Quantum Dots as a Potential Multifunctional Material for the Enhancement of Clinical Diagnosis Strategies and Cancer Treatments
Abstract
1. Introduction
2. Properties of QDs
2.1. Physical Properties of QDs
2.2. Common Synthesis Methods of QDs
| Method | Advantages | Disadvantages | References | |
|---|---|---|---|---|
| Top-down | Laser ablation | Controllable shape and size | Complex operation and high cost | [8,23] |
| Electrochemical oxidation | High purity, high yield, controllable size, and good reproducibility | Complex operation | [24,25,26] | |
| Chemical oxidation | Easy to operate, large-scale production, no need for sophisticated equipment | Uneven size distribution | [27] | |
| Ultrasonic treatment | Easy to operate | Instrument waste and high energy costs | [28,29,30] | |
| Microwave | Shortened reaction time, increased yield and purity | High energy costs | [31,32,33] | |
| Hydrothermal synthesis | Relatively simple, quick response | Low yield | [34,35,36] | |
| Bottom-up | Cage opening | Strong luminescent properties | Complex operation | [37] |
| Thermal decomposition | Simple operation, solvent-free, low cost, and large-scale production | Uneven size distribution | [38] | |
| Precursor pyrolysis | Stable and strong excitation-dependent photoluminescence | Complex operation and high cost | [39,40,41] | |
| Ultraviolet irradiation | Mild, clean, and efficient | High energy costs | [42] |
2.3. Cellular Uptake Modes of QDs
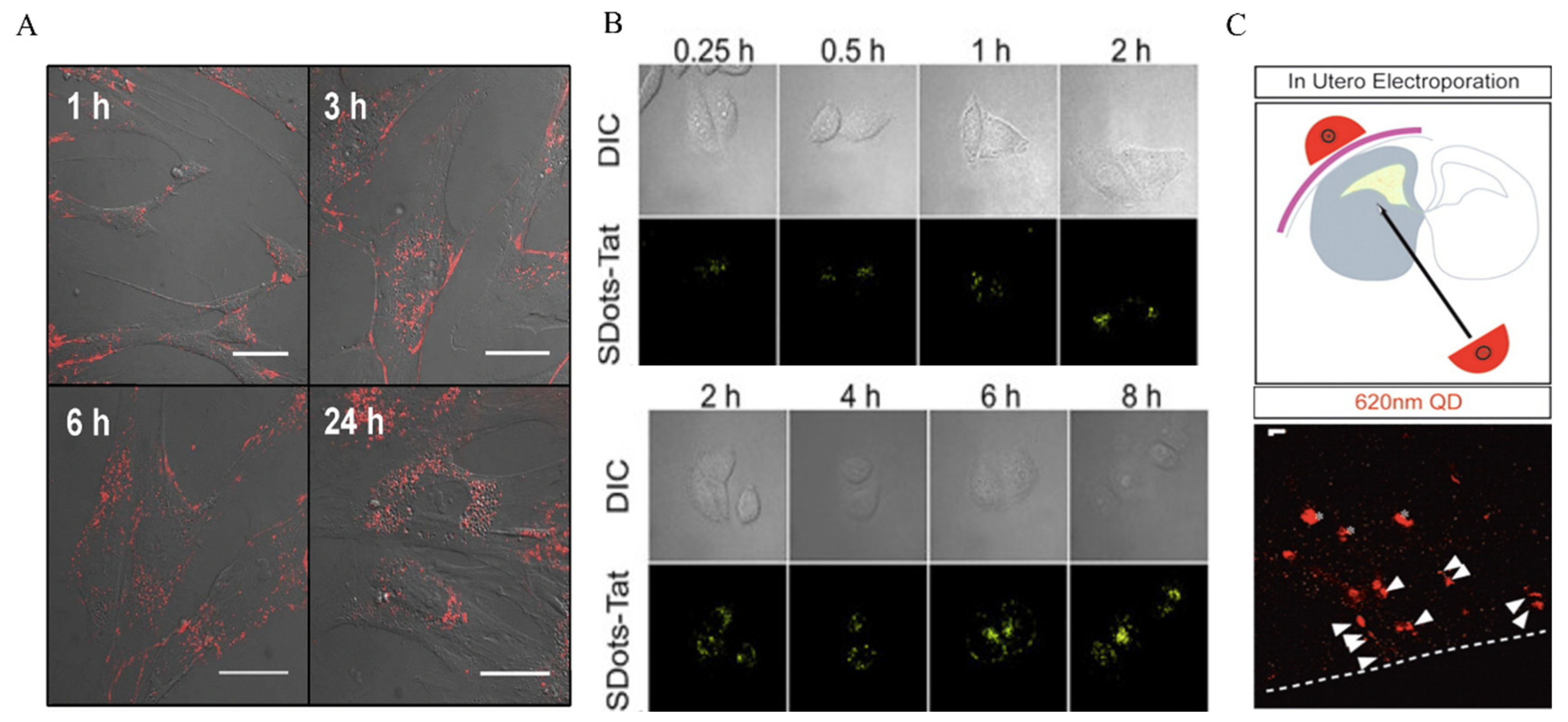
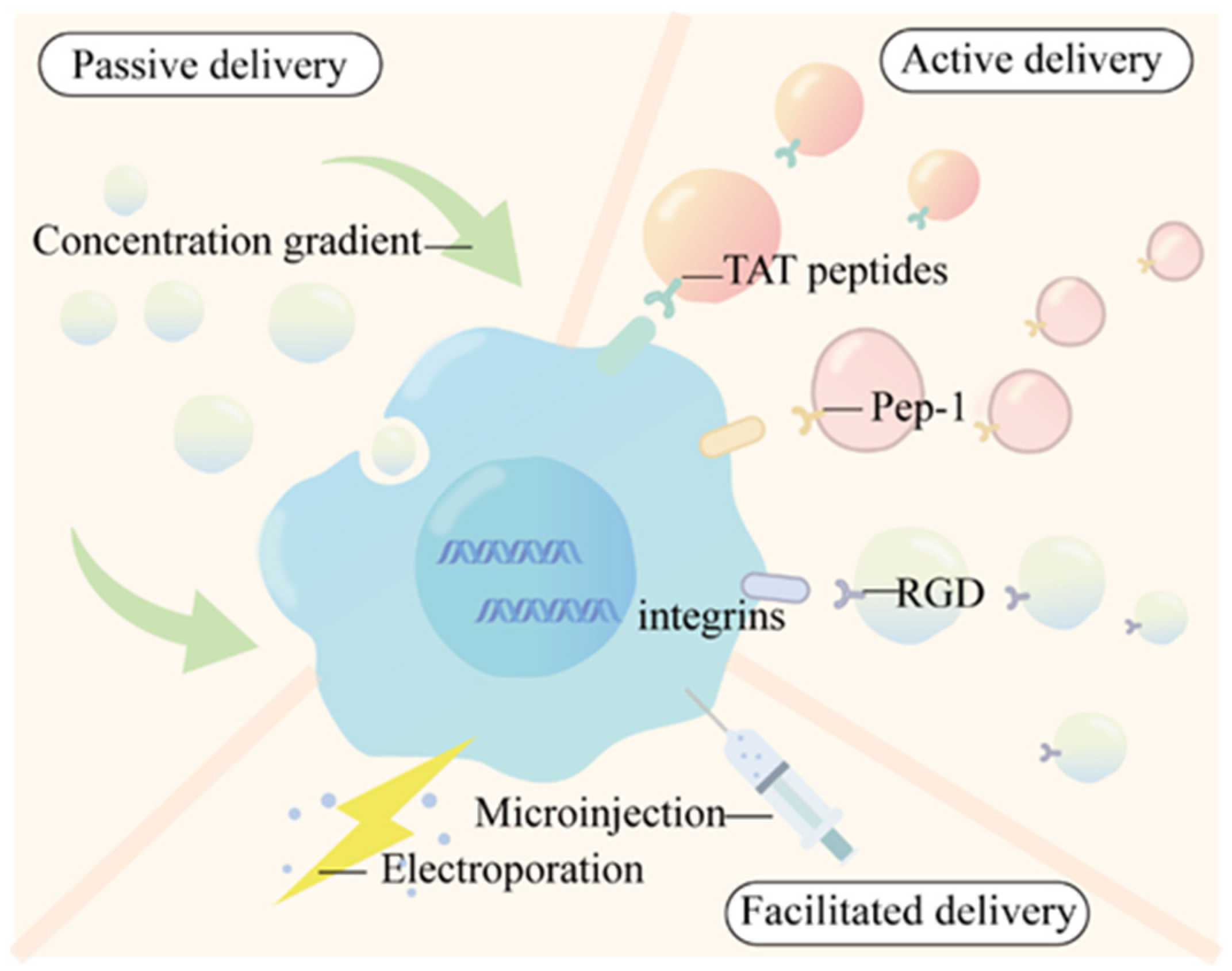
3. The Application of QDs in Tumor Diagnoses
3.1. In Vitro Imaging
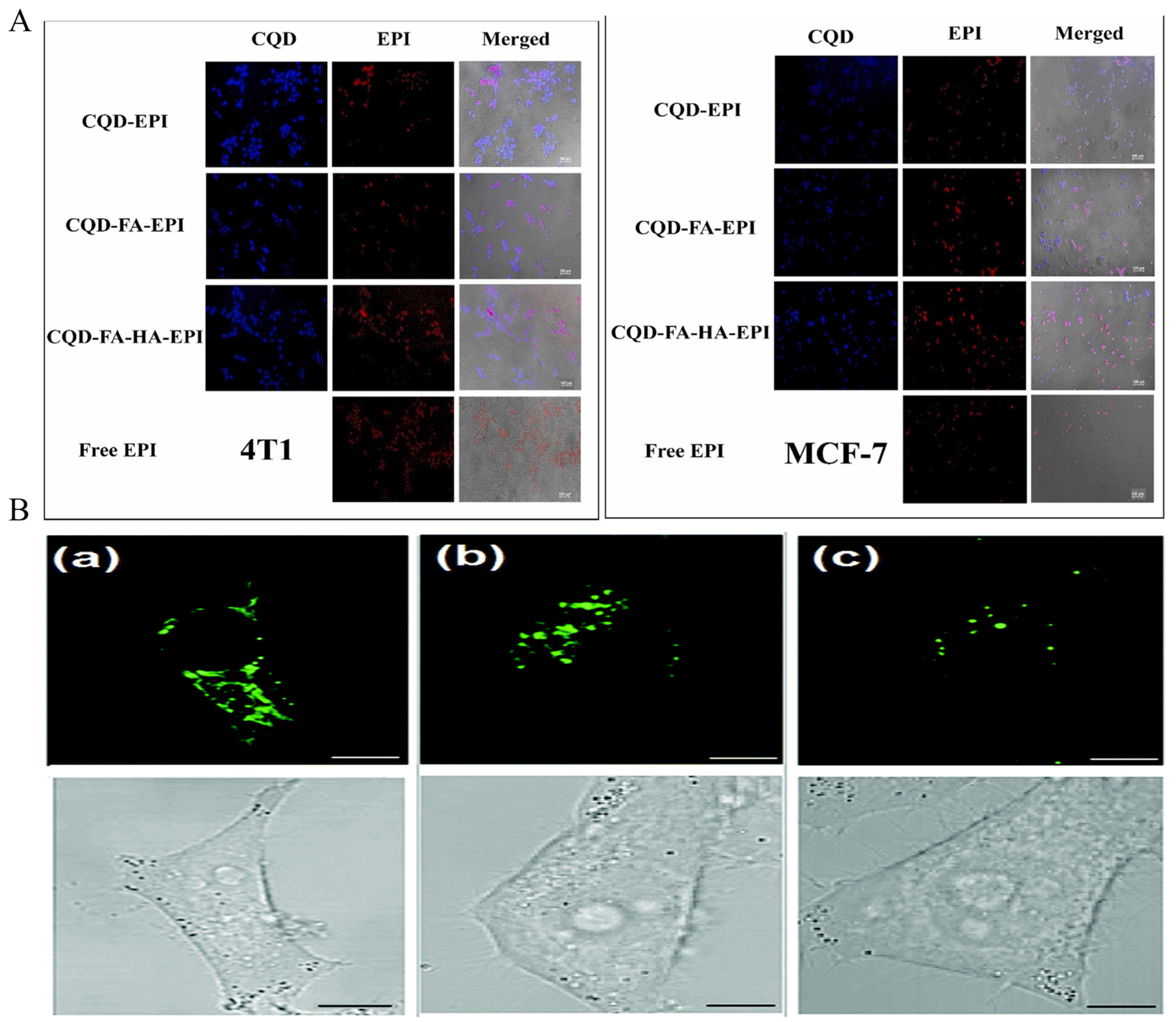
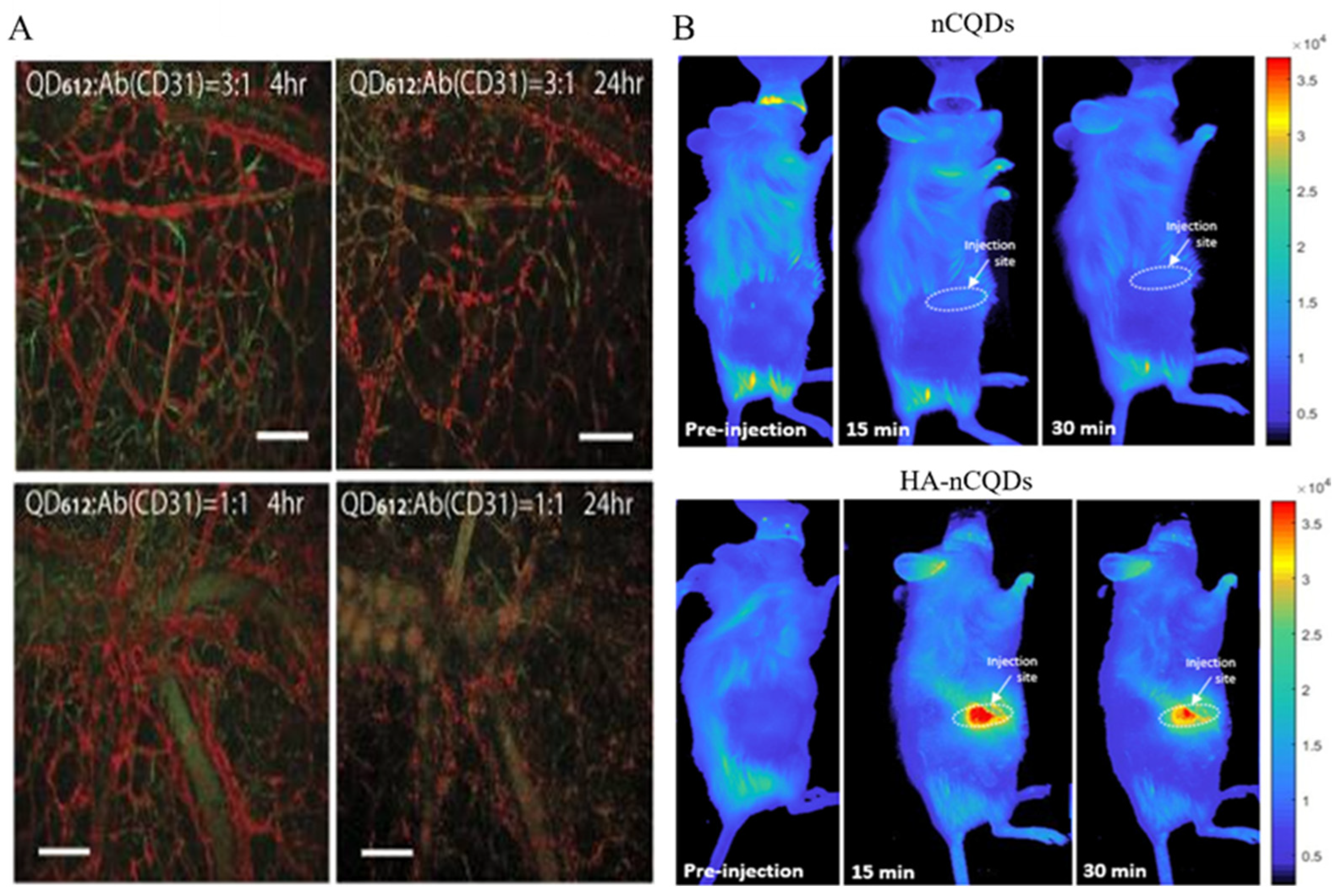
3.2. In Vivo Imaging
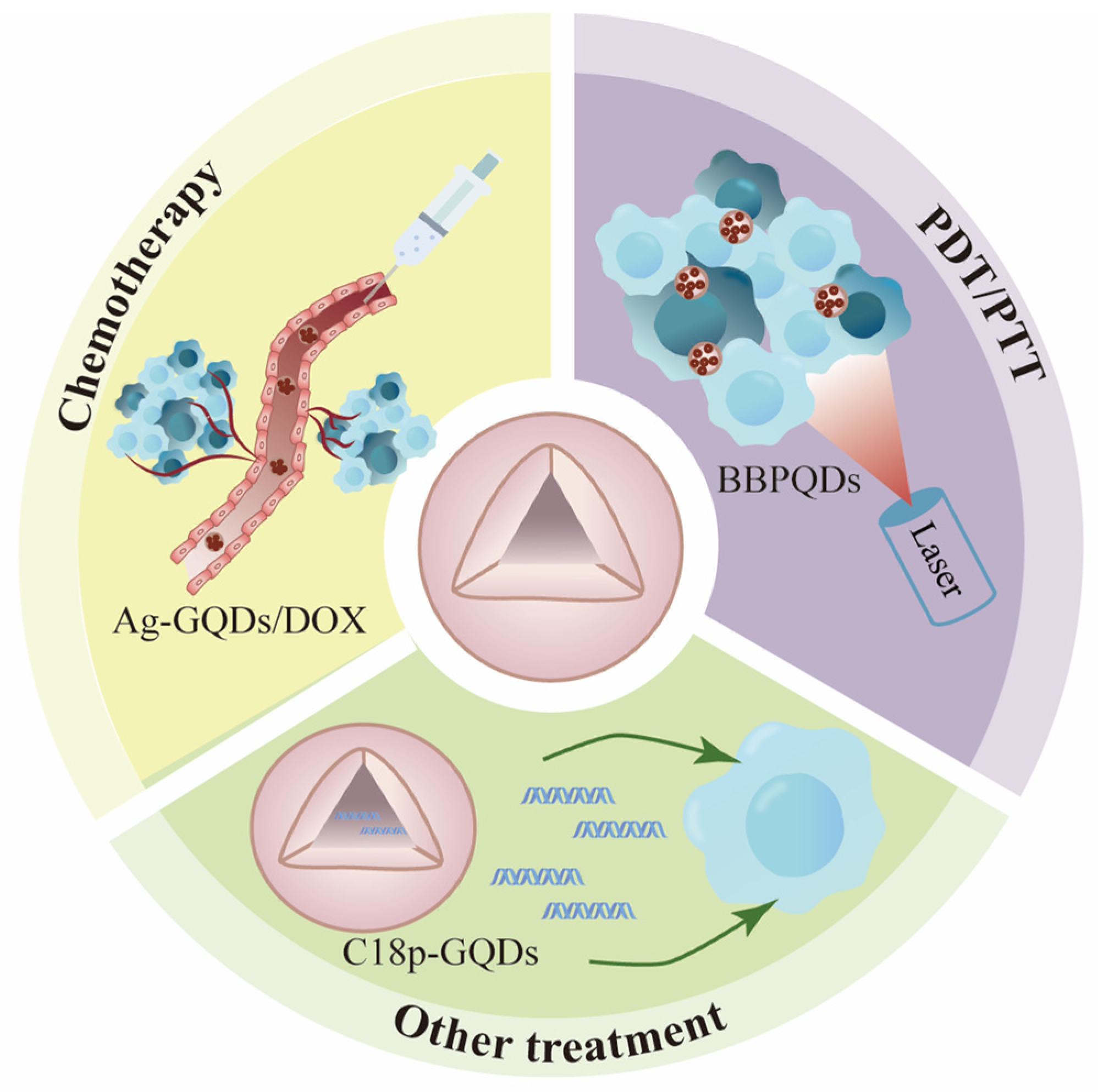
4. The Potential Application of QDs in Tumor-Targeted Therapy
4.1. Non-Functionalized Modified QDs
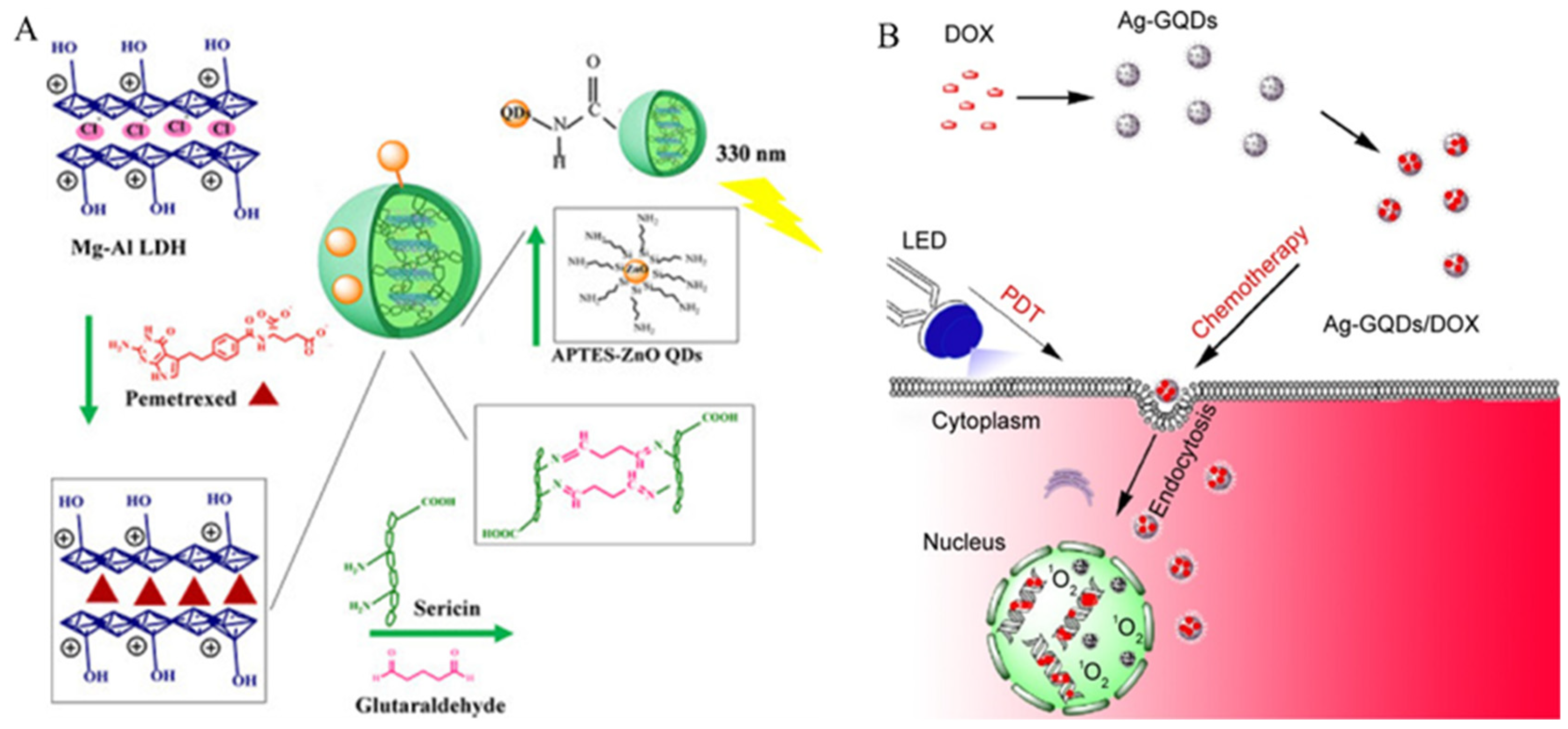
4.1.1. Chemotherapy
4.1.2. Photothermal and Photodynamic Therapy from Single-Mode to Dual-Mode
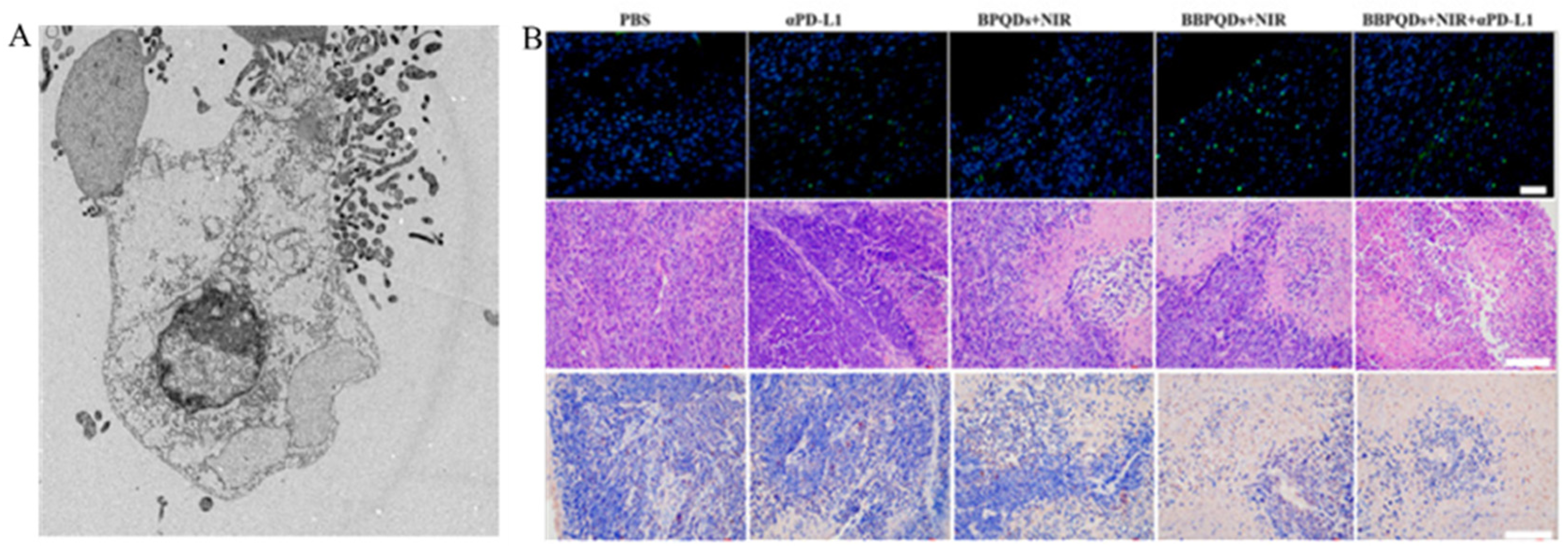
4.1.3. Other Treatments
| Modification of QDs | Modalities of Treatment | QDs | Types of Cancer |
|---|---|---|---|
| Non-functionalized modified QDs | Chemotherapy | QDs carry doxorubicin [103,104,105,106,107,108] | Breast cancer Lung cancer Cervical cancer Hepatocarcinoma Glioblastoma |
| QDs carry paclitaxel [109,110] | Hepatocarcinoma Prostate cancer | ||
| QDs carry pemetrexed [111] | Breast cancer | ||
| QDs carry 5-fluorouracil [112] | Lung cancer | ||
| PTT/PDT | CdSe/ZnS QDs [120] | Pancreatic cancer | |
| MoSe2 NDs [121] | Cervical cancer | ||
| Cu-Sec QDs [122] | Hepatocarcinoma | ||
| DPP-CTS-CQDs [124] | Hepatocarcinoma | ||
| CNQD-CN [127] | Cervical cancer | ||
| GQDs [129] | Glioblastoma Colon cancer | ||
| MXene-MOD [138] | Cervical cancer | ||
| N-B-GQDs [139] | Glioblastoma | ||
| BBPQDs [140] | Breast cancer | ||
| Other treatments | C18p-GQDs [146] | Breast cancer | |
| GQD-PEG-P [147] | Lung cancer | ||
| ChA CQDs [148] | Hepatocarcinoma |
4.2. Functionalized QDs
4.2.1. Lipid and Polysaccharide Modification
4.2.2. Protein Modification
4.2.3. Organic Polymer Modification
4.2.4. Other Modifications
| Modification of QDs | Type of Modification | QDs | Types of Cancer | Treatment |
|---|---|---|---|---|
| Functionalized QDs | Lipid and polysaccharide modification | BPQDs@EXO [149] | Bladder cancer | PTT |
| Ce6-HA-GQDs [150] | Non-small-cell carcinoma | PDT | ||
| Protein modification | Peptide E5-modified CdSe/ZnS QDs [151] | Cervical cancer | Ligand-receptor-specific binding | |
| L-cysteine-capped CdSe QDs coupled with methotrexate (MTX) [152] | Oral epidermal carcinoma | Chemotherapy | ||
| IL-13-modified CdSe QDs [153] | Glioma | Ligand-receptor-specific binding | ||
| CdTe/CdS QDs with GP73 [155] | Hepatocarcinoma | Specific binding of antigen to antibody | ||
| BPQDs@DOX@ss-Fe3O4@C-EGFR NPs [86] | Breast cancer | PDT | ||
| Organic polymer modification | CdSe-aza-BODIPY QDs [157] | Cervical cancer | PDT | |
| FA-Cys-CdTe/CdS [158] | Breast cancer | Ligand-receptor-specific binding | ||
| INOP-Mn CdS@ZnS [66] | Breast cancer | Chemotherapy | ||
| mPEG-OAL/N-CQDs [159] | Cervical cancer | Chemotherapy | ||
| TRITC-UCNP-GQDs [160] | Breast cancer | PDT | ||
| BPQDs/PLGA NS [161] | Breast cancer glioma | PTT | ||
| BP Ve-Ag QDs [162] | Breast cancer | PDT | ||
| siRNA-BPQDs [163] | Ovarian teratocarcinoma | PDT | ||
| Other modification | amino-N-GQDs [139] | Oral epidermal carcinoma | PDT | |
| BPNd [164] | Glioblastoma | PDT |
5. Challenges and Summary
Author Contributions
Funding
Conflicts of Interest
References
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Gao, X.; Su, J.Z.; Nie, S. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nat. Biotechnol. 2001, 19, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Dabbousi, B.O.; RodriguezViejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS core-shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Zhai, C.X.; Zhang, H.; Du, N.; Chen, B.D.; Huang, H.; Wu, Y.L.; Yang, D.R. One-Pot Synthesis of Biocompatible CdSe/CdS Quantum Dots and Their Applications as Fluorescent Biological Labels. Nanoscale Res. Lett. 2011, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.W.; Lee, W.Z.; Shu, I.J.; Shen, J.L.; Lin, J.C.A.; Chang, W.H.; Ruaan, R.C.; Chou, W.C. Photoluminescence of colloidal CdSe/ZnS quantum dots under oxygen atmosphere. IEEE Trans. Nanotechnol. 2005, 4, 632–636. [Google Scholar] [CrossRef]
- Wang, Y.F.; Hu, A.G. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.F.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, C.; Liu, Y.; Yang, D.; Zhang, Y.; Ren, Z.; Li, Q.; Hao, J.; Hu, W.; Wu, Y.; et al. Constructing Heterogeneous Photocatalysts Based on Carbon Nitride Nanosheets and Graphene Quantum Dots for Highly Efficient Photocatalytic Hydrogen Generation. Materials 2022, 15, 5390. [Google Scholar] [CrossRef]
