Microwave-Responsive Metal-Organic Frameworks (MOFs) for Enhanced In Vitro Controlled Release of Doxorubicin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization Tests
2.4. Reactive Oxygen Species (ROS)
2.5. DOX Encapsulation and In Vitro Release
2.6. Microwave Treatment and Cytotoxicity Assays
Cytotoxicity Studies with Free MIL-53(Al) MOFs
3. Results and Discussion
3.1. Characterization Tests
3.1.1. Morphology
3.1.2. X-ray Diffraction (XRD)
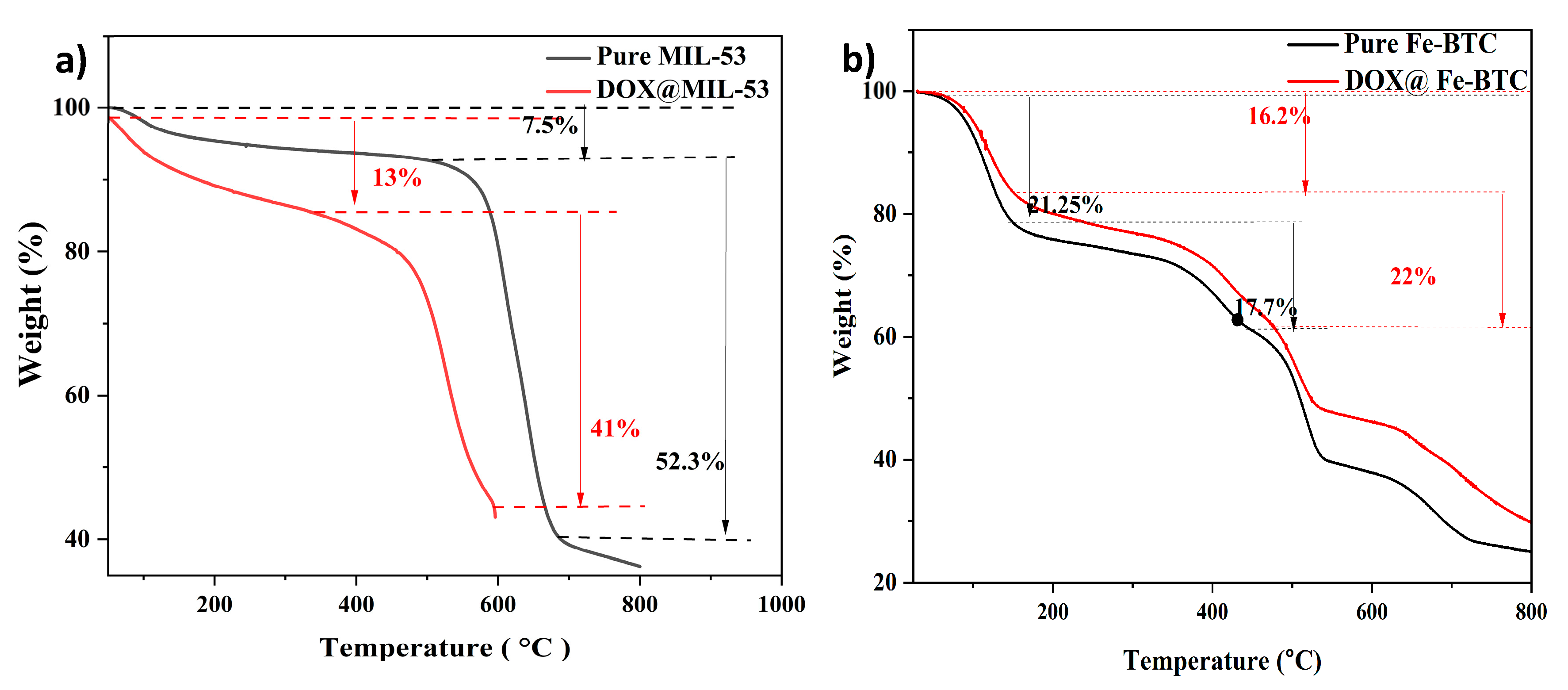
3.1.3. Thermal Gravimetric Analysis (TGA)
3.1.4. N2 Adsorption/Desorption Isotherms
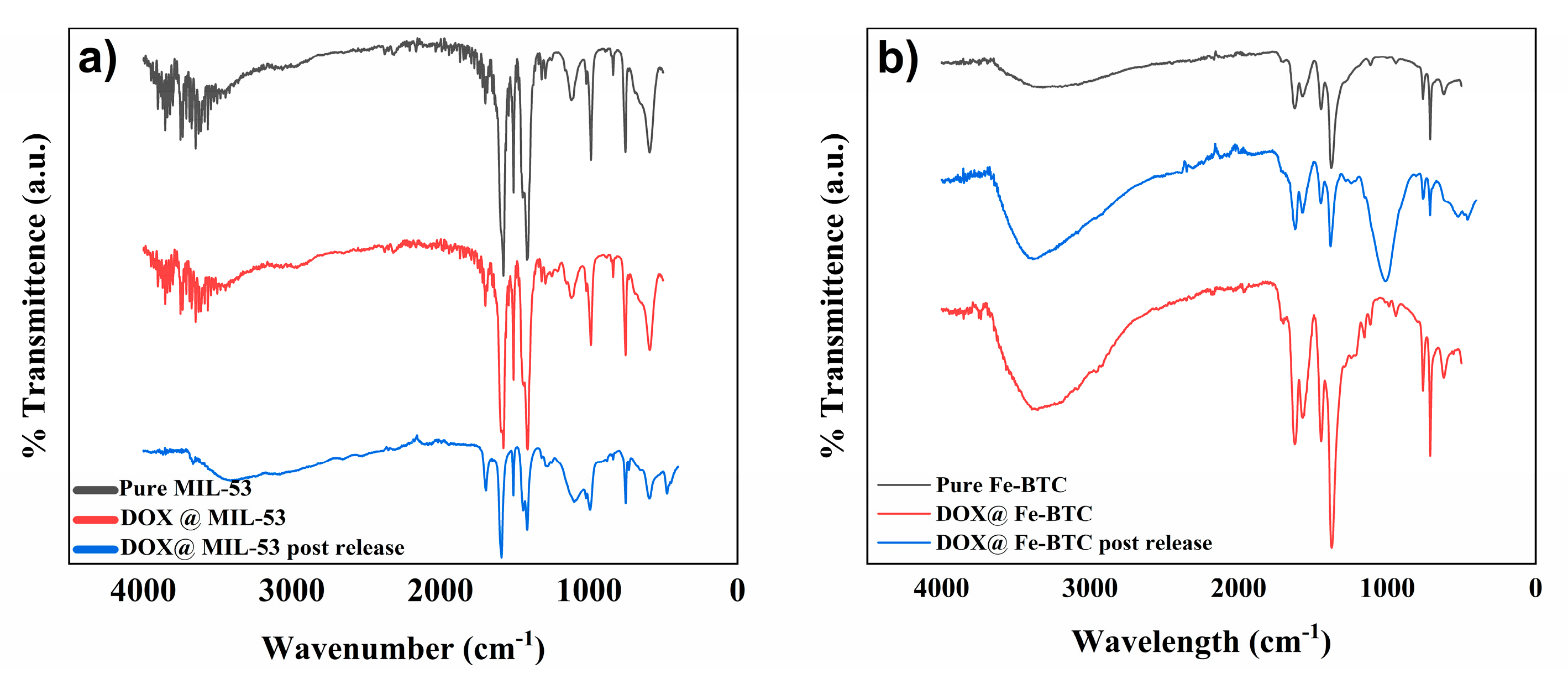
3.1.5. Fourier Transform Infrared (FTIR)
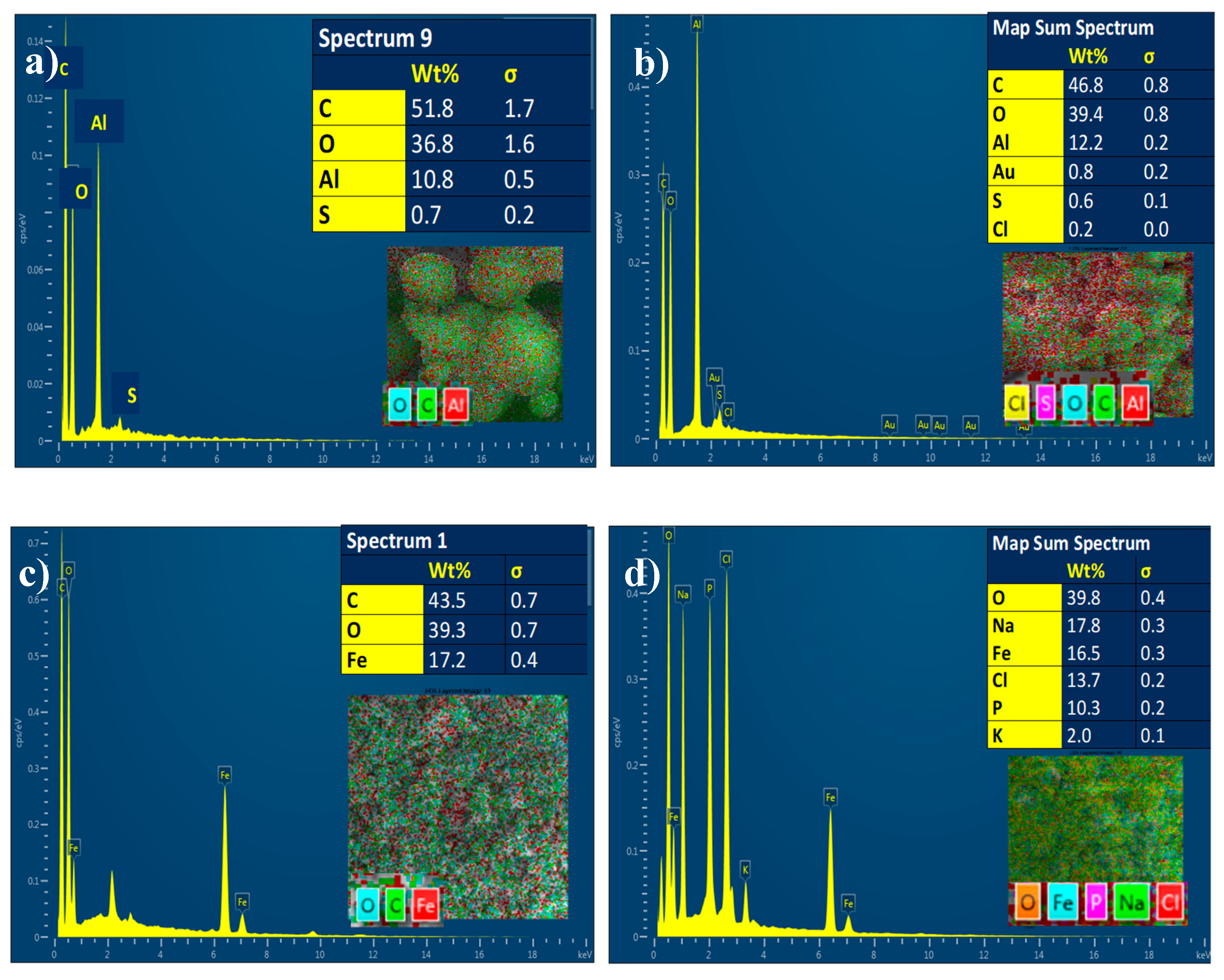
3.1.6. Energy Dispersive X-ray Analysis (EDX)
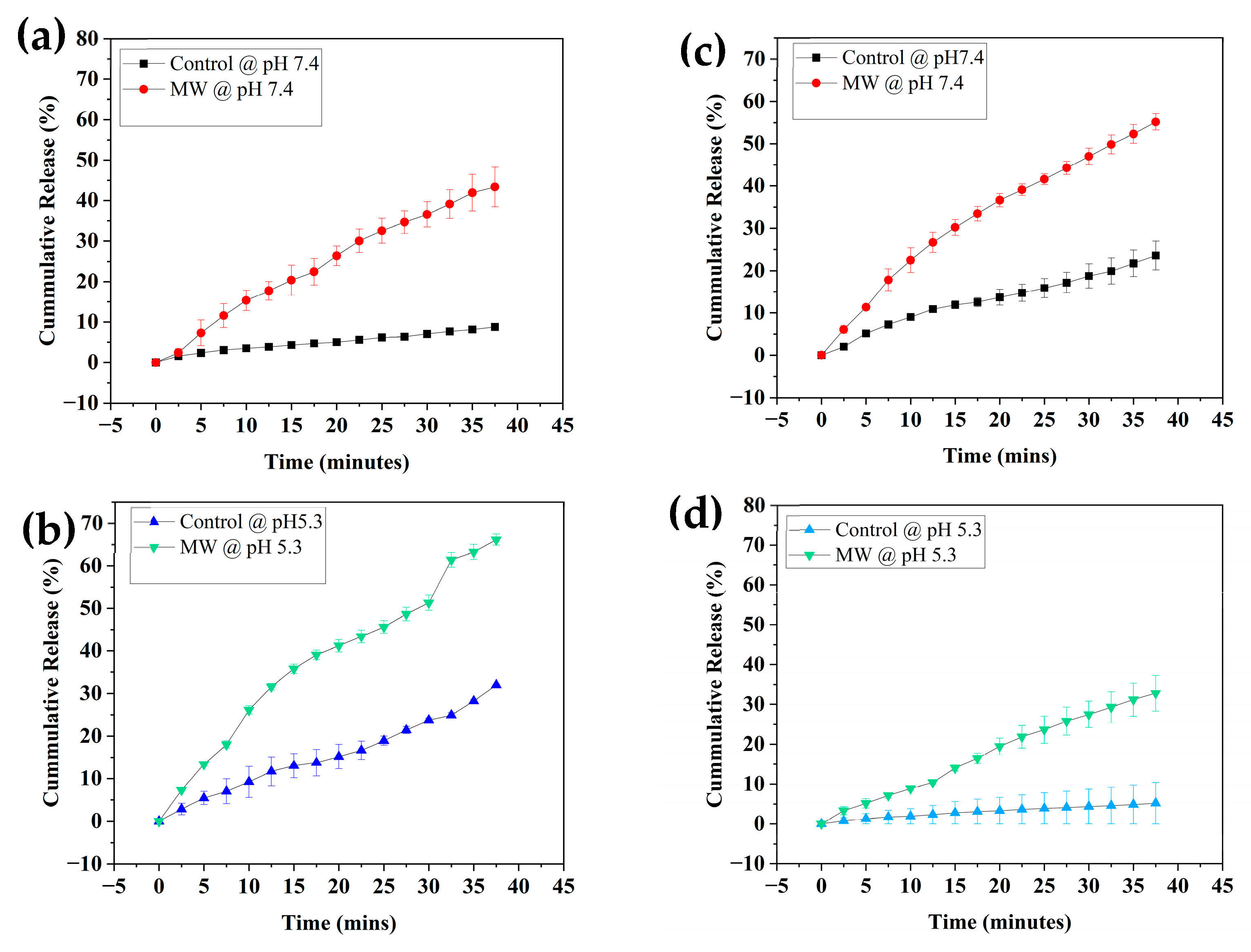
