Magnetic Barium Hexaferrite Nanoparticles with Tunable Coercivity as Potential Magnetic Heating Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Particle Synthesis
2.2. Particle Characterization
2.2.1. Vibrating Sample Magnetometry (VSM)
2.2.2. X-ray Diffraction (XRD)
2.2.3. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Variation of Synthesis Parameters
3.1.1. Phase Composition
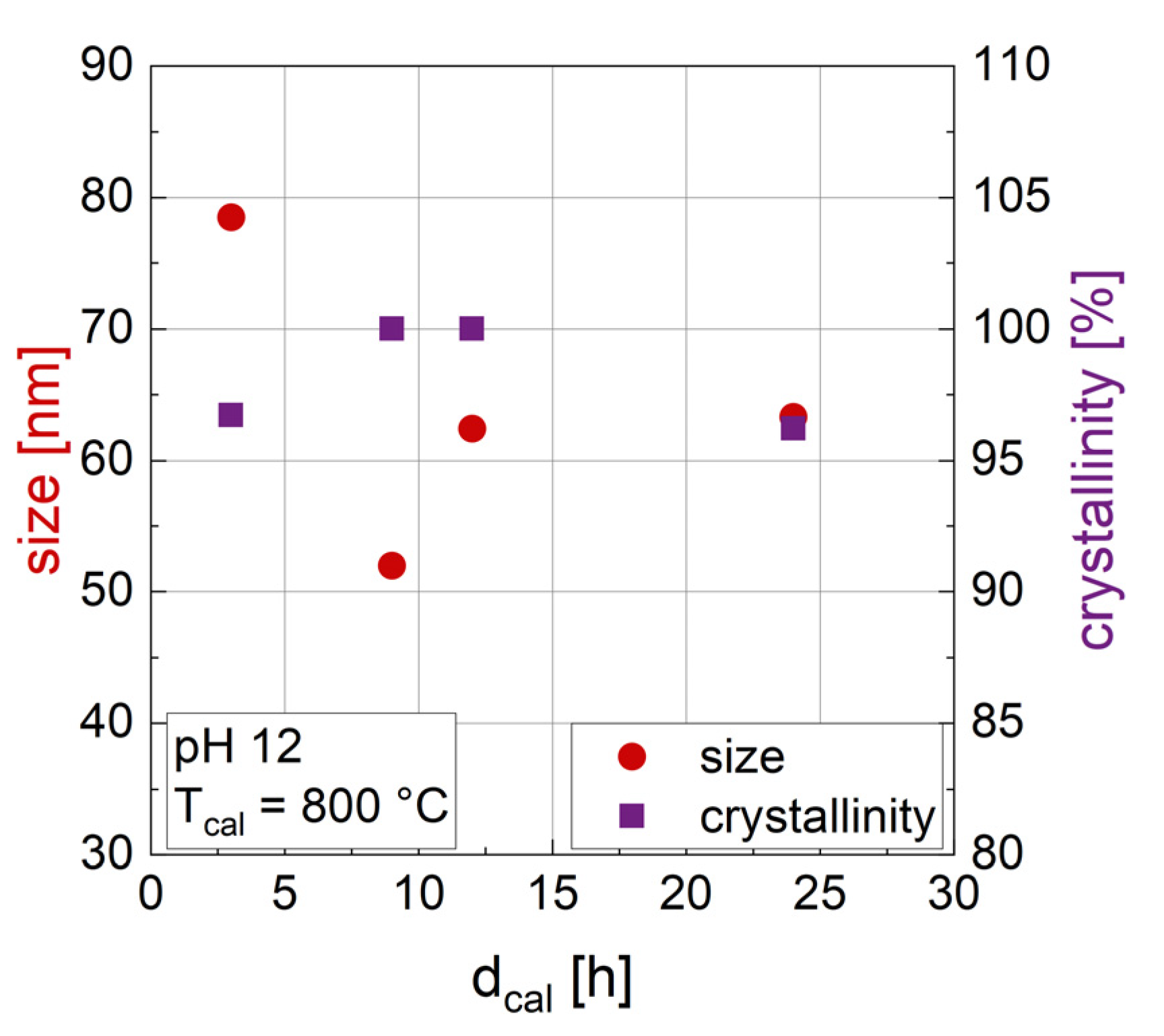
3.1.2. Influence of the Duration of Calcination dcal
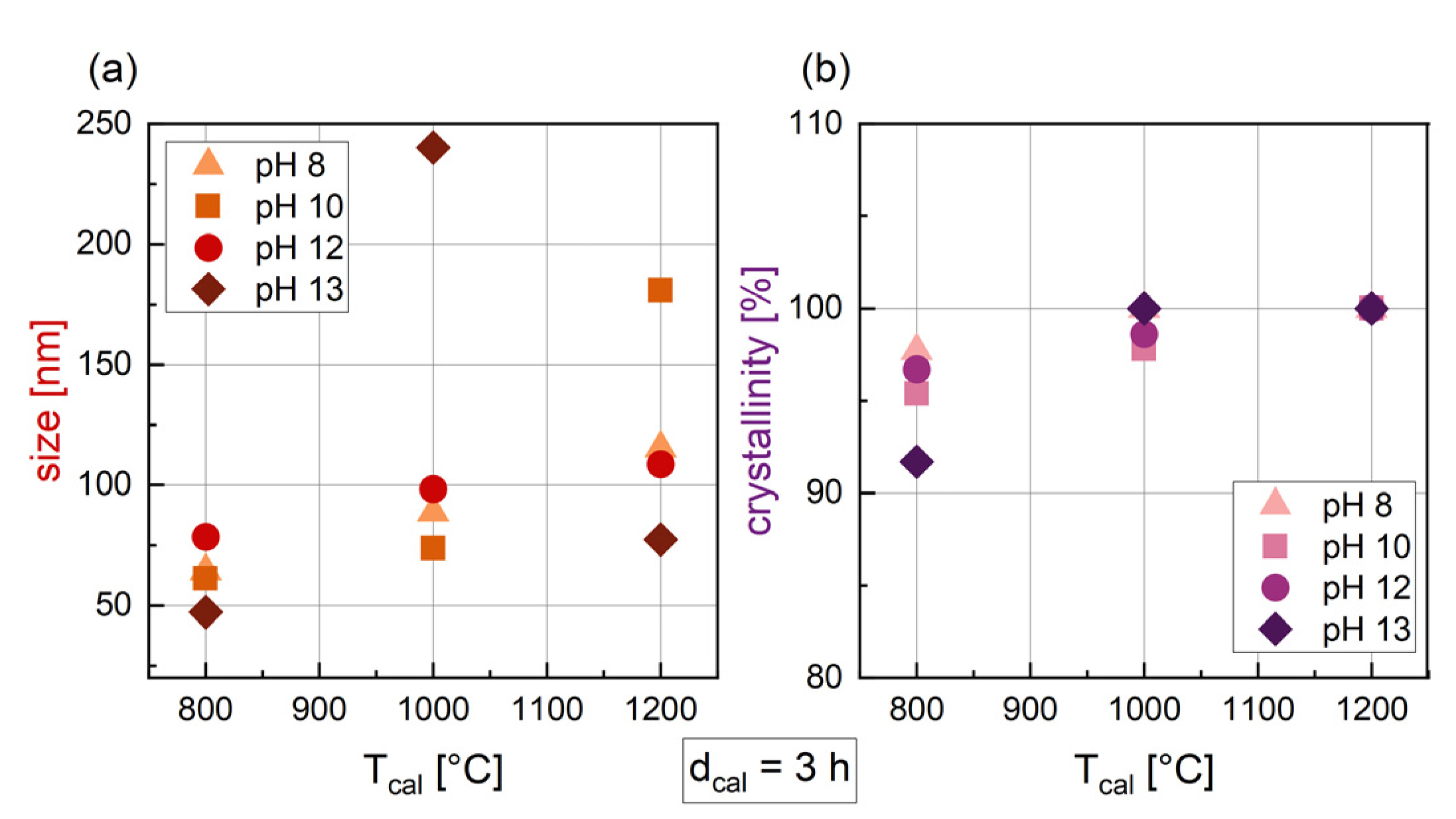
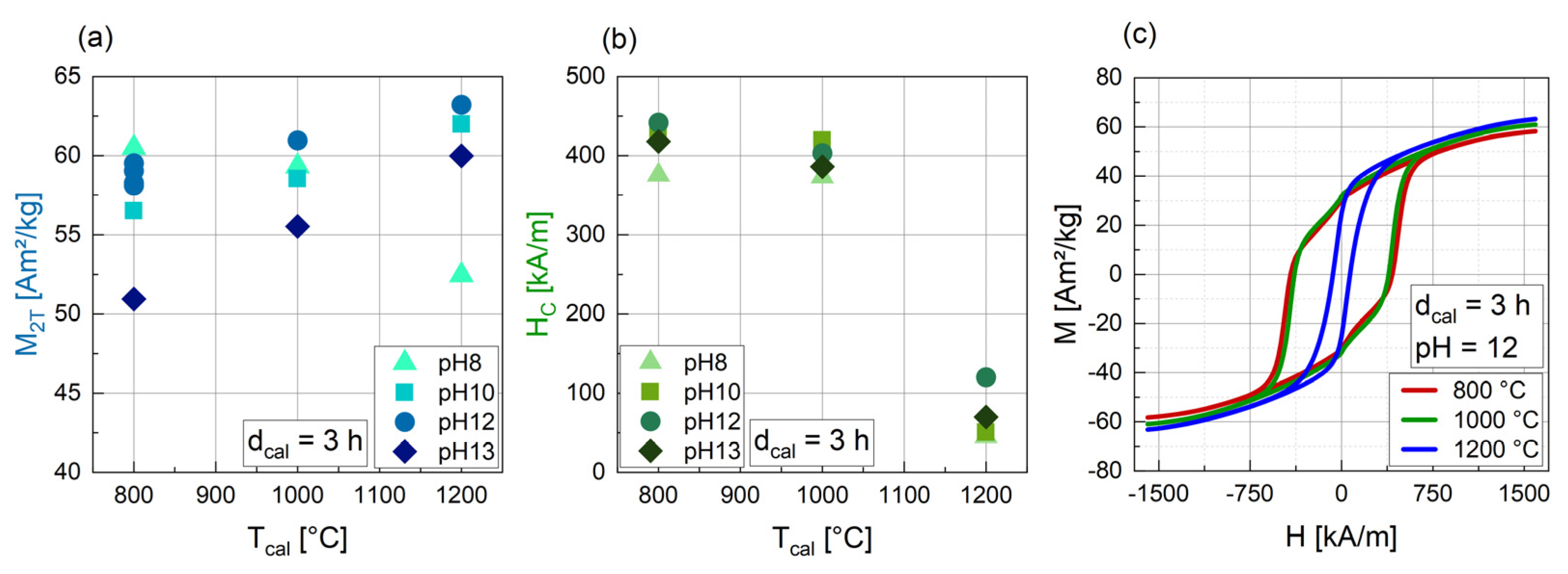
3.1.3. Influence of the Temperature of Calcination (Tcal)
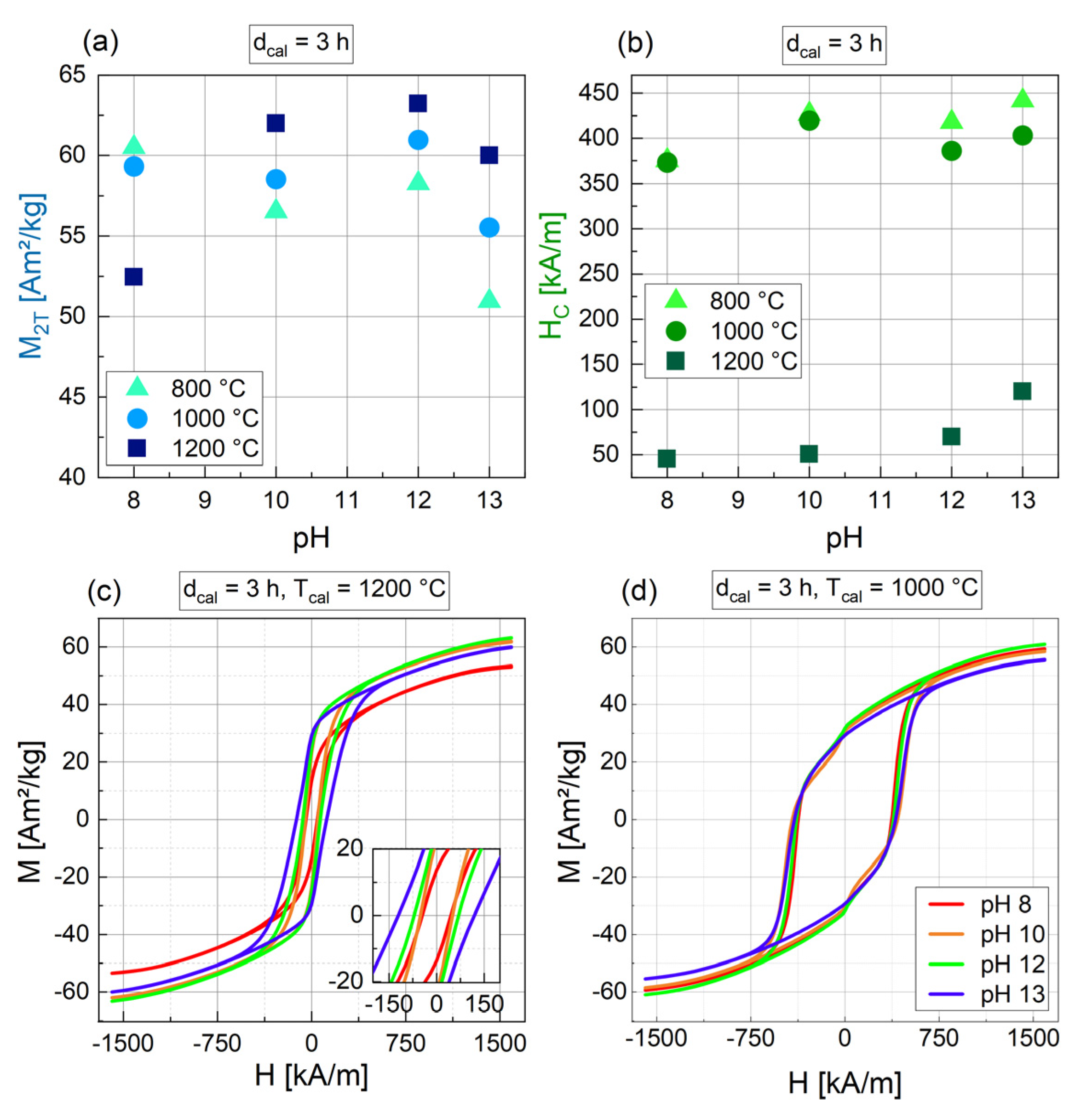
3.1.4. Influence of the pH
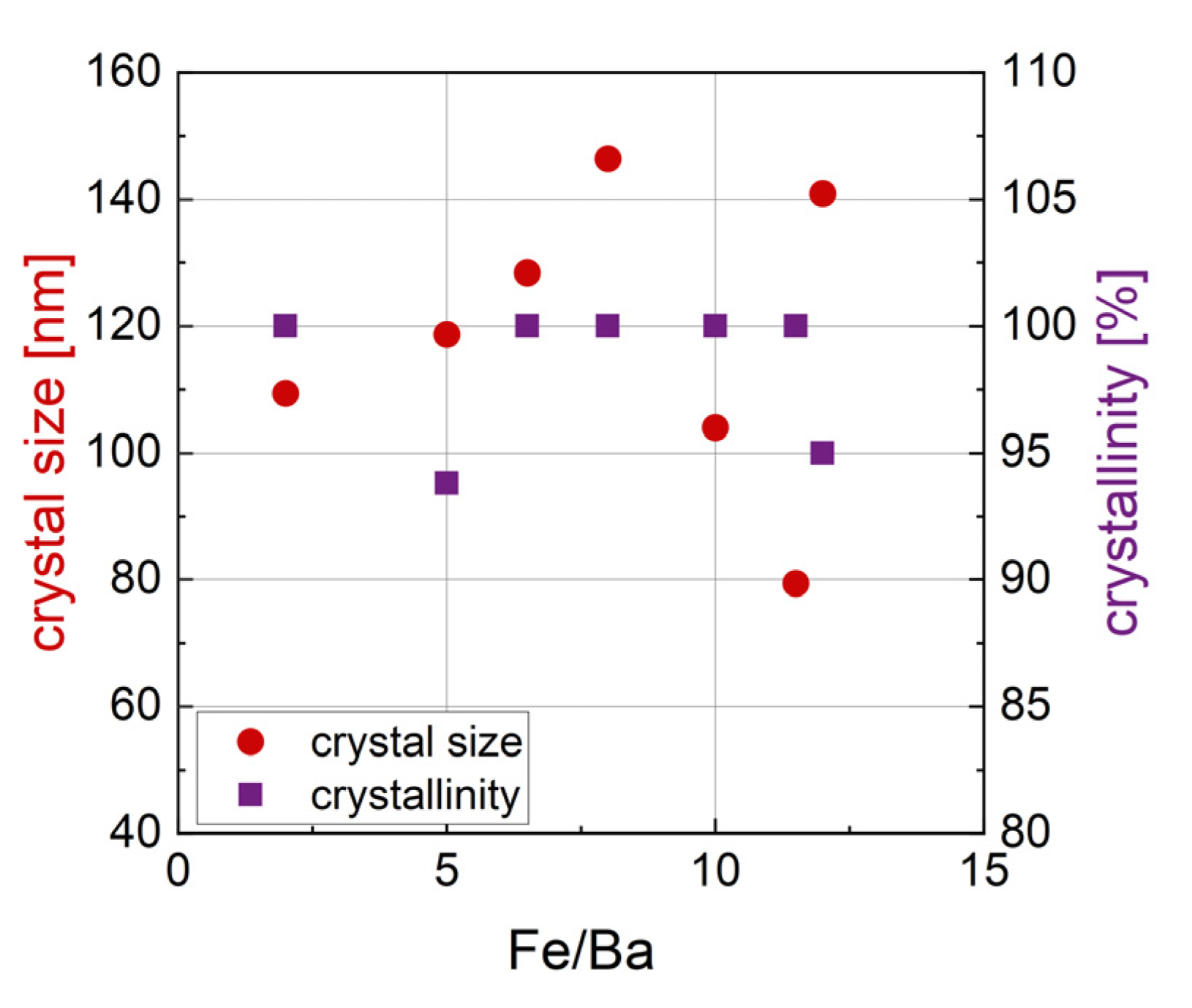
3.1.5. Relation of Synthesis Parameters and Crystal Size
3.2. Variation of Fe/Ba Ratio (r)
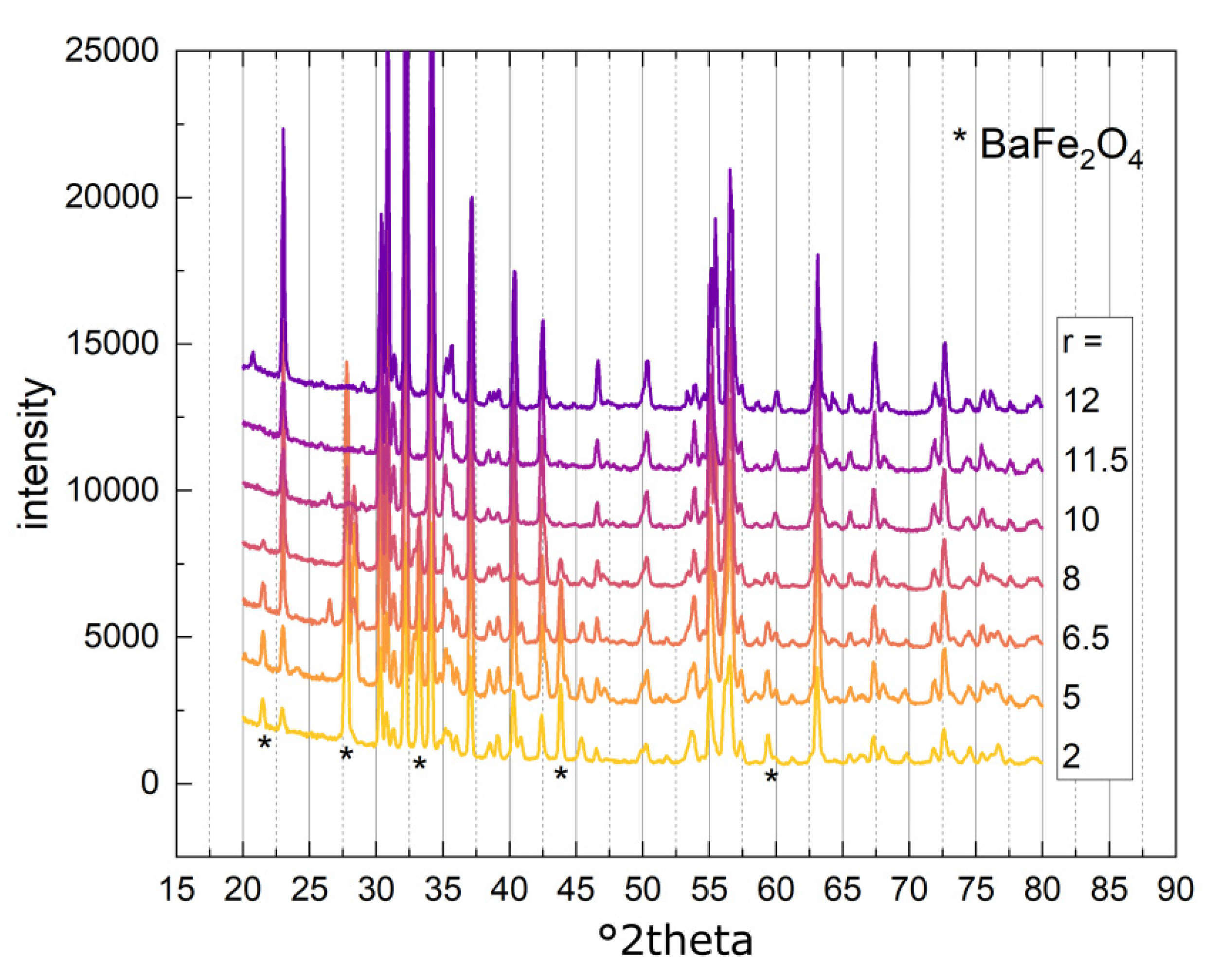
3.2.1. Phase Composition
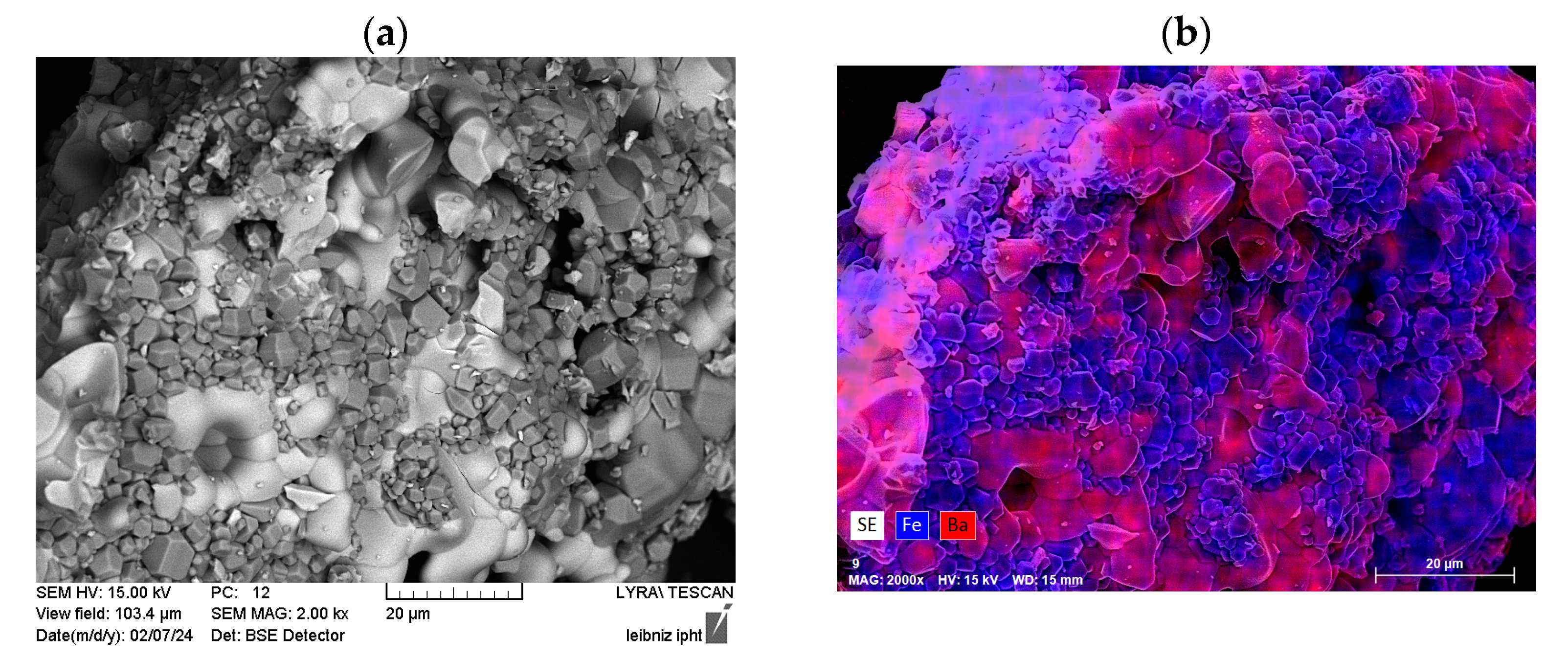
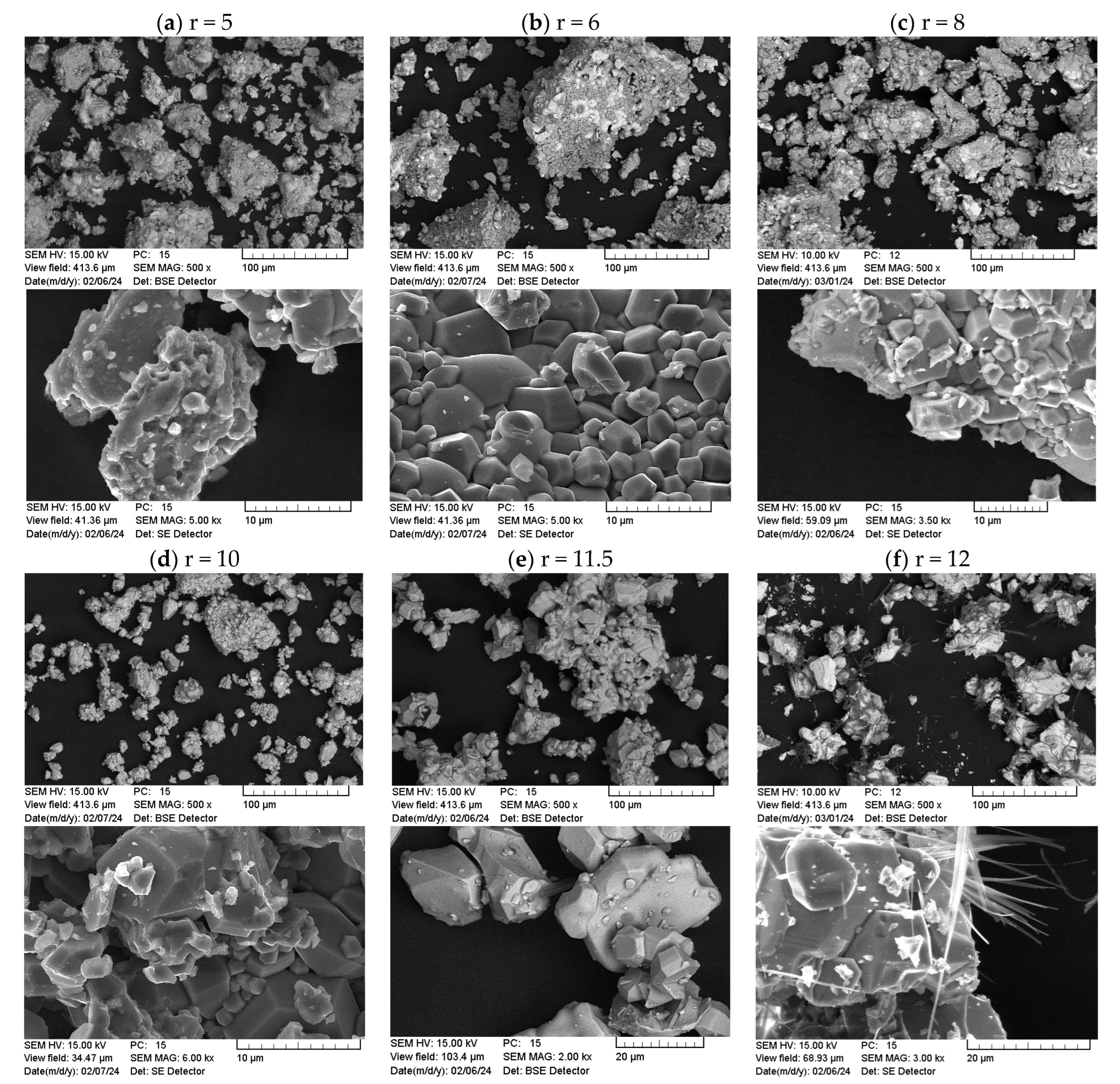
3.2.2. Morphology and Structural Characterization
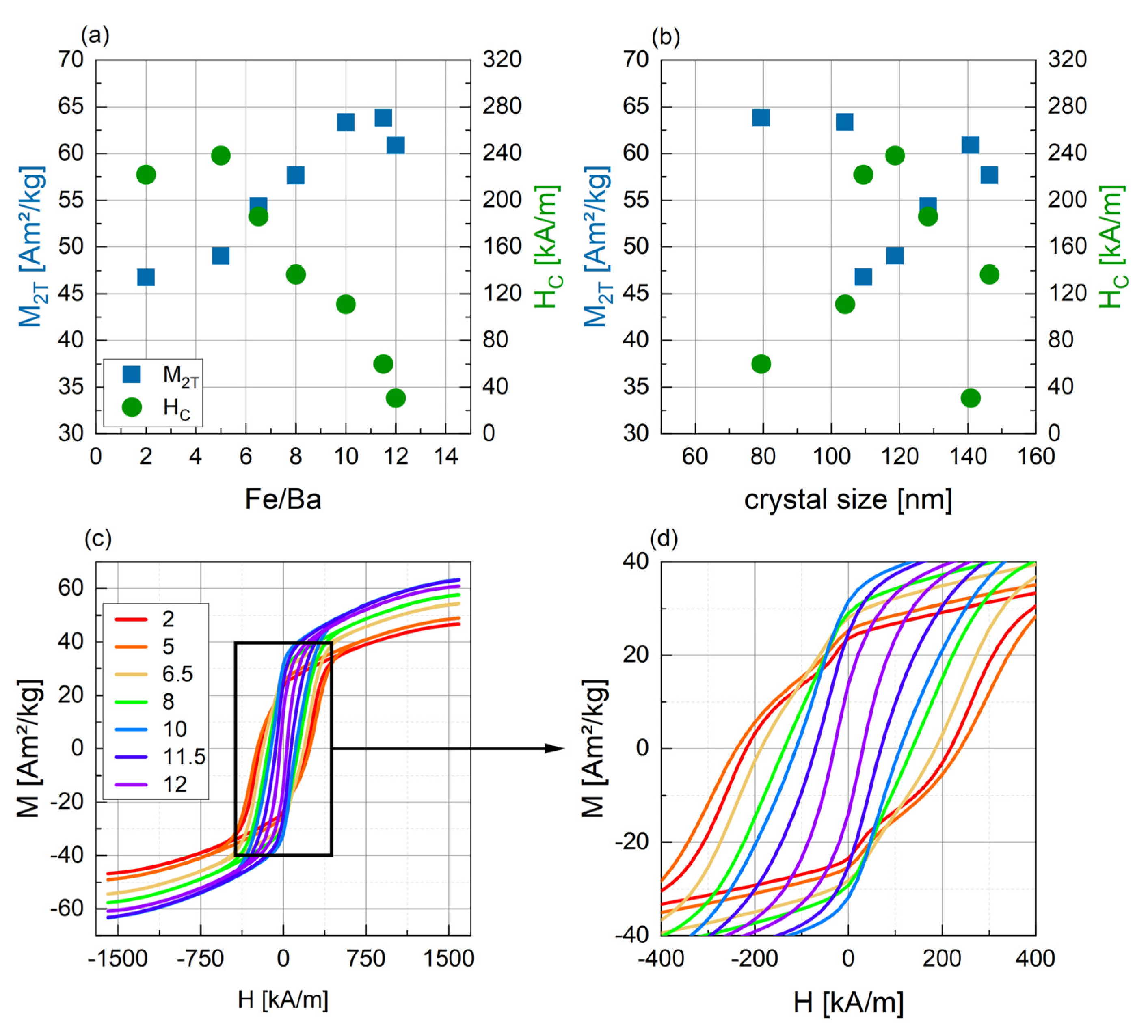
3.2.3. Magnetic Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, H.F.; Capistrano, G.; Bakuzis, A.F. In vivo magnetic nanoparticle hyperthermia: A review on preclinical studies, low-field nano-heaters, noninvasive thermometry and computer simulations for treatment planning. Int. J. Hyperth. 2020, 37, 76–99. [Google Scholar] [CrossRef]
- Hensley, D.; Tay, Z.W.; Dhavalikar, R.; Zheng, B.; Goodwill, P.; Rinaldi, C.; Conolly, S. Combining magnetic particle imaging and magnetic fluid hyperthermia in a theranostic platform. Phys. Med. Biol. 2017, 62, 3483–3500. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
- Müller, R.; Dutz, S.; Neeb, A.; Cato, A.C.B.; Zeisberger, M. Magnetic heating effect of nanoparticles with different sizes and size distributions. J. Magn. Magn. Mater. 2013, 328, 80–85. [Google Scholar] [CrossRef]
- Schmidt, A.M. Electromagnetic Activation of Shape Memory Polymer Networks Containing Magnetic Nanoparticles. Macromol. Rapid Commun. 2006, 27, 1168–1172. [Google Scholar] [CrossRef]