- Chen, C.F.; Park, C.H.; Boudouris, B.W.; Horng, J.; Geng, B.; Girit, C.; Zettl, A.; Crommie, M.F.; Segalman, R.A.; Louie, S.G.; et al. Controlling inelastic light scattering quantum pathways in graphene. Nature 2011, 471, 617–620. [Google Scholar] [CrossRef]
- Zhu, S.J.; Wang, L.; Li, B.; Song, Y.B.; Zhao, X.H.; Zhang, G.Y.; Zhang, S.T.; Lu, S.; Zhang, J.H.; Wang, H.Y.; et al. Investigation of photoluminescence mechanism of graphene quantum dots and evaluation of their assembly into polymer dots. Carbon 2014, 77, 462–472. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Zhang, Y.H.; Liu, S.F. Recent advance in black phosphorus: Properties and applications. Mater. Chem. Phys. 2017, 189, 215–229. [Google Scholar] [CrossRef]
- Li, L.K.; Yu, Y.J.; Ye, G.J.; Ge, Q.Q.; Ou, X.D.; Wu, H.; Feng, D.L.; Chen, X.H.; Zhang, Y.B. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Dai, J.; Zeng, X.C. Electron-Transport Properties of Few-Layer Black Phosphorus. J. Phys. Chem. Lett. 2015, 6, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Wang, H.; Huang, S.X.; Xia, F.N.; Dresselhaus, M.S. The renaissance of black phosphorus. Proc. Natl. Acad. Sci. USA 2015, 112, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.T.; Najmaei, S.; Zhang, Z.H.; Zhang, J.; Lei, S.D.; Ajayan, P.M.; Yakobson, B.I.; Lou, J. Photoluminescence Quenching and Charge Transfer in Artificial Heterostacks of Monolayer Transition Metal Dichalcogenides and Few-Layer Black Phosphorus. ACS Nano 2015, 9, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.B.; Xie, H.H.; Tang, S.Y.; Yu, X.F.; Guo, Z.N.; Shao, J.D.; Zhang, H.; Huang, H.; Wang, H.Y.; Chu, P.K. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Zhang, L.W.; Cai, P.; Xue, X.G.; Wang, M.K.; Zhang, J.; Tu, G.L. Enhancing stability of red perovskite nanocrystals through copper substitution for efficient light-emitting diodes. Nano Energy 2019, 62, 434–441. [Google Scholar] [CrossRef]
- Wu, H.S.; Zhang, W.W.; Wu, J.J.; Chi, Y.W. A Visual Solar UV Sensor Based on Paraffin-Perovskite Quantum Dot Composite Film. Acs Appl. Mater. Inter. 2019, 11, 16713–16719. [Google Scholar] [CrossRef]
- Liu, Y.F.; Tang, X.S.; Zhu, T.; Deng, M.; Ikechukwu, I.P.; Huang, W.; Yin, G.L.; Bai, Y.Z.; Qu, D.R.; Huang, X.B.; et al. All-inorganic CsPbBr perovskite quantum dots as a photoluminescent probe for ultrasensitive Cu detection. J. Mater. Chem. C 2018, 6, 4793–4799. [Google Scholar] [CrossRef]
- Rainò, G.; Nedelcu, G.; Protesescu, L.; Bodnarchuk, M.I.; Kovalenko, M.V.; Mahrt, R.F.; Stöferle, T. Single Cesium Lead Halide Perovskite Nanocrystals at Low Temperature: Fast Single Photon Emission, Reduced Blinking, and Exciton Fine Structure. ACS Nano 2016, 10, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, Y.; Zhang, C.Y. Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application. Chem. Rev. 2015, 115, 11669–11717. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.S.; Wang, H.F.; Luo, P.J.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yang, J.X.; Wang, J.Z.; Lim, A.L.; Wang, S.; Loh, K.P. One-Pot Synthesis of Fluorescent Carbon Nanoribbons, Nanoparticles, and Graphene by the Exfoliation of Graphite in Ionic Liquids. ACS Nano 2009, 3, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.G.; Booker, C.; Li, R.Y.; Zhou, X.T.; Sham, T.K.; Sun, X.L.; Ding, Z.F. An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; He, X.D.; Kang, Z.H.; Huang, H.; Liu, Y.; Liu, J.L.; Lian, S.Y.; Tsang, C.H.A.; Yang, X.B.; Lee, S.T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Hu, S.L.; Tian, R.X.; Wu, L.L.; Zhao, Q.; Yang, J.L.; Liu, J.; Cao, S.R. Chemical Regulation of Carbon Quantum Dots from Synthesis to Photocatalytic Activity. Chem.-Asian J. 2013, 8, 1035–1041. [Google Scholar] [CrossRef]
- Zhuo, S.J.; Shao, M.W.; Lee, S.T. Upconversion and Downconversion Fluorescent Graphene Quantum Dots: Ultrasonic Preparation and Photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, H.M.; Liu, Z.D.; Tan, C.L.; Luo, Z.M.; Li, H.; Lin, J.D.; Sun, L.Q.; Chen, W.; Xu, Z.C.; et al. Black Phosphorus Quantum Dots. Angew. Chem. Int. Ed. 2015, 54, 3653–3657. [Google Scholar] [CrossRef]
- Lee, H.U.; Park, S.Y.; Lee, S.C.; Choi, S.; Seo, S.; Kim, H.; Won, J.; Choi, K.; Kang, K.S.; Park, H.G.; et al. Black Phosphorus (BP) Nanodots for Potential Biomedical Applications. Small 2016, 12, 214–219. [Google Scholar] [CrossRef]
- Li, L.L.; Ji, J.; Fei, R.; Wang, C.Z.; Lu, Q.; Zhang, J.R.; Jiang, L.P.; Zhu, J.J. A Facile Microwave Avenue to Electrochemiluminescent Two-Color Graphene Quantum Dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Nair, R.V.; Thomas, R.T.; Sankar, V.; Muhammad, H.; Dong, M.D.; Pillai, S. Rapid, Acid-Free Synthesis of High-Quality Graphene Quantum Dots for Aggregation Induced Sensing of Metal Ions and Bioimaging. Acs Omega 2017, 2, 8051–8061. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene Quantum Dots from: Application in Near Infrared Bioimaging and Intracellular Nanothermometry. ACS Sustain. Chem. Eng. 2017, 5, 1382–1391. [Google Scholar] [CrossRef]
- Pan, D.Y.; Zhang, J.C.; Li, Z.; Wu, M.H. Hydrothermal Route for Cutting Graphene Sheets into Blue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.Y.; Guo, L.; Zhang, J.C.; Xi, C.; Xue, Q.; Huang, H.; Li, J.H.; Zhang, Z.W.; Yu, W.J.; Chen, Z.W.; et al. Cutting spclusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J. Mater. Chem. 2012, 22, 3314–3318. [Google Scholar] [CrossRef]