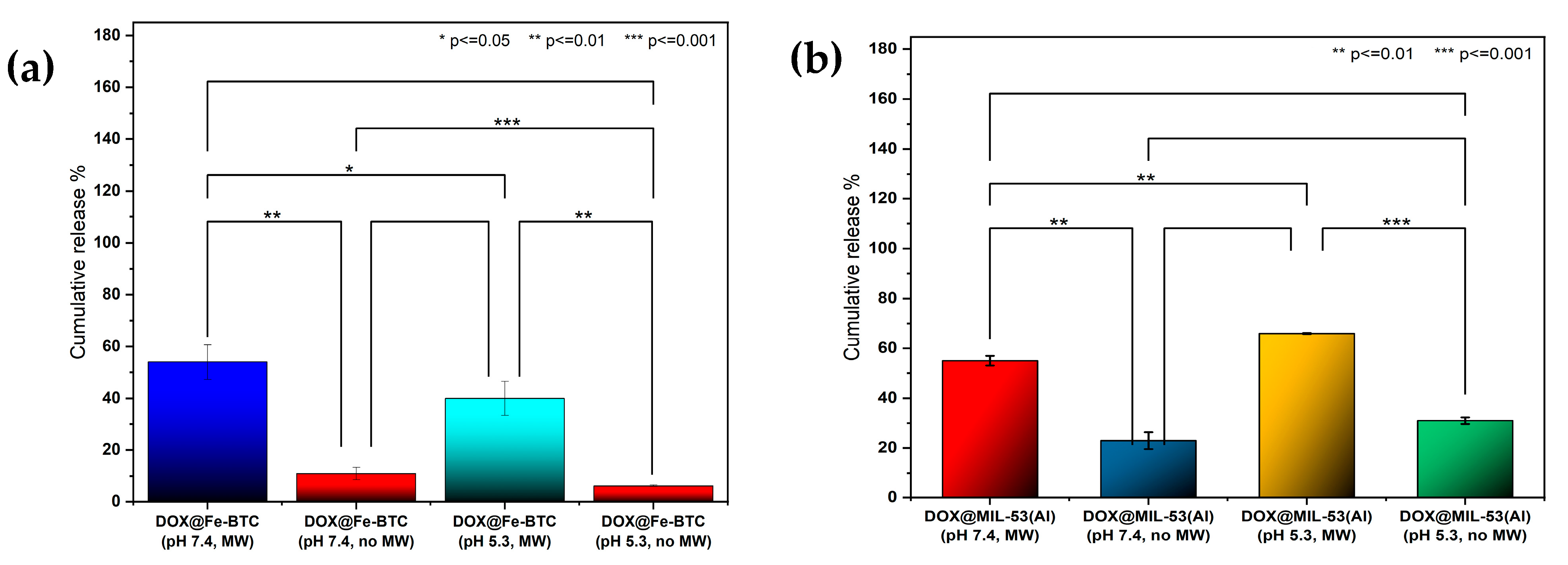
3.2. Release Profiles
3.3. Reactive Oxygen Species (ROS)
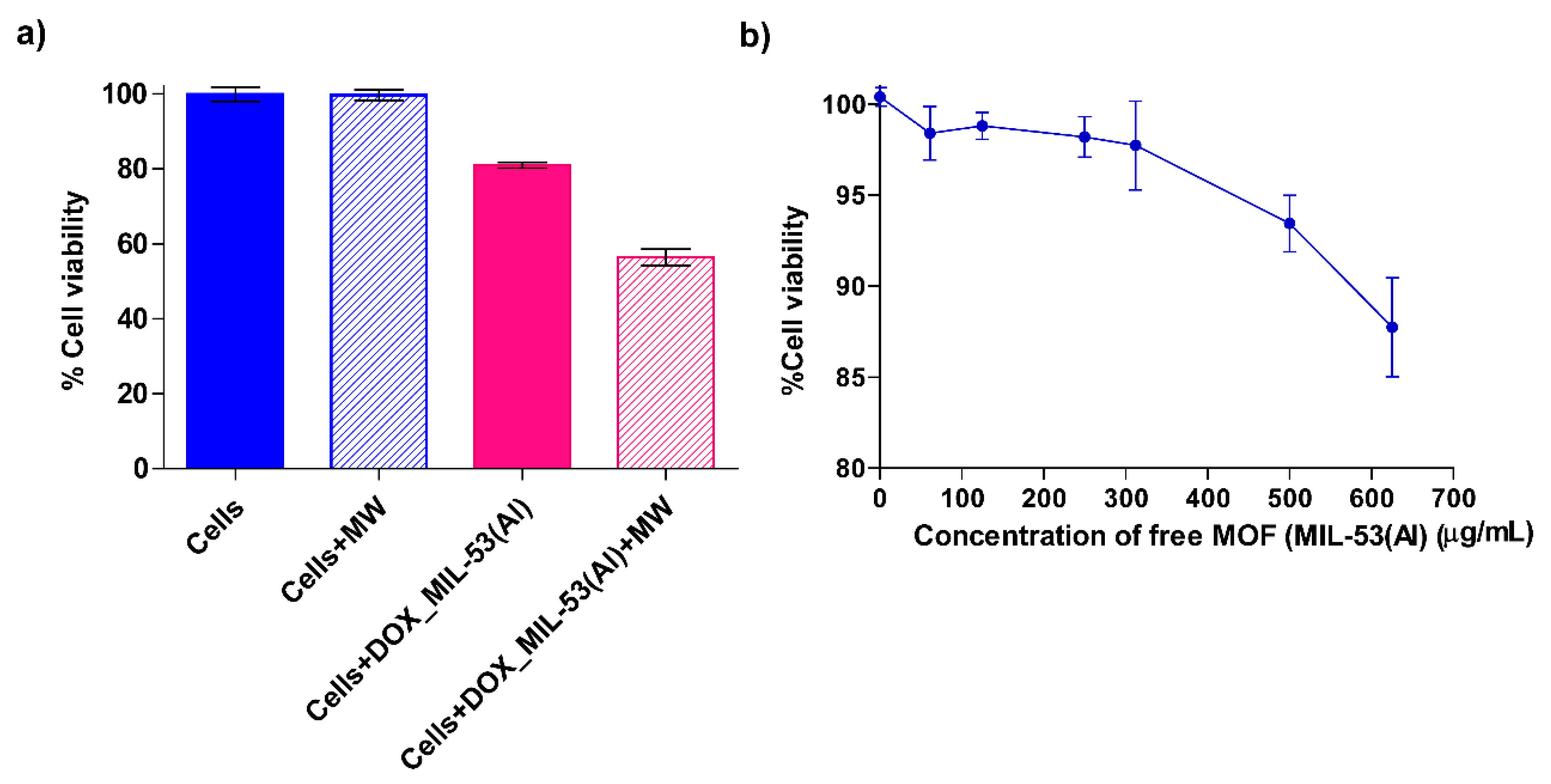
3.4. Cell Viability and Cytotoxicity Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Observatory. Malaysia Cancer Statistics. 2019. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/458-malaysia-fact-sheet.pdf (accessed on 29 November 2023).
