Ultra-Mild Fabrication of Highly Concentrated SWCNT Dispersion Using Spontaneous Charging in Solvated Electron System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Electron Solution and Charged SWCNT Dispersion
2.3. Fabrication of SWCNT Film, Fiber, Membrane, Thermal Composite, and SWCNT-Coated Microparticle
2.3.1. SWCNT Film Fabrication
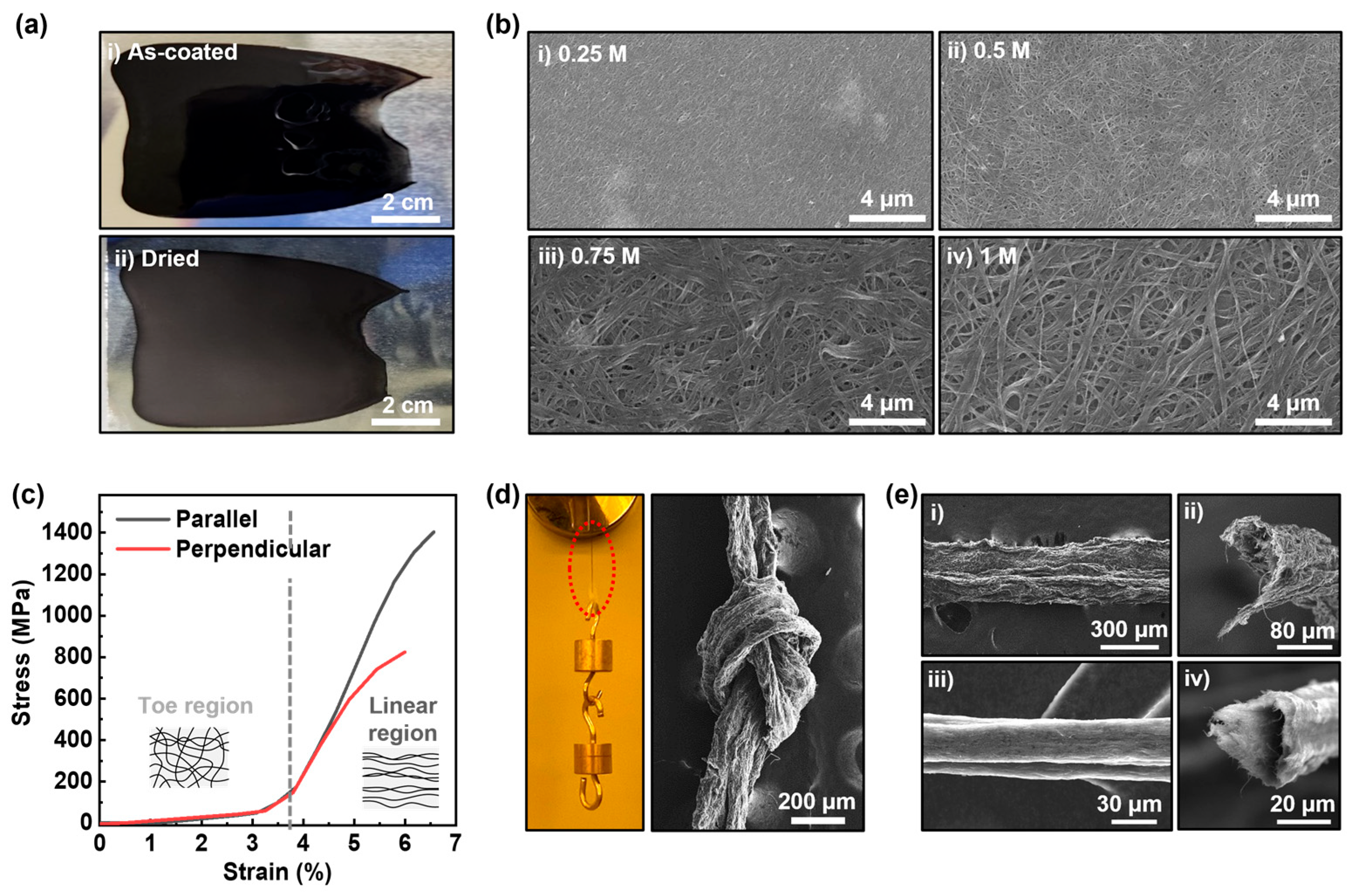
2.3.2. SWCNT Fiber Fabrication
2.3.3. SWCNT Membrane Fabrication
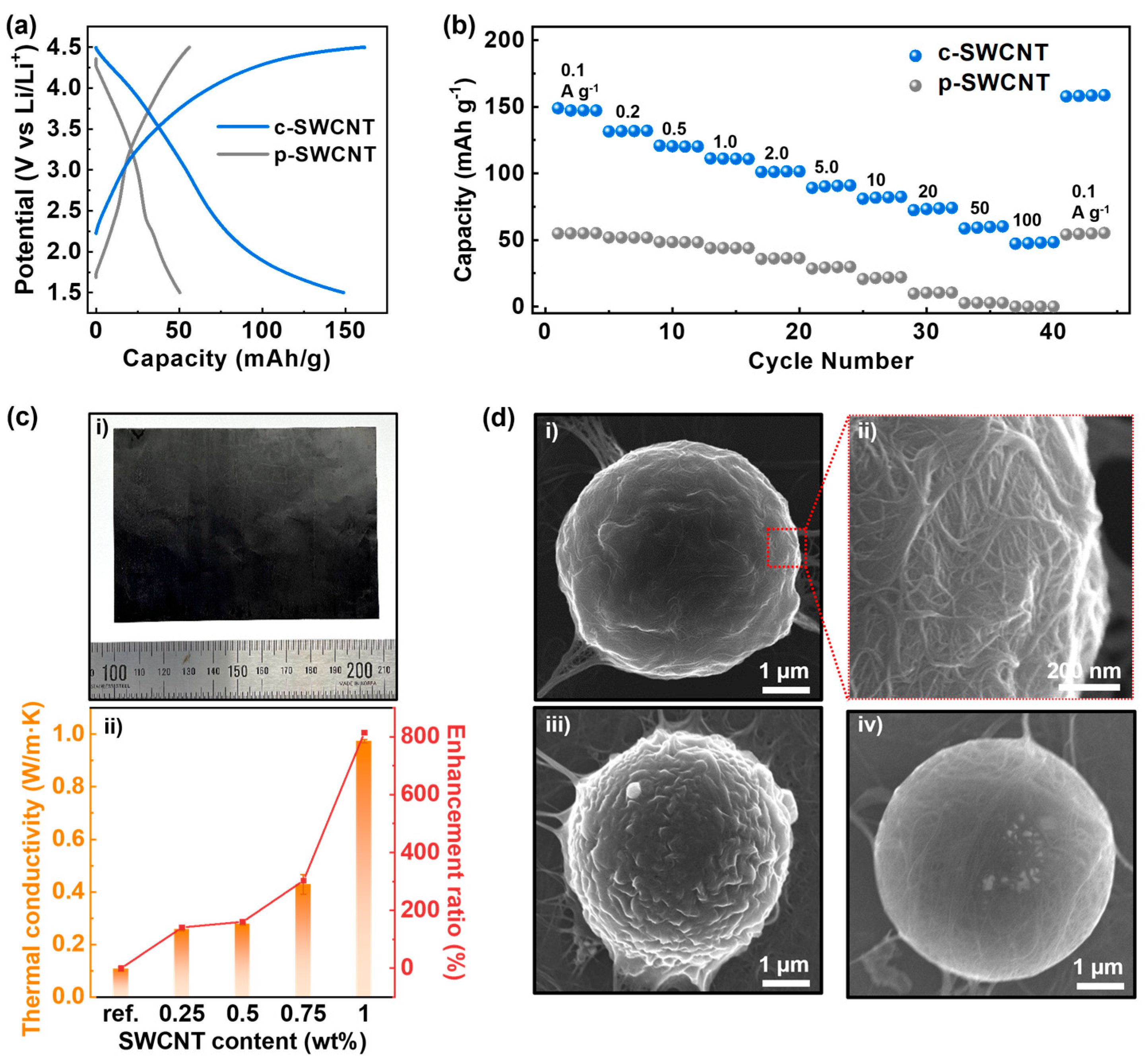
2.3.4. SWCNT Thermal Composite Fabrication
2.3.5. SWCNT-Coated Microparticle Fabrication
2.4. Characterizations and Measurements
2.4.1. Analysis of SWCNTs Exposed to the Electron Solution
2.4.2. Morphology and Crystallographic Analyses
2.4.3. Analysis of Electrical Conductivity of SWCNT Thin Film
2.4.4. Electrochemical Characterization
3. Results and Discussion
3.1. Charging of SWCNTs in Solvated Electron System
3.2. Defect-Free Dispersion of Charged SWCNTs
3.3. Fabrication of Free-Standing Film and Fiber Using c-SWCNT Dispersion
3.4. Versatile Applications of c-SWCNT Dispersion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon Nanotube–Polymer Composites: Chemistry, Processing, Mechanical and Electrical Properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Sabaruddin, F.A.; Harussani, M.M.; Kamarudin, S.H.; Rayung, M.; Asyraf, M.R.M.; Aisyah, H.A.; Norrrahim, M.N.F.; Ilyas, R.A.; Abdullah, N.; et al. Mechanical Performance and Applications of Cnts Reinforced Polymer Composites-a Review. Nanomaterials 2021, 11, 2186. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lei, L.; Zeng, K.; Wu, K.; Yang, N. Surface Chemistry Determined Electrochemical Sensing Performance of Red Phosphorus and Single Walled Carbon Nanotube Composites. Adv. Funct. Mater. 2022, 33, 2211335. [Google Scholar] [CrossRef]
- Ge, X.; Tay, G.; Hou, Y.; Zhao, Y.; Sugumaran, P.J.; Thai, B.Q.; Ang, C.K.; Zhai, W.; Yang, Y. Flexible and Leakage-Proof Phase Change Composite for Microwave Attenuation and Thermal Management. Carbon 2023, 210, 118084. [Google Scholar] [CrossRef]
