Biomass-Derived Carbon Utilization for Electrochemical Energy Enhancement in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Method of Nitrogen-Doped Carbon-Coated LFP
2.2. Electrochemical Measurements
2.3. Material Characterizations
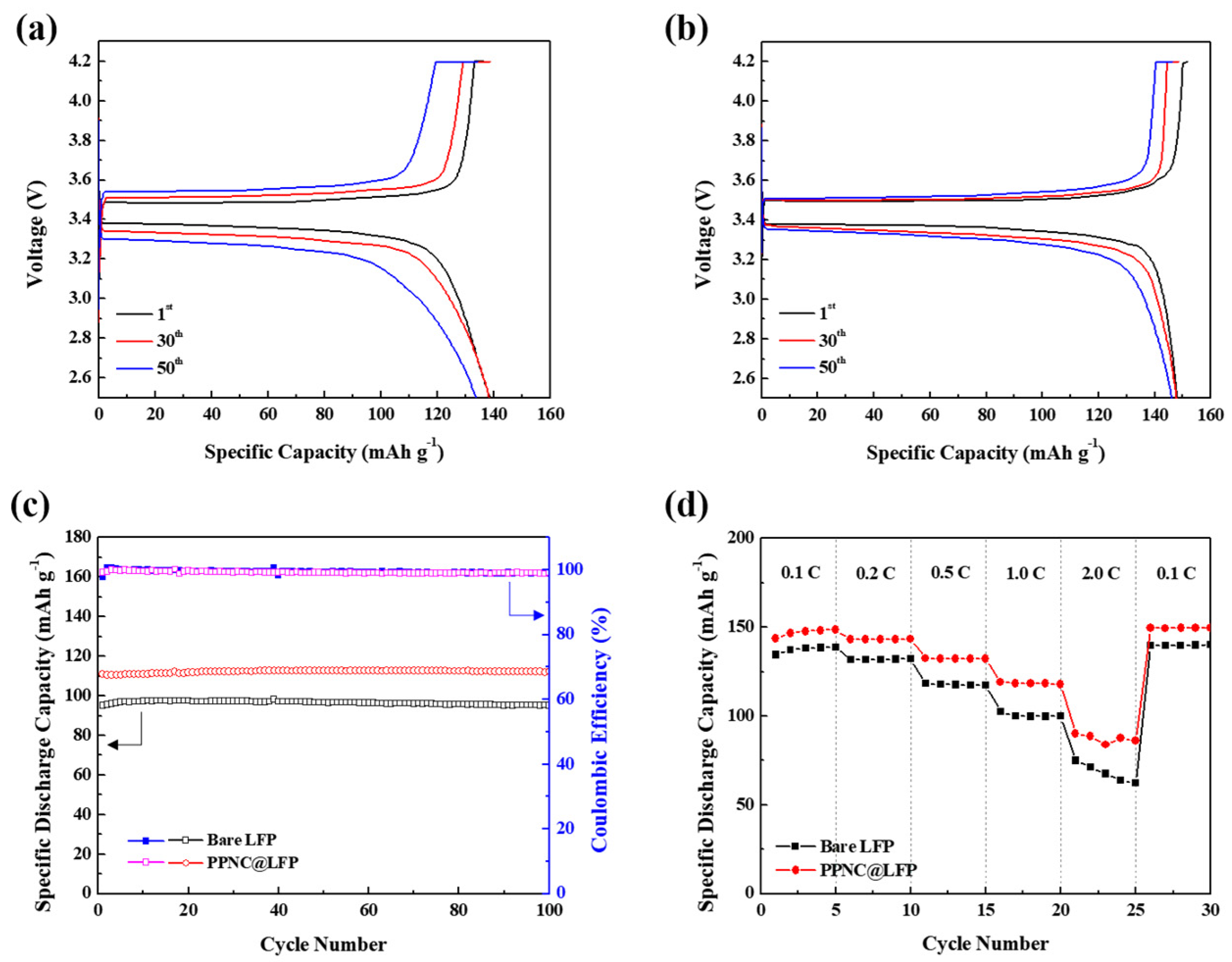
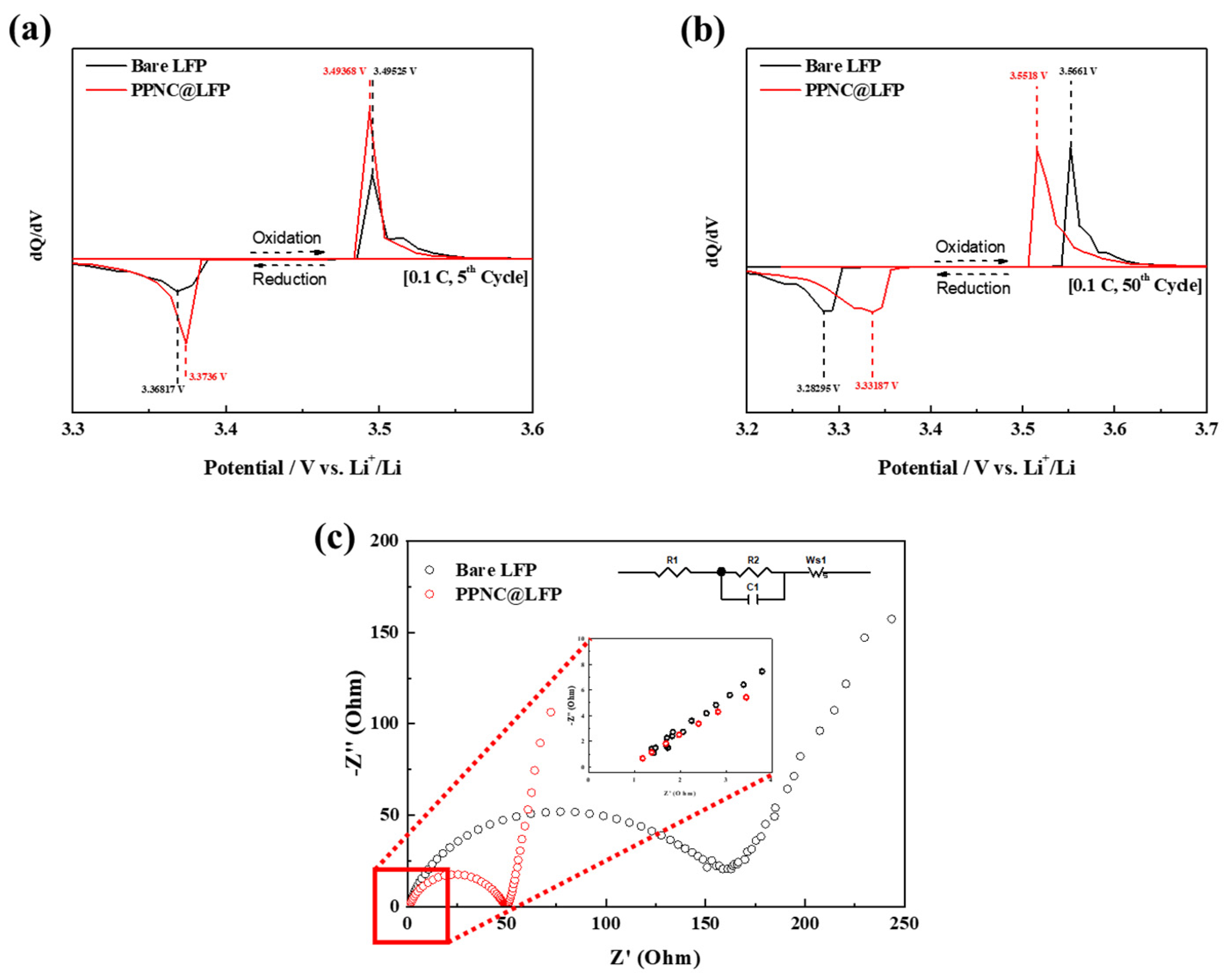
3. Results
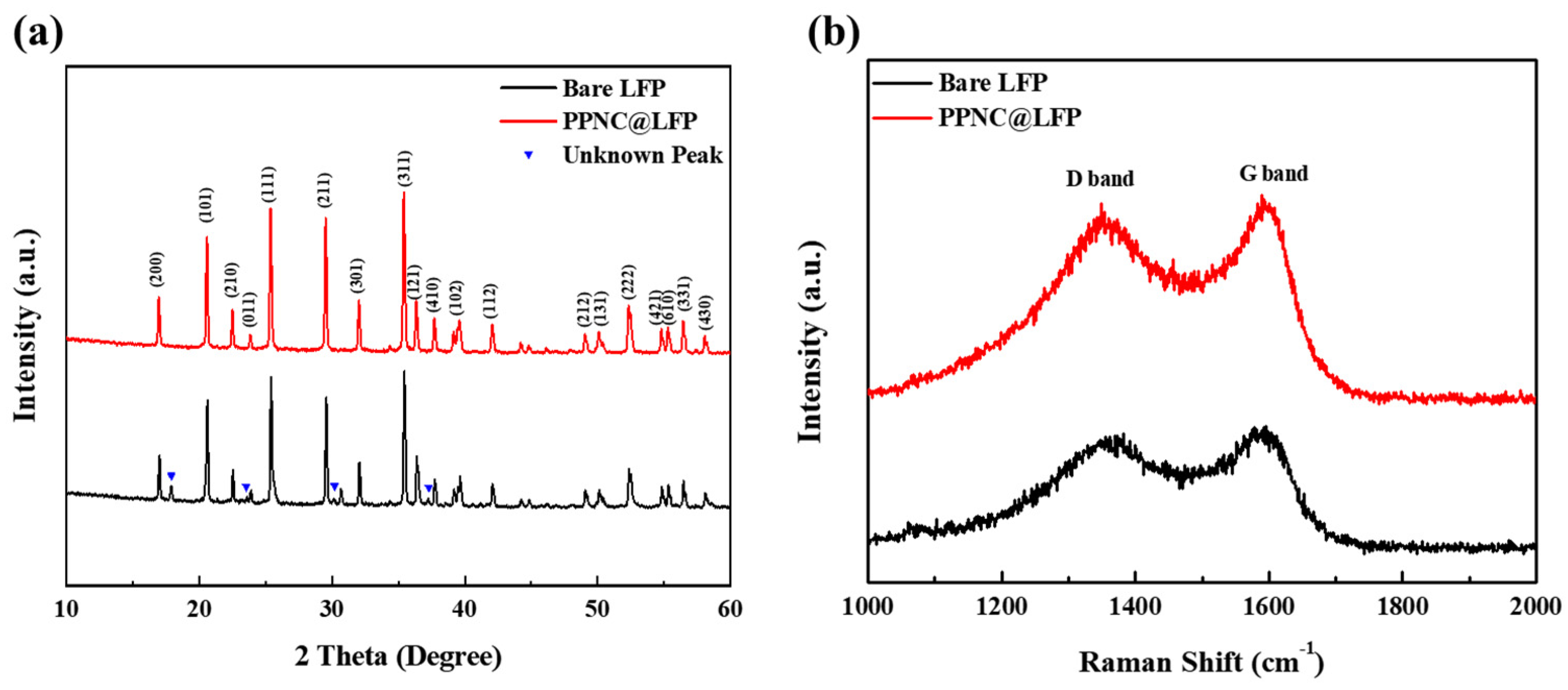
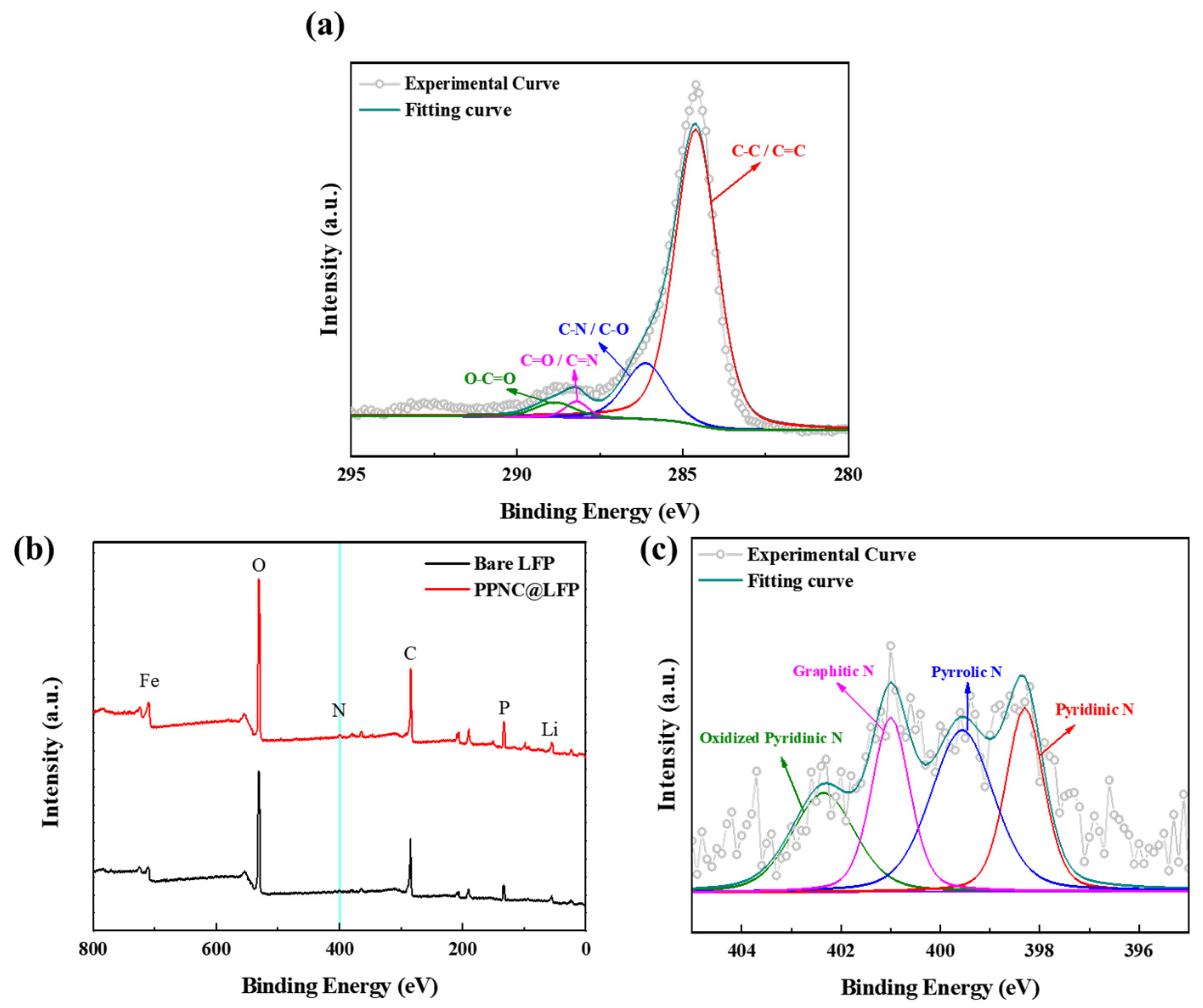
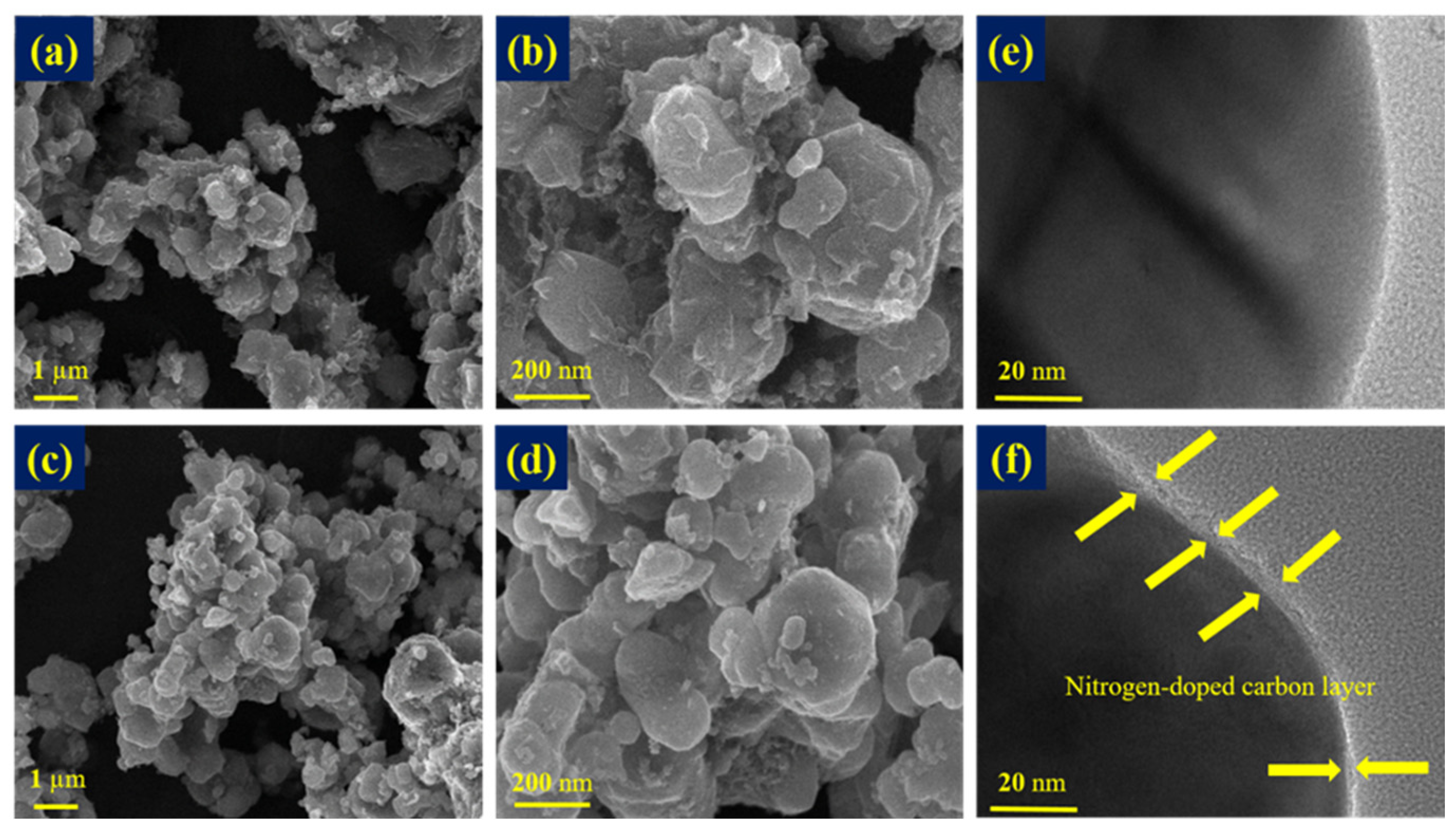
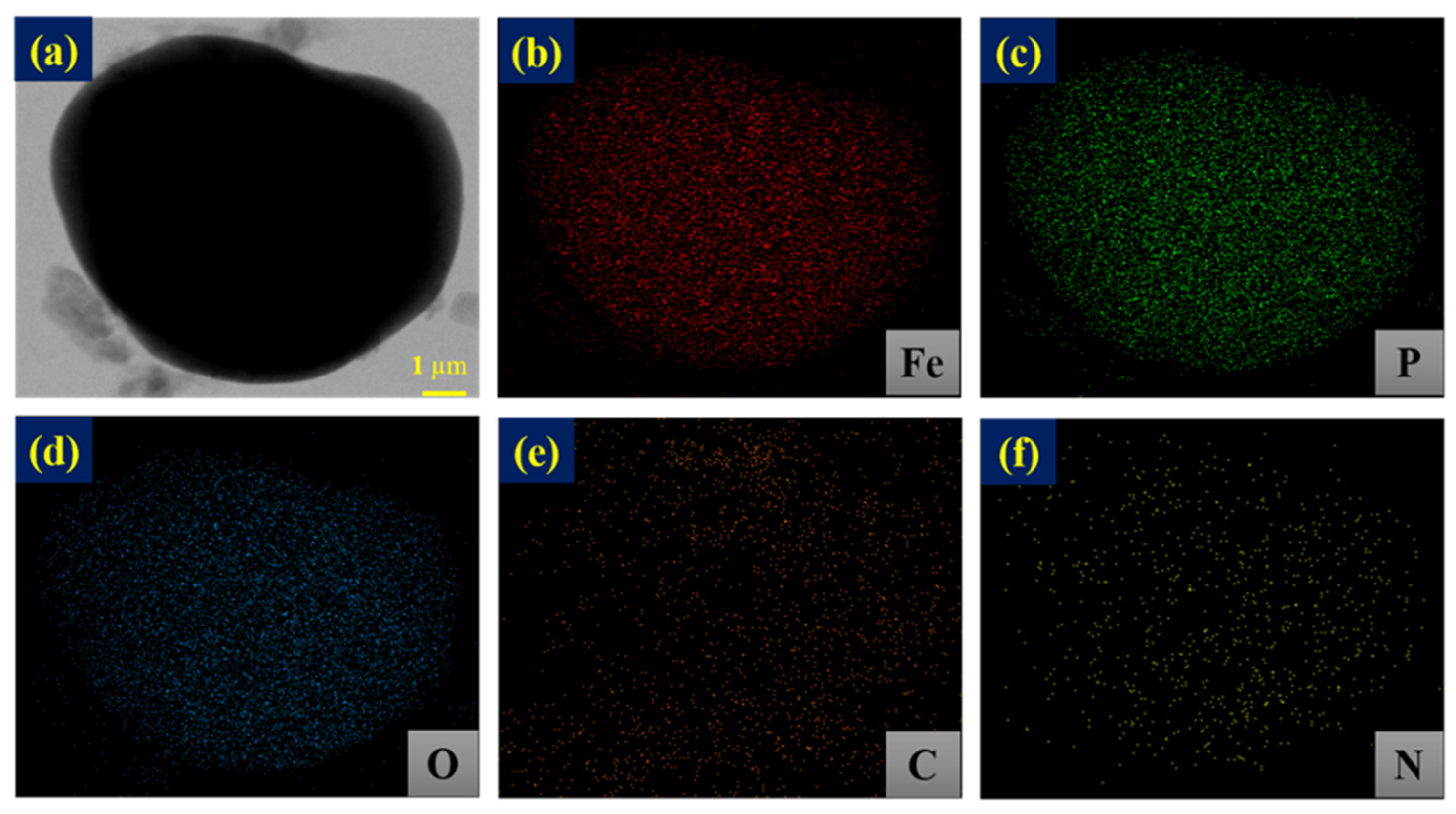
3.1. Physical and Chemical Characterizations
3.2. Electrochemical Performances
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Croguennec, L.; Palacin, M.R. Recent Achievements on Inorganic Electrode Materials for Lithium-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 3140–3156. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Bloom, I.; Jones, S.A.; Battaglia, V.S.; Henriksen, G.L.; Christophersen, J.P.; Wright, R.B.; Ho, C.D.; Belt, J.R.; Motloch, C.G. Effect of cathode composition on capacity fade, impedance rise and power fade in high-power, lithium-ion cells. J. Power Sources 2003, 124, 538–550. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Sim, G.S.; Shaji, N.; Santhoshkumar, P.; Park, J.W.; Ho, C.W.; Nanthagopal, M.; Kim, H.K.; Lee, C.W. Silkworm Protein-Derived Nitrogen-Doped Carbon-Coated Li[Ni0.8Co0.15Al0.05]O2 for Lithium-Ion Batteries. Nanomaterials 2022, 12, 1166. [Google Scholar] [CrossRef]
