Promoting the Photoelectrochemical Properties of BiVO4 Photoanode via Dual Modification with CdS Nanoparticles and NiFe-LDH Nanosheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Photoanodes
2.3. Measurements
2.4. Photoelectrochemical Analysis
3. Results
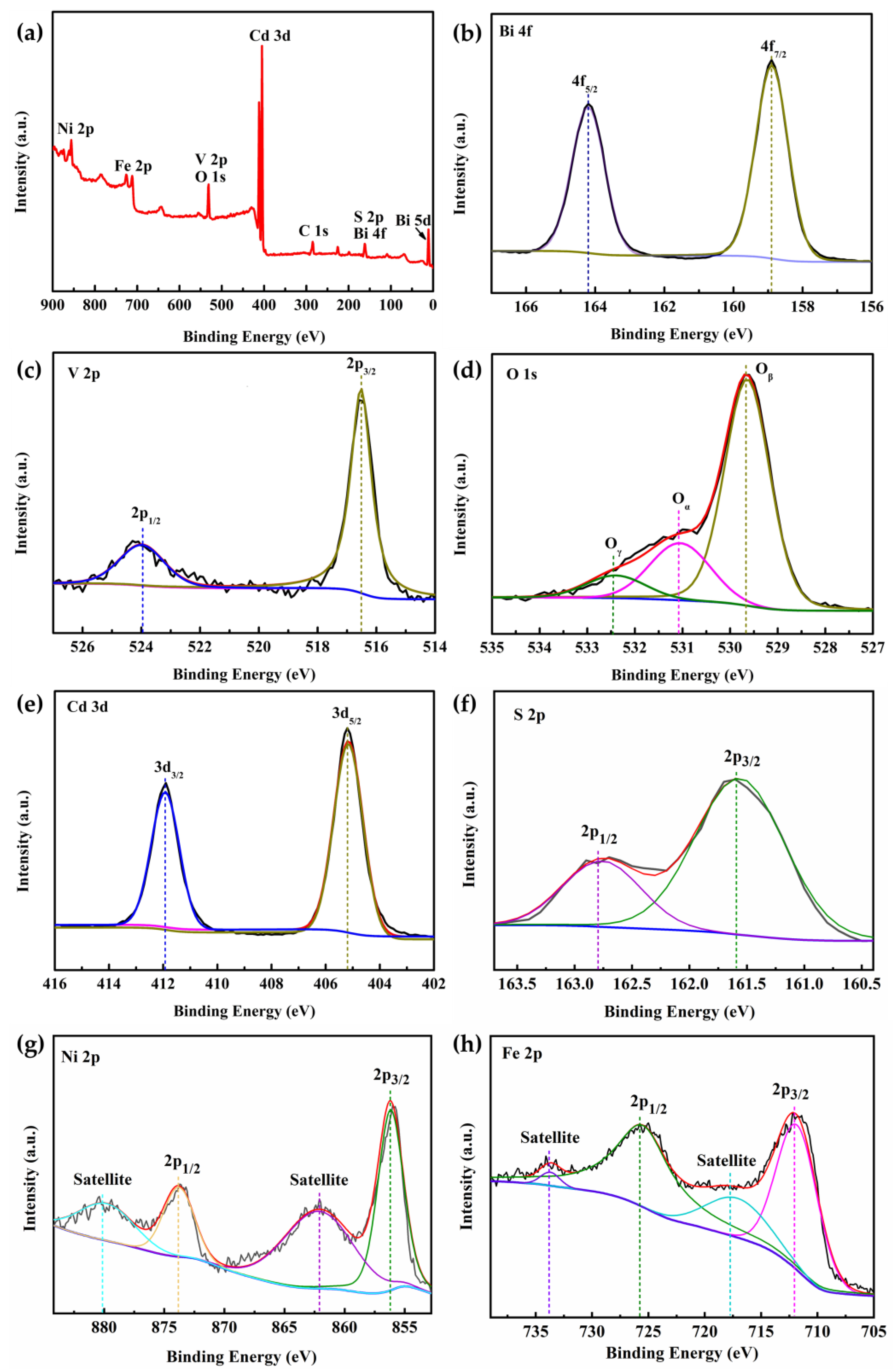
3.1. Characterization of the Photoanode Materials
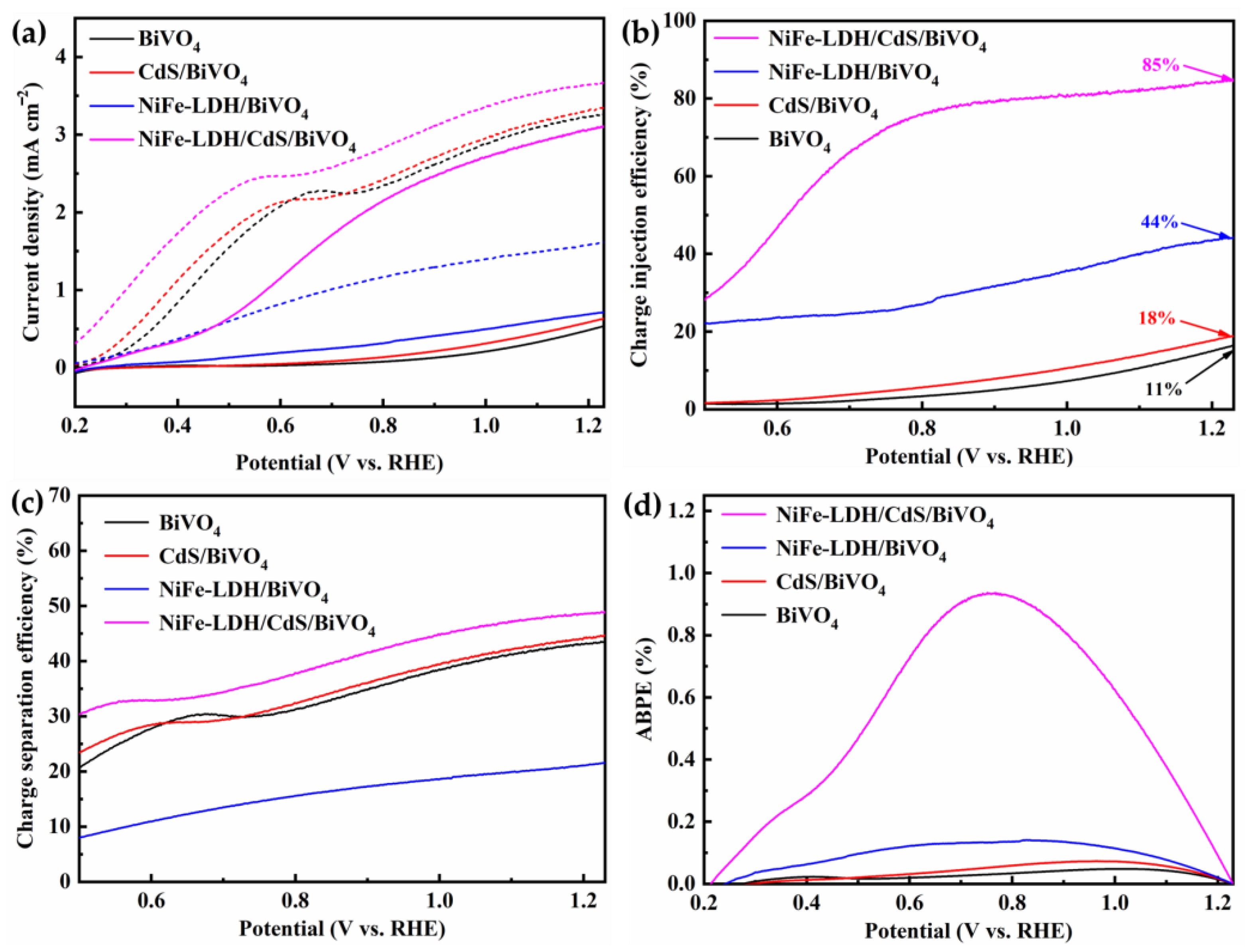
3.2. PEC Properties of the Photoanodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chiari, L.; Zecca, A. Constraints of Fossil Fuels Depletion on Global Warming Projections. Energy Policy 2011, 39, 5026–5034. [Google Scholar] [CrossRef]
- Yusaf, T.; Fernandes, L.; Talib, A.R.A.; Altarazi, Y.S.M.; Alrefae, W.; Kadirgama, K.; Ramasamy, D.; Jayasuriya, A.; Brown, G.; Mamat, R.; et al. Sustainable Aviation—Hydrogen Is the Future. Sustainability 2022, 14, 548. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical Devices for Solar Water Splitting-Materials and Challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef]
- Ros, C.; Andreu, T.; Morante, J.R. Photoelectrochemical Water Splitting: A Road from Stable Metal Oxides to Protected Thin Film Solar Cells. J. Mater. Chem. A 2020, 8, 10625–10669. [Google Scholar] [CrossRef]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective Preparation of Monoclinic and Tetragonal BiVO4 with Scheelite Structure and Their Photocatalytic Properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Abdi, F.F.; Firet, N.; van de Krol, R. Efficient BiVO4 Thin Film Photoanodes Modified with Cobalt Phosphate Catalyst and W-Doping. ChemCatChem 2013, 5, 490–496. [Google Scholar] [CrossRef]
- Yao, X.; Wang, D.; Zhao, X.; Ma, S.; Bassi, P.S.; Yang, G.; Chen, W.; Chen, Z.; Sritharan, T. Scale-Up of BiVO4 Photoanode for Water Splitting in a Photoelectrochemical Cell: Issues and Challenges. Energy Technol. 2017, 1, 100–109. [Google Scholar] [CrossRef]
- Wang, T.; Pei, H.; Zhang, Y.; Li, R.; Zhang, J.; Peng, T. Efficient Strategy for Boosting the Solar-Driven Water Oxidation of the BiVO4 Photoanode by Using a CuPc Derivative as a Hole Transport Highway. ACS Appl. Energy Mater. 2022, 5, 11271–11282. [Google Scholar] [CrossRef]
- Abdi, F.F.; Savenije, T.J.; May, M.M.; Dam, B. The Origin of Slow Carrier Transport in BiVO4 Thin Film Photoanodes: A Time-Resolved Microwave Conductivity Study. J. Phys. Chem. Lett. 2013, 4, 2752–2757. [Google Scholar] [CrossRef]