- Mohr, R.; Kratz, K.; Weigel, T.; Lucka-Gabor, M.; Moneke, M.; Lendlein, A. Initiation of shape-memory effect by inductive heating of magnetic nanoparticles in thermoplastic polymers. Proc. Natl. Acad. Sci. USA 2006, 103, 3540–3545. [Google Scholar] [CrossRef]
- Noh, S.H.; Na, W.; Jang, J.T.; Lee, J.H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.S.; Cheon, J. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.L.; Lv, Y.B.; Herng, T.S.; Xu, X.H.; Xia, W.X.; Zhang, T.S.; Fang, J.; Xiao, W.; Ding, J. Orientation Mediated Enhancement on Magnetic Hyperthermia of Fe3O4 Nanodisc. Adv. Funct. Mater. 2015, 25, 812–820. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Martinez, L.; Khurshid, H.; Garaio, E.; Garcia, J.; Phan, M.; Srikanth, H. Enhanced magnetic hyperthermia in iron oxide nano-octopods: Size and anisotropy effects. J. Phys. Chem. C 2016, 120, 8370–8379. [Google Scholar] [CrossRef]
- Zahn, D.; Landers, J.; Buchwald, J.; Diegel, M.; Salamon, S.; Müller, R.; Köhler, M.; Ecke, G.; Wende, H.; Dutz, S. Ferrimagnetic Large Single Domain Iron Oxide Nanoparticles for Hyperthermia Applications. Nanomaterials 2022, 12, 343. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Wilhelm, C.; Siaugue, J.M.; Horner, O.; Bacri, J.C.; Gazeau, F. Magnetically induced hyperthermia: Size-dependent heating power of gamma-Fe2O3 nanoparticles. J. Phys. Condens. Matter 2008, 20, 204133. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kawashita, M.; Araki, N.; Mitsumori, M.; Hiraoka, M. Preparation of Size-Controlled Magnetite Nanoparticles for Hyperthermia of Cancer. Trans. Mater. Res. Soc. Jpn. 2009, 34, 77–80. [Google Scholar] [CrossRef]
- Zahn, D.; Landers, J.; Diegel, M.; Salamon, S.; Stihl, A.; Schacher, F.H.; Wende, H.; Dellith, J.; Dutz, S. Optimization of Magnetic Cobalt Ferrite Nanoparticles for Magnetic Heating Applications in Biomedical Technology. Nanomaterials 2023, 13, 1673. [Google Scholar] [CrossRef] [PubMed]
