Supramolecular Self-Assembled Nanostructures Derived from Amplified Structural Isomerism of Zn(II)−Sn(IV)−Zn(II) Porphyrin Triads and Their Visible Light Photocatalytic Degradation of Pollutants †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 5,10-bis(3-pyridyl)-15,20-bis(phenyl)porphyrin H2P
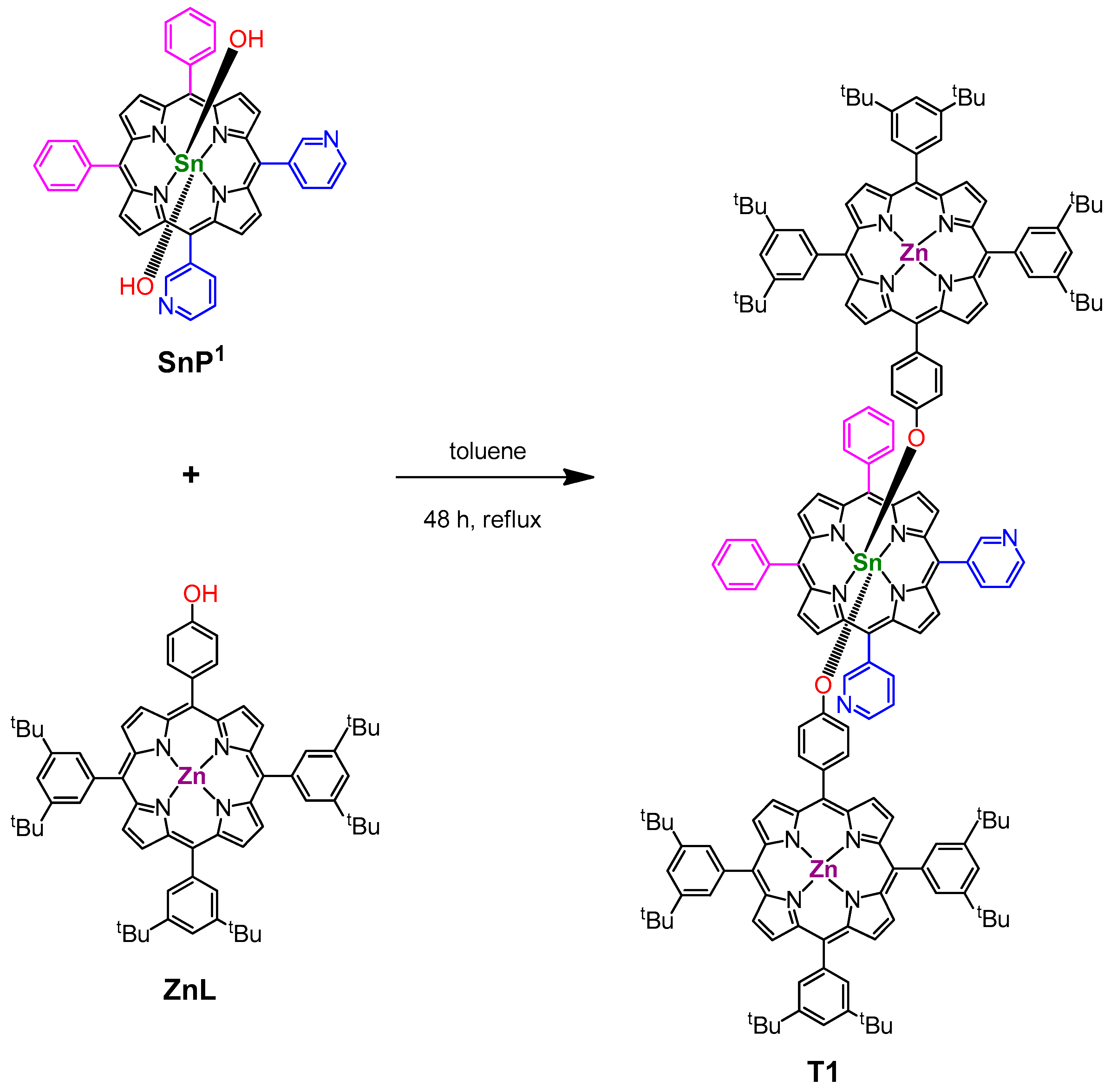
2.2. Synthesis of SnP1
2.3. Synthesis of triad 1 (T1)
2.4. Photoelectrochemical Measurement
2.5. Photocatalytic Degradation Experiment
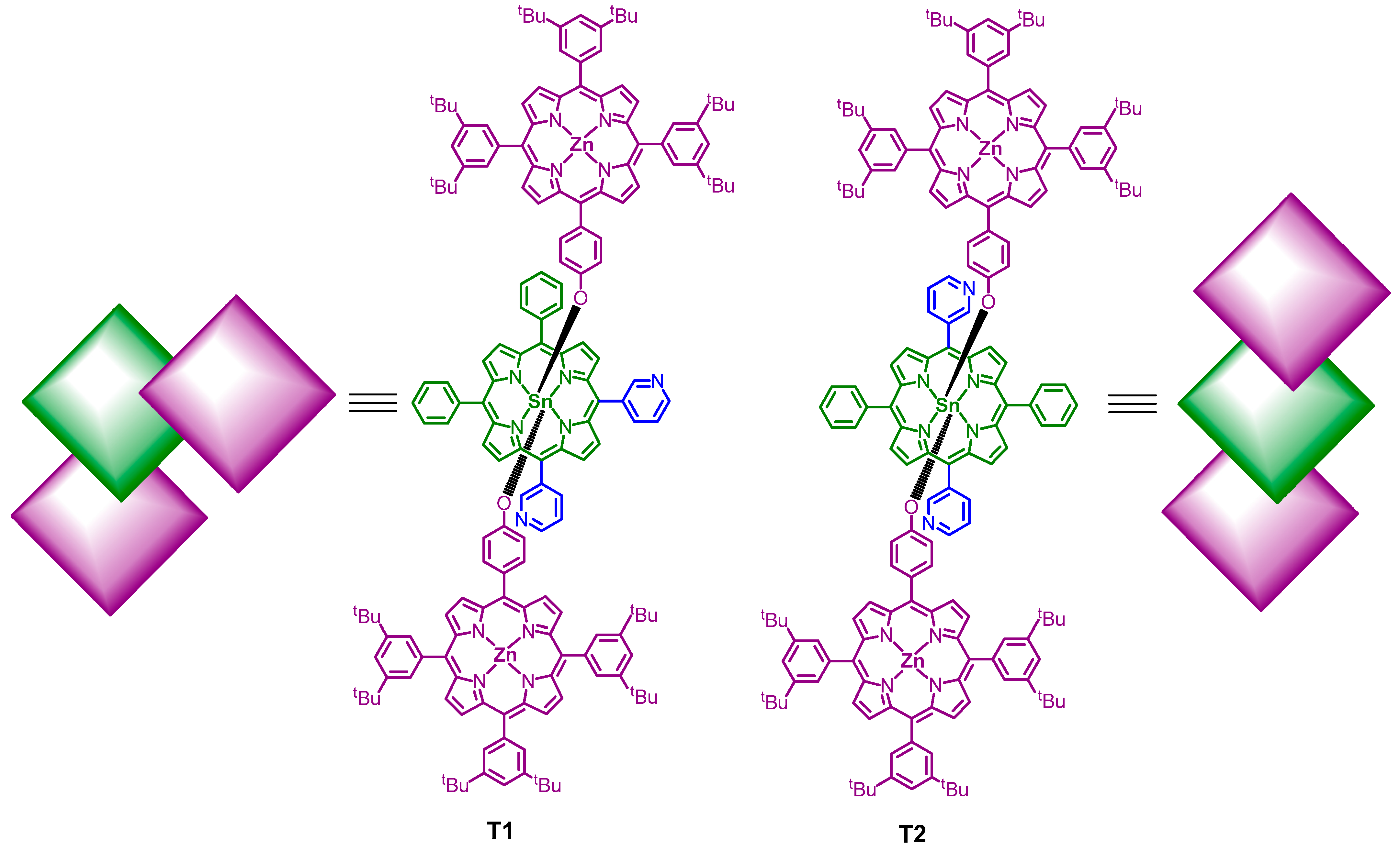
3. Results and Discussion
3.1. Syntheses
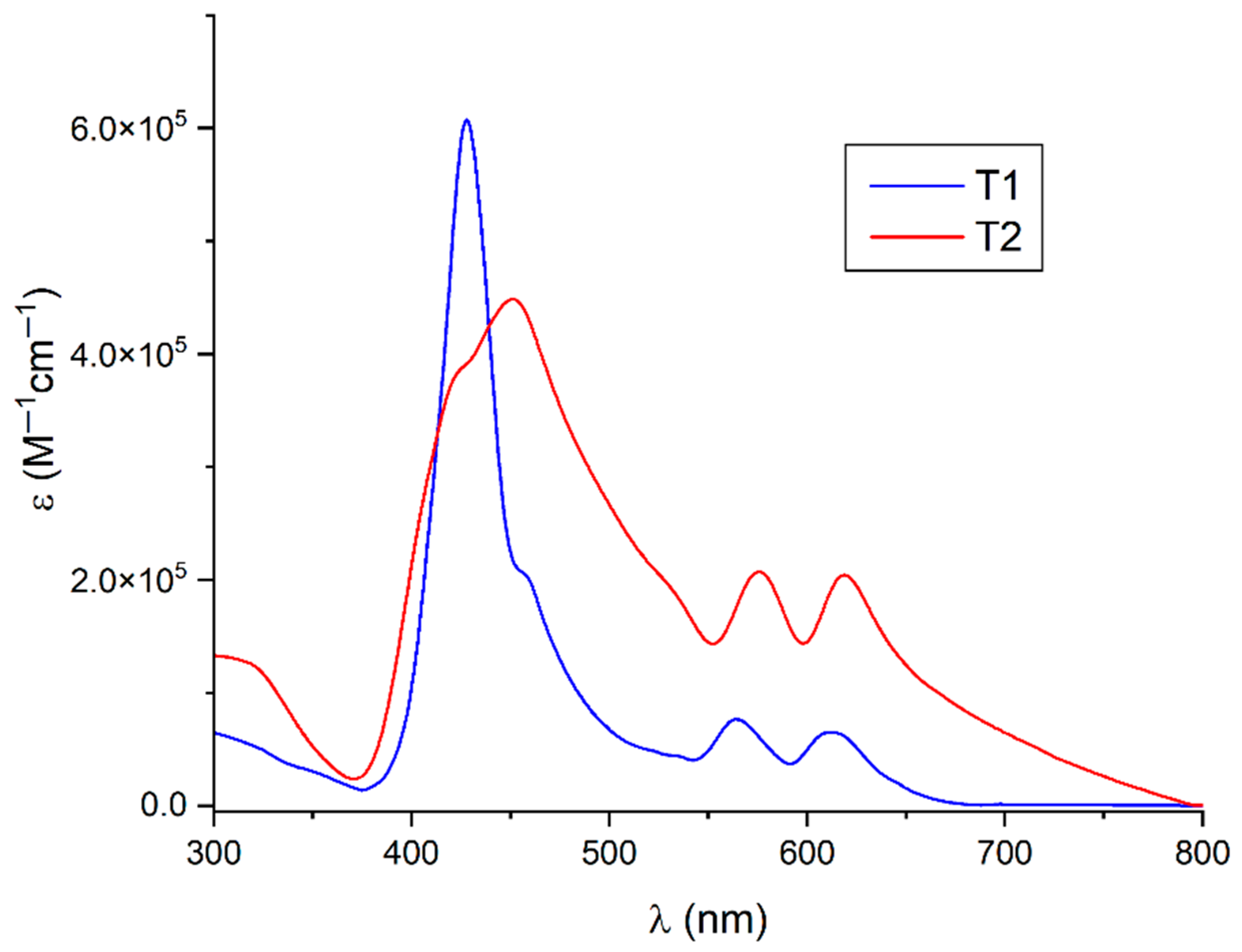
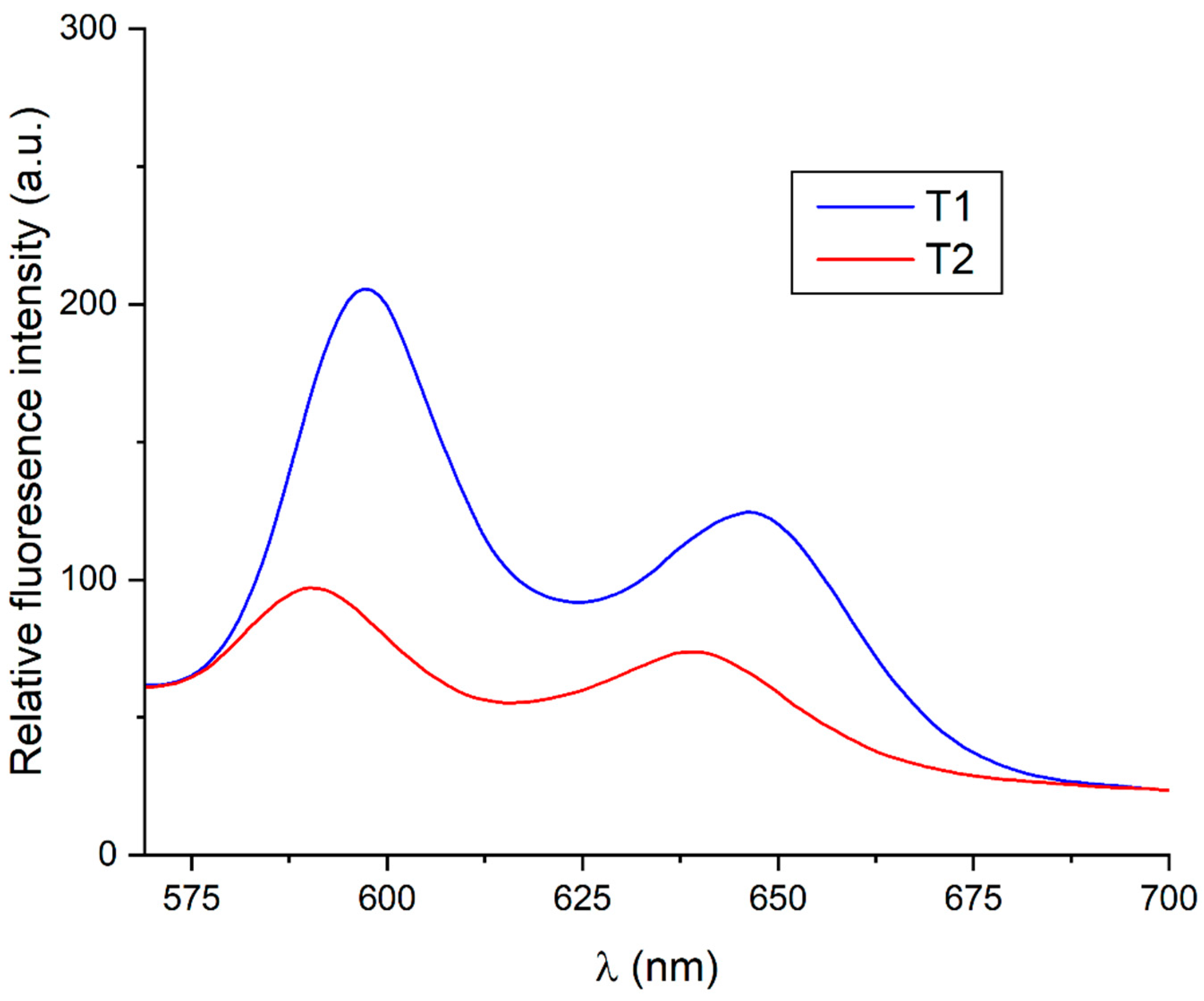
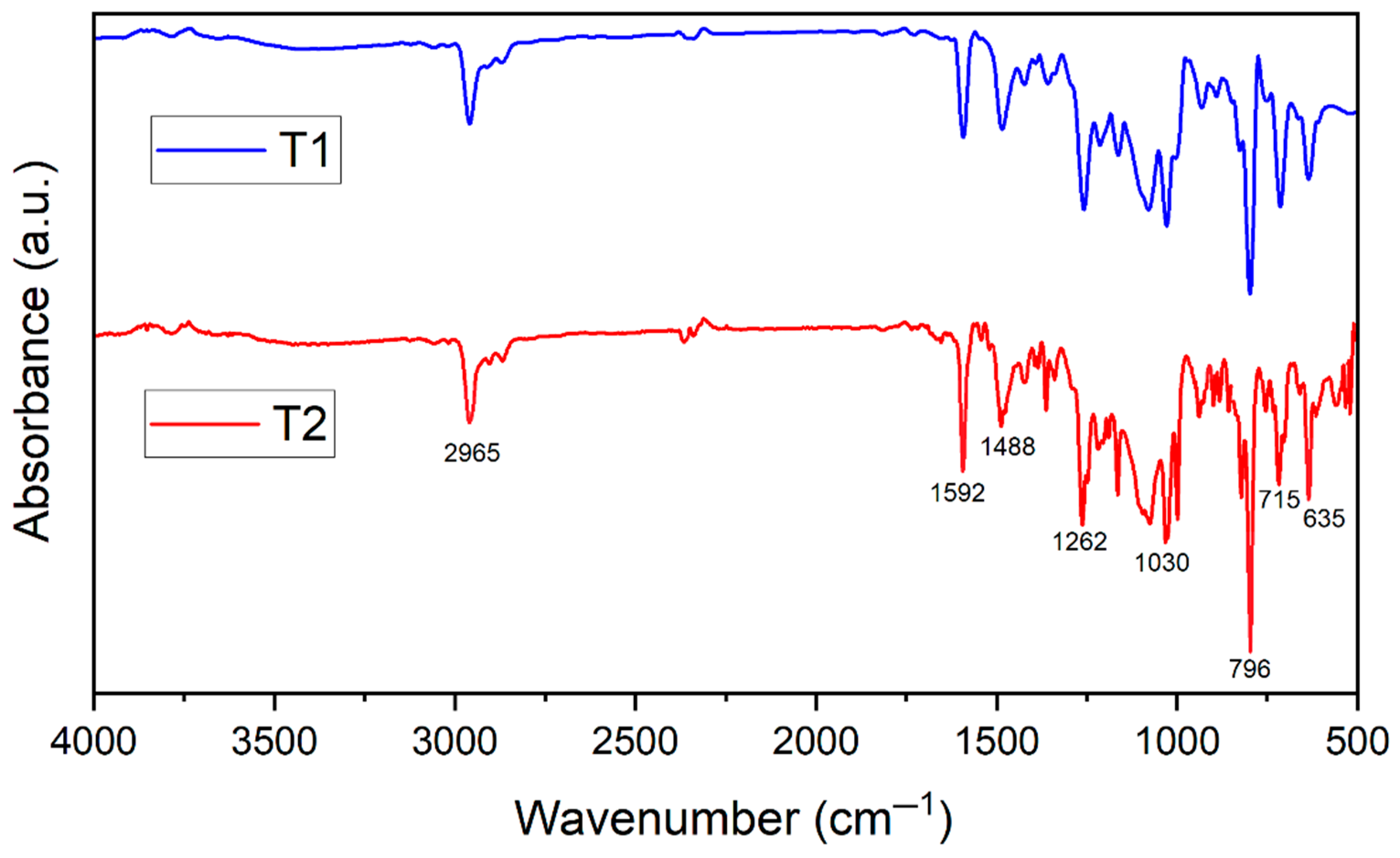
3.2. Spectroscopic Characterization
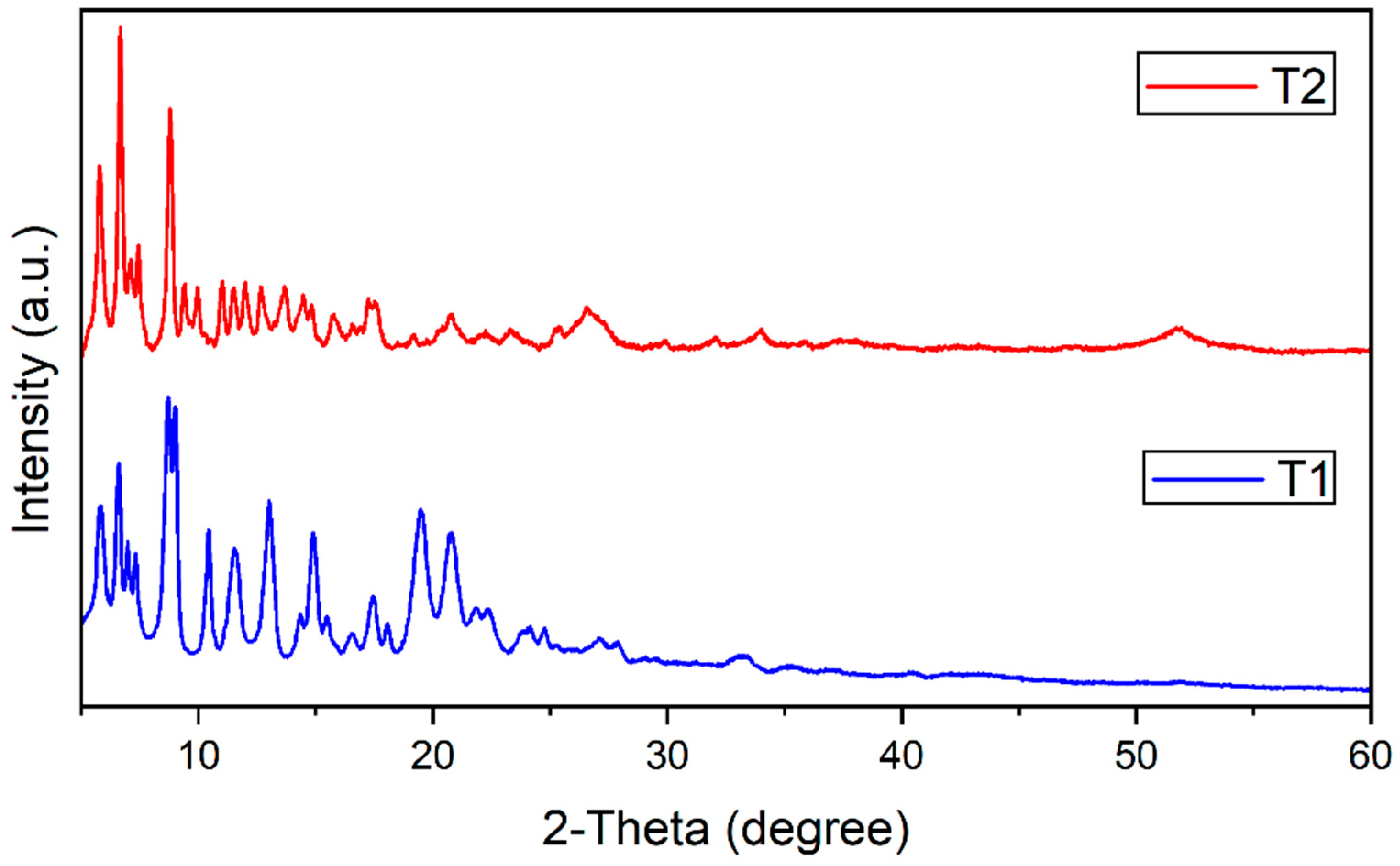
3.3. Microscopic Investigation
3.4. Photocatalytic Degradation of Pollutants
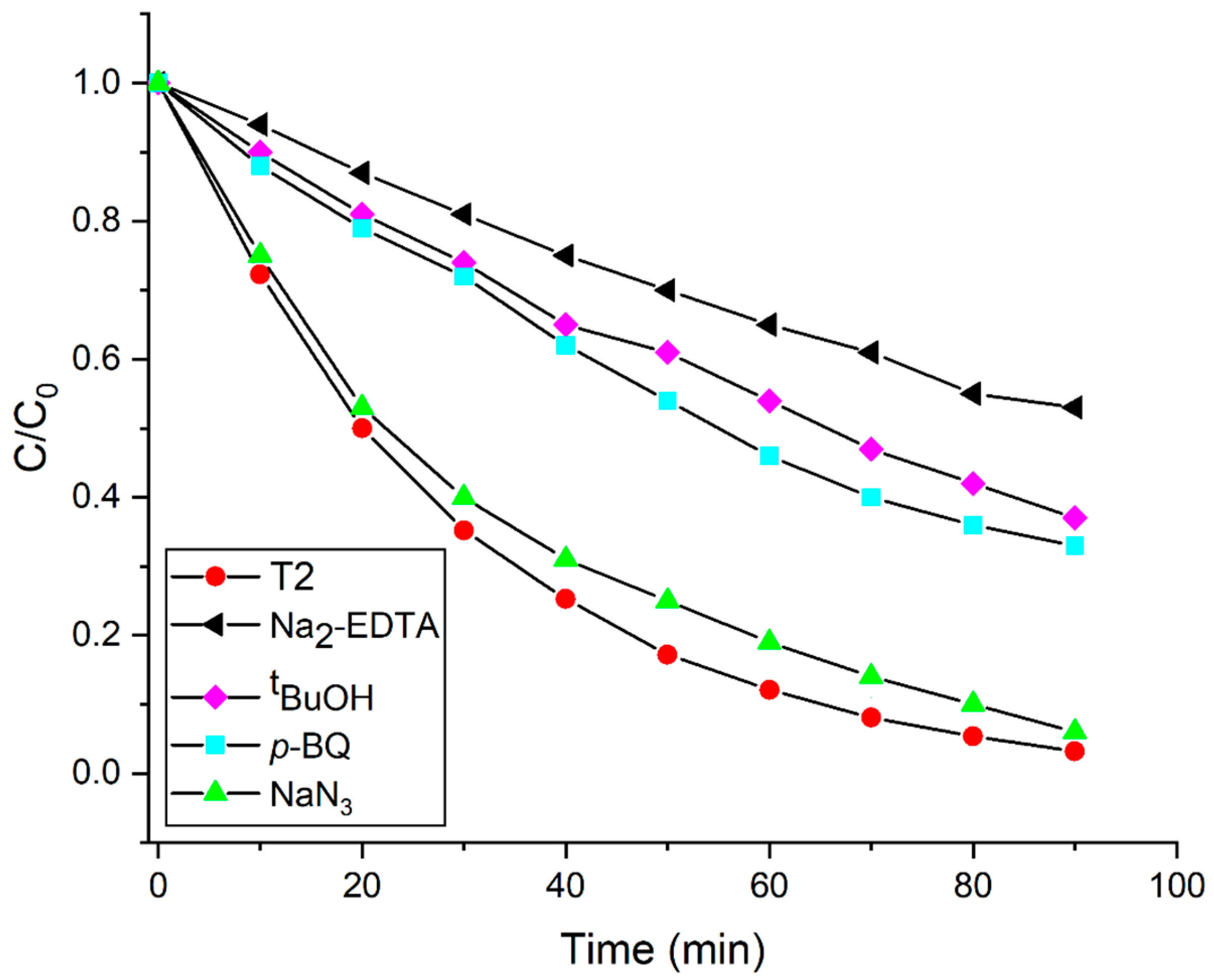
3.5. Possible Mechanism of the Photocatalytic Degradation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for Energy Conversion and Storage. Chem. Soc. Rev. 2013, 42, 3127–3171. