, 10, 1060–1066. [Google Scholar] [CrossRef]
- Hu, J.L.; Zhao, B.J.; Wen, R.R.; Zhang, X.B.; Zhang, Y.X.; Kohane, D.S.; Liu, Q. Squaraine Dye-Sensitized Upconversion with Enhanced Stability and Minimized Aggregation-Caused Quenching. Nano Lett. 2023, 23, 5209–5216. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ke, J.X.; Li, X.J.; Tu, D.T.; Chen, X.Y. Luminescent nano-bioprobes based on NIR dye/lanthanide nanoparticle composites. Aggregate 2021, 2, e59. [Google Scholar] [CrossRef]
- Ke, J.X.; Lu, S.; Shang, X.Y.; Liu, Y.; Guo, H.H.; You, W.W.; Li, X.J.; Xu, J.; Li, R.F.; Chen, Z.; et al. A Strategy of NIR Dual-Excitation Upconversion for Ratiometric Intracellular Detection. Adv. Sci. 2019, 6, 1901874. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yuan, C.; Jia, X.J.; Zheng, Z.Y.; Yang, X.J.; Yang, J.T.; Kayitmazer, A.B.; Ahmad, A.; Ramzan, N.; Xu, Y.S. Efficient ROS activation by highly stabilized aqueous ICG encapsulated upconversion nanoparticles for tumor cell imaging and therapeutics. Chem. Eng. J. 2023, 452, 139343. [Google Scholar] [CrossRef]
- Ansari, A.A.; Nazeeruddin, M.K.; Tavakoli, M.M. Organic-inorganic upconversion nanoparticles hybrid in dye-sensitized solar cells. Coord. Chem. Rev. 2021, 436, 213805. [Google Scholar]
- Nguyen, T.N.; Ebrahim, F.M.; Stylianou, K.C. Photoluminescent, upconversion luminescent and nonlinear optical metal-organic frameworks: From fundamental photophysics to potential applications. Coord. Chem. Rev. 2018, 377, 259–306. [Google Scholar] [CrossRef]
- Kalmbach, J.; Wang, C.; You, Y.; Förster, C.; Schubert, H.; Heinze, K.; Resch-Genger, U.; Seitz, M. Near-IR to Near-IR Upconversion Luminescence in Molecular Chromium Ytterbium Salts. Angew. Chem. Int. Ed. 2020, 59, 18804–18808. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.Q.; Visser, C.; Maduro, J.A.; Pshenichnikov, M.S.; Hummelen, J.C. Broadband dye-sensitized upconversion of near-infrared light. Nat. Photonics 2012, 6, 560–564. [Google Scholar] [CrossRef]
- Chen, G.Y.; Damasco, J.; Qiu, H.L.; Shao, W.; Ohulchanskyy, T.Y.; Valiev, R.R.; Wu, X.; Han, G.; Wang, Y.; Yang, C.H.; et al. Energy-Cascaded Upconversion in an Organic Dye-Sensitized Core/Shell Fluoride Nanocrystal. Nano Lett. 2015, 15, 7400–7407. [Google Scholar] [CrossRef]
- Shao, W.; Chen, G.Y.; Kuzmin, A.; Kutscher, H.L.; Pliss, A.; Ohulchanskyy, T.Y.; Prasad, P.N. Tunable Narrow Band Emissions from Dye-Sensitized Core/Shell/Shell Nanocrystals in the Second Near-Infrared Biological Window. J. Am. Chem. Soc. 2016, 138, 16192–16195. [Google Scholar] [CrossRef]
- Wang, D.; Wang, D.P.; Kuzmin, A.; Pliss, A.; Shao, W.; Xia, J.; Qu, J.L.; Prasad, P.N. ICG-Sensitized NaYF4: Er Nanostructure for Theranostics. Adv. Opt. Mater. 2018, 6, 1701142. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, Z.H.; Hu, H.T.; Kang, B.W.; Zhang, B.B.; Mi, X.H.; Guo, L.; Zhang, C.Y.; Li, J.P.; Lu, J.B.; et al. Sub-50-ns ultrafast upconversion luminescence of a rare-earth-doped nanoparticle. Nat. Photonics 2022, 16, 651–657. [Google Scholar] [CrossRef]