- Yadav, M.D.; Joshi, H.M.; Sawant, S.V.; Dasgupta, K.; Patwardhan, A.W.; Joshi, J.B. Advances in the Application of Carbon Nanotubes as Catalyst Support for Hydrogenation Reactions. Chem. Eng. Sci. 2023, 272, 118586. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Torres, C.E.I.; González, L.T.; Kharisov, B.I. All-Carbon Hybrid Aerogels: Synthesis, Properties, and Applications. Ind. Eng. Chem. Res. 2019, 58, 16258–16286. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Fan, Y.; Hou, W.; Zhao, T.; Hu, Z.; Shi, E.; Lv, J.A. Adaptive Nanotube Networks Enabling Omnidirectionally Deformable Electro-Driven Liquid Crystal Elastomers Towards Artificial Muscles. Mater. Horiz. 2024, 11, 1877–1888. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Graphene/Reduced Graphene Oxide-Carbon Nanotubes Composite Electrodes: From Capacitive to Battery-Type Behaviour. Nanomaterials 2021, 11, 1240. [Google Scholar] [CrossRef] [PubMed]
- Kumanek, B.; Janas, D. Thermal Conductivity of Carbon Nanotube Networks: A Review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Chen, P.; Xia, R.; Qian, J. Covalently Interconnected Carbon Nanotubes Network Enhancing Thermal Conductivity of Ep-Based Composite. Compos. Commun. 2023, 40, 101591. [Google Scholar] [CrossRef]
- Liu, D.; Yang, Z.; Zhang, Y.; Wang, S.; Niu, Y.; Yang, J.; Yang, X.; Fu, H.; Chen, L.; Yong, Z.; et al. Tailoring Aligned and Densified Carbon Nanotube@Graphene Coaxial Yarn for 3d Thermally Conductive Networks. Carbon 2023, 208, 322–329. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Chow, S.; To, K.; Tang, Z.; Kim, J.-K. Correlations between Percolation Threshold, Dispersion State, and Aspect Ratio of Carbon Nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, K.; Dixit, A.R. A Review on the Mechanical Properties of Polymer Composites Reinforced by Carbon Nanotubes and Graphene. Carbon Lett. 2020, 31, 149–165. [Google Scholar] [CrossRef]
- Singh, I.; Verma, A.; Kaur, I.; Bharadwaj, L.M.; Bhatia, V.; Jain, V.K.; Bhatia, C.S.; Bhatnagar, P.K.; Mathur, P.C. The Effect of Length of Single-Walled Carbon Nanotubes (Swnts) on Electrical Properties of Conducting Polymer–Swnt Composites. J. Polym. Sci. Part B Polym. Phys. 2009, 48, 89–95. [Google Scholar] [CrossRef]