- Hausman, D.M. What is cancer? Perspect. Biol. Med. 2019, 62, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Geisberg, C.A.; Sawyer, D.B. Mechanisms of anthracycline cardiotoxicity and strategies to decrease cardiac damage. Curr. Hypertens. Rep. 2010, 12, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim. Biophys. Acta-Rev. Cancer 2014, 1845, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.M.; Marina, N.; Hudson, M.M.; Hodgson, D.C.; Adams, M.J.; Landier, W.; Bhatia, S.; Meeske, K.; Chen, M.H.; Kinahan, K.E.; et al. Monitoring for Cardiovascular Disease in Survivors of Childhood Cancer: Report From the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics 2008, 121, e387–e396. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.; Wheatley, P.S.; Quartapelle-Procopio, E.; Gil, B.; Marzalek, B.; Morris, R.E.; Barea, E. Metal-organic frameworks as potential multi-carriers of drugs. CrystEngComm 2013, 15, 9364–9367. [Google Scholar] [CrossRef]
- Oh, J.Y.; Sim, Y.; Yang, G.; Park, M.-H.; Kim, K.; Ryu, J.-H. Surface functionalization of metal–organic framework nanoparticle for overcoming biological barrier in cancer therapy. Inorg. Chem. Front. 2024, 11, 3119–3135. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug deliveryand imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Cai, M.; Qin, L.; You, L.; Yao, Y.; Wu, H.; Zhang, Z.; Zhang, L.; Yin, X.; Ni, J. Functionalization of MOF-5 with mono-substituents: Effects on drug delivery behavior. RSC Adv. 2020, 10, 36862–36872. [Google Scholar] [CrossRef]
- Seki, K.; Takamizawa, S.; Mori, W. Characterization of microporous copper(II) dicarboxylates (fumarate, terephthalate, and trans-1,4-cyclohexanedicarboxylate) by gas adsorption. Chem. Lett. 2001, 30, 122–123. [Google Scholar] [CrossRef]
- Wyszogrodzka, G.; Dorożyński, P.; Gil, B.; Roth, W.J.; Strzempek, M.; Marszałek, B.; Węglarz, W.P.; Menaszek, E.; Strzempek, W.; Kulinowski, P. Iron-Based Metal-Organic Frameworks as a Theranostic Carrier for Local Tuberculosis Therapy. Pharm. Res. 2018, 35, 144. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Pooresmaeil, M.; Hashemi, H.; Namazi, H. Carboxymethylcellulose capsulated Cu-based metal-organic framework-drug nanohybrid as a pH-sensitive nanocomposite for ibuprofen oral delivery. Int. J. Biol. Macromol. 2018, 119, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.R.M.; Velander, J.; Perez, M.D.; Joseph, L.; Mattsson, V.; Asan, N.B.; Huss, F.; Augustine, R. Improved Sensor for Non-invasive Assessment of Burn Injury Depth Using Microwave Reflectometry. In Proceedings of the 13th European Conference on Antennas and Propagation, EuCAP 2019, Krakow, Poland, 31 March–5 April 2019. [Google Scholar]
- Yalamandala, B.N.; Shen, W.; Min, S.; Chiang, W.; Chang, S.; Hu, S. Advances in Functional Metal-Organic Frameworks Based On-Demand Drug Delivery Systems for Tumor Therapeutics. Adv. Nanobiomed Res. 2021, 1, 2100014. [Google Scholar] [CrossRef]
- Jin, Y.; Liang, X.; An, Y.; Dai, Z. Microwave-Triggered Smart Drug Release from Liposomes Co-encapsulating Doxorubicin and Salt for Local Combined Hyperthermia and Chemotherapy of Cancer. Bioconjugate Chem. 2016, 27, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhou, H.; Tan, L.; Huang, Z.; Wu, Q.; Ren, X.; Ren, J.; Meng, X. Microwave-Activated Mn-Doped Zirconium Metal-Organic Framework Nanocubes for Highly Effective Combination of Microwave Dynamic and Thermal Therapies Against Cancer. ACS Nano 2018, 12, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Cui, B.; Zhao, W.; Yang, Z. Preparation of multifunctional Fe3O4@ZnAl2O4:Eu3+@mSiO2-APTES drug-carrier for microwave controlled release of anticancer drugs. RSC Adv. 2017, 7, 55489–55495. [Google Scholar] [CrossRef]