- Murugan, C.; Pandikumar, A. Reinforcement of Visible-Light Harvesting and Charge-Transfer Dynamics of BiVO4 Photoanode via Formation of p–n Heterojunction with CuO for Efficient Photoelectrocatalytic Water Splitting. ACS Appl. Energy Mater. 2022, 5, 6618–6632. [Google Scholar] [CrossRef]
- Cheng, C.; Fang, Q.; Long, R. Controlling Charge Carrier Trapping and Recombination in BiVO4 with the Oxygen Vacancy Oxidation State. J. Phys. Chem. Lett. 2021, 12, 3514–3521. [Google Scholar] [CrossRef]
- Sayama, K.; Nomura, A.; Arai, T.; Sugita, T.; Abe, R.; Yanagida, M.; Oi, T.; Iwasaki, Y.; Abe, Y.; Sugihara, H. Photoelectrochemical Decomposition of Water into H2 and O2 on Porous BiVO4 Thin-Film Electrodes under Visible Light and Significant Effect of Ag Ion Treatment. J. Phys. Chem. B 2006, 110, 11352–11360. [Google Scholar] [CrossRef]
- Chatchai, P.; Murakami, Y.; Kishioka, S.; Nosaka, A.Y.; Nosaka, Y. FTO/SnO2/BiVO4 Composite Photoelectrode for Water Oxidation under Visible Light Irradiation. Electrochem. Solid-State Lett. 2008, 11, H160–H163. [Google Scholar] [CrossRef]
- Berglund, S.P.; Flaherty, D.W.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Photoelectrochemical Oxidation of Water Using Nanostructured BiVO4 Films. J. Phys. Chem. C 2011, 115, 3794–3802. [Google Scholar] [CrossRef]
- Dall’Antonia, L.H.; de Tacconi, N.R.; Chanmanee, W.; Timmaji, H.; Myung, N.; Rajeshwar, K. Electrosynthesis of Bismuth Vanadate Photoelectrodes. Electrochem. Solid-State Lett. 2010, 13, D29–D32. [Google Scholar] [CrossRef]
- Li, M.; Zhao, L.; Guo, L. Preparation and photoelectrochemical study of BiVO4 thin films deposited by ultrasonic spray pyrolysis. Int. J. Hydrogen Energy 2010, 35, 7127–7133. [Google Scholar] [CrossRef]
- Long, M.; Cai, W.; Kisch, H. Visible Light Induced Photoelectrochemical Properties of n-BiVO4 and n-BiVO4/p-Co3O4. J. Phys. Chem. C 2008, 112, 548–554. [Google Scholar] [CrossRef]
- Luo, W.; Yang, Z.; Li, Z.; Zhang, J.; Liu, J.; Zhao, Z.; Wang, Z.; Yan, S.; Yu, T.; Zou, Z. Solar Hydrogen Generation from Seawater with a Modified BiVO4 Photoanode. Energy Environ. Sci. 2011, 4, 4046–4051. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Kanan, M.W.; Nocera, D.G. In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+. Science 2008, 321, 1072–1075. [Google Scholar] [CrossRef]