- Chen, W.F.; Li, D.J.; Tian, L.; Xiang, W.; Wang, T.Y.; Hu, W.M.; Hu, Y.L.; Chen, S.N.; Chen, J.F.; Dai, Z.X. Synthesis of graphene quantum dots from natural polymer starch for cell imaging. Green Chem. 2018, 20, 4438–4442. [Google Scholar] [CrossRef]
- Lu, J.; Yeo, P.S.E.; Gan, C.K.; Wu, P.; Loh, K.P. Transforming C molecules into graphene quantum dots. Nat. Nanotechnol. 2011, 6, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Martindale, B.C.M.; Hutton, G.A.M.; Caputo, C.A.; Reisner, E. Solar Hydrogen Production Using Carbon Quantum Dots and a Molecular Nickel Catalyst. J. Am. Chem. Soc. 2015, 137, 6018–6025. [Google Scholar] [CrossRef] [PubMed]
- Gevaerd, A.; Banks, C.E.; Bergamini, M.F.; Marcolino, L.H. Graphene Quantum Dots Modified Screen-printed Electrodes as Electroanalytical Sensing Platform for Diethylstilbestrol. Electroanalysis 2019, 31, 838–843. [Google Scholar] [CrossRef]
- Wu, X.; Tian, F.; Wang, W.X.; Chen, J.; Wu, M.; Zhao, J.X. Fabrication of highly fluorescent graphene quantum dots using L-glutamic acid for in vitro/in vivo imaging and sensing. J. Mater. Chem. C 2013, 1, 4676–4684. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.S.; Li, Z.Q.; Shen, J.P.; Yang, Y.T.; Cui, X.D.; Yang, G.C. Bottom-Up Fabrication of Single-Layered Nitrogen-Doped Graphene Quantum Dots through Intermolecular Carbonization Arrayed in a 2D Plane. Chem.-Eur. J. 2016, 22, 272–278. [Google Scholar] [CrossRef]
- Zhu, J.L.; Tang, Y.F.; Wang, G.; Mao, J.R.; Liu, Z.D.; Sun, T.M.; Wang, M.; Chen, D.; Yang, Y.C.; Li, J.P.; et al. Green, Rapid, and Universal Preparation Approach of Graphene Quantum Dots under Ultraviolet Irradiation. ACS Appl. Mater. Inter. 2017, 9, 14470–14477. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, J.K.; Mattoussi, H.; Mauro, J.M.; Simon, S.M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 2003, 21, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Nabiev, I.; Mitchell, S.; Davies, A.; Williams, Y.; Kelleher, D.; Moore, R.; Gun’ko, Y.K.; Byrne, S.; Rakovich, Y.P.; Donegan, J.F.; et al. Nonfunctionalized nanocrystals can exploit a cell’s active transport machinery delivering them to specific nuclear and cytoplasmic compartments. Nano Lett. 2007, 7, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Kundrotas, G.; Karabanovas, V.; Pleckaitis, M.; Juraleviciute, M.; Steponkiene, S.; Gudleviciene, Z.; Rotomskis, R. Uptake and distribution of carboxylated quantum dots in human mesenchymal stem cells: Cell growing density matters. J. Nanobiotech. 2019, 17, 39. [Google Scholar] [CrossRef]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Pons, T.; Medintz, I.L.; Higashiya, S.; Brunel, F.M.; Dawson, P.E.; Mattoussi, H. Kinetics of metal-affinity driven self-assembly between proteins or peptides and CdSe-ZnS quantum dots. J. Phys. Chem. C 2007, 111, 11528–11538. [Google Scholar] [CrossRef]
- Delehanty, J.B.; Medintz, I.L.; Pons, T.; Brunel, F.M.; Dawson, P.E.; Mattoussi, H. Self-assembled quantum dot-peptide bioconjugates for selective intracellular delivery. Bioconjug Chem. 2006, 17, 920–927. [Google Scholar] [CrossRef]
- Dai, J.; Wang, J.; Yang, X.; Xu, Z.; Ruan, G. Examining the Cellular Transport Pathway of Fusogenic Quantum Dots Conjugated with Tat Peptide. Front. Bioeng. Biotechnol. 2022, 10, 831379. [Google Scholar] [CrossRef]
- Chang, J.C.; Su, H.L.; Hsu, S.H. The use of peptide-delivery to protect human adipose-derived adult stem cells from damage caused by the internalization of quantum dots. Biomaterials 2008, 29, 925–936. [Google Scholar] [CrossRef]
- Smith, B.R.; Cheng, Z.; De, A.; Rosenberg, J.; Gambhir, S.S. Dynamic visualization of RGD-quantum dot binding to tumor neovasculature and extravasation in multiple living mouse models using intravital microscopy. Small 2010, 6, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.A.; de Carvalho, S.M.; Mansur, H.S. Bioengineered quantum dot/chitosan-tripeptide nanoconjugates for targeting the receptors of cancer cells. Int. J. Biol. Macromol. 2016, 82, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Intracellular delivery of quantum dots for live cell labeling and organelle tracking. Adv. Mater. 2004, 16, 961–966. [Google Scholar] [CrossRef]
- Chen, F.Q.; Gerion, D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett. 2004, 4, 1827–1832. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, T.Y.; Lin, Y.; Tang, M.; Zhai, C.H.; Xia, H.F.; Wang, J.; Zhang, Z.L.; Xie, Z.X.; Chen, G.; et al. Transformation of Viral Light Particles into Near-Infrared Fluorescence Quantum Dot-Labeled Active Tumor-Targeting Nanovectors for Drug Delivery. Nano Lett. 2019, 19, 7035–7042. [Google Scholar] [CrossRef] [PubMed]
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, J.R.; Chakrabarti, L.; Dai, H.N.; Carney, R.S.E.; Hirata, T.; Bregman, B.S.; Gallicano, G.I.; Corbin, J.G.; Haydar, T.F. In vivo quantum dot Labeling of mammalian stem and progenitor cells. Dev. Dynam 2007, 236, 3393–3401. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Larson, D.R.; Zipfel, W.R.; Williams, R.M.; Clark, S.W.; Bruchez, M.P.; Wise, F.W.; Webb, W.W. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 2003, 300, 1434–1436. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Liu, J.; Haley, K.N.; Treadway, J.A.; Larson, J.P.; Ge, N.; Peale, F.; Bruchez, M.P. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 2003, 21, 41–46. [Google Scholar] [CrossRef]
- Lin, Z.; Ma, Q.; Fei, X.; Zhang, H.; Su, X. A novel aptamer functionalized CuInS2 quantum dots probe for daunorubicin sensing and near infrared imaging of prostate cancer cells. Anal. Chim. Acta 2014, 818, 54–60. [Google Scholar] [CrossRef]