- Chen, P.; Cui, B.; Cui, X.; Zhao, W.; Bu, Y.; Wang, Y. A microwave-triggered controllable drug delivery system based on hollow-mesoporous cobalt ferrite magnetic nanoparticles. J. Alloys Compd. 2017, 699, 526–533. [Google Scholar] [CrossRef]
- Zhang, F.-M.; Dong, H.; Zhang, X.; Sun, X.-J.; Liu, M.; Yang, D.-D.; Liu, X.; Wei, J.-Z. Postsynthetic Modification of ZIF-90 for Potential Targeted Codelivery of Two Anticancer Drugs. ACS Appl. Mater. Interfaces 2017, 9, 27332–27337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shang, Y.; Li, Y.-H.; Sun, S.-K.; Yin, X.-B. Smart Metal-Organic Framework-Based Nanoplatforms for Imaging-Guided Precise Chemotherapy. ACS Appl. Mater. Interfaces 2019, 11, 1886–1895. [Google Scholar] [CrossRef]
- Nejadshafiee, V.; Naeimi, H.; Goliaei, B.; Bigdeli, B.; Sadighi, A.; Dehghani, S.; Lotfabadi, A.; Hosseini, M.; Nezamtaheri, M.S.; Amanlou, M.; et al. Magnetic bio-metal–organic framework nanocomposites decorated with folic acid conjugated chitosan as a promising biocompatible targeted theranostic system for cancer treatment. Mater. Sci. Eng. C 2019, 99, 805–815. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Gumma, S.; Purkait, M.K. Fe3O4 promoted metal organic framework MIL-100(Fe) for the controlled release of doxorubicin hydrochloride. Microporous Mesoporous Mater. 2018, 259, 203–210. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Ataei, F.; Morsali, A.; Carpenter-Warren, C.L.; Mehdizadeh, K.; Slawin, A.M.Z. Chitosan Immobilization on Bio-MOF Nanostructures: A Biocompatible pH-Responsive Nanocarrier for Doxorubicin Release on MCF-7 Cell Lines of Human Breast Cancer. Inorg. Chem. 2018, 57, 13364–13379. [Google Scholar] [CrossRef]
- Wu, T.; Prasetya, N.; Li, K. Recent advances in aluminium-based metal-organic frameworks (MOF) and its membrane applications. J. Membr. Sci. 2020, 615, 118493. [Google Scholar] [CrossRef]
- Haydar, M.A.L.; Abid, H.R.; Sunderland, B.; Wang, S. Metal organic frameworks as a drug delivery system for flurbiprofen. Drug Des. Dev. Ther. 2017, 11, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, A.A.C.; García, E.R.; Medina, R.L.; Larios, J.L.C.; Parra, R.S.; Franco, A.M.M. Selective adsorption of aqueous diclofenac sodium, naproxen sodium, and ibuprofen using a stable Fe3O4-FeBTC metal-organic framework. Materials 2021, 14, 2293. [Google Scholar] [CrossRef]
- Ahmed, A.; Karami, A.; Sabouni, R.; Husseini, G.A.; Paul, V. pH and ultrasound dual-responsive drug delivery system based on PEG–folate-functionalized Iron-based metal–organic framework for targeted doxorubicin delivery. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127062. [Google Scholar] [CrossRef]
- Ibrahim, M.; Sabouni, R.; Husseini, G.A.; Karami, A.; Bai, R.G.; Mukhopadhyay, D. Facile Ultrasound-Triggered Release of Calcein and Doxorubicin from Iron-Based Metal-Organic Frameworks. J. Biomed. Nanotechnol. 2020, 16, 1359–1369. [Google Scholar] [CrossRef]
- Karami, A.; Ahmed, A.; Sabouni, R.; Husseini, G.A.; Paul, V. Combined and Single Doxorubicin Naproxen Drug Loading and Dual-Responsive pH Ultrasound Release from Flexible Metal-Organic Framework Nanocarriers. J. Biomed. Nanotechnol. 2022, 18, 1770–1781. [Google Scholar] [CrossRef]