- Ye, H.; Park, H.S.; Bard, A.J. Screening of Electrocatalysts for Photoelectrochemical Water Oxidation on W-Doped BiVO4 Photocatalysts by Scanning Electrochemical Microscopy. J. Phys. Chem. C 2011, 115, 12464–12470. [Google Scholar] [CrossRef]
- Zhong, D.K.; Choi, S.; Gamelin, D.R. Near-Complete Suppression of Surface Recombination in Solar Photoelectrolysis by “Co-Pi” Catalyst-Modified W:BiVO4. J. Am. Chem. Soc. 2011, 133, 18370–18377. [Google Scholar] [CrossRef] [PubMed]
- Pilli, S.K.; Furtak, T.E.; Brown, L.D.; Deutsch, T.G.; Turner, J.A.; Herring, A.M. Cobalt-Phosphate (Co-Pi) Catalyst Modified Mo-Doped BiVO4 Photoelectrodes for Solar Water Oxidation. Energy Environ. Sci. 2011, 4, 5028–5034. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, X.; Zhang, Y.; Lu, G.; Chou, L.; Bi, Y. Unveiling the Activity and Stability Origin of BiVO4 Photoanodes with FeNi Oxyhydroxides for Oxygen Evolution. Angew. Chem. Int. Ed. 2020, 59, 18990–18995. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yu, Y.; Yu, Y.; Huang, Y.; Zhao, B.; Zhang, B. Boosting Photoelectrochemical Water Oxidation Activity and Stability of Mo-doped BiVO4 through the Uniform Assembly Coating of NiFe-Phenolic Networks. ACS Energy Lett. 2018, 3, 1648–1654. [Google Scholar] [CrossRef]
- Wang, S.; He, T.; Chen, P.; Du, A.; Ostrikov, K.; Huang, W.; Wang, L. In Situ Formation of Oxygen Vacancies Achieving Near-Complete Charge Separation in Planar BiVO4 Photoanodes. Adv. Mater. 2020, 32, 2001385. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhao, J.; Li, H.; Park, J.; Cho, I.S.; Han, H.S.; Zheng, X. One-Step Hydrothermal Deposition of Ni:FeOOH onto Photoanodes for Enhanced Water Oxidation. ACS Energy Lett. 2016, 1, 624–632. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Bai, J.; Zeng, Q.; Xia, L.; Zhang, Y.; Chen, S.; Xu, Q.; Zhou, B. Serial Hole Transfer Layers for A BiVO4 Photoanode with Enhanced Photoelectrochemical Water Splitting. Nanoscale 2018, 10, 18378–18386. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.J.A.; Zhu, J.; Tang, J. 1D Co-Pi Modified BiVO4/ZnO Junction Cascade for Efficient Photoelectrochemical Water Cleavage. Adv. Energy Mater. 2014, 4, 1301590. [Google Scholar] [CrossRef]