- Nanthagopal, M.; Santhoshkumar, P.; Shaji, N.; Praveen, S.; Kang, H.S.; Senthil, C.; Lee, C.W. Nitrogen-doped carbon-coated Li[Ni0.8Co0.1Mn0.1]O2 cathode material for enhanced lithium-ion storage. Appl. Surf. Sci. 2019, 492, 871–878. [Google Scholar] [CrossRef]
- Thackeray, M.M. Manganese oxides for lithium batteries. Prog. Solid State Chem. 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Xin, Y.-M.; Xu, H.-Y.; Ruan, J.-H.; Li, D.-C.; Wang, A.-G.; Sun, D.-S. A Review on Application of LiFePO4 based composites as electrode materials for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2021, 16, 210655. [Google Scholar] [CrossRef]
- Wang, Y.; He, P.; Zhou, H. Olivine LiFePO4: Development and future. Energy Environ. Sci. 2010, 4, 805–817. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X. Olivine LiFePO4: The remaining challenges for future energy storage. Energy Environ. Sci. 2015, 8, 1110–1138. [Google Scholar] [CrossRef]
- Zhang, W.-J. Structure and performance of LiFePO4 cathode materials: A review. J. Power Sources 2011, 196, 2962–2970. [Google Scholar] [CrossRef]