- Cedeño-Mattei, Y.; Perales-Pérez, O.; Uwakweh, O.N.C. Synthesis of high-coercivity non-stoichiometric cobalt ferrite nanocrystals: Structural and magnetic characterization. Mater. Chem. Phys. 2012, 132, 999–1006. [Google Scholar] [CrossRef]
- Babukutty, B.; Kalarikkal, N.; Nair, S.S. Studies on structural, optical and magnetic properties of cobalt substituted magnetite fluids (CoxFe1−xFe2O4). Mater. Res. Express 2017, 4, 035906. [Google Scholar] [CrossRef]
- Kumar, P.; Pathak, S.; Singh, A.; Kuldeep; Khanduri, H.; Wang, X.; Basheed, G.A.; Pant, R.P. Optimization of cobalt concentration for improved magnetic characteristics and stability of CoxFe3−xO4 mixed ferrite nanomagnetic fluids. Mater. Chem. Phys. 2021, 265, 124476. [Google Scholar] [CrossRef]
- Fang, H.; Yang, Z.; Ong, C.; Li, Y.; Wang, C. Preparation and magnetic properties of (Zn–Sn) substituted barium hexaferrite nanoparticles for magnetic recording. J. Magn. Magn. Mater. 1998, 187, 129–135. [Google Scholar] [CrossRef]
- de Julian Fernandez, C.; Sangregorio, C.; de la Figuera, J.; Belec, B.; Makovec, D.; Quesada, A. Topical Review: Progress and Prospects of Hard Hexaferrites for Permanent Magnet Applications. J. Phys. D Appl. Phys. 2020, 54, 153001. [Google Scholar] [CrossRef]
- Zhong, W.; Ding, W.; Zhang, N.; Hong, J.; Yan, Q.; Du, Y. Key step in synthesis of ultrafine BaFe12O19 by sol-gel technique. J. Magn. Magn. Mater. 1997, 168, 196–202. [Google Scholar] [CrossRef]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Drofenik, M.; Ban, I.; Makovec, D.; Žnidaršič, A.; Jagličić, Z.; Hanžel, D.; Lisjak, D. The hydrothermal synthesis of super-paramagnetic barium hexaferrite particles. Mater. Chem. Phys. 2011, 127, 415–419. [Google Scholar] [CrossRef]
- Torabi, Z.; Arab, A.; Ghanbari, F. Structural, Magnetic and Microwave Absorption Properties of Hydrothermally Synthesized (Gd, Mn, Co) Substituted Ba-Hexaferrite Nanoparticles. J. Electron. Mater. 2017, 47, 1259–1270. [Google Scholar] [CrossRef]
- Sürig, C.; Hempel, K.A.; Bonnenberg, D. Formation and microwave absorption of barium and strontium ferrite prepared by sol-gel technique. Appl. Phys. Lett. 1993, 63, 2836–2838. [Google Scholar] [CrossRef]