- Chatchai, P.; Murakami, Y.; Kishioka, S.; Nosaka, A.Y.; Nosaka, Y. Efficient Photocatalytic Activity of Water Oxidation over WO3/BiVO4 Composite under Visible Light Irradiation. Electrochim. Acta 2009, 54, 1147–1152. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Uemura, J.; Kazoe, Y.; Kosar, S.; Makita, K.; Sugaya, T.; Matsui, T.; Fujita, D.; et al. Photocatalytic Generation of Hydrogen by Core-Shell WO3/BiVO4 Nanorods with Ultimate Water Splitting Efficiency. Sci. Rep. 2015, 5, 11141. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Lee, C.S.; Jung, H.; Joo, O.; Min, B.K.; Kim, J.H.; Hwang, Y.J. Insight into Charge Separation in WO3/BiVO4 Heterojunction for Solar Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 19780–19790. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, M.; Chiarello, G.L.; Vassallo, E.; Selli, E. Multilayer WO3/BiVO4 Photoanodes for Solar-Driven Water Splitting Prepared by RF-Plasma Sputtering. Surfaces 2020, 3, 105–115. [Google Scholar] [CrossRef]
- Saito, R.; Miseki, Y.; Sayama, K. Highly Efficient Photoelectrochemical Water Splitting Using A Thin Film Photoanode of BiVO4/SnO2/WO3 Multi-Composite in a Carbonate Electrolyte. Chem. Commun. 2012, 48, 3833–3835. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Kim, B.J.; Han, G.S.; Hwang, S.W.; Kim, D.R.; Cho, I.S.; Jung, H.S. BiVO4/WO3/SnO2 Double-Heterojunction Photoanode with Enhanced Charge Separation and Visible-Transparency for Bias-Free Solar Water-Splitting with a Perovskite Solar Cell. ACS Appl. Mater. Interfaces 2017, 9, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Yang, J.-S.; Wu, J.-J. Three-Dimensional Undoped Crystalline SnO2 Nanodendrite Arrays Enable Efficient Charge Separation in BiVO4/SnO2 Heterojunction Photoanodes for Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2018, 1, 2143–2149. [Google Scholar] [CrossRef]
- Dong, G.; Xie, F.; Kou, F.; Chen, T.; Wang, F.; Zhou, Y.; Wu, K.; Du, S.; Fang, M.; Ho, J.C. NiFe-Layered Double Hydroxide Arrays for Oxygen Evolution Reaction in Fresh Water and Seawater. Mater. Today Energy 2021, 22, 100883. [Google Scholar] [CrossRef]
- She, H.; Yue, P.; Ma, X.; Huang, J.; Wang, L.; Wang, Q. Fabrication of BiVO4 Photoanode Cocatalyzed with NiCo-Layered Double Hydroxide for Enhanced Photoactivity of Water Oxidation. Appl. Catal. B: Environ. 2020, 263, 118280. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, T.; Wang, L.; Huang, J.; She, H. NiFe Layered Double-Hydroxide Nanoparticles for Efficiently Enhancing Performance of BiVO4 Photoanode in Photoelectrochemical Water Splitting. Chin. J. Catal. 2018, 39, 613–618. [Google Scholar] [CrossRef]