- Xue, Y.X.; Ding, C.J.; Rong, Y.Y.; Ma, Q.; Pan, C.D.; Wu, E.; Wu, B.T.; Zeng, H.P. Tuning Plasmonic Enhancement of Single Nanocrystal Upconversion Luminescence by Varying Gold Nanorod Diameter. Small 2017, 13, 1701155. [Google Scholar] [CrossRef] [PubMed]
- Saboktakin, M.; Ye, X.C.; Oh, S.J.; Hong, S.H.; Fafarman, A.T.; Chettiar, U.K.; Engheta, N.; Murray, C.B.; Kagan, C.R. Metal-Enhanced Upconversion Luminescence Tunable through Metal Nanoparticle-Nanophosphor Separation. ACS Nano 2012, 6, 8758–8766. [Google Scholar] [CrossRef]
- Kwon, S.J.; Lee, G.Y.; Jung, K.; Jang, H.S.; Park, J.S.; Ju, H.; Han, I.; Ko, H. A Plasmonic Platform with Disordered Array of Metal Nanoparticles for Three-Order Enhanced Upconversion Luminescence and Highly Sensitive Near-Infrared Photodetector. Adv. Mater. 2016, 28, 7899–7909. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Jung, K.; Kwon, S.J.; Jang, H.S.; Byun, D.; Han, I.K.; Ko, H. Plasmonic Nanowire-Enhanced Upconversion Luminescence for Anticounterfeit Devices. Adv. Funct. Mater. 2016, 26, 7836–7846. [Google Scholar] [CrossRef]
- Deng, K.R.; Xu, L.L.; Guo, X.; Wu, X.T.; Liu, Y.L.; Zhu, Z.M.; Li, Q.; Zhan, Q.Q.; Li, C.X.; Quan, Z.W. Binary Nanoparticle Superlattices for Plasmonically Modulating Upconversion Luminescence. Small 2020, 16, 2002066. [Google Scholar] [CrossRef]
- Goddati, M.; Nguyen, M.C.T.; Nguyen, H.Q.; Kang, H.; Lee, S.Y.; Yee, K.J.; Lee, J. Metal plasmon-enhanced lanthanide fluorescent nanoparticles for monitoring aqueous copper ions. Mater. Today Nano 2023, 24, 100380. [Google Scholar]
- Das, A.; Mao, C.C.; Cho, S.; Kim, K.; Park, W. Over 1000-fold enhancement of upconversion luminescence using water-dispersible metal-insulator-metal nanostructures. Nat. Commun. 2018, 9, 4828. [Google Scholar] [CrossRef]
- Xu, J.H.; Dong, Z.G.; Asbahi, M.; Wu, Y.M.; Wang, H.; Liang, L.L.; Ng, R.J.H.; Liu, H.L.; Vallée, R.A.L.; Yang, J.K.W.; et al. Multiphoton Upconversion Enhanced by Deep Subwavelength Near-Field Confinement. Nano Lett. 2021, 21, 3044–3051. [Google Scholar] [CrossRef]
- Xu, W.; Xu, S.; Zhu, Y.S.; Liu, T.; Bai, X.; Dong, B.A.; Xu, L.; Song, H.W. Ultra-broad plasma resonance enhanced multicolor emissions in an assembled Ag/NaYF4: Yb,Er nano-film. Nanoscale 2012, 4, 6971–6973. [Google Scholar] [CrossRef]
- Ahn, I.H.; Yeo, S.J.; Jung, K.; Kang, G.; Shin, D.H.; Jang, H.S.; Kim, B.; Nam, M.; Kwon, S.J.; Ko, D.H. A Multi-Functional Highly Efficient Upconversion Luminescent Film with an Array of Dielectric Microbeads Decorated with Metal Nanoparticles. Adv. Funct. Mater. 2020, 30, 1909445. [Google Scholar] [CrossRef]
- Sahu, S.P.; Cates, S.L.; Kim, H.I.; Kim, J.H.; Cates, E.L. The Myth of Visible Light Photocatalysis Using Lanthanide Upconversion Materials. Environ. Sci. Technol. 2018, 52, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Xu, J.H.; Poh, E.T.; Liang, L.L.; Liu, H.L.; Yang, J.K.W.; Qiu, C.W.; Vallée, R.A.L.; Liu, X.G. Upconversion superburst with sub-2 μs lifetime. Nat. Nanotechnol. 2019, 14, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Liu, E.Y.; Wang, Y.B.; Hua, Y.J.; Zhang, J.J.; Chen, J.J.; Mao, R.D.; Jia, G.H.; Xu, S.Q. Amplifying Upconversion by Engineering Interfacial Density of State in Sub-10 nm Colloidal Core/Shell Fluoride Nanoparticles. Nano Lett. 2021, 21, 10222–10229. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Sun, D.P.; Tu, L.P.; Wu, Y.N.; Cao, Y.H.; Xue, B.; Zhang, Y.L.; Chang, Y.L.; Liu, X.M.; Kong, X.G.; et al. Precisely Tailoring Upconversion Dynamics via Energy Migration in Core-Shell Nanostructures. Angew. Chem. Int. Ed. 2018, 57, 3054–3058. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Johnson, N.J.J.; Huu, V.A.N.; Cory, E.; Huang, Y.R.; Sah, R.L.; Jokerst, J.V.; Almutairi, A. Simultaneous Enhancement of Photoluminescence, MRI Relaxivity, and CT Contrast by Tuning the Interfacial Layer of Lanthanide Heteroepitaxial Nanoparticles. Nano Lett. 2017, 17, 4873–4880. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.M.; Liu, C.W.; Ge, R.; Fang, Y.; Wei, J.; Chen, X.; Chen, Q.S.; Chen, X.M. Paper-supported near-infrared-light-triggered photoelectrochemical platform for monitoring Escherichia coli O157:H7 based on silver nanoparticles-sensitized-upconversion nanophosphors. Biosens. Bioelectron. 2022, 203, 114022. [Google Scholar] [CrossRef]
- Zhou, L.J.; Zheng, X.P.; Gu, Z.J.; Yin, W.Y.; Zhang, X.; Ruan, L.F.; Yang, Y.B.; Hu, Z.B.; Zhao, Y.L. Mesoporous NaYbF4@NaGdF4 core-shell up-conversion nanoparticles for targeted drug delivery and multimodal imaging. Biomaterials 2014, 35, 7666–7678. [Google Scholar] [CrossRef]
- Xu, W.X.; Zheng, Y.; Liu, X.F.; Zhou, M.; Deng, R.R.; Yang, Y.M.; Qiu, J.R. Manipulation of Coupled X-Ray-Excited Persistent Luminescence and Upconversion in Er3+ Doped Fluoride Nanoparticles for Multifaceted Applications. Laser Photon. Rev. 2024, 2400147. [Google Scholar] [CrossRef]
- Ding, M.Y.; Cui, S.S.; Fang, L.; Lin, Z.X.; Lu, C.H.; Yang, X.F. NIR-I-Responsive Single-Band Upconversion Emission through Energy Migration in Core-Shell-Shell Nanostructures. Angew. Chem. Int. Ed. 2022, 61, e202203631. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.K.; Liu, Y.T.; Lin, J.D.; Wang, S.X.; Huang, F.; Chen, D.Q. Lanthanide-Doped Core@Multishell Nanoarchitectures: Multimodal Excitable Upconverting/Downshifting Luminescence and High-Level Anti-Counterfeiting. Small 2020, 16, 2000708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tao, L.L.; Chai, Y.; Lau, S.P.; Zhang, Q.Y.; Tsang, Y.H. Constructing Interfacial Energy Transfer for Photon Up- and Down-Conversion from Lanthanides in a Core-Shell Nanostructure. Angew. Chem. Int. Ed. 2016, 55, 12356–12360. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.D.; Peng, Z.X.; Zhan, Q.Q.; Huang, X.J.; Peng, X.Y.; Guo, X.; Dong, G.P.; Qiu, J.R. Anisotropic Excitation Polarization Response from a Single White Light-Emitting β-NaYF4:Yb3+, Pr3+ Microcrystal. Small 2019, 15, 1904298. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.K.; Wang, S.H.; Shen, M.H.; Xie, J.C.; Tang, H.X. Controlling single rare earth ion emission in an electro-optical nanocavity. Nat. Commun. 2023, 14, 1718. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.A.; Jain, N.; Popescu, R.; Busko, D.; Madirov, E.; Arús, B.A.; Gerthsen, D.; De Backer, A.; Bals, S.; Bruns, O.T.; et al. Preventing cation intermixing enables 50% quantum yield in sub-15 nm short-wave infrared-emitting rare-earth based core-shell nanocrystals. Nat. Commun. 2023, 14, 4462. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.H.; Bagheri, N.; Wang, L.; Ågren, H.; Zhang, J.L.; Pu, R.; Zhan, Q.Q.; Jing, Y.H.; Xu, W.; Widengren, J.; et al. Suppression of Cation Intermixing Highly Boosts the Performance of Core-Shell Lanthanide Upconversion Nanoparticles. J. Am. Chem. Soc. 2023, 145, 17621–17631. [Google Scholar] [CrossRef] [PubMed]