- Alibolandi, M.; Abnous, K.; Ramezani, M.; Hosseinkhani, H.; Hadizadeh, F. Synthesis of AS1411-aptamer-conjugated CdTe quantum dots with high fluorescence strength for probe labeling tumor cells. J. Fluoresc. 2014, 24, 1519–1529. [Google Scholar] [CrossRef]
- Xu, N.; Piao, M.; Arkin, K.; Ren, L.; Zhang, J.; Hao, J.; Zheng, Y.; Shang, Q. Imaging of water soluble CdTe/CdS core-shell quantum dots in inhibiting multidrug resistance of cancer cells. Talanta 2019, 201, 309–316. [Google Scholar] [CrossRef]
- Louie, A. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef]
- Bai, W.F.; Zhang, K.S.; Yu, S.H.; Zhang, J.P.; Jin, L. The preparation of MnO/BSA/CdTe quantum dots complex for ratiometric fluorescence/T-weighted MRI detection of HO. Talanta 2023, 252, 123774. [Google Scholar] [CrossRef]
- Mitra, R.N.; Doshi, M.; Zhang, X.; Tyus, J.C.; Bengtsson, N.; Fletcher, S.; Page, B.D.; Turkson, J.; Gesquiere, A.J.; Gunning, P.T.; et al. An activatable multimodal/multifunctional nanoprobe for direct imaging of intracellular drug delivery. Biomaterials 2012, 33, 1500–1508. [Google Scholar] [CrossRef]
- Radchanka, A.; Iodchik, A.; Terpinskaya, T.; Balashevich, T.; Yanchanka, T.; Palukoshka, A.; Sizova, S.; Oleinikov, V.; Feofanov, A.; Artemyev, M. Emitters with different dimensionality: 2D cadmium chalcogenide nanoplatelets and 0D quantum dots in non-specific cell labeling and two-photon imaging. Nanotechnology 2020, 31, 435102. [Google Scholar] [CrossRef]
- Du, F.; Min, Y.; Zeng, F.; Yu, C.; Wu, S. A targeted and FRET-based ratiometric fluorescent nanoprobe for imaging mitochondrial hydrogen peroxide in living cells. Small 2014, 10, 964–972. [Google Scholar] [CrossRef]
- Irmania, N.; Dehvari, K.; Gedda, G.; Tseng, P.J.; Chang, J.Y. Manganese-doped green tea-derived carbon quantum dots as a targeted dual imaging and photodynamic therapy platform. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1616–1625. [Google Scholar] [CrossRef]
- Yang, W.; Fan, H.; Gao, X.; Gao, S.; Karnati, V.V.; Ni, W.; Hooks, W.B.; Carson, J.; Weston, B.; Wang, B. The first fluorescent diboronic acid sensor specific for hepatocellular carcinoma cells expressing sialyl Lewis X. Chem. Biol. 2004, 11, 439–448. [Google Scholar] [CrossRef]
- Das, R.K.; Mohapatra, S. Highly luminescent, heteroatom-doped carbon quantum dots for ultrasensitive sensing of glucosamine and targeted imaging of liver cancer cells. J. Mater. Chem. B 2017, 5, 2190–2197. [Google Scholar] [CrossRef]
- Gao, N.; Yang, W.; Nie, H.; Gong, Y.; Jing, J.; Gao, L.; Zhang, X. Turn-on theranostic fluorescent nanoprobe by electrostatic self-assembly of carbon dots with doxorubicin for targeted cancer cell imaging, in vivo hyaluronidase analysis, and targeted drug delivery. Biosens. Bioelectron. 2017, 96, 300–307. [Google Scholar] [CrossRef]
- Ziaee, N.; Farhadian, N.; Abnous, K.; Matin, M.M.; Khoshnood, A.; Yaghoobi, E. Dual targeting of Mg/N doped-carbon quantum dots with folic and hyaluronic acid for targeted drug delivery and cell imaging. Biomed. Pharmacother. 2023, 164, 114971. [Google Scholar] [CrossRef]
- Zheng, M.; Ruan, S.; Liu, S.; Sun, T.; Qu, D.; Zhao, H.; Xie, Z.; Gao, H.; Jing, X.; Sun, Z. Self-Targeting Fluorescent Carbon Dots for Diagnosis of Brain Cancer Cells. ACS Nano 2015, 9, 11455–11461. [Google Scholar] [CrossRef]
- Zou, F.; Zhou, H.; Tan, T.V.; Kim, J.; Koh, K.; Lee, J. Dual-Mode SERS-Fluorescence Immunoassay Using Graphene Quantum Dot Labeling on One-Dimensional Aligned Magnetoplasmonic Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 12168–12175. [Google Scholar] [CrossRef]
- Ananthanarayanan, A.; Wang, Y.; Routh, P.; Sk, M.A.; Than, A.; Lin, M.; Zhang, J.; Chen, J.; Sun, H.; Chen, P. Nitrogen and phosphorus co-doped graphene quantum dots: Synthesis from adenosine triphosphate, optical properties, and cellular imaging. Nanoscale 2015, 7, 8159–8165. [Google Scholar] [CrossRef]
- Chen, S.; Hai, X.; Xia, C.; Chen, X.W.; Wang, J.H. Preparation of excitation-independent photoluminescent graphene quantum dots with visible-light excitation/emission for cell imaging. Chemistry 2013, 19, 15918–15923. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Chen, C.Q.; Zheng, X.T.; Gao, L.L.; Cui, Z.M.; Yang, H.B.; Guo, C.X.; Chi, Y.W.; Li, C.M. One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J. Mater. Chem. 2012, 22, 8764–8766. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Nafiujjaman, M.; Joon, H.; Kwak, K.S.; Lee, Y.K. Synthesis of Nitrogen- and Chlorine-Doped Graphene Quantum Dots for Cancer Cell Imaging. J. Nanosci. Nanotechnol. 2018, 18, 3793–3799. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Lao, J.; He, H.; Cheng, T.; Wang, M.; Wang, S.; Huang, F. Multifunctional graphene quantum dots for simultaneous targeted cellular imaging and drug delivery. Colloids Surf. B Biointerfaces 2014, 122, 638–644. [Google Scholar] [CrossRef]
- Luo, Z.M.; Yang, D.L.; Yang, C.; Wu, X.Y.; Hu, Y.L.; Zhang, Y.; Yuwen, L.H.; Yeow, E.K.L.; Weng, L.X.; Huang, W.; et al. Graphene quantum dots modified with adenine for efficient two-photon bioimaging and white light-activated antibacteria. Appl. Surf. Sci. 2018, 434, 155–162. [Google Scholar] [CrossRef]
- Wu, P.C.; Wang, J.Y.; Wang, W.L.; Chang, C.Y.; Huang, C.H.; Yang, K.L.; Chang, J.C.; Hsu, C.L.L.L.; Chen, S.Y.; Chou, T.M.; et al. Efficient two-photon luminescence for cellular imaging using biocompatible nitrogen-doped graphene quantum dots conjugated with polymers. Nanoscale 2018, 10, 109–117. [Google Scholar] [CrossRef]