- Reddy, D.A.; Reddy, K.A.J.; Gopannagari, M.; Kim, Y.; Rangappa, A.P.; Kumar, D.P.; Kim, T.K. Exposure of NiFe-LDH Active Sites by Cation-Exchange to Promote Photoelectrochemical Water Splitting Performance. Appl. Surf. Sci. 2021, 570, 151134. [Google Scholar] [CrossRef]
- Cong, S.; Yu, J.; Liu, B.; Teng, W.; Tang, Y. Preparing a Dual-Function BiVO4/NiFe-LDH Composite Photoanode for Enhanced Photoelectrocatalytic Wastewater Treatment and Simultaneous Hydrogen Evolution. New J. Chem. 2022, 46, 15227–15243. [Google Scholar] [CrossRef]
- Han, B.; Liu, S.; Xu, Y.-J.; Tang, Z.-R. 1D CdS Nanowires-2D BiVO4 Nanosheets Heterostructures toward Photocatalytic Selective Fine-Chemical Synthesis. RSC Adv. 2015, 5, 16476–16483. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, M.; Li, R.; Ma, L.; Guo, L. Fabricating CdS/BiVO4 and BiVO4/CdS Heterostructured Film Photoelectrodes for Photoelectrochemical Applications. Int. J. Hydrogen Energy 2013, 38, 13069–13076. [Google Scholar] [CrossRef]
- Zhou, F.; Fan, J.; Xu, Q.; Min, Y. BiVO4 Nanowires Decorated with CdS Nanoparticles as Z-scheme Photocatalyst with Enhanced H2 Generation. Appl. Catal. B Environ. 2017, 201, 77–83. [Google Scholar] [CrossRef]
- McDonald, K.J.; Choi, K.-S. A New Electrochemical Synthesis Route for A BiOI Electrode and Its Conversion to A Highly Efficient Porous BiVO4 Photoanode for Solar Water Oxidation. Energy Environ. Sci. 2012, 5, 8553–8557. [Google Scholar] [CrossRef]
- Chen, Z.; Qu, Q.; Li, X.; Srinivas, K.; Chen, Y.; Zhu, M. Room-Temperature Synthesis of Carbon-Nanotube-Interconnected Amorphous NiFe-Layered Double Hydroxides for Boosting Oxygen Evolution Reaction. Molecules 2023, 28, 7289. [Google Scholar] [CrossRef]
- Li, Z.; Chen, D.; Jiao, X. Monoclinic Structured BiVO4 Nanosheets: Hydrothermal Preparation, Formation Mechanism, and Coloristic and Photocatalytic Properties. J. Phys. Chem. 2006, 110, 2668–2673. [Google Scholar]
- Xia, L.; Li, J.; Bai, J.; Li, L.; Zeng, Q.; Xu, Q.; Zhou, B. Preparation of A BiVO4 Nanoporous Photoanode Based on Peroxovanadate Reduction and Conversion for Efficient Photoelectrochemical Performance. Nanoscale 2018, 10, 2848–2855. [Google Scholar] [CrossRef]