- Hudry, D.; Busko, D.; Popescu, R.; Gerthsen, D.; Abeykoon, A.M.M.; Kübel, C.; Bergfeldt, T.; Richards, B.S. Direct Evidence of Significant Cation Intermixing in Upconverting Core@Shell Nanocrystals: Toward a New Crystallochemical Model. Chem. Mater. 2017, 29, 9238–9246. [Google Scholar] [CrossRef]
- Hudry, D.; De Backer, A.; Popescu, R.; Busko, D.; Howard, I.A.; Bals, S.; Zhang, Y.; Pedrazo-Tardajos, A.; Van Aert, S.; Gerthsen, D.; et al. Interface Pattern Engineering in Core-Shell Upconverting Nanocrystals: Shedding Light on Critical Parameters and Consequences for the Photoluminescence Properties. Small 2021, 17, 2104441. [Google Scholar] [CrossRef] [PubMed]
- Hudry, D.; Popescu, R.; Busko, D.; Diaz-Lopez, M.; Abeykoon, M.; Bordet, P.; Gerthsen, D.; Howard, I.A.; Richards, B.S. Interface disorder in large single- and multi-shell upconverting nanocrystals. J. Mater. Chem. C 2019, 7, 1164–1172. [Google Scholar] [CrossRef]
- Zhao, J.X.; Chen, X.; Chen, B.; Luo, X.; Sun, T.Y.; Zhang, W.W.; Wang, C.J.; Lin, J.; Su, D.; Qiao, S.; et al. Accurate Control of Core-Shell Upconversion Nanoparticles through Anisotropic Strain Engineering. Adv. Funct. Mater. 2019, 29, 1903295. [Google Scholar] [CrossRef]
- Johnson, N.J.J.; Van Veggel, F.C.J.M. Lanthanide-Based Heteroepitaxial Core-Shell Nanostructures: Compressive versus Tensile Strain Asymmetry. ACS Nano 2014, 8, 10517–10527. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.X.; Chen, B.; Chen, X.; Zhang, X.; Sun, T.Y.; Su, D.; Wang, F. Tuning epitaxial growth on NaYbF4 upconversion nanoparticles by strain management. Nanoscale 2020, 12, 13973–13979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lee, J.Y. Prevalence of Anisotropic Shell Growth in Rare Earth Core-Shell Upconversion Nanocrystals. ACS Nano 2013, 7, 4393–4402. [Google Scholar] [CrossRef] [PubMed]
- Stouwdam, J.W.; van Veggel, F. Near-infrared emission of redispersible Er3+, Nd3+, and Ho3+ doped LaF3 nanoparticles. Nano Lett. 2002, 2, 733–737. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y.D. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wan, Y.; Yu, T.; Zhang, F.Q.; Shi, Y.F.; Xie, S.H.; Li, Y.G.; Xu, L.; Tu, B.; Zhao, D.Y. Uniform nanostructured arrays of sodium rare-earth fluorides for highly efficient multicolor upconversion luminescence. Angew. Chem. Int. Ed. 2007, 46, 7976–7979. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.-Y.; Dong, H.; Yang, X.-F.; Huang, L.; Xu, Y.-J.; Wu, K.; Sun, L.-D.; Yan, C.-H. Phase-Transition-Driven Regional Distribution of Rare-Earth Ions for Multiplexed Upconversion Emissions. JACS Au 2023, 3, 860–867. [Google Scholar] [CrossRef]