- Gupta, A.S. Role of particle size, shape, and stiffness in design of intravascular drug delivery systems: Insights from computations, experiments, and nature. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 255–270. [Google Scholar] [CrossRef]
- Jiang, J.X.; Keating, J.J.; De Jesus, E.M.; Judy, R.P.; Madajewski, B.; Venegas, O.; Okusanya, O.T.; Singhal, S. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 390–400. [Google Scholar]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjugate Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Seoane, B.; Sorribas, S.; Mayoral, Á.; Téllez, C.; Coronas, J. Real-time monitoring of breathing of MIL-53(Al) by environmental SEM. Microporous Mesoporous Mater. 2015, 203, 17–23. [Google Scholar] [CrossRef]
- Liu, J.-F.; Mu, J.-C.; Qin, R.-X.; Ji, S.-F. Pd nanoparticles immobilized on MIL-53(Al) as highly effective bifunctional catalysts for oxidation of liquid methanol to methyl formate. Pet. Sci. 2019, 16, 901–911. [Google Scholar] [CrossRef]
- Li, A.; Yang, X.; Chen, J. A novel route to size-controlled MIL-53(Fe) metal–organic frameworks for combined chemodynamic therapy and chemotherapy for cancer. RSC Adv. 2021, 11, 10540–10547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, X.; Yang, T.; Yue, C.; Pu, Q.; Zhang, Y. Facile synthesis of polyoxometalates tethered to post Fe-BTC frameworks for esterification of free fatty acids to biodiesel. RSC Adv. 2019, 9, 8113–8120. [Google Scholar] [CrossRef]
- Majano, G.; Ingold, O.; Yulikov, M.; Jeschke, G.; Pérez-Ramírez, J. Room-temperature synthesis of Fe-BTC from layered iron hydroxides: The influence of precursor organisation. CrystEngComm 2013, 15, 9885–9892. [Google Scholar] [CrossRef]
- Nguyen, P.T.H.; Le, B.T.; Ninh, H.D.; La, D.D. Ultrasonic-Assisted Synthesis of Fe-BTC-PEG Metal-Organic Complex: An Effective and Safety Nanocarrier for Anticancer Drug Delivery. ACS Omega 2021, 6, 33419–33427. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, L.W.; da Silva, C.T.P.; de Lima, H.H.C.; Moises, M.P.; Rinaldi, A.W. Evaluation of the synthetic methods for preparing metal organic frameworks with transition metals. AIMS Mater. Sci. 2018, 5, 467–478. [Google Scholar] [CrossRef]
- Milakin, K.A.; Gavrilov, N.; Pašti, I.A.; Morávková, Z.; Acharya, U.; Unterweger, C.; Breitenbach, S.; Zhigunov, A.; Bober, P. Polyaniline-metal organic framework (Fe-BTC) composite for electrochemical applications. Polymer 2020, 208, 122945. [Google Scholar] [CrossRef]
- Karami, A.; Ahmed, A.; Sabouni, R.; Husseini, G.A.; Al Sharabati, M.; AlSawaftah, N.; Paul, V. Hybrid liposome/metal–organic framework as a promising dual-responsive nanocarriers for anticancer drug delivery. Colloids Surf. B Biointerfaces 2022, 217, 112599. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Jiang, S.; Ji, S.; Shi, D.; Cheng, H. Metal-organic framework MIL-53(Al): Synthesis, catalytic performance for the Friedel-Crafts acylation, and reaction mechanism. Sci. China Chem. 2015, 58, 1544–1552. [Google Scholar] [CrossRef]
- Ahadi, N.; Askari, S.; Fouladitajar, A.; Akbari, I. Facile synthesis of hierarchically structured MIL-53(Al) with superior properties using an environmentally-friendly ultrasonic method for separating lead ions from aqueous solutions. Sci. Rep. 2022, 12, 2649. [Google Scholar] [CrossRef] [PubMed]