- Chen, S.-P.; Lv, D.; Chen, J.; Zhang, Y.-H.; Shi, F.-N. Review on Defects and Modification Methods of LiFePO4 Cathode Material for Lithium-Ion Batteries. Energy Fuels 2022, 36, 1232–1251. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, Z.; Zhang, S.; Liu, J.; Zhong, S. A review of the Doping Modification of LiFePO4 as a Cathode Material for Lithium Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 12041–12067. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, G.; Li, Z.; Sheng, W.; Zhang, Y.; Gu, J.; Zheng, X.; Cao, F. Improved Electrochemical Performance of LiFePO4@N-Doped Carbon Nanocomposites Using Polybenzoxazine as Nitrogen and Carbon Sources. ACS Appl. Mater. Interfaces 2016, 8, 26908–26915. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Q.; Guo, S. Composites of graphene and LiFePO4 as cathode materials for lithium-ion battery: A mini-review. Nano-Micro Lett. 2014, 6, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, L.; Bewlay, S.; Chen, Y.; Liu, H.; Ahn, J. Electrochemical properties of carbon coated LiFePO4 cathode materials. J. Power Sources 2005, 146, 521–524. [Google Scholar] [CrossRef]
- Gong, C.; Xue, Z.; Wen, S.; Ye, Y.; Xie, X. Advanced carbon materials/olivine LiFePO4 composites cathode for lithium ion batteries. J. Power Sources 2016, 318, 93–112. [Google Scholar] [CrossRef]
- Stenina, I.; Minakova, P.; Kulova, T.; Yaroslavtsev, A. Electrochemical Properties of LiFePO4 Cathodes: The Effect of Carbon Additives. Batteries 2022, 8, 111. [Google Scholar] [CrossRef]