- Li, X.M.; Shen, D.K.; Yang, J.P.; Yao, C.; Che, R.C.; Zhang, F.; Zhao, D.Y. Successive Layer-by-Layer Strategy for Multi-Shell Epitaxial Growth: Shell Thickness and Doping Position Dependence in Upconverting Optical Properties. Chem. Mater. 2013, 25, 106–112. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Huang, L.; Liu, X.G. Unraveling Epitaxial Habits in the NaLnF4 System for Color Multiplexing at the Single-Particle Level. Angew. Chem. Int. Ed. 2016, 55, 5718–5722. [Google Scholar] [CrossRef]
- Liu, D.M.; Xu, X.X.; Du, Y.; Qin, X.; Zhang, Y.H.; Ma, C.S.; Wen, S.H.; Ren, W.; Goldys, E.M.; Piper, J.A.; et al. Three-dimensional controlled growth of monodisperse sub-50 nm heterogeneous nanocrystals. Nat. Commun. 2016, 7, 10254. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zheng, W.; Kong, J.T.; Liu, Y.; Huang, P.; Zhou, S.Y.; Chen, Z.; Shi, J.L.; Chen, X.Y. Rechargeable and LED-activated ZnGa2O4: Cr3+ near-infrared persistent luminescence nanoprobes for background-free biodetection. Nanoscale 2017, 9, 6846–6853. [Google Scholar] [CrossRef]
- Mai, H.X.; Zhang, Y.W.; Sun, L.D.; Yan, C.H. Highly efficient multicolor up-conversion emissions and their mechanisms of monodisperse NaYF4: Yb, Er core and core/shell-structured nanocrystals. J. Phys. Chem. C 2007, 111, 13721–13729. [Google Scholar] [CrossRef]
- Qian, H.S.; Zhang, Y. Synthesis of Hexagonal-Phase Core−Shell NaYF4 Nanocrystals with Tunable Upconversion Fluorescence. Langmuir 2008, 24, 12123–12125. [Google Scholar] [CrossRef]
- Wang, F.; Deng, R.R.; Liu, X.G. Preparation of core-shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat. Protoc. 2014, 9, 1634–1644. [Google Scholar] [CrossRef]
- Wen, S.H.; Liu, Y.T.; Wang, F.; Lin, G.G.; Zhou, J.J.; Shi, B.Y.; Suh, Y.D.; Jin, D.Y. Nanorods with multidimensional optical information beyond the diffraction limit. Nat. Commun. 2020, 11, 6047. [Google Scholar] [CrossRef]
- Li, X.M.; Wang, R.; Zhang, F.; Zhao, D.Y. Engineering Homogeneous Doping in Single Nanoparticle to Enhance Upconversion Efficiency. Nano Lett. 2014, 14, 3634–3639. [Google Scholar] [CrossRef]
- Li, X.M.; Guo, Z.Z.; Zhao, T.C.; Lu, Y.; Zhou, L.; Zhao, D.Y.; Zhang, F. Filtration Shell Mediated Power Density Independent Orthogonal Excitations-Emissions Upconversion Luminescence. Angew. Chem. Int. Ed. 2016, 55, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Peng, D.F.; Chen, X.; Qiao, X.S.; Fan, X.P.; Wang, F. Establishing the Structural Integrity of Core-Shell Nanoparticles against Elemental Migration using Luminescent Lanthanide Probes. Angew. Chem. Int. Ed. 2015, 54, 12788–12790. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Sun, L.D.; Li, L.D.; Si, R.; Liu, R.; Yan, C.H. Selective Cation Exchange Enabled Growth of Lanthanide Core/Shell Nanoparticles with Dissimilar Structure. J. Am. Chem. Soc. 2017, 139, 18492–18495. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.Q.; Wei, H.L.; Liu, Y.C.; Chen, C.H.; Guan, M.; Wang, S.; Su, Y.; Wang, H.F.; Chen, Z.G.; Jin, D.Y. Six-photon upconverted excitation energy lock-in for ultraviolet-C enhancement. Nat. Commun. 2021, 12, 4367. [Google Scholar] [CrossRef]
- Dühnen, S.; Haase, M. Study on the Intermixing of Core and Shell in NaEuF4/NaGdF4 Core/Shell Nanocrystals. Chem. Mater. 2015, 27, 8375–8386. [Google Scholar] [CrossRef]