- Shen, S.-Y.; Zheng, H.; Zheng, P.; Wu, Q.; Deng, J.-X.; Ying, Z.-H.; Zheng, L. Microstructure, magnetic properties of hexagonal barium ferrite powder based on calcination temperature and holding time. Rare Met. 2018, 40, 981–986. [Google Scholar] [CrossRef]
- Xu, P.; Han, X.; Wang, M. Synthesis and magnetic properties of BaFe12O19 hexaferrite nanoparticles by a reverse microemulsion technique. J. Phys. Chem. C 2007, 111, 5866–5870. [Google Scholar] [CrossRef]
- Roos, W. Formation of chemically coprecipitated barium ferrite. J. Am. Ceram. Soc. 1980, 63, 601–603. [Google Scholar] [CrossRef]
- Lisjak, D.; Drofenik, M. The low-temperature formation of barium hexaferrites. J. Eur. Ceram. Soc. 2006, 26, 3681–3686. [Google Scholar] [CrossRef]
- Pashkova, E.V.; Solovyova, E.D.; Kotenko, I.E.; Kolodiazhnyi, T.V.; Belous, A.G. Effect of preparation conditions on fractal structure and phase transformations in the synthesis of nanoscale M-type barium hexaferrite. J. Magn. Magn. Mater. 2011, 323, 2497–2503. [Google Scholar] [CrossRef]
- Haneda, K.; Miyakawa, C.; Kojima, H. Preparation of High-Coercivity BaFe12O19. J. Am. Ceram. Soc. 2006, 57, 354–357. [Google Scholar] [CrossRef]
- Radwan, M.; Rashad, M.M.; Hessien, M.M. Synthesis and characterization of barium hexaferrite nanoparticles. J. Mater. Process. Technol. 2007, 181, 106–109. [Google Scholar] [CrossRef]
- Du, Y.; Gao, H.; Liu, X.; Wang, J.; Xu, P.; Han, X. Solvent-free synthesis of hexagonal barium ferrite (BaFe12O19) particles. J. Mater. Sci. 2010, 45, 2442–2448. [Google Scholar] [CrossRef]
- Ataie, A.; Heshmati-Manesh, S.; Kazempour, H. Synthesis of barium hexaferrite by the co-precipitation method using acetate precursor. J. Mater. Sci. 2002, 37, 2125–2128. [Google Scholar] [CrossRef]
- Dudziak, S.; Ryzynska, Z.; Bielan, Z.; Ryl, J.; Klimczuk, T.; Zielinska-Jurek, A. Pseudo-superparamagnetic behaviour of barium hexaferrite particles. RSC Adv. 2020, 10, 18784–18796. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, A.; Jin, C. Synthesis, structure and magnetic properties of hexagonal BaFe12O19 ferrite obtained via a hydrothermal method. J. Mater. Sci. Mater. Electron. 2016, 27, 10864–10868. [Google Scholar] [CrossRef]