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sun, L.; Zhang, Q.; Zhou, Y.; Zhang, J.; Yang, B.; Xu, B.; Xu, Q. Nanomaterials-Based Ion-Imprinted Electrochemical Sensors for Heavy Metal Ions Detection: A Review. Biosensors 2022, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Lyu, F.; Yin, Y. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef]
- Ryabchikova, E. Advances in Nanomaterials in Biomedicine. Nanomaterials 2021, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ren, J.; Qu, X. Carbon Nanomaterials and DNA: From Molecular Recognition to Applications. Acc. Chem. Res. 2016, 49, 461–470. [Google Scholar] [CrossRef]
- Martell, S.A.; Lai, Y.; Traver, E.; MacInnis, J.; Richards, D.D.; MacQuarrie, S.; Dasog, M. High Surface Area Mesoporous Silicon Nanoparticles Prepared via Two-Step Magnesiothermic Reduction for Stoichiometric CO2 to CH3OH Conversion. ACS Appl. Nano Mater. 2019, 2, 5713–5719. [Google Scholar] [CrossRef]
- Gould, A.L.; Kadkhodazadeh, S.; Wagner, J.B.; Catlow, C.R.A.; Logsdail, A.J.; Di Vece, M. Understanding the thermal stability of silver nanoparticles embedded in a-Si. J. Phys. Chem. C 2015, 119, 23767–23773. [Google Scholar] [CrossRef]
- Minea, A.A. A review on electrical conductivity of nanoparticle-enhanced fluids. Nanomaterials 2019, 9, 1592. [Google Scholar] [CrossRef] [PubMed]