- Sun, Z.B.; Zhao, Y.T.; Li, Z.B.; Cui, H.D.; Zhou, Y.Y.; Li, W.H.; Tao, W.; Zhang, H.; Wang, H.Y.; Chu, P.K.; et al. TiL-Coordinated Black Phosphorus Quantum Dots as an Efficient Contrast Agent for In Vivo Photoacoustic Imaging of Cancer. Small 2017, 13, 1602896. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, H.; Gui, R. Visual bio-detection and versatile bio-imaging of zinc-ion-coordinated black phosphorus quantum dots with improved stability and bright fluorescence. Biosens. Bioelectron. 2020, 165, 112390. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Wu, F.; Graveran, K.; Zhang, J.; Wu, C. Black Phosphorus Quantum Dots Gated, Carbon-Coated Fe3O4 Nanocapsules (BPQDs@ss-Fe3O4@C) with Low Premature Release Could Enable Imaging-Guided Cancer Combination Therapy. Chemistry 2018, 24, 12890–12901. [Google Scholar] [CrossRef]
- Jana, A.; Kim, K.S. Water-Stable, Fluorescent Organic Inorganic Hybrid and Fully Inorganic Perovskites. Acs Energy Lett. 2018, 3, 2120–2126. [Google Scholar] [CrossRef]
- Zheng, L.L.; Ma, Y.Z.; Xiao, L.X.; Zhang, F.Y.; Wang, Y.H.; Yang, H.X. Water-Soluble Polymeric Interfacial Material for Planar Perovskite Solar Cells. ACS Appl. Mater. Inter. 2017, 9, 14129–14135. [Google Scholar] [CrossRef]
- Pramanik, A.; Gates, K.; Patibandla, S.; Davis, D.; Begum, S.; Iftekhar, R.; Alamgir, S.; Paige, S.; Porter, M.M.; Ray, P.C. Water-Soluble and Bright Luminescent Cesium-Lead-Bromide Perovskite Quantum Dot-Polymer Composites for Tumor-Derived Exosome Imaging. Acs Appl. Bio Mater. 2019, 2, 5872–5879. [Google Scholar] [CrossRef]
- Getachew, G.; Korupalli, C.; Rasal, A.S.; Dirersa, W.B.; Fahmi, M.Z.; Chang, J.Y. Highly Luminescent, Stable, and Red-Emitting CsMgPbI Quantum Dots for Dual-Modal Imaging-Guided Photodynamic Therapy and Photocatalytic Activity. ACS Appl. Mater. Inter. 2022, 14, 278–296. [Google Scholar] [CrossRef]
- Han, H.S.; Niemeyer, E.; Huang, Y.; Kamoun, W.S.; Martin, J.D.; Bhaumik, J.; Chen, Y.; Roberge, S.; Cui, J.; Martin, M.R.; et al. Quantum dot/antibody conjugates for in vivo cytometric imaging in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 1350–1355. [Google Scholar] [CrossRef]
- Yao, F.; Wang, Z.G.; Liu, S.L.; Wang, H.; Zhu, J.; He, R.; Yang, X.; Liu, X.; Wu, Q.; Wu, J.K. Purified fluorescent nanohybrids based on quantum dot-HER2-antibody for breast tumor target imaging. Talanta 2023, 260, 124560. [Google Scholar] [CrossRef]
- Mansur, A.A.; Mansur, H.S.; Soriano-Araujo, A.; Lobato, Z.I. Fluorescent nanohybrids based on quantum dot-chitosan-antibody as potential cancer biomarkers. ACS Appl. Mater. Interfaces 2014, 6, 11403–11412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yue, J.; Cui, R.; Ma, Z.; Wan, H.; Wang, F.; Zhu, S.; Zhou, Y.; Kuang, Y.; Zhong, Y.; et al. Bright quantum dots emitting at approximately 1,600 nm in the NIR-IIb window for deep tissue fluorescence imaging. Proc. Natl. Acad. Sci. USA 2018, 115, 6590–6595. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Y.; Peng, F.; Zhong, Y.; Zhou, Y.; Jiang, X.; Su, Y.; He, Y. Photostable water-dispersible NIR-emitting CdTe/CdS/ZnS core-shell-shell quantum dots for high-resolution tumor targeting. Biomaterials 2013, 34, 9509–9518. [Google Scholar] [CrossRef]
- Gil, H.M.; Price, T.W.; Chelani, K.; Bouillard, J.G.; Calaminus, S.D.J.; Stasiuk, G.J. NIR-quantum dots in biomedical imaging and their future. iScience 2021, 24, 102189. [Google Scholar] [CrossRef] [PubMed]
- Karakocak, B.B.; Laradji, A.; Primeau, T.; Berezin, M.Y.; Li, S.; Ravi, N. Hyaluronan-Conjugated Carbon Quantum Dots for Bioimaging Use. ACS Appl. Mater. Interfaces 2021, 13, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Luo, Y.C.; Lv, Y.Y.; Zhang, Y.Y.; Wu, Y.Z.; Yao, S.H.; Zhou, J.; Chen, Y.B.; Chen, Y.F. Electrical scattering mechanism evolution in un-doped and halogen-doped BiOSe single crystals. J. Phys.-Condens. Mat. 2020, 32, 365705. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Liu, M.; You, B.; Luo, G.; Chen, Y.; Liu, B.; Jiang, Z.; Chu, P.K.; Shao, J.; Yu, X.F. Biodegradable Bi2O2Se Quantum Dots for Photoacoustic Imaging-Guided Cancer Photothermal Therapy. Small 2020, 16, e1905208. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Li, Y.; Li, X.; Zhu, J.; Fan, L.; Yang, S. Exceptionally High Payload of the IR780 Iodide on Folic Acid-Functionalized Graphene Quantum Dots for Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 22332–22341. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, B.; Rao, Z.; Zhang, B.; Gong, J.R. Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett. 2013, 13, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Zhou, S.X.; Fan, L.Z.; Fan, H. Synthesis of red fluorescent graphene quantum dot-europium complex composites as a viable bioimaging platform. Microchim. Acta 2016, 183, 2605–2613. [Google Scholar] [CrossRef]
- Abdelgalil, R.M.; Khattab, S.N.; Ebrahim, S.; Elkhodairy, K.A.; Teleb, M.; Bekhit, A.A.; Sallam, M.A.; Elzoghby, A.O. Engineered Sericin-Tagged Layered Double Hydroxides for Combined Delivery of Pemetrexed and ZnO Quantum Dots as Biocompatible Cancer Nanotheranostics. ACS Omega 2023, 8, 5655–5671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liu, X.; Li, Y.; Zhang, M.; He, J.; Zhang, X.; Liu, H.; Wang, X.; Gu, H. Fluorescence and drug loading properties of ZnSe:Mn/ZnS-Paclitaxel/SiO2 nanocapsules templated by F127 micelles. J. Colloid Interface Sci. 2017, 490, 436–443. [Google Scholar] [CrossRef]
- Habiba, K.; Encarnacion-Rosado, J.; Garcia-Pabon, K.; Villalobos-Santos, J.C.; Makarov, V.I.; Avalos, J.A.; Weiner, B.R.; Morell, G. Improving cytotoxicity against cancer cells by chemo-photodynamic combined modalities using silver-graphene quantum dots nanocomposites. Int. J. Nanomed. 2016, 11, 107–119. [Google Scholar] [CrossRef]