- Farboudi, A.; Mahboobnia, K.; Chogan, F.; Karimi, M.; Askari, A.; Banihashem, S.; Davaran, S.; Irani, M. UiO-66 metal organic framework nanoparticles loaded carboxymethyl chitosan/poly ethylene oxide/polyurethane core-shell nanofibers for controlled release of doxorubicin and folic acid. Int. J. Biol. Macromol. 2020, 150, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Delpiano, G.R.; Tocco, D.; Medda, L.; Magner, E.; Salis, A. Adsorption of malachite green and alizarin red s dyes using fe-btc metal organic framework as adsorbent. Int. J. Mol. Sci. 2021, 22, 788. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Li, L.; Li, M.; Si, R. Adsorption mechanism on metal organic frameworks of Cu-BTC, Fe-BTC and ZIF-8 for CO2 capture investigated by X-ray absorption fine structure. RSC Adv. 2016, 6, 62705–62716. [Google Scholar] [CrossRef]
- Pangkumhang, B.; Jutaporn, P.; Sorachoti, K.; Khamdahsag, P.; Tanboonchuy, V. Applicability of iron (III) Trimesic (Fe-BTC) to enhance lignin separation from pulp and paper wastewater. Sains Malays. 2019, 48, 199–208. [Google Scholar] [CrossRef]
- Chen, X.Y.; Hoang, V.-T.; Rodrigue, D.; Kaliaguine, S. Optimization of continuous phase in amino-functionalized metal-organic framework (MIL-53) based co-polyimide mixed matrix membranes for CO2/CH4 separation. RSC Adv. 2013, 3, 24266–24279. [Google Scholar] [CrossRef]
- Yılmaz, E.; Sert, E.; Atalay, F.S. Synthesis, characterization of a metal organic framework: MIL-53 (Fe) and adsorption mechanisms of methyl red onto MIL-53 (Fe). J. Taiwan Inst. Chem. Eng. 2016, 65, 323–330. [Google Scholar] [CrossRef]
- Zhao, Z.; Pang, J.; Liu, W.; Lin, T.; Ye, F.; Zhao, S. A bifunctional metal organic framework of type Fe(III)-BTC for cascade (enzymatic and enzyme-mimicking) colorimetric determination of glucose. Microchim. Acta 2019, 186, 295. [Google Scholar] [CrossRef] [PubMed]
- Dorosti, F.; Alizadehdakhel, A. Fabrication and investigation of PEBAX/Fe-BTC, a high permeable and CO2 selective mixed matrix membrane. Chem. Eng. Res. Des. 2018, 136, 119–128. [Google Scholar] [CrossRef]
- Blahovec, J.; Yanniotis, S. Modified classification of sorption isotherms. J. Food Eng. 2009, 91, 72–77. [Google Scholar] [CrossRef]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Férey, G. A Rationale for the Large Breathing of the Porous Aluminum Terephthalate (MIL-53) Upon Hydration. Chem.-A Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.; Leo, P.; Orcajo, G.; Díaz-García, M.; Sanchez-Sanchez, M.; Calleja, G. Sustainable Fe-BTC catalyst for efficient removal of mehylene blue by advanced fenton oxidation. Catal. Today 2018, 313, 6–11. [Google Scholar] [CrossRef]
- Adhikari, C.; Das, A.; Chakraborty, A. Zeolitic Imidazole Framework (ZIF) Nanospheres for Easy Encapsulation and Controlled Release of an Anticancer Drug Doxorubicin under Different External Stimuli: A Way toward Smart Drug Delivery System. Mol. Pharm. 2015, 12, 3158–3166. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-J.; Yu, X.-Y.; Jia, Y.; Peng, F.-M.; Sun, B.; Zhang, M.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Iron and 1,3,5-benzenetricarboxylic metal-organic coordination polymers prepared by solvothermal method and their application in efficient as(V) removal from aqueous solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- McKinstry, C.; Cussen, E.J.; Fletcher, A.J.; Patwardhan, S.V.; Sefcik, J. Scalable continuous production of high quality HKUST-1 via conventional and microwave heating. Chem. Eng. J. 2017, 326, 570–577. [Google Scholar] [CrossRef]