- Oh, J.; Lee, J.; Hwang, T.; Kim, J.M.; Seoung, K.-D.; Piao, Y. Dual Layer Coating Strategy Utilizing N-doped Carbon and Reduced Graphene Oxide for High-Performance LiFePO4 Cathode Material. Electrochim. Acta 2017, 231, 85–93. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wu, M.; Wei, Z.; Cui, C.; Mao, M.; Zhang, J.; Han, X.; Liu, Q.; Ma, J. Nitrogen, Fluorine, and Boron Ternary Doped Carbon Fibers as Cathode Electrocatalysts for Zinc–Air Batteries. Small 2018, 14, e1800737. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Y.; Xia, J.; Hu, Q.; Ke, X.; Ren, G.; Zhu, F. F-doped LiFePO4@N/B/F-doped carbon as high performance cathode materials for Li-ion batteries. Appl. Surf. Sci. 2019, 476, 761–768. [Google Scholar] [CrossRef]
- Mao, Y.; Duan, H.; Xu, B.; Zhang, L.; Hu, Y.; Zhao, C.; Wang, Z.; Chen, L.; Yang, Y. Lithium storage in nitrogen-rich mesoporous carbon materials. Energy Environ. Sci. 2012, 5, 7950–7955. [Google Scholar] [CrossRef]
- Shaji, N.; Ho, C.W.; Nanthagopal, M.; Santhoshkumar, P.; Sim, G.S.; Lee, C.W. Biowaste-derived heteroatoms-doped carbon for sustainable sodium-ion storage. J. Alloys Compd. 2021, 872, 159670. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The recent progress of nitrogen-doped carbon nanomaterials for electrochemical batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.