- Simien, D.; Fagan, J.A.; Luo, W.; Douglas, J.F.; Kalman, M.; Obrzut, J. Influence of Nanotube Length on the Optical and Conductivity Properties of Thin Single Wall Carbon Nanotube Networks. ACS Nano 2008, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Setaro, A. Advanced Carbon Nanotubes Functionalization. J. Phys. Condens. Matter 2017, 29, 423003. [Google Scholar] [CrossRef] [PubMed]
- Han, J.T.; Cho, J.Y.; Kim, J.H.; Jang, J.I.; Kim, J.S.; Lee, H.J.; Park, J.H.; Chae, J.S.; Roh, K.C.; Lee, W.; et al. Structural Recovery of Highly Oxidized Single-Walled Carbon Nanotubes Fabricated by Kneading and Electrochemical Applications. Chem. Mater. 2019, 31, 3468–3475. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Jin, J.; Wang, Y.; Zhang, Z.; Chen, Z.; Wang, Q.; Chen, Y.; Paek, E.; Mitlin, D. Synergy of Epoxy Chemical Tethers and Defect-Free Graphene in Enabling Stable Lithium Cycling of Silicon Nanoparticles. Angew. Chem. Int. Ed. 2019, 58, 16590–16600. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhou, D.; Jiang, Y.; Cheng, F.; Sun, J.; Guo, Q.; Luo, Y.; Chen, Y.; Liu, W. Lightweight Zn-Philic 3d-Cu Scaffold for Customizable Zinc Ion Batteries. Adv. Funct. Mater. 2024, 34, 2312664. [Google Scholar] [CrossRef]
- Fagan, J.A.; Bauer, B.J.; Hobbie, E.K.; Becker, M.L.; Walker, A.R.H.; Simpson, J.R.; Chun, J.; Obrzut, J.; Bajpai, V.; Phelan, F.R.; et al. Carbon Nanotubes: Measuring Dispersion and Length. Adv. Mater. 2011, 23, 338–348. [Google Scholar] [CrossRef]
- Liang, L.; Xie, W.; Fang, S.; He, F.; Yin, B.; Tlili, C.; Wang, D.; Qiu, S.; Li, Q. High-Efficiency Dispersion and Sorting of Single-Walled Carbon Nanotubes Via Non-Covalent Interactions. J. Mater. Chem. C 2017, 5, 11339–11368. [Google Scholar] [CrossRef]
- Ramesh, S.; Ericson, L.M.; Davis, V.A.; Saini, R.K.; Kittrell, C.; Pasquali, M.; Billups, W.E.; Adams, W.W.; Hauge, R.H.; Smalley, R.E. Dissolution of Pristine Single Walled Carbon Nanotubes in Superacids by Direct Protonation. J. Phys. Chem. B 2004, 108, 8794–8798. [Google Scholar] [CrossRef]