- Maity, N.; Ghosh, R.; Nandi, A.K. Optoelectronic Properties of Self-Assembled Nanostructures of Polymer Functionalized Polythiophene and Graphene. Langmuir 2018, 34, 7585–7597. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Djurišić, A.; Chen, B.; Leung, X.; Ng, Y.H. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2012, 22, 6526–6535. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [PubMed]
- Kolahalam, L.A.; Viswanath, I.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, F.; Li, L.; Zhang, J. Self-Assembled Organic Nanomaterials for Drug Delivery, Bioimaging, and Cancer Therapy. ACS Biomater. Sci. Eng. 2020, 6, 4816–4833. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Huang, Z.-H.; Wang, L.-N.; Kang, F. Porphyrin-based nanostructures for photocatalytic applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef]
- Magna, G.; Monti, D.; Di Natale, C.; Paolesse, R.; Stefanelli, M. The Assembly of Porphyrin Systems in Well-Defined Nanostructures: An Update. Molecules 2019, 24, 4307. [Google Scholar] [CrossRef]
- La, D.D.; Ngo, H.H.; Nguyen, D.D.; Tran, N.T.; Vo, H.T.; Nguyen, X.H.; Chang, S.W.; Chung, W.J.; Nguyen, M.D. Advances and prospects of porphyrin-based nanomaterials via self-assembly for photocatalytic applications in environmental treatment. Coord. Chem. Rev. 2022, 463, 214543. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Recent Developments in Porphyrin-Based Metal-Organic Framework Materials for Water Remediation under Visible-Light Irradiation. Int. J. Mol. Sci. 2024, 25, 4183. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Porphyrin-Based Nanomaterials for the Photocatalytic Remediation of Wastewater: Recent Advances and Perspectives. Molecules 2024, 29, 611. [Google Scholar] [CrossRef]
- Lehn, J.-M. Perspectives in Supramolecular Chemistry—From Molecular Recognition towards Molecular Information Processing and Self-Organization. Angew. Chem. Int. Ed. Engl. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Drain, C.M.; Varotto, A.; Radivojevic, I. Self-Organized Porphyrinic Materials. Chem. Rev. 2009, 109, 1630–1658. [Google Scholar] [CrossRef] [PubMed]
- Hasobe, T. Photo- and electro-functional self-assembled architectures of porphyrins. Phys. Chem. Chem. Phys. 2012, 14, 15975–15987. [Google Scholar] [CrossRef]