-X.; Dutta, S.; Ok, Y.S.; Wu, K.C.-W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2021, 9, 3703–3728. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, J.; Tian, J.; Yu, J.-S. How do nitrogen-doped carbon dots generate from molecular precursors? An investigation of the formation mechanism and a solution-based large-scale synthesis. J. Mater. Chem. B 2015, 3, 5608–5614. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Wesley, R.J.; Durairaj, A.; Ramanathan, S.; Obadiah, A.; Justinabraham, R.; Lv, X.; Vasanthkumar, S. Potato peels biochar composite with copper phthalocyanine for energy storage application. Diam. Relat. Mater. 2021, 115, 108360. [Google Scholar] [CrossRef]
- Wu, D. Recycle Technology for Potato Peel Waste Processing: A Review. Procedia Environ. Sci. 2016, 31, 103–107. [Google Scholar] [CrossRef]
- Gebrechristos, H.Y.; Chen, W. Utilization of potato peel as eco-friendly products: A review. Food Sci. Nutr. 2018, 6, 1352–1356. [Google Scholar] [CrossRef]
- Osman, A.I.; Blewitt, J.; Abu-Dahrieh, J.K.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Rooney, D.W. Production and characterisation of activated carbon and carbon nanotubes from potato peel waste and their application in heavy metal removal. Environ. Sci. Pollut. Res. 2019, 26, 37228–37241. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Yang, Y.; Wang, H.; Jia, B.; Ma, G.; Yu, D.; Guo, L.; Yang, S. Direct chitin conversion to N-doped amorphous carbon nanofibers for high-performing full sodium-ion batteries. Nano Energy 2018, 45, 220–228. [Google Scholar] [CrossRef]
- Sim, G.S.; Nanthagopal, M.; Santhoshkumar, P.; Park, J.W.; Ho, C.W.; Shaji, N.; Kim, H.K.; Lee, C.W. Biomass-derived nitrogen-doped carbon on LiFePO4 material for energy storage applications. J. Alloys Compd. 2022, 902, 163720. [Google Scholar] [CrossRef]
- Ho, C.W.; Shaji, N.; Kim, H.K.; Park, J.W.; Nanthagopal, M.; Lee, C.W. Thermally assisted conversion of biowaste into environment-friendly energy storage materials for lithium-ion batteries. Chemosphere 2022, 286, 131654. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Artemenko, A.; Shchukarev, A.; Štenclová, P.; Wågberg, T.; Segervald, J.; Jia, X.; Kromka, A. Reference XPS spectra of amino acids. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1050, 012001. [Google Scholar] [CrossRef]
- Ayiania, M.; Smith, M.; Hensley, A.J.; Scudiero, L.; McEwen, J.-S.; Garcia-Perez, M. Deconvoluting the XPS spectra for nitrogen-doped chars: An analysis from first principles. Carbon 2020, 162, 528–544. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Pels, J.; Kapteijn, F.; Moulijn, J.; Zhu, Q.; Thomas, K. Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 1995, 33, 1641–1653. [Google Scholar] [CrossRef]