- Xie, Y.; Song, Y.P.; Sun, G.T.; Hu, P.F.; Bednarkiewicz, A.; Sun, L.N. Lanthanide-doped heterostructured nanocomposites toward advanced optical anti-counterfeiting and information storage. Light Sci. Appl. 2022, 11, 1264–1273. [Google Scholar] [CrossRef]
- Bloembergen, N. Solid State Infrared Quantum Counters. Phys. Rev. Lett. 1959, 2, 84–85. [Google Scholar] [CrossRef]
- Su, Q.Q.; Han, S.Y.; Xie, X.J.; Zhu, H.M.; Chen, H.Y.; Chen, C.K.; Liu, R.S.; Chen, X.Y.; Wang, F.; Liu, X.G. The Effect of Surface Coating on Energy Migration-Mediated Upconversion. J. Am. Chem. Soc. 2012, 134, 20849–20857. [Google Scholar] [CrossRef]
- Deng, R.R.; Wang, J.; Chen, R.F.; Huang, W.; Liu, X.G. Enabling Forster Resonance Energy Transfer from Large Nanocrystals through Energy Migration. J. Am. Chem. Soc. 2016, 138, 15972–15979. [Google Scholar] [CrossRef] [PubMed]
- Chivian, J.S.; Case, W.E.; Eden, D.D. The photon avalanche: A new phenomenon in Pr3+-based infrared quantum counters. Appl. Phys. Lett. 1979, 35, 124–125. [Google Scholar] [CrossRef]
- Wang, F.; Deng, R.R.; Wang, J.; Wang, Q.X.; Han, Y.; Zhu, H.M.; Chen, X.Y.; Liu, X.G. Tuning upconversion through energy migration in core-shell nanoparticles. Nat. Mater. 2011, 10, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Chen, D.; Chen, W.; Zhang, W.; Xie, R.J. X-ray-charged bright persistent luminescence in NaYF4: Ln3+@NaYF4 nanoparticles for multidimensional optical information storage. Light. Sci. Appl. 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Chen, Y.; Sun, C.X.; Fan, Y.; Yang, Y.M.; Liu, X.; Lu, L.F.; Zhao, M.Y.; Zhang, H.X.; Zhao, D.Y.; et al. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 2021, 16, 1011. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Huang, J.; An, Z.; Zhang, Q.; Zhou, B. Spatiotemporal control of photochromic upconversion through interfacial energy transfer. Nat. Commun. 2024, 15, 1923. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, L.; Zhao, J.; Liu, B.; Han, M.Y.; Zhang, Z. White-Light Emission from an Integrated Upconversion Nanostructure: Toward Multicolor Displays Modulated by Laser Power. Angew. Chem. Int. Ed. 2015, 54, 11531–11535. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.F.; Zheng, W.; Huang, P.; Zhang, M.R.; Zhang, W.; Shao, Z.Q.; Zhang, W.; Yi, X.D.; Chen, X.Y. A sandwiched luminescent heterostructure based on lanthanide-doped Gd2O2S@NaYF4 core/shell nanocrystals. Aggregate 2023, 4, e387. [Google Scholar] [CrossRef]
- Sun, T.Y.; Chen, B.; Guo, Y.; Zhu, Q.; Zhao, J.X.; Li, Y.H.; Chen, X.; Wu, Y.K.; Gao, Y.B.; Jin, L.M.; et al. Ultralarge anti-Stokes lasing through tandem upconversion. Nat. Commun. 2022, 13, 1032. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Huang, J.S.; Yan, L.; Liu, X.L.; Song, N.; Tao, L.L.; Zhang, Q.Y. Probing Energy Migration through Precise Control of Interfacial Energy Transfer in Nanostructure. Adv. Mater. 2019, 31, 1806308. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Li, Q.; Huang, J.; Tao, L.; Zhou, B. Selectively Manipulating Interactions between Lanthanide Sublattices in Nanostructure toward Orthogonal Upconversion. Nano Lett. 2023, 23, 6241–6248. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, S.S.; Zhang, H.J.; Wu, Y.H.; Zhang, J.; Liu, J.L.; Zhu, X.H.; Zhang, Y. Unraveling the Growth Process of Core-Shell Structured Upconversion Nanoparticles: Implications for Color Tuning of Upconversion Luminescence. ACS Appl. Nano Mater. 2023, 6, 6398–6406. [Google Scholar] [CrossRef]