- Wang, S.-P.; Lin, W.; Wang, X.; Cen, T.-Y.; Xie, H.; Huang, J.; Zhu, B.-Y.; Zhang, Z.; Song, A.; Hao, J.; et al. Controllable hierarchical self-assembly of porphyrin-derived supra-amphiphiles. Nat. Commun. 2019, 10, 1399–1411. [Google Scholar] [CrossRef]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin Nanotubes by Ionic Self-Assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Medforth, C.J.; Shelnutt, J.A. Self-assembly and self-metallization of porphyrin nanosheets. J. Am. Chem. Soc. 2007, 129, 2440–2441. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Complementary metalloporphyrin-based nanostructure decorated with silver nanoparticles for photocatalytic degradation of organic dyes. Inorg. Chem. Commun. 2024, 163, 112252. [Google Scholar] [CrossRef]
- Medforth, C.J.; Wang, Z.; Martin, K.E.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 47, 7261–7277. [Google Scholar] [CrossRef]
- Tian, Y.; Busani, T.; Uyeda, G.H.; Martin, K.E.; van Swol, F.; Medforth, C.J.; Montan, G.A.; Shelnutt, J.A. Hierarchical cooperative binary ionic porphyrin nanocomposites. Chem. Commun. 2012, 48, 4863–4865. [Google Scholar] [CrossRef]
- Xie, M.-H.; Yang, X.-L.; Zou, C.; Wu, C.-D. A SnIV–Porphyrin-Based Metal-Organic Framework for the Selective Photo-Oxygenation of Phenol and Sulfides. Inorg. Chem. 2011, 50, 5318–5320. [Google Scholar] [CrossRef]