- Chiu, S.H.; Gedda, G.; Girma, W.M.; Chen, J.K.; Ling, Y.C.; Ghule, A.V.; Ou, K.L.; Chang, J.Y. Rapid fabrication of carbon quantum dots as multifunctional nanovehicles for dual-modal targeted imaging and chemotherapy. Acta Biomater. 2016, 46, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jiang, H.; Dong, J.; Zhang, W.; Dang, G.; Yang, M.; Li, Y.; Chen, H.; Ji, H.; Dong, L. PEGylated MoS2 quantum dots for traceable and pH-responsive chemotherapeutic drug delivery. Colloids Surf. B Biointerfaces 2020, 185, 110590. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, K.; Zhu, P.; Zou, X.; Ma, G.; Zhang, W.; Wang, D.; Wan, J.; Ma, Y.; Sun, X.; et al. A near-infrared triggered upconversion/MoS2 nanoplatform for tumour-targeted chemo-photodynamic combination therapy. Colloids Surf. B Biointerfaces 2022, 213, 112393. [Google Scholar] [CrossRef] [PubMed]
- Olerile, L.D.; Liu, Y.; Zhang, B.; Wang, T.; Mu, S.; Zhang, J.; Selotlegeng, L.; Zhang, N. Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Colloids Surf. B Biointerfaces 2017, 150, 121–130. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Z.; Lin, Z.; Su, X. CuInS2 quantum dots/poly((L)-glutamic acid)-drug conjugates for drug delivery and cell imaging. Analyst 2014, 139, 831–836. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A.; Abdullah, J.; Alitheen, N.B.; Hussein, M.Z.; Abu, N.; Mohammed, N.E.; Nordin, N.; Zamberi, N.R.; et al. In vivo tumor targeting and anti-tumor effects of 5-fluororacil loaded, folic acid targeted quantum dot system. J. Colloid Interface Sci. 2016, 480, 146–158. [Google Scholar] [CrossRef]
- Kurniawan, D.; Mathew, J.; Rahardja, M.R.; Pham, H.P.; Wong, P.C.; Rao, N.V.; Ostrikov, K.K.; Chiang, W.H. Plasma-Enabled Graphene Quantum Dot Hydrogels as Smart Anticancer Drug Nanocarriers. Small 2023, 19, e2206813. [Google Scholar] [CrossRef] [PubMed]
- Zrazhevskiy, P.; Gao, X. Multifunctional Quantum Dots for Personalized Medicine. Nano Today 2009, 4, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Wilson, B.C. Photodynamic therapy for cancer: Principles. Can. J. Gastroenterol. 2002, 16, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.K.; Park, I.K.; Hwang, S.R. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Bakalova, R.; Ohba, H.; Zhelev, Z.; Ishikawa, M.; Baba, Y. Quantum dots as photosensitizers? Nat. Biotechnol. 2004, 22, 1360–1361. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, P.; Li, L.; Yang, M. Biochemistry and biomedicine of quantum dots: From biodetection to bioimaging, drug discovery, diagnostics, and therapy. Acta Biomater. 2018, 74, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Samia, A.C.; Chen, X.; Burda, C. Semiconductor quantum dots for photodynamic therapy. J. Am. Chem. Soc. 2003, 125, 15736–15737. [Google Scholar] [CrossRef]
- He, S.J.; Cao, J.; Li, Y.S.; Yang, J.C.; Zhou, M.; Qu, C.Y.; Zhang, Y.; Shen, F.; Chen, Y.; Li, M.M.; et al. CdSe/ZnS quantum dots induce photodynamic effects and cytotoxicity in pancreatic cancer cells. World J. Gastroenterol. 2016, 22, 5012–5022. [Google Scholar] [CrossRef]
- Yuwen, L.; Zhou, J.; Zhang, Y.; Zhang, Q.; Shan, J.; Luo, Z.; Weng, L.; Teng, Z.; Wang, L. Aqueous phase preparation of ultrasmall MoSe2 nanodots for efficient photothermal therapy of cancer cells. Nanoscale 2016, 8, 2720–2726. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, Y.; Pan, S.; Tan, Y.; Sun, C.; Cao, M.; Xu, H. Copper-Selenocysteine Quantum Dots for NIR-II Photothermally Enhanced Chemodynamic Therapy. ACS Appl. Bio Mater. 2022, 5, 1794–1803. [Google Scholar] [CrossRef]
- Fan, J.X.; Liu, M.D.; Li, C.X.; Hong, S.; Zheng, D.W.; Liu, X.H.; Chen, S.; Cheng, H.; Zhang, X.Z. A metal-semiconductor nanocomposite as an efficient oxygen-independent photosensitizer for photodynamic tumor therapy. Nanoscale Horiz. 2017, 2, 349–355. [Google Scholar] [CrossRef]
- He, H.; Zheng, X.; Liu, S.; Zheng, M.; Xie, Z.; Wang, Y.; Yu, M.; Shuai, X. Diketopyrrolopyrrole-based carbon dots for photodynamic therapy. Nanoscale 2018, 10, 10991–10998. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Xu, W.; Niandu, W.; Wang, Z.K.; Wang, Y.; Zhang, Y.; Zhong, W.; Cai, H.L.; Wu, X.S. Bridging and bonding: Zinc and potassium co-assisted crystalline g-CN for significant highly efficient upon photocatalytic hydrogen evolution. Appl. Surf. Sci. 2021, 542, 148620. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Zhou, X.; Li, M.M.; Wang, Y.Q.; Zhou, B.Y.; Wu, N.D.; Zhong, W.; Cai, H.L.; Wu, X.S. 2D/3D interface engineering: Direct Z-scheme g-CN/YMnO heterojunction for reinforced visible-light photocatalytic oxidation. J. Mater. Sci-Mater. 2019, 30, 17601–17611. [Google Scholar] [CrossRef]
- Liu, H.; Lv, X.; Qian, J.; Li, H.; Qian, Y.; Wang, X.; Meng, X.; Lin, W.; Wang, H. Graphitic Carbon Nitride Quantum Dots Embedded in Carbon Nanosheets for Near-Infrared Imaging-Guided Combined Photo-Chemotherapy. ACS Nano 2020, 14, 13304–13315. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Ristic, B.Z.; Arsikin, K.M.; Klisic, D.G.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepic, D.P.; Kravic-Stevovic, T.K.; Jovanovic, S.P.; Milenkovic, M.M.; et al. Graphene quantum dots as autophagy-inducing photodynamic agents. Biomaterials 2012, 33, 7084–7092. [Google Scholar] [CrossRef]
- Ruan, J.; Wang, Y.; Li, F.; Jia, R.; Zhou, G.; Shao, C.; Zhu, L.; Cui, M.; Yang, D.P.; Ge, S. Graphene Quantum Dots for Radiotherapy. ACS Appl. Mater. Interfaces 2018, 10, 14342–14355. [Google Scholar] [CrossRef]
- Nolsøe, C.P.; Torp-Pedersen, S.; Burcharth, F.; Horn, T.; Pedersen, S.; Christensen, N.E.; Olldag, E.S.; Andersen, P.H.; Karstrup, S.; Lorentzen, T.; et al. Interstitial hyperthermia of colorectal liver metastases with a US-guided Nd-YAG laser with a diffuser tip: A pilot clinical study. Radiology 1993, 187, 333–337. [Google Scholar] [CrossRef]