- Davis, V.A.; Parra-Vasquez, A.N.; Green, M.J.; Rai, P.K.; Behabtu, N.; Prieto, V.; Booker, R.D.; Schmidt, J.; Kesselman, E.; Zhou, W.; et al. True Solutions of Single-Walled Carbon Nanotubes for Assembly into Macroscopic Materials. Nat. Nanotechnol. 2009, 4, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Pekker, S.; Salvetat, J.P.; Jakab, E.; Bonard, J.M.; Forró, L. Hydrogenation of Carbon Nanotubes and Graphite in Liquid Ammonia. J. Phys. Chem. B 2001, 105, 7938–7943. [Google Scholar] [CrossRef]
- Pénicaud, A.; Poulin, P.; Derré, A.; Anglaret, E.; Petit, P. Spontaneous Dissolution of a Single-Wall Carbon Nanotube Salt. J. Am. Chem. Soc. 2005, 127, 8–9. [Google Scholar] [CrossRef]
- Fogden, S.; Howard, C.A.; Heenan, R.K.; Skipper, N.T.; Shaffer, M.S.P. Scalable Method for the Reductive Dissolution, Purification, and Separation of Single-Walled Carbon Nanotubes. ACS Nano 2012, 6, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoon, G.; Kim, J.; Yoon, H.; Baek, J.; Lee, J.H.; Kang, K.; Jeon, S. Extremely Large, Non-Oxidized Graphene Flakes Based on Spontaneous Solvent Insertion into Graphite Intercalation Compounds. Carbon 2018, 139, 309–316. [Google Scholar] [CrossRef]
- Kraus, C.A. Solutions of Metals in Non-Metallic Solvents; I. General Properties of Solutions of Metals in Liquid Ammonia. J. Am. Chem. Soc. 1907, 29, 1557–1571. [Google Scholar] [CrossRef]
- Feng, H.; Cheng, R.; Zhao, X.; Duan, X.; Li, J. A Low-Temperature Method to Produce Highly Reduced Graphene Oxide. Nat. Commun. 2013, 4, 1539. [Google Scholar] [CrossRef]
- Pramanik, C.; Gissinger, J.R.; Kumar, S.; Heinz, H. Carbon Nanotube Dispersion in Solvents and Polymer Solutions: Mechanisms, Assembly, and Preferences. ACS Nano 2017, 11, 12805–12816. [Google Scholar] [CrossRef]
- Jorio, A.; Saito, R. Raman Spectroscopy for Carbon Nanotube Applications. J. Appl. Phys. 2021, 129, 021102. [Google Scholar] [CrossRef]
- Rao, A.M.; Eklund, P.C.; Bandow, S.; Thess, A.; Smalley, R.E. Evidence for Charge Transfer in Doped Carbon Nanotube Bundles from Raman Scattering. Nature 1997, 388, 257–259. [Google Scholar] [CrossRef]