- Wang, S.; Shen, B.; Wei, H.L.; Liu, Z.X.; Chen, Z.G.; Zhang, Y.; Su, Y.; Zhang, J.Z.; Wang, H.F.; Su, Q.Q. Comparative investigation of the optical spectroscopic and thermal effect in Nd3+-doped nanoparticles. Nanoscale 2019, 11, 10220–10228. [Google Scholar] [CrossRef]
- Zhou, M.Z.; Wang, X.Y.; Tian, X.L.; Ren, L.T.; Li, W.W.; Su, Q.Q. Downshifting luminescence amplification by multi-phase energy harvesting. Chem. Eng. J. 2023, 475, 146433. [Google Scholar] [CrossRef]
- Chen, X.; Jin, L.M.; Kong, W.; Sun, T.Y.; Zhang, W.F.; Liu, X.H.; Fan, J.; Yu, S.F.; Wang, F. Confining energy migration in upconversion nanoparticles towards deep ultraviolet lasing. Nat. Commun. 2016, 7, 10304. [Google Scholar] [CrossRef]
- Li, F.; Tu, L.P.; Zhang, Y.Q.; Huang, D.X.; Liu, X.X.; Zhang, X.R.; Du, J.R.; Fan, R.W.; Yang, C.H.; Kraemer, K.W.; et al. Size-dependent lanthanide energy transfer amplifies upconversion luminescence quantum yields. Nat. Photonics 2024, 18, 440–449. [Google Scholar] [CrossRef]
- Ortgies, D.H.; Tan, M.; Ximendes, E.C.; del Rosal, B.; Hu, J.; Xu, L.; Wang, X.; Rodriguez, E.M.; Jacinto, C.; Fernandez, N.; et al. Lifetime-Encoded Infrared-Emitting Nanoparticles for in Vivo Multiplexed Imaging. ACS Nano 2018, 12, 4362–4368. [Google Scholar] [CrossRef]
- Tan, M.L.; del Rosal, B.; Zhang, Y.Q.; Rodríguez, E.M.; Hu, J.; Zhou, Z.G.; Fan, R.W.; Ortgies, D.H.; Fernández, N.; Chaves-Coira, I.; et al. Rare-earth-doped fluoride nanoparticles with engineered long luminescence lifetime for time-gated in vivo optical imaging in the second biological window. Nanoscale 2018, 10, 17771–17780. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fan, Y.; Wang, R.; Li, X.M.; Fan, L.L.; Zhang, F. High-Capacity Upconversion Wavelength and Lifetime Binary Encoding for Multiplexed Biodetection. Angew. Chem. Int. Ed. 2018, 57, 12824–12829. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, P.Y.; Lu, Y.Q.; Wang, R.; Zhou, L.; Zheng, X.L.; Li, X.M.; Piper, J.A.; Zhang, F. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat. Nanotechnol. 2018, 13, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.Y.; Qin, X.; Huang, B.L.; Zan, J.; Wu, Q.X.; Hong, Z.Z.; Xie, L.L.; Bian, H.Y.; Yi, Z.G.; Chen, X.F.; et al. High-resolution X-ray luminescence extension imaging. Nature 2021, 590, 410–415. [Google Scholar] [CrossRef] [PubMed]

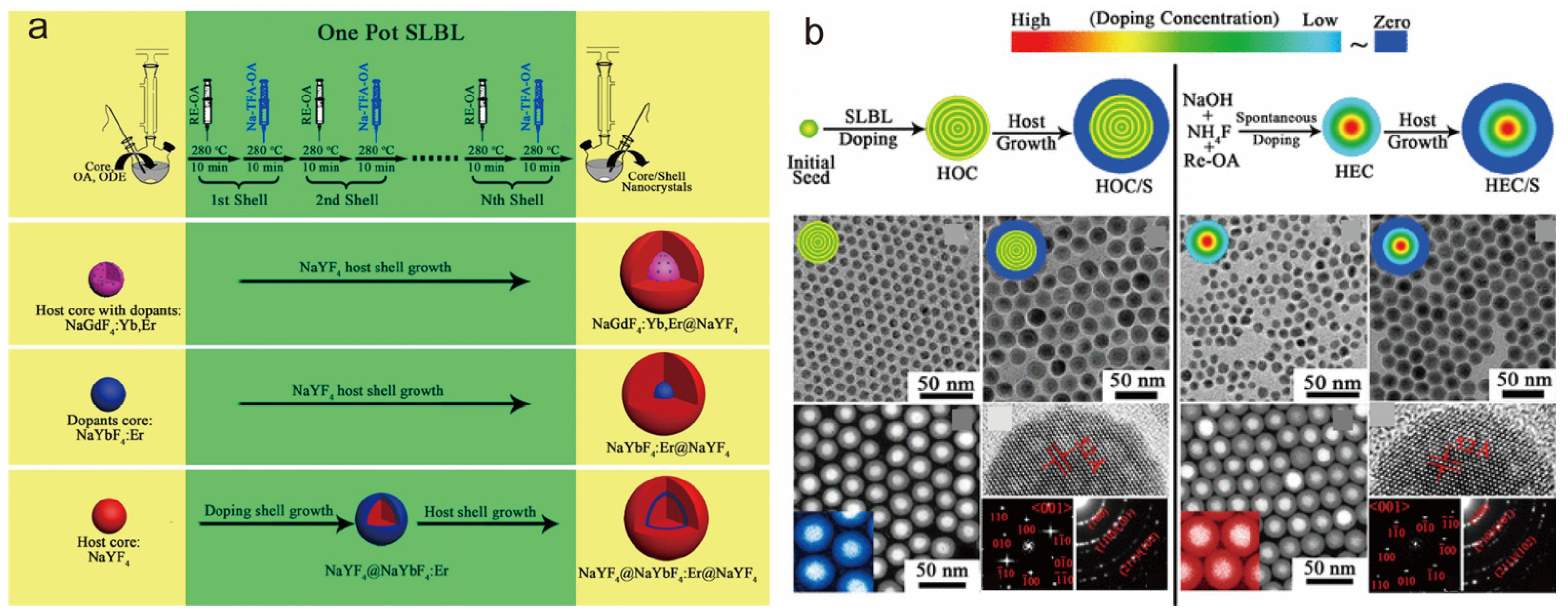


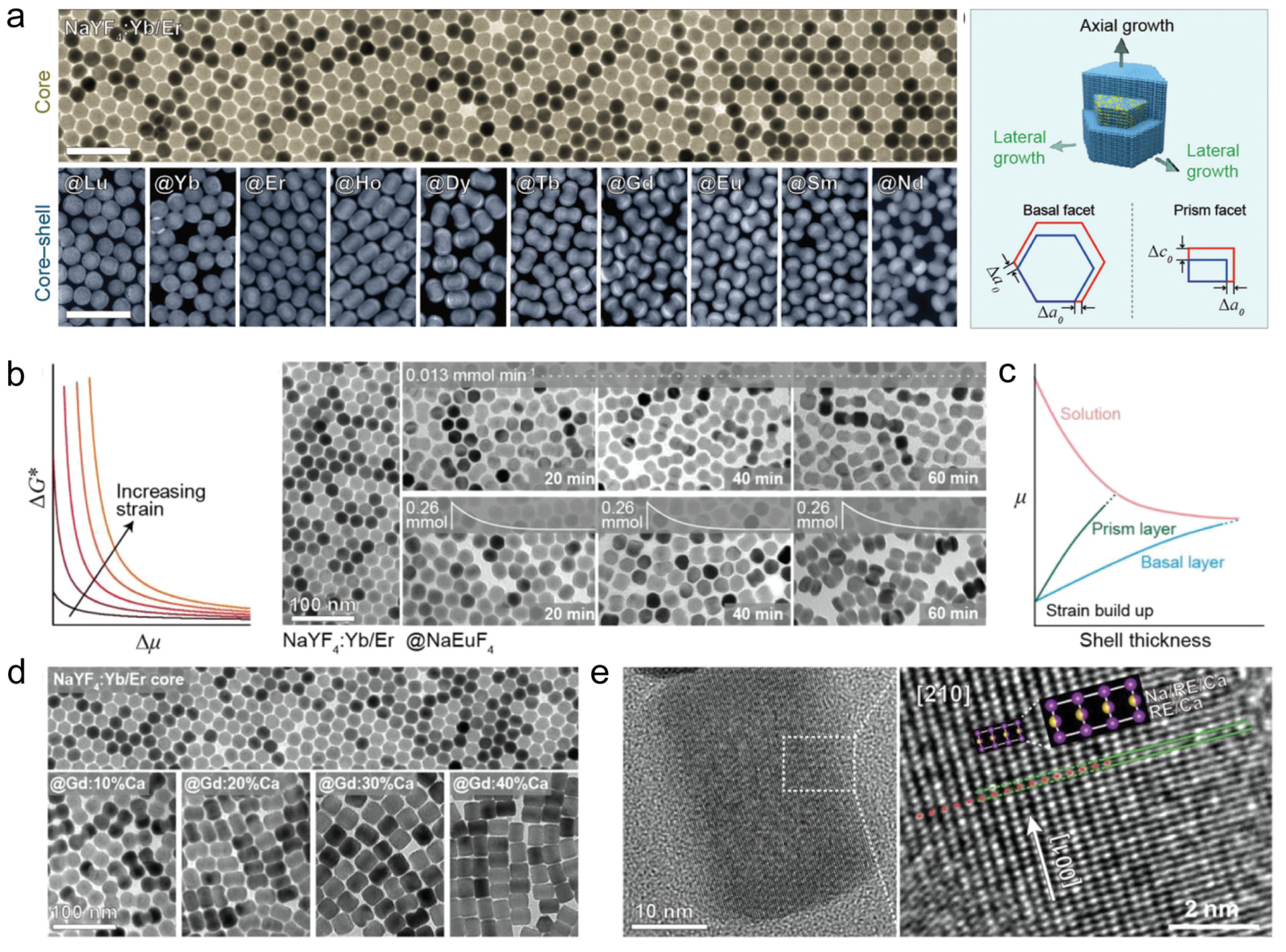
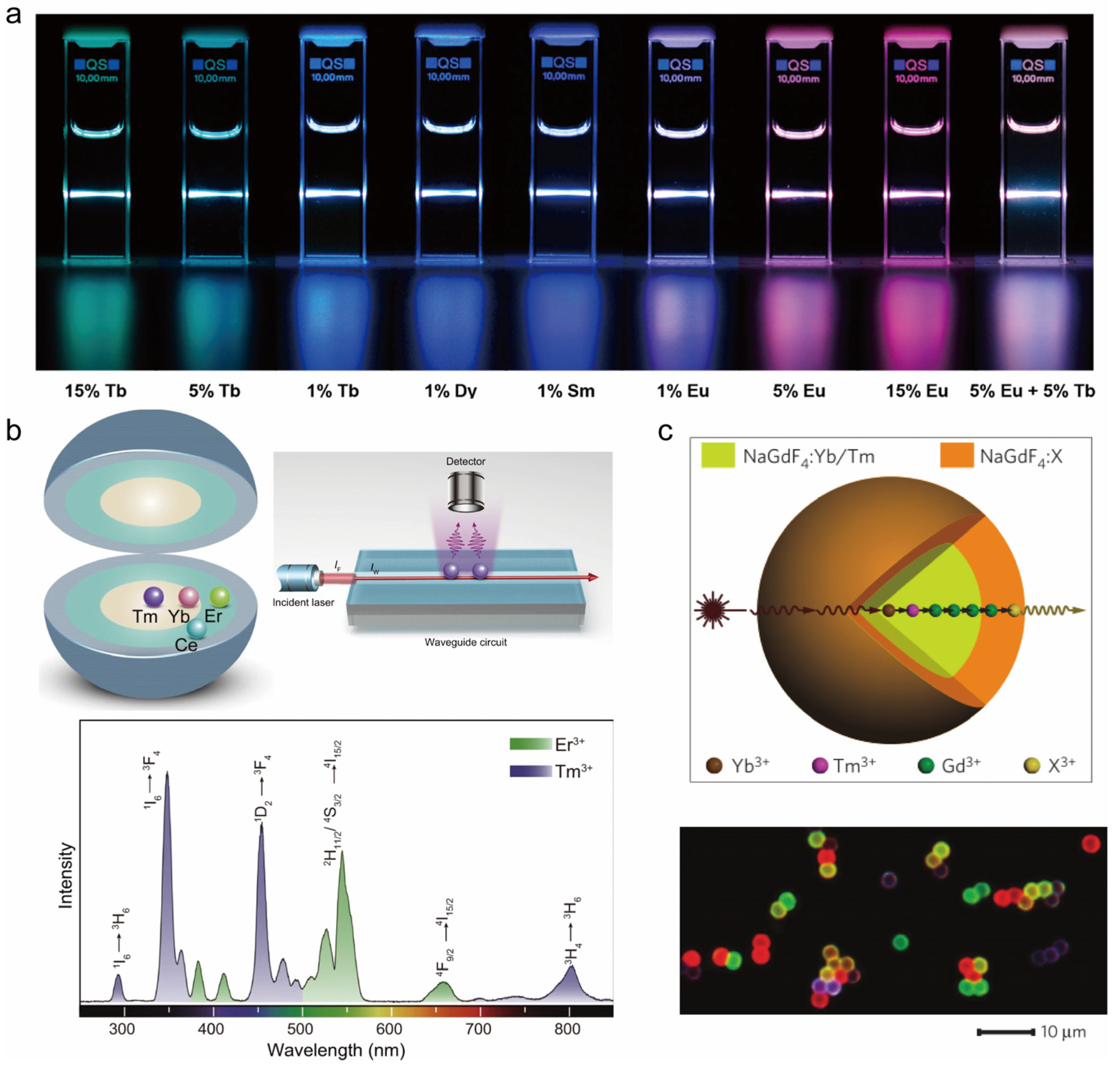

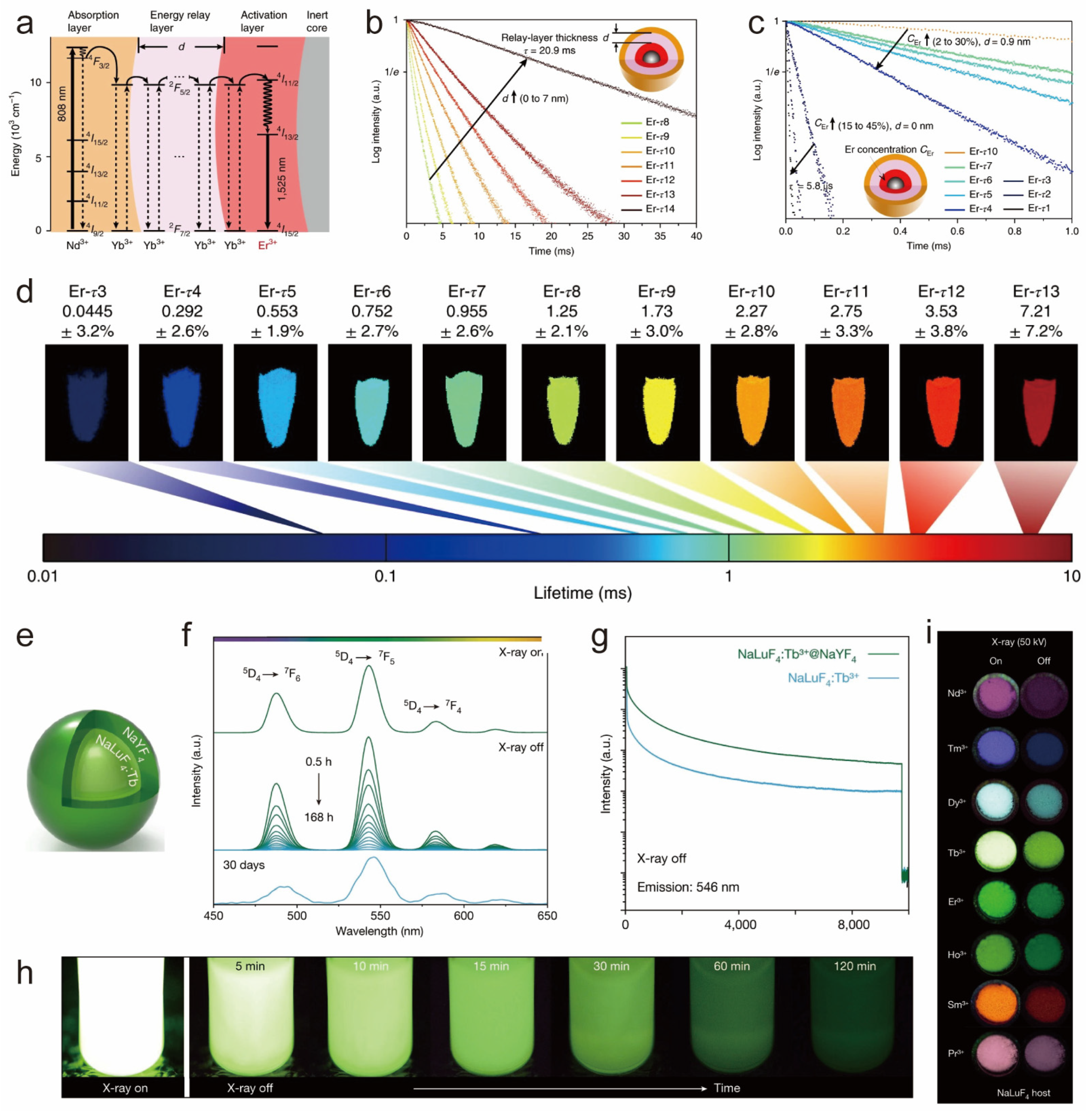
| Synthesis Method | Thermal Synthesis in Water (Solvent) | Co-Precipitation Method | Thermal Injection Method |
|---|---|---|---|
| Advantages | 1. Higher purity at lower temperatures. 2. Suitability for heat-sensitive compounds. | 1. Simple operation, low cost. 2. No high-end equipment required. | 1. Shell layer thickness can be precisely adjusted. |