- McMillan, K.; Perepelitsyn, I.; Wang, Z.; Shapshay, S.M. Tumor growth inhibition and regression induced by photothermal vascular targeting and angiogenesis inhibitor retinoic acid. Cancer Lett. 1999, 137, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Liu, J.; Gu, S.; Yuan, Q.; Ren, J.; Qu, X. Long-circulating Er3+-doped Yb2O3 up-conversion nanoparticle as an in vivo X-Ray CT imaging contrast agent. Biomaterials 2012, 33, 6748–6757. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold Nanomaterials at Work in Biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.Y.; Wu, Y.; Xu, J.; Xiong, W.; Xu, W.; Li, J.; Sun, Z.; Lv, Z.; Wu, X.S.; Jiang, Q.; et al. Niobium carbide (MXene) reduces UHMWPE particle-induced osteolysis. Bioact. Mater. 2022, 8, 435–448. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Zhou, X.; Song, R.; Tang, W.; Yang, D.; Wang, Y.; Lv, Z.; Zhong, W.; Cai, H.L.; et al. Piezo-Activated Atomic-Thin Molybdenum Disulfide/MXene Nanoenzyme for Integrated and Efficient Tumor Therapy via Ultrasound-Triggered Schottky Electric Field. Small 2023, 19, e2205053. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cai, X.; Cui, H.; Lee, S.W.; Yu, X.F.; Liu, B. Fluorine-free preparation of titanium carbide MXene quantum dots with high near-infrared photothermal performances for cancer therapy. Nanoscale 2017, 9, 17859–17864. [Google Scholar] [CrossRef]
- Kuo, W.S.; Shao, Y.T.; Huang, K.S.; Chou, T.M.; Yang, C.H. Antimicrobial Amino-Functionalized Nitrogen-Doped Graphene Quantum Dots for Eliminating Multidrug-Resistant Species in Dual-Modality Photodynamic Therapy and Bioimaging under Two-Photon Excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446. [Google Scholar] [CrossRef]
- Zhao, P.; Xu, Y.; Ji, W.; Zhou, S.; Li, L.; Qiu, L.; Qian, Z.; Wang, X.; Zhang, H. Biomimetic black phosphorus quantum dots-based photothermal therapy combined with anti-PD-L1 treatment inhibits recurrence and metastasis in triple-negative breast cancer. J. Nanobiotech. 2021, 19, 181. [Google Scholar] [CrossRef]
- Amer, M.H. Gene therapy for cancer: Present status and future perspective. Mol. Cell. Ther. 2014, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Pasi, K.J.; Rangarajan, S.; Mitchell, N.; Lester, W.; Symington, E.; Madan, B.; Laffan, M.; Russell, C.B.; Li, M.; Pierce, G.F.; et al. Multiyear Follow-up of AAV5-hFVIII-SQ Gene Therapy for Hemophilia A. N. Engl. J. Med. 2020, 382, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Hinderer, C.; Katz, N.; Buza, E.L.; Dyer, C.; Goode, T.; Bell, P.; Richman, L.K.; Wilson, J.M. Severe Toxicity in Nonhuman Primates and Piglets Following High-Dose Intravenous Administration of an Adeno-Associated Virus Vector Expressing Human SMN. Hum. Gene Ther. 2018, 29, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Kafshdooz, T.; Bakhtiary, M.; Nikzamir, N.; Nikzamir, N.; Nikzamir, M.; Mohammadian, M.; Akbarzadeh, A. Biomedical and biological applications of quantum dots. Artif. Cells Nanomed. Biotechnol. 2016, 44, 885–891. [Google Scholar] [CrossRef] [PubMed]
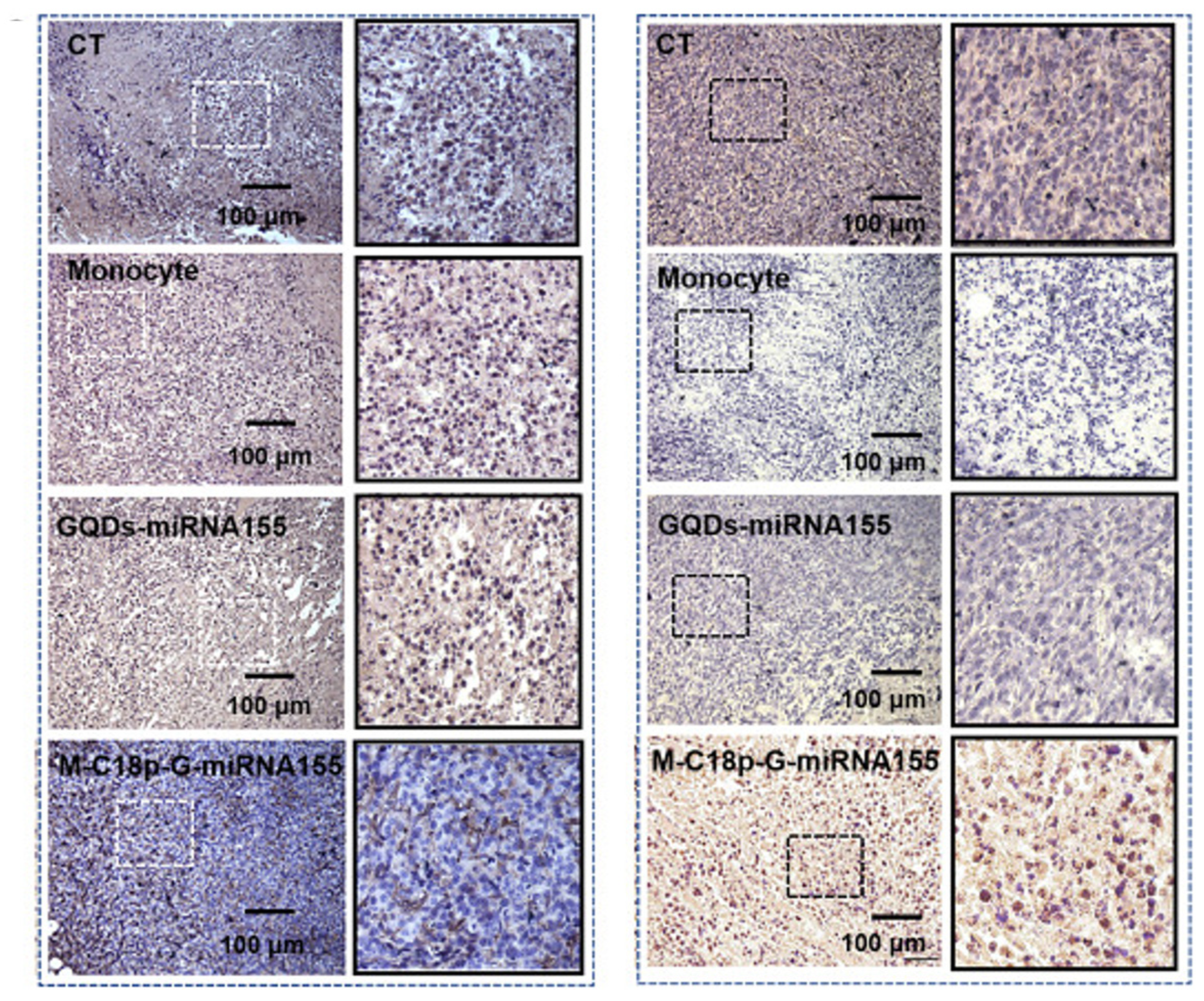
- Xia, Q.; Tang, Y.; Li, W.; Liang, T.; Zhou, Y.; Liu, J.; Liu, F. Surface-Engineered Monocyte Immunotherapy Combined Graphene Quantum Dots Effective Against Solid Tumor Targets. Int. J. Nanomed. 2023, 18, 2127–2140. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, H.; Yang, Z.; Zhong, X.; Chen, Y.; Dai, W.; Zhang, X. Aptamer-Conjugated Graphene Quantum Dots/Porphyrin Derivative Theranostic Agent for Intracellular Cancer-Related MicroRNA Detection and Fluorescence-Guided Photothermal/Photodynamic Synergetic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhao, M.M.; Luo, Q.W.; Zhang, Y.C.; Liu, T.T.; Yang, Z.; Liao, M.; Tu, P.; Zeng, K.W. Carbon Quantum Dots-Based Nanozyme from Coffee Induces Cancer Cell Ferroptosis to Activate Antitumor Immunity. ACS Nano 2022, 16, 9228–9239. [Google Scholar] [CrossRef]
- Liu, J.; Yi, K.; Zhang, Q.; Xu, H.; Zhang, X.; He, D.; Wang, F.; Xiao, X. Strong Penetration-Induced Effective Photothermal Therapy by Exosome-Mediated Black Phosphorus Quantum Dots. Small 2021, 17, e2104585. [Google Scholar] [CrossRef]
- Nafiujjaman, M.; Revuri, V.; Park, H.K.; Kwon, I.K.; Cho, K.J.; Lee, Y.K. Enhanced Photodynamic Properties of Graphene Quantum Dot Conjugated Ce6 Nanoparticles for Targeted Cancer Therapy and Imaging. Chem. Lett. 2016, 45, 997–999. [Google Scholar] [CrossRef]
- Zu, R.; Fang, X.; Lin, Y.; Xu, S.; Meng, J.; Xu, H.; Yang, Y.; Wang, C. Peptide-enabled receptor-binding-quantum dots for enhanced detection and migration inhibition of cancer cells. J. Biomater. Sci. Polym. Ed. 2020, 31, 1604–1621. [Google Scholar] [CrossRef]
- Johari-Ahar, M.; Barar, J.; Alizadeh, A.M.; Davaran, S.; Omidi, Y.; Rashidi, M.R. Methotrexate-conjugated quantum dots: Synthesis, characterisation and cytotoxicity in drug resistant cancer cells. J. Drug Target. 2016, 24, 120–133. [Google Scholar] [CrossRef]