- Lisjak, D.; Drofenik, M. The influence of the coprecipitation conditions on the low-temperature formation of barium hexaferrite. J. Mater. Sci. 2007, 42, 8606–8612. [Google Scholar] [CrossRef]
- Janasi, S.; Emura, M.; Landgraf, F.; Rodrigues, D. The effects of synthesis variables on the magnetic properties of coprecipitated barium ferrite powders. J. Magn. Magn. Mater. 2002, 238, 168–172. [Google Scholar] [CrossRef]
- Ogasawara, T.; Oliveira, M. Microstructure and hysteresis curves of the barium hexaferrite from co-precipitation by organic agent. J. Magn. Magn. Mater. 2000, 217, 147–154. [Google Scholar] [CrossRef]
- Matutes-Aquino, J.; Dıaz-Castanón, S.; Mirabal-Garcıa, M.; Palomares-Sanchez, S. Synthesis by coprecipitation and study of barium hexaferrite powders. Scr. Mater. 2000, 42, 295–299. [Google Scholar] [CrossRef]
- Yensano, R.; Phokha, S. Effect of pH on single phase BaFe12O19 nanoparticles and their improved magnetic properties. J. Mater. Sci. Mater. Electron. 2020, 31, 11764–11773. [Google Scholar] [CrossRef]
- Hu, S.L.; Liu, J.; Yu, H.Y.; Liu, Z.W. Synthesis and properties of barium ferrite nano-powders by chemical co-precipitation method. J. Magn. Magn. Mater. 2019, 473, 79–84. [Google Scholar] [CrossRef]
- Kwak, J.-Y.; Lee, C.-S.; Kim, D.; Kim, Y.-I. Characteristics of Barium Hexaferrite Nanoparticles Prepared by Temperature-Controlled Chemical Coprecipitation. J. Korean Chem. Soc. 2012, 56, 609–616. [Google Scholar] [CrossRef]
- Rashad, M.M.; Ibrahim, I.A. Improvement of the magnetic properties of barium hexaferrite nanopowders using modified co-precipitation method. J. Magn. Magn. Mater. 2011, 323, 2158–2164. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Gan, L.-M.; Ng, S.-C. Improving the magnetic properties of hydrothermally synthesized barium ferrite. J. Magn. Magn. Mater. 1999, 195, 452–459. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The highscore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Toraya, H. A new method for quantitative phase analysis using X-ray powder diffraction: Direct derivation of weight fractions from observed integrated intensities and chemical compositions of individual phases. J. Appl. Crystallogr. 2016, 49, 1508–1516. [Google Scholar] [CrossRef]
- Rösler, M.; Görnert, P.; Sinn, E. Structural and magnetic properties of Ba-ferrite fine particles grown by glass crystallization. Z. Phys. D At. Mol. Clust. 1991, 19, 279–281. [Google Scholar] [CrossRef]
- Palla, B.J.; Shah, D.O.; Garcia-Casillas, P.; Matutes-Aquino, J. Preparation of Nanoparticles of Barium Ferrite from Precipitation in Microemulsions. J. Nanopart. Res. 1999, 1, 215–221. [Google Scholar] [CrossRef]
- Gjørup, F.H.; Saura-Múzquiz, M.; Ahlburg, J.V.; Andersen, H.L.; Christensen, M. Coercivity enhancement of strontium hexaferrite nano-crystallites through morphology controlled annealing. Materialia 2018, 4, 203–210. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods at different acquisition geometries for determination of crystallite size in nano-scale materials. Mater. Charact. 2013, 85, 111–123. [Google Scholar] [CrossRef]
- Marzban, S.; Khorrami, S.A. pH and Properties of Synthesized Barium Hexa-Ferrite by Co-precipitation Method. Int. J. Bio-Inorg. Hybrid Nanomater. 2013, 2, 491–494. [Google Scholar]
- Hsiang, H.I.; Yen, F.S. Effect of crystallite size on the ferroelectric domain growth of ultrafine BaTiO3 powders. J. Am. Ceram. Soc. 1996, 79, 1053–1060. [Google Scholar] [CrossRef]
- Pesch, J.A. Direct Correlation between Domain Size and Coercive Force in Iron Alloys. J. Appl. Phys. 1966, 37, 2627–2630. [Google Scholar] [CrossRef]
- Singh, V.P.; Jasrotia, R.; Kumar, R.; Raizada, P.; Thakur, S.; Batoo, K.M.; Singh, M. A Current Review on the Synthesis and Magnetic Properties of M-Type Hexaferrites Material. World J. Condens. Matter Phys. 2018, 08, 36–61. [Google Scholar] [CrossRef]