- Manke, A.M.; Geisel, K.; Fetzer, A.; Kurz, P. A Water-Soluble Tin(IV) Porphyrin as a Bioinspired Photosensitiser for Light-Driven Proton-Reduction. Phys. Chem. Chem. Phys. 2014, 16, 12029–12042. [Google Scholar] [CrossRef] [PubMed]
- Zadehahmadi, F.; Ahmadi, F.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Kardanpour, R. Catalytic CO2 fixation using tin porphyrin supported on organic and inorganic materials under mild conditions. J. Mol. Catal. A: Chem. 2015, 398, 1–10. [Google Scholar] [CrossRef]
- Shee, N.K.; Park, B.-H.; Kim, H.-J. Hybrid Composite of Sn(IV)-Porphyrin and Mesoporous Structure for Enhanced Visible Light Photocatalytic Degradation of Organic Dyes. Molecules 2023, 28, 1886. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Lee, C.-J.; Kim, H.-J. Hexacoordinated Sn(IV) porphyrin-based square-grid frameworks exhibiting selective uptake of CO2 over N2. Bull. Korean Chem. Soc. 2022, 43, 103–109. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Supramolecular squares of Sn(IV)porphyrins with Re(I)-corners for the fabrication of self-assembled nanostructures performing photocatalytic degradation of Eriochrome Black T dye. Inorg. Chem. Front. 