- Bai, S.; Li, Q.; Han, J.; Yang, X.; Shu, X.; Sun, J.; Sun, L.; Luo, R.; Li, D.; Chen, A. Photoanode of LDH Catalyst Decorated Semiconductor Heterojunction of BiVO4/CdS to Enhance PEC Water Splitting Efficiency. Int. J. Hydrogen Energy 2019, 44, 24642–24652. [Google Scholar] [CrossRef]
- Guo, J.; Yang, X.; Bai, S.; Xiang, X.; Luo, R.; He, J.; Chen, A. Effect of Mo Doping and NiFe-LDH Cocatalyst on PEC Water Oxidation Efficiency. J. Colloid Interface Sci. 2019, 540, 9–19. [Google Scholar] [CrossRef]
- Wang, D.; Shen, H.; Guo, L.; Fu, F.; Liang, Y. Design and Construction of the Sandwich-Like Z-scheme Multicomponent CdS/Ag/Bi2MoO6 Heterostructure with Enhanced Photocatalytic Performance in RhB Photodegradation. New J. Chem. 2016, 40, 8614–8624. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Zhang, P.; Zhang, J.; Li, A.; Gong, J. Enhanced Surface Reaction Kinetics and Charge Separation of p-n Heterojunction Co3O4/BiVO4 Photoanodes. J. Am. Chem. Soc. 2015, 137, 8356–8359. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Kubendhiran, S.; Chung, R.-J.; Yougbaré, S.; Lin, L.-Y.; Wu, Y.-F. Enhanced Photoelectrochemical Water Oxidation on BiVO4 by Addition of ZnCo-MOFs As Effective Hole Transfer Co-Catalyst. Int. J. Hydrogen Energ. 2023, 48, 101–112. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Kong, W.; Liu, H.; Fan, H.; Wang, M. Reducing the Surface Recombination during Light-Driven Water Oxidation by Core-Shell BiVO4@Ni:FeOOH. Electrochim. Acta 2019, 300, 77–84. [Google Scholar] [CrossRef]
- Bai, H.; Wang, F.; You, Z.; Sun, D.; Cui, J.; Fan, W. Fabrication of Zn-MOF Decorated BiVO4 Photoanode for Water Splitting. Colloid Surf. A 2022, 640, 128412. [Google Scholar] [CrossRef]
- She, H.; Yue, P.; Huang, J.; Wang, L.; Wang, Q. One-Step Hydrothermal Deposition of F:FeOOH onto BiVO4 Photoanode for Enhanced Water Oxidation. Chem. Eng. J. 2020, 392, 123703. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Wang, J.; Zhang, Y.; Li, J.; Bai, J.; Zhou, B. Dramatically Enhanced Solar-Driven Water Splitting of BiVO4 Photoanode via Strengthening Hole Transfer and Light Harvesting by Co-Modification of CQDs and Ultrathin β-FeOOH Layers. Chem. Eng. J. 2021, 403, 126350. [Google Scholar] [CrossRef]