- Fan, L.; Shi, Z.; Ren, Q.; Yan, L.; Zhang, F.; Fan, L. Nitrogen-doped lignin based carbon microspheres as anode material for high performance sodium ion batteries. Green Energy Environ. 2021, 6, 220–228. [Google Scholar] [CrossRef]
- Kim, H.-J.; Bae, G.-H.; Lee, S.-M.; Ahn, J.-H.; Kim, J.-K. Properties of lithium iron phosphate prepared by biomass-derived carbon coating for flexible lithium ion batteries. Electrochim. Acta 2019, 300, 18–25. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Lou, J.J.; Teng, X.J.; Lu, X.X.; Liu, S.Y.; Chi, H.Z.; Ji, Z.G. Controllable synthesis of N-C@LiFePO4 nanospheres as advanced cathode of lithium ion batteries. J. Alloys Compd. 2018, 743, 377–382. [Google Scholar] [CrossRef]
- Han, B.; Meng, X.; Ma, L.; Nan, J. Nitrogen-doped carbon decorated LiFePO4 composite synthesized via a microwave heating route using polydopamine as carbon–nitrogen precursor. Ceram. Int. 2016, 42, 2789–2797. [Google Scholar] [CrossRef]
| Sample | a (Å) | b (Å) | c (Å) | V (Å3) |
|---|---|---|---|---|
| Bare LFP | 10.336 | 6.007 | 4.694 | 291.451 |
| PPNC@LFP | 10.316 | 6.001 | 4.688 | 290.242 |