- Madhankumar, A.B.; Mrowczynski, O.D.; Patel, S.R.; Weston, C.L.; Zacharia, B.E.; Glantz, M.J.; Siedlecki, C.A.; Xu, L.C.; Connor, J.R. Interleukin-13 conjugated quantum dots for identification of glioma initiating cells and their extracellular vesicles. Acta Biomater. 2017, 58, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.S.; Wu, D.W.; Liang, S.; Miao, X.Y. GP73, a resident Golgi glycoprotein, is sensibility and specificity for hepatocellular carcinoma of diagnosis in a hepatitis B-endemic Asian population. Med. Oncol. 2010, 27, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, A.X.; Xu, G.H.; Wei, F.D.; Yang, J.; Hu, Q. Manganese modified CdTe/CdS quantum dots as an immunoassay biosensor for the detection of Golgi protein-73. J. Pharm. Biomed. 2016, 117, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ke, J.; Zhou, X.E.; Yi, W.; Brunzelle, J.S.; Li, J.; Yong, E.L.; Xu, H.E.; Melcher, K. Structural basis for molecular recognition of folic acid by folate receptors. Nature 2013, 500, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Tian, J.W.; Hu, F.R.; Wang, X.Y.; Shen, Z. A near IR photosensitizer based on self-assembled CdSe quantum dot-aza-BODIPY conjugate coated with poly(ethylene glycol) and folic acid for concurrent fluorescence imaging and photodynamic therapy. RSC Adv. 2016, 6, 113991–113996. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Fei, X.; Li, J.; Zheng, Y.; Li, B.; Zhang, T. Identification and elimination of cancer cells by folate-conjugated CdTe/CdS Quantum Dots Chiral Nano-Sensors. Biochem. Biophys. Res. Commun. 2021, 560, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Ma, H.B.; Wang, N.; He, Z.H. pH-sensitive carbon quantum dots-doxorubicin nanoparticles for tumor cellular targeted drug delivery. Polym. Adv. Technol. 2019, 30, 2664–2673. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, L.; Huang, R.; Wang, H.; Hu, X.; Xing, D. Mitochondrial specific photodynamic therapy by rare-earth nanoparticles mediated near-infrared graphene quantum dots. Biomaterials 2018, 153, 14–26. [Google Scholar] [CrossRef]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.F.; Zhao, Y.; Zhang, H.; et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Q.; Ye, J.; Ge, X.; Wang, J.; Song, J.; Yang, H. Ag(+) -Coupled Black Phosphorus Vesicles with Emerging NIR-II Photoacoustic Imaging Performance for Cancer Immune-Dynamic Therapy and Fast Wound Healing. Angew. Chem. Int. Ed. Engl. 2020, 59, 22202–22209. [Google Scholar] [CrossRef]
- Yin, F.; Hu, K.; Chen, S.; Wang, D.Y.; Zhang, J.N.; Xie, M.S.; Yang, D.; Qiu, M.; Zhang, H.; Li, Z.G. Black phosphorus quantum dot based novel siRNA delivery systems in human pluripotent teratoma PA-1 cells. J. Mater. Chem. B 2017, 5, 5433–5440. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, C.; Fu, Q.; Ye, J.; Su, L.; Ge, X.; Chen, L.; Song, J.; Yang, H. Neodymium (3+)-Coordinated Black Phosphorus Quantum Dots with Retrievable NIR/X-Ray Optoelectronic Switching Effect for Anti-Glioblastoma. Small 2022, 18, e2105160. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. Review of in vitro toxicological research of quantum dot and potentially involved mechanisms. Sci. Total Environ. 2018, 625, 940–962. [Google Scholar] [CrossRef]
- Lovric, J.; Cho, S.J.; Winnik, F.M.; Maysinger, D. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chem. Biol. 2005, 12, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, Y.F.; Song, B.; Zhong, Y.L.; Wu, S.C.; Cui, R.R.; Cong, H.X.; Su, Y.Y.; Zhang, H.M.; He, Y. Linking Subcellular Disturbance to Physiological Behavior and Toxicity Induced by Quantum Dots in. Small 2016, 12, 3143–3154. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Steinbrück, A.; Gao, J.; Doggett, N.; Hollingsworth, J.A.; Iyer, R. Comprehensive Analysis of the Effects of CdSe Quantum Dot Size, Surface Charge, and Functionalization on Primary Human Lung Cells. ACS Nano 2012, 6, 4748–4762. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Sarsons, C.D.; Gomez-Garcia, M.J.; Cramb, D.T.; Rinker, K.D.; Childs, S.J. Quantum dot interactions and flow effects in angiogenic zebrafish (Danio rerio) vessels and human endothelial cells. Nanomed.-Nanotechnol. 2017, 13, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Niu, A.; Li, J.; Fu, J.W.; Xu, Q.; Pei, D.S. characterization of hair and skin derived carbon quantum dots with high quantum yield as long-term bioprobes in zebrafish. Sci. Rep. 2016, 6, 37860. [Google Scholar] [CrossRef]
- Wang, J.L.; Sun, H.B.; Meng, P.J.; Wang, M.M.; Tian, M.; Xiong, Y.M.; Zhang, X.Y.; Huang, P.L. Dose and time effect of CdTe quantum dots on antioxidant capacities of the liver and kidneys in mice. Int. J. Nanomed. 2017, 12, 6425–6435. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.W.; Rosenkrans, Z.T.; Ni, D.L.; Lin, J.; Huang, P.; Cai, W.B. Nanomedicines for Renal Management: From Imaging to Treatment. Acc. Chem. Res. 2020, 53, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Du, B.J.; Jiang, X.Y.; Das, A.; Zhou, Q.H.; Yu, M.X.; Jin, R.C.; Zheng, J. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 2017, 12, 1096–1102. [Google Scholar] [CrossRef]
- Kim, W.; Ly, N.K.; He, Y.Y.; Li, Y.Z.; Yuan, Z.Y.; Yeo, Y. Protein corona: Friend or foe? Co-opting serum proteins for nanoparticle delivery. Adv. Drug Deliv. Rev. 2023, 192, 114635. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Song, X.; Liu, J.; Liu, W.; Chu, X.; Lei, Z. Quantum Dots as a Potential Multifunctional Material for the Enhancement of Clinical Diagnosis Strategies and Cancer Treatments. Nanomaterials 2024, 14, 1088. https://doi.org/10.3390/nano14131088
Guo W, Song X, Liu J, Liu W, Chu X, Lei Z. Quantum Dots as a Potential Multifunctional Material for the Enhancement of Clinical Diagnosis Strategies and Cancer Treatments. Nanomaterials. 2024; 14(13):1088. https://doi.org/10.3390/nano14131088
Chicago/Turabian StyleGuo, Wenqi, Xueru Song, Jiaqi Liu, Wanyi Liu, Xiaoyuan Chu, and Zengjie Lei. 2024. "Quantum Dots as a Potential Multifunctional Material for the Enhancement of Clinical Diagnosis Strategies and Cancer Treatments" Nanomaterials 14, no. 13: 1088. https://doi.org/10.3390/nano14131088
APA StyleGuo, W., Song, X., Liu, J., Liu, W., Chu, X., & Lei, Z. (2024). Quantum Dots as a Potential Multifunctional Material for the Enhancement of Clinical Diagnosis Strategies and Cancer Treatments. Nanomaterials, 14(13), 1088. https://doi.org/10.3390/nano14131088