- Peymanfar, R.; Rahmanisaghieh, M. Preparation of neat and capped BaFe2O4 nanoparticles and investigation of morphology, magnetic, and polarization effects on its microwave and optical performance. Mater. Res. Express 2018, 5, 105012. [Google Scholar] [CrossRef]
- Jaafar, A.; Arekat, S.; Al-Saie, A.; Bououdina, M. Strucutre and magnetic properties of nanosized BaFe2O4 material. Int. J. Nanosci. 2011, 09, 575–577. [Google Scholar] [CrossRef]
- Dilip, R.; Ravikumar, K.; Chandekar, K.V.; Shkir, M.; Krishnan, V.G.; Shinde, K.S.; Ashraf, I.M. Enhancement of magnetic and dielectrics performance of BaFe2O4 nanoparticles influenced by tri-sodium citrate as surfactant. J. Mater. Sci. Mater. Electron. 2022, 33, 23841–23850. [Google Scholar] [CrossRef]
- Dimri, M.C.; Khanduri, H.; Agarwal, P.; Pahapill, J.; Stern, R. Structural, magnetic, microwave permittivity and permeability studies of barium monoferrite (BaFe2O4). J. Magn. Magn. Mater. 2019, 486, 165278. [Google Scholar] [CrossRef]




| Sample | dcal | Tcal | pH |
|---|---|---|---|
| [h] | [°C] | ||
| 3_800_8 | 3 | 800 | 8 |
| 3_1000_8 | 3 | 1000 | 8 |
| 3_1200_8 | 3 | 1200 | 8 |
| 3_800_10 | 3 | 800 | 10 |
| 3_1000_10 | 3 | 1000 | 10 |
| 3_1200_10 | 3 | 1200 | 10 |
| 3_800_12 | 3 | 800 | 12 |
| 3_1000_12 | 3 | 1000 | 12 |
| 3_1200_12 | 3 | 1200 | 12 |
| 9_800_12 | 9 | 800 | 12 |
| 12_800_12 | 12 | 800 | 12 |
| 24_800_12 | 24 | 800 | 12 |
| 3_800_13 | 3 | 800 | 13 |
| 3_1000_13 | 3 | 1000 | 13 |
| 3_1200_13 | 3 | 1200 | 13 |
| dcal [h] | Tcal [°C] | pH | Phase 1 [wt%] | Phase 2 [wt%] | Phase 3 [wt%] | Size [nm] | Crystal-Linity [%] | M2T [Am2/kg] | HC [kA/m] |
|---|---|---|---|---|---|---|---|---|---|
| 3 | 800 | 8 | BaFe12O19 (96.8) | α-Fe2O3 (3.2) | 64.3 | 97.7 | 60.5 | 375.6 | |
| 3 | 1000 | 8 | BaFe12O19 (95.9) | α-Fe2O3 (4.1) | 88.8 | 100 | 59.3 | 373.3 | |
| 3 | 1200 | 8 | BaFe12O19 (87.1) | α-Fe2O3 (10.5) | Ba(OH)2 (2.4) | 115.3 | 100 | 52.5 | 45.4 |
| 3 | 800 | 10 | BaFe12O19 (100) | 61.2 | 95.4 | 56.5 | 427.0 | ||
| 3 | 1000 | 10 | BaFe12O19 (100) | 73.8 | 97.8 | 58.5 | 419.6 | ||
| 3 | 1200 | 10 | BaFe12O19 (100) | 180.8 | 100 | 62.0 | 50.6 | ||
| 3 | 800 | 12 | BaFe12O19 (94.6) | BaFe18O27 (5.4) | 78.5 | 96.7 | 58.3 | 418.0 | |