| Carbon Precursor | Cathode Active Material | Discharge Capacity (mAh g−1) | Cycling Performance | Ionic Conductivity (S cm−1) | Ref. |
|---|---|---|---|---|---|
| Orange peel | LFP | 147.3 at 0.5 C | 95% after 50 cycles at 0.5 C | 4.80 × 10−4 | [42] |
| Eggshell membrane | LFP | 155.4 at 0.1 C | 99% after 100 cycles at 1.0 C | 1.04 × 10−3 | [35] |
| Dopamine | LFP | 163 at 0.1 C | 97.9% after 50 cycles at 0.1 C | - | [43] |
| Potato peel | LFP | 150 at 0.1 C | 99% after 100 cycles at 1.0 C | 2.52 × 10−3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, B.J.; Jiang, F.; Sung, J.Y.; Jung, S.P.; Oh, D.W.; Gnanamuthu, R.; Vediappan, K.; Lee, C.W. Biomass-Derived Carbon Utilization for Electrochemical Energy Enhancement in Lithium-Ion Batteries. Nanomaterials 2024, 14, 999. https://doi.org/10.3390/nano14120999
Jeong BJ, Jiang F, Sung JY, Jung SP, Oh DW, Gnanamuthu R, Vediappan K, Lee CW. Biomass-Derived Carbon Utilization for Electrochemical Energy Enhancement in Lithium-Ion Batteries. Nanomaterials. 2024; 14(12):999. https://doi.org/10.3390/nano14120999
Chicago/Turabian StyleJeong, Byeong Jin, Feng Jiang, Jae Yoon Sung, Soon Phil Jung, Dae Won Oh, RM. Gnanamuthu, Kumaran Vediappan, and Chang Woo Lee. 2024. "Biomass-Derived Carbon Utilization for Electrochemical Energy Enhancement in Lithium-Ion Batteries" Nanomaterials 14, no. 12: 999. https://doi.org/10.3390/nano14120999