| Disadvantages | 1. Closed system hampers real-time monitoring of morphological changes. | 1. More challenging in morphology control. 2. Product may contain more impurities. | 1. High technical requirements: precise control of the injection volume and reaction conditions are required. 2. Equipment for precise control of temperature and injection rate is required. |
| Key variables and effects on characteristics | 1. Temperature and pressure: dissolves insoluble materials; affects crystallization rate. 2. Solvent/ratio adjustments: control crystal growth direction, morphology, and phase. 3. Reaction time: determines phase transitions and product formation. | 1. Reaction temperature: influences crystal growth rate and crystallinity, crucial for upconversion efficiency. 2. Solvent composition: enables fine tuning of epitaxial growth dynamics of the shell. | 1. Injection volume: affects the thickness of the shell layer. 2. Reaction parameters: e.g., ratio of OA− to OAH, amount of precursor, regulates the direction and homogeneity of the shell layer growth. 3. Temperature: affects the phase structure and degree of crystallinity of the product. |
| Core–Shell Structural Design | Type of Activator | Emission Output | Application | Ref. | ||
|---|---|---|---|---|---|---|
| Altered reactive ion distribution | Homogeneous core–shell structure | NaYF4:Ln3+@ NaYF4 | Tb, Er, Dy, Ho, Eu, Nd | Green, Blue, Red, Yellow, NIR | Multi-optical storage | [89] |
| NaYF4:Nd3+/Er3+/Ho3+/Tm3+@NaYF4 | Er, Tm, Ho, Nd | NIR | Vascular resolution, tumor imaging | [90] | ||
| Heterogeneous core–shell structure | NaGdF4:Yb/Tm@NaGdF4:A@NaYF4 (A: active ion) | Tb, Dy, Sm, Eu, | Green, Blue, Red | / | [85] | |
| NaErF4:Ho(0.5 mol%)@NaYF4:Yb (10 mol%)@NaYF4 | Ho, Er | Green, Yellow, Red | Optical security, speed detection | [91] | ||
| NaGdF4: Yb/Tm/Er@ NaGdF4:Eu @NaYF4 | Tm, Er, Eu | Green, Cyan, White, Red | Displays | [92] | ||
| Gd2O2S:Ln3+@ NaYF4 | Tb, Eu, Sm, Dy, Tm, Er, Ho | Green, Red, Pink, Cyan | / | [93] | ||
| NaYF4: Yb/Tm@NaErF4: Ce@NaYF4 | Tm | UV | Toroid microcavity laser | [94] | ||
| Active core–active shell structure | NaGdF4: Yb/Tm@NaGdF4: Tb/Eu/Dy/Sm | Tb, Eu, Dy, Sm | Red, Yellow, Blue, Green | / | [88] | |
| NaYbF4:Tm/Gd@NaYF4:Tb@NaYF4:Eu | Tb, Eu | Red, Blue, | Information security | [95] | ||
| Shell thickness | NaErF4:Tm@ NaYF4@NaYbF4@NaYF4 | Er, Tm | Red, Green | Information encryption | [96] | |
| NaErF4@NaYF4 | Er | Red, Green, Orange | / | [97] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Tian, X.; Ren, L.; Su, Y.; Su, Q. Understanding of Lanthanide-Doped Core–Shell Structure at the Nanoscale Level. Nanomaterials 2024, 14, 1063. https://doi.org/10.3390/nano14121063
Zhao Q, Tian X, Ren L, Su Y, Su Q. Understanding of Lanthanide-Doped Core–Shell Structure at the Nanoscale Level. Nanomaterials. 2024; 14(12):1063. https://doi.org/10.3390/nano14121063
Chicago/Turabian StyleZhao, Qing, Xinle Tian, Langtao Ren, Yan Su, and Qianqian Su. 2024. "Understanding of Lanthanide-Doped Core–Shell Structure at the Nanoscale Level" Nanomaterials 14, no. 12: 1063. https://doi.org/10.3390/nano14121063