- Basso, A.; Sinigoi, L.; Gardossi, L.; Flitsch, S. Effect of Microwave Radiation on Enzymatic and Chemical Peptide Bond Synthesis on Solid Phase. Int. J. Pept. 2009, 2009, 362482. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ma, S.; Xu, R.; Wei, Y.; Zhang, J.; Zuo, T.; Wang, Z.; Deng, H.; Yang, N.; Shen, Q. Smart biomimetic metal organic frameworks based on ROS-ferroptosis-glycolysis regulation for enhanced tumor chemo-immunotherapy. J. Control. Release 2021, 334, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Gao, Y.; Liu, J.; Wen, Y.; Zhao, Y.; Zhang, K.; Yu, G. Photoreactivity of Metal-Organic Frameworks in Aqueous Solutions: Metal Dependence of Reactive Oxygen Species Production. Environ. Sci. Technol. 2016, 50, 3634–3640. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Yadian, B.; Wu, R.; Long, Y.; Zhou, K.; Zhu, B.; Huang, Y. Structure stability of metal-organic framework MIL-53 (Al) in aqueous solutions. Int. J. Hydrogen Energy 2013, 38, 16710–16715. [Google Scholar] [CrossRef]
- Yang, J.; Dai, D.; Zhang, X.; Teng, L.; Ma, L.; Yang, Y.-W. Multifunctional metal-organic framework (MOF)-based nanoplatforms for cancer therapy: From single to combination therapy. Theranostics 2023, 13, 295–323. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Namazi, H. Fabrication of biocompatible magnetic maltose/MIL-88 metal–organic frameworks decorated with folic acid-chitosan for targeted and pH-responsive controlled release of doxorubicin. Int. J. Pharm. 2023, 634, 122675. [Google Scholar] [CrossRef]
- Virlley, S.; Shukla, S.; Arora, S.; Shukla, D.; Nagdiya, D.; Bajaj, T.; Kujur, S.; Garima; Kumar, A.; Bhatti, J.S.; et al. Recent advances in microwave-assisted nanocarrier based drug delivery system: Trends and technologies. J. Drug Deliv. Sci. Technol. 2023, 87, 104842. [Google Scholar] [CrossRef]
- Sharker, S.M. Interaction of microwave and nanomaterials for thermoresponsive drug delivery and hyperthermal cancer therapy. Inorg. Chem. Commun. 2023, 156, 111152. [Google Scholar] [CrossRef]

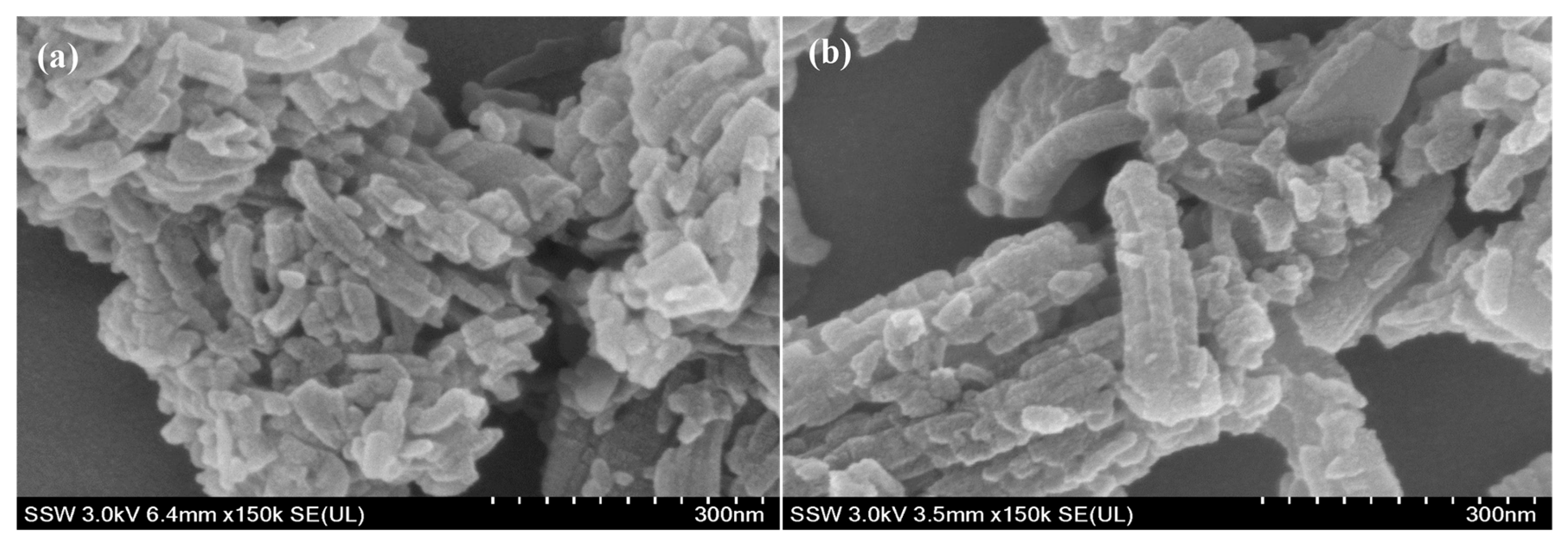
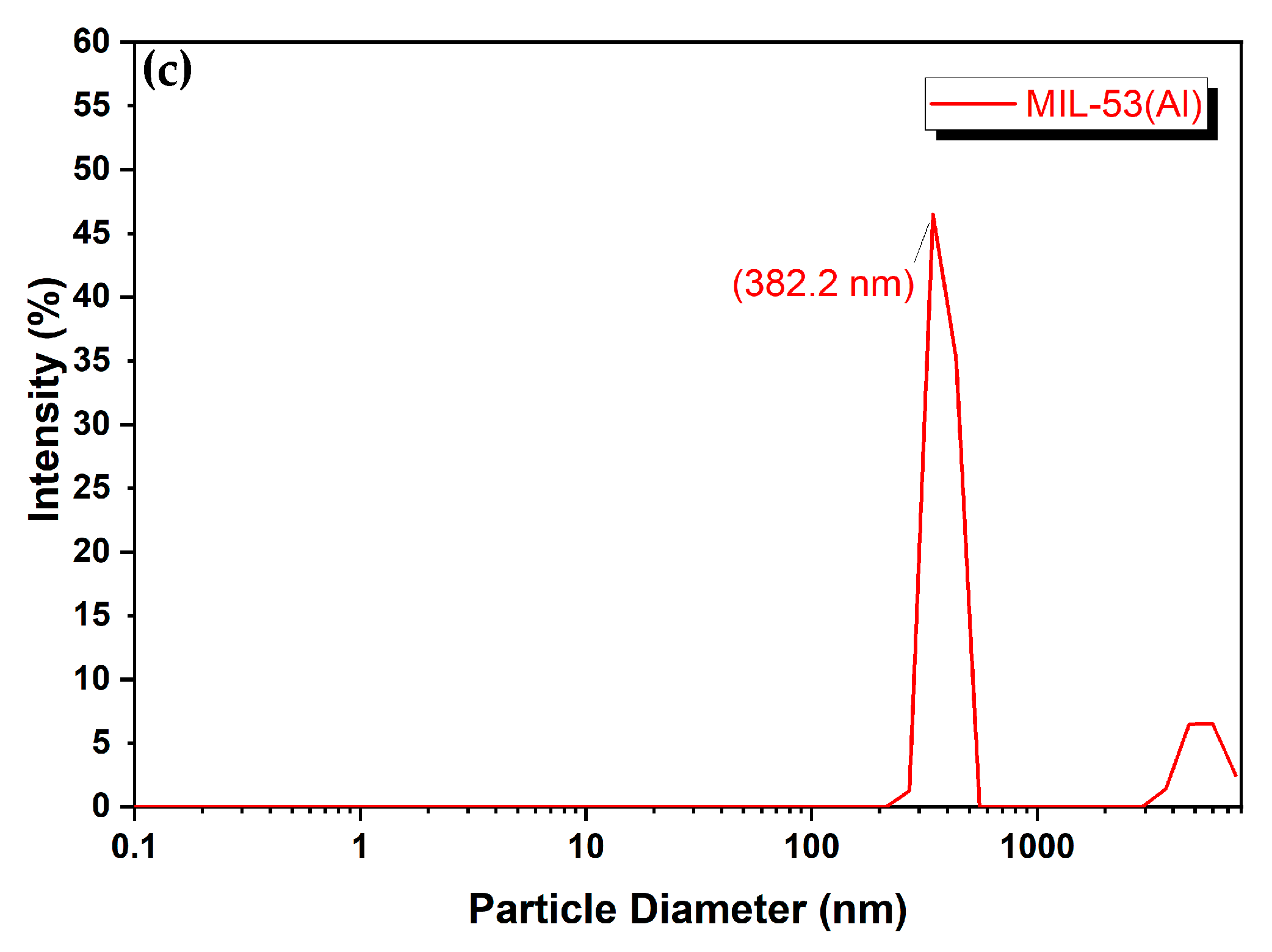

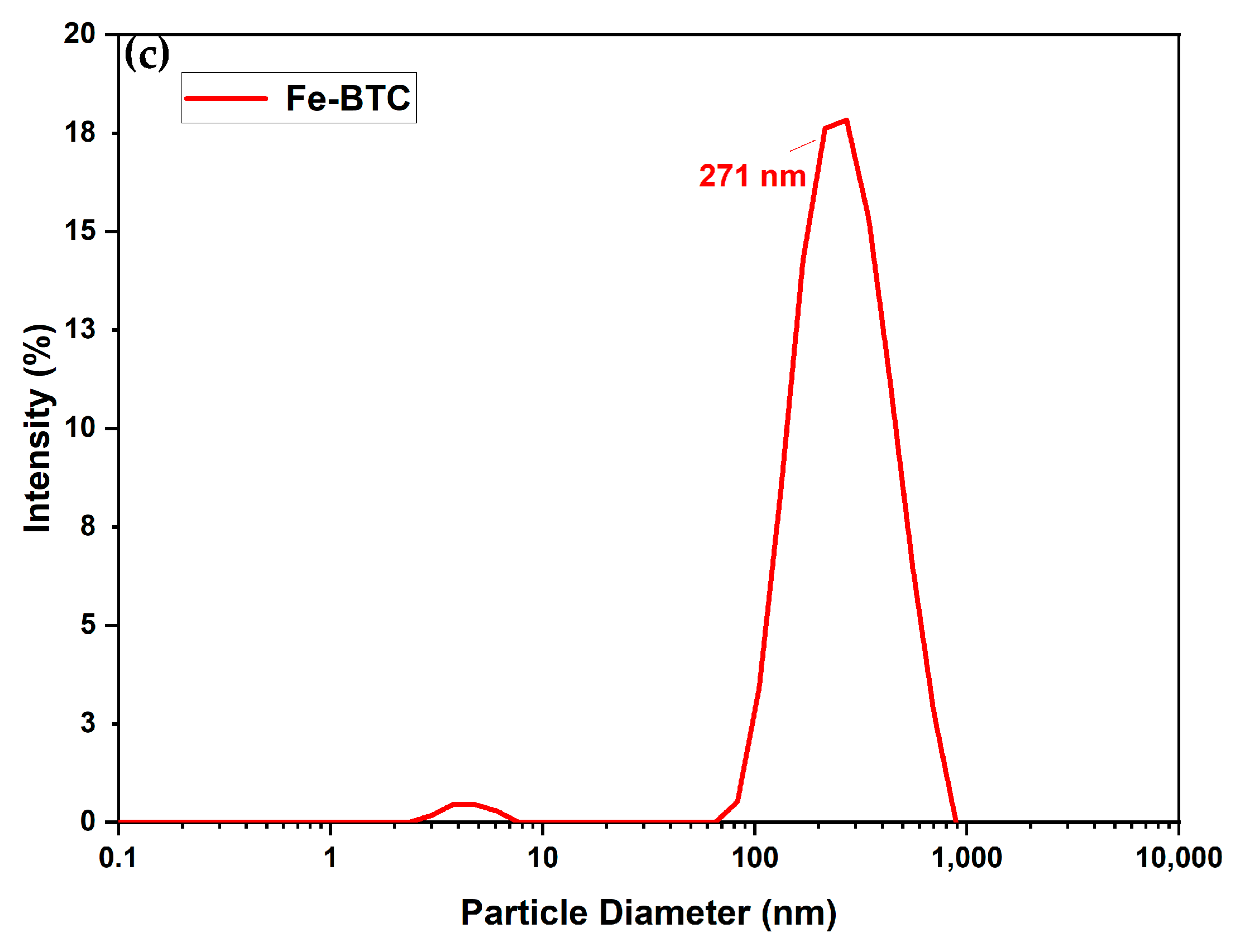
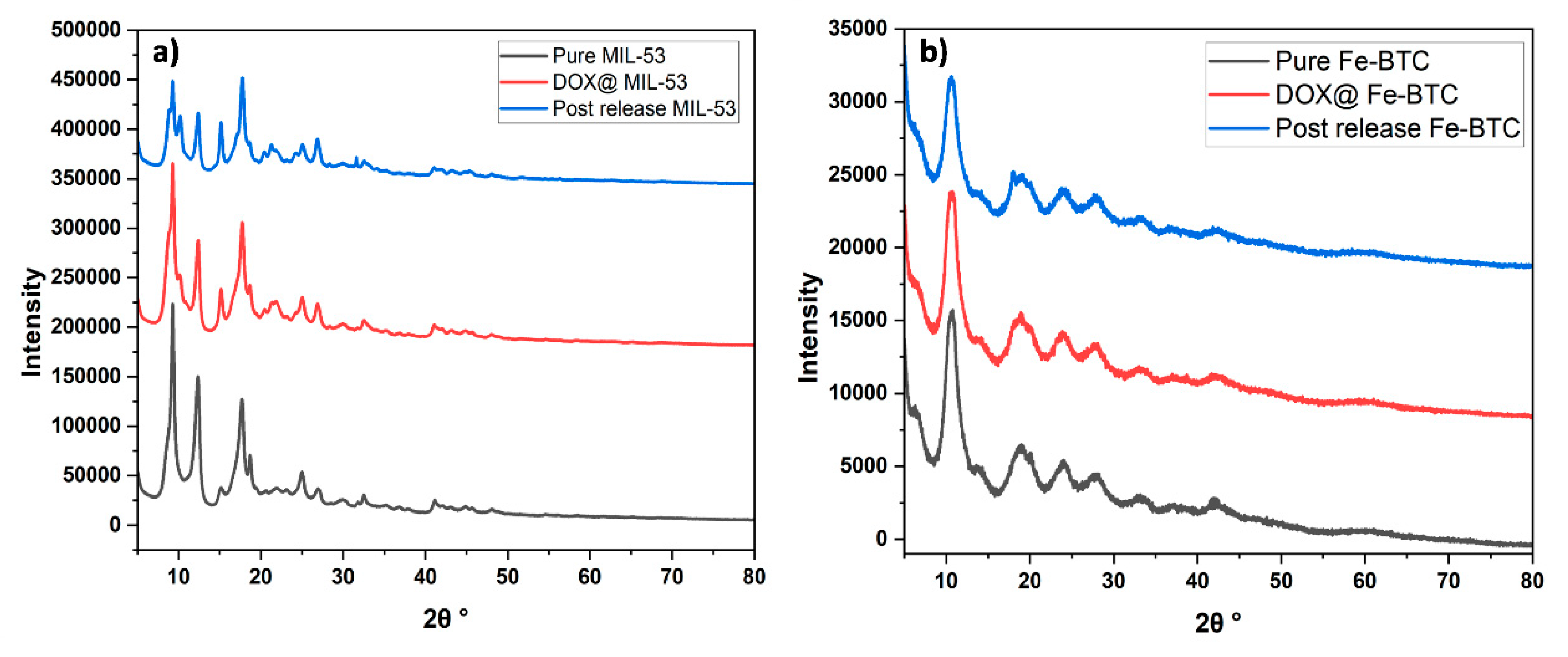
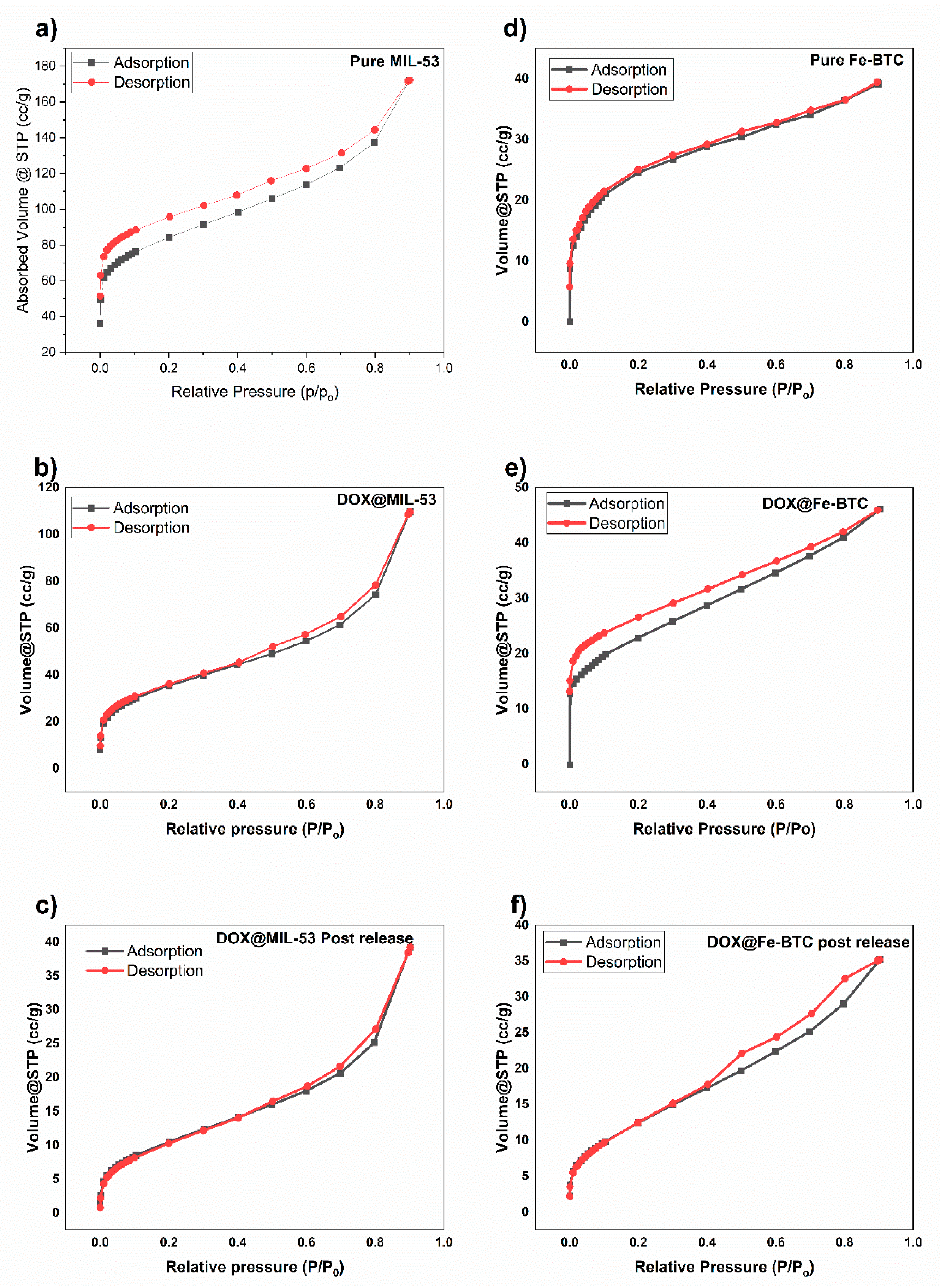

| Sample | Pore Volume cm3/g | Pore Diameter Å | Surface Area BET m2/g |
|---|---|---|---|
| MIL-5(Al) | 0.24 | 12.318 | 329.741 |
| DOX@MIL-53(Al) | 0.186 | 12.318 | 203.435 |
| DOX@MIL-53(Al) + MW | 0.055 | 17.656 | 22.811 |
| Fe-BTC | 0.168 | 6.159 | 247.801 |
| DOX@Fe-BTC | 0.066 | 6.159 | 81.402 |
| DOX@Fe-BTC + MW | 0.051 | 36.274 | 27.654 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, S.F.; Sabouni, R.; Husseini, G.; Paul, V.; Gomaa, H.; Radha, R. Microwave-Responsive Metal-Organic Frameworks (MOFs) for Enhanced In Vitro Controlled Release of Doxorubicin. Nanomaterials 2024, 14, 1081. https://doi.org/10.3390/nano14131081
Fatima SF, Sabouni R, Husseini G, Paul V, Gomaa H, Radha R. Microwave-Responsive Metal-Organic Frameworks (MOFs) for Enhanced In Vitro Controlled Release of Doxorubicin. Nanomaterials. 2024; 14(13):1081. https://doi.org/10.3390/nano14131081
Chicago/Turabian StyleFatima, Syeda Fiza, Rana Sabouni, Ghaleb Husseini, Vinod Paul, Hassan Gomaa, and Remya Radha. 2024. "Microwave-Responsive Metal-Organic Frameworks (MOFs) for Enhanced In Vitro Controlled Release of Doxorubicin" Nanomaterials 14, no. 13: 1081. https://doi.org/10.3390/nano14131081