- Yue, P.; She, H.; Zhang, L.; Niu, B.; Lian, R.; Huang, J.; Wang, L. Super-Hydrophilic CoAl-LDH on BiVO4 for Enhanced Photoelectrochemical Water Oxidation Activity. Appl. Catal. B Environ. 2021, 286, 119875. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Zhang, Y.; Ding, Y.; Bi, Y. Ultrathin FeOOH Nanolayers with Abundant Oxygen Vacancies on BiVO4 Photoanodes for Efficient Water Oxidation. Angew. Chem. Int. Ed. 2018, 57, 2248–2252. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Ding, Q.; Yu, Y.; Huo, P.; Shi, W.; Xu, D. MOF Derived NiO Thin Film Formed p-n Heterojunction with BiVO4 Photoelectrode for Enhancement of PEC Performance. Colloid Surf. A 2022, 655, 130282. [Google Scholar] [CrossRef]
- Fang, G.; Liu, Z.; Han, C. Enhancing the PEC Water Splitting Performance of BiVO4 Co-Modifying with NiFeOOH and Co-Pi Double Layer Cocatalysts. Appl. Surf. Sci. 2020, 515, 146095. [Google Scholar] [CrossRef]
- Huang, J.; Luo, W.; Yuan, X.; Wang, J. Bimetallic MOF-Derived Oxides Modified BiVO4 for Enhanced Photoelectrochemical Water Oxidation Performance. J. Alloy. Compd. 2023, 930, 167397. [Google Scholar] [CrossRef]
- Wang, D.; Gu, J.; Wang, H.; Liu, M.; Liu, Y.; Zhang, X. Promoting Photoelectrochemical Water Oxidation of BiVO4 Photoanode via Co-MOF-Derived Heterostructural Cocatalyst. Appl. Surf. Sci. 2023, 619, 156710. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, J.; Wang, Q.; Jiang, X.; Shen, Y. Enhanced Photoelectrochemical Water Splitting Using a Cobalt-Sulfide-Decorated BiVO4 Photoanode. Chin. J. Catal. 2022, 43, 433–441. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, G.; Chen, T.; Kou, F.; Xie, F.; Xiao, C.; Liang, J.; Lou, C.; Zhuang, J.; Du, S. Promoting the Photoelectrochemical Properties of BiVO4 Photoanode via Dual Modification with CdS Nanoparticles and NiFe-LDH Nanosheets. Nanomaterials 2024, 14, 1100. https://doi.org/10.3390/nano14131100
Dong G, Chen T, Kou F, Xie F, Xiao C, Liang J, Lou C, Zhuang J, Du S. Promoting the Photoelectrochemical Properties of BiVO4 Photoanode via Dual Modification with CdS Nanoparticles and NiFe-LDH Nanosheets. Nanomaterials. 2024; 14(13):1100. https://doi.org/10.3390/nano14131100
Chicago/Turabian StyleDong, Guofa, Tingting Chen, Fangxia Kou, Fengyan Xie, Caihong Xiao, Jiaqi Liang, Chenfang Lou, Jiandong Zhuang, and Shaowu Du. 2024. "Promoting the Photoelectrochemical Properties of BiVO4 Photoanode via Dual Modification with CdS Nanoparticles and NiFe-LDH Nanosheets" Nanomaterials 14, no. 13: 1100. https://doi.org/10.3390/nano14131100