| 3 | 1000 | 12 | BaFe12O19 (100) | 98.4 | 98.6 | 61.0 | 386.0 | ||
| 3 | 1200 | 12 | BaFe12O19 (99.0) | BaFe2O4 (1.0) | 108.7 | 100 | 63.2 | 70.0 | |
| 9 | 800 | 12 | BaFe12O19 (93.7) | α-Fe2O3 (6.3) | 52 | 100 | 58.1 | 408.1 | |
| 12 | 800 | 12 | BaFe12O19 (99.7) | BaFe2O4 (0.3) | 62.9 | 100 | 59.5 | 408.6 | |
| 24 | 800 | 12 | BaFe12O19 (100) | 63.3 | 96.2 | 59.0 | 400.6 | ||
| 3 | 800 | 13 | BaFe12O19 (99.3) | FeNaO2 (0.7) | 47.3 | 91.7 | 51.0 | 441.7 | |
| 3 | 1000 | 13 | BaFe12O19 (100) | 240.1 | 100 | 55.5 | 403.2 | ||
| 3 | 1200 | 13 | BaFe12O19 (100) | 77.4 | 100 | 60.0 | 120.1 |
| r = Fe/Ba | Phase 1 [wt%] | Phase 2 [wt%] | Phase 3 [wt%] | Size [nm] | Crystal- Linity [%] | M2T [Am2/kg] | HC [kA/m] |
|---|---|---|---|---|---|---|---|
| 2 | BaFe12O19 (80.1) | BaFe2O4 (19.9) | 109.4 | 100 | 46.8 | 221.9 | |
| 5 | BaFe12O19 (72.5) | BaFe2O4 (25.5) | Fe21.4O32 (2.0) | 118.7 | 93.8 | 49.0 | 238.4 |
| 6.5 | BaFe12O19 (89.0) | BaFe2O4 (11.0) | 128.4 | 100 | 54.4 | 186.2 | |
| 8 | BaFe12O19 (94.6) | BaFe2O4 (5.4) | 146.4 | 100 | 57.7 | 136.6 | |
| 10 | BaFe12O19 (93.0) | BaFeO2.67 (0.6) | BaFeO2.89 (6.4) | 104.0 | 100 | 63.3 | 111.1 |
| 11.5 | BaFe12O19 (100) | 79.4 | 100 | 63.8 | 60.03 | ||
| 12 | BaFe12O19 (99.5) | Fe12Na4O20 (0.5) | 140.9 | 95 | 60.8 | 30.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahn, D.; Diegel, M.; Valitova, A.; Dellith, J.; Dutz, S. Magnetic Barium Hexaferrite Nanoparticles with Tunable Coercivity as Potential Magnetic Heating Agents. Nanomaterials 2024, 14, 992. https://doi.org/10.3390/nano14120992
Zahn D, Diegel M, Valitova A, Dellith J, Dutz S. Magnetic Barium Hexaferrite Nanoparticles with Tunable Coercivity as Potential Magnetic Heating Agents. Nanomaterials. 2024; 14(12):992. https://doi.org/10.3390/nano14120992
Chicago/Turabian StyleZahn, Diana, Marco Diegel, Alina Valitova, Jan Dellith, and Silvio Dutz. 2024. "Magnetic Barium Hexaferrite Nanoparticles with Tunable Coercivity as Potential Magnetic Heating Agents" Nanomaterials 14, no. 12: 992. https://doi.org/10.3390/nano14120992