- Davis, V.A. Anisotropic Nanomaterial Liquid Crystals: From Fiber Spinning to Additive Manufacturing. Langmuir 2023, 39, 3829–3836. [Google Scholar] [CrossRef]
- Vobornik, D.; Chen, M.; Zou, S.; Lopinski, G.P. Measuring the Diameter of Single-Wall Carbon Nanotubes Using Afm. Nanomaterials 2023, 13, 477. [Google Scholar] [CrossRef]
- Kriener, K.; Lala, R.; Homes, R.A.; Finley, H.; Sinclair, K.; Williams, M.K.; Midwinter, M.J. Mechanical Characterization of the Human Abdominal Wall Using Uniaxial Tensile Testing. Bioengineering 2023, 10, 1213. [Google Scholar] [CrossRef]
- Guo, T.; Wan, Z.; Yu, Y.; Chen, H.; Wang, Z.; Li, D.; Song, J.; Rojas, O.J.; Jin, Y. Mechanisms of Strain-Induced Interfacial Strengthening of Wet-Spun Filaments. ACS Appl. Mater. Interfaces 2022, 14, 16809–16819. [Google Scholar] [CrossRef]


| Point | Thickness (μm) | Sheet Resistance 1 (Ω/□) | Electrical Conductivity (S/m) |
|---|---|---|---|
| 1 | 4 | 0.349 | 716,332 |
| 2 | 4 | 0.408 | 612,745 |
| 3 | 4 | 0.744 | 336,021 |
| 4 | 4 | 0.201 | 1,243,781 |
| 5 | 4 | 0.436 | 573,394 |
| Avg. | 4 | 0.4276 | 696,455 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Kim, J.H.; Lee, J.; Lee, S.; Park, J.H.; Jeong, S.Y.; Jeong, H.J.; Han, J.T.; Seo, S.H.; Lee, S.-K.; et al. Ultra-Mild Fabrication of Highly Concentrated SWCNT Dispersion Using Spontaneous Charging in Solvated Electron System. Nanomaterials 2024, 14, 1094. https://doi.org/10.3390/nano14131094
Shin J, Kim JH, Lee J, Lee S, Park JH, Jeong SY, Jeong HJ, Han JT, Seo SH, Lee S-K, et al. Ultra-Mild Fabrication of Highly Concentrated SWCNT Dispersion Using Spontaneous Charging in Solvated Electron System. Nanomaterials. 2024; 14(13):1094. https://doi.org/10.3390/nano14131094
Chicago/Turabian StyleShin, Junho, Jung Hoon Kim, Jungeun Lee, Sangyong Lee, Jong Hwan Park, Seung Yol Jeong, Hee Jin Jeong, Joong Tark Han, Seon Hee Seo, Seoung-Ki Lee, and et al. 2024. "Ultra-Mild Fabrication of Highly Concentrated SWCNT Dispersion Using Spontaneous Charging in Solvated Electron System" Nanomaterials 14, no. 13: 1094. https://doi.org/10.3390/nano14131094