2022, 10, 174–183. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Sn(IV)-Porphyrin-Based Nanostructures Featuring Pd(II)-Mediated Supramolecular Arrays and Their Photocatalytic Degradation of Acid Orange 7 Dye. Int. J. Mol. Sci. 2022, 23, 13702. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. (trans-Dihydroxo)Sn(IV)-[5,10,15,20-tetrakis(2-pyridyl)porphyrin]. Molbank 2023, 2023, M1669. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Supramolecular Self-Assembly of the Zwitterionic Sn(IV)-Porphyrin Complex. Molbank 2023, 2023, M1723. [Google Scholar] [CrossRef]
- Shee, N.K.; Lee, G.-S.; Kim, H.-J. Sn(IV)porphyrin-Incorporated TiO2 Nanotubes for Visible Light-Active Photocatalysis. Molecules 2024, 29, 1612. [Google Scholar] [CrossRef]
- Lu, J.; Li, Z.; An, W.; Liu, L.; Cui, W. Tuning the Supramolecular Structures of Metal-Free Porphyrin via Surfactant Assisted Self-Assembly to Enhance Photocatalytic Performance. Nanomaterials 2019, 9, 1321. [Google Scholar] [CrossRef] [PubMed]
- Nikoloudakis, E.; Pigiaki, M.; Polychronaki, M.N.; Margaritopoulou, A.; Charalambidis, G.; Serpetzoglou, E.; Mitraki, A.; Loukakos, P.A.; Coutsolelos, A.G. Self-Assembly of Porphyrin Dipeptide Conjugates toward Hydrogen Production. ACS Sustain. Chem. Eng. 2021, 9, 7781–7791. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Morphology-controlled self-assembled nanostructures of complementary metalloporphyrin triads through intermolecular coordination tuning and their photocatalytic degradation for Orange II. Inorg. Chem. Front. 2022, 9, 5538–5548. [Google Scholar] [CrossRef]
- Hasobe, T.; Oki, H.; Sandanayakaa, A.S.D.; Murata, H. Sonication-assisted supramolecular nanorods of meso-diaryl-substituted porphyrins. Chem. Commun. 2008, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Sn(IV) Porphyrin-Based Ionic Self-Assembled Nanostructures and Their Application in Visible Light Photo-Degradation of Malachite Green. Catalysts 2022, 12, 799. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Supramolecular Porphyrin Nanostructures Based on Coordination-Driven Self-Assembly and Their Visible Light Catalytic Degradation of Methylene Blue Dye. Nanomaterials 2020, 10, 2314. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Self-Assembled Nanomaterials Based on Complementary Sn(IV) and Zn(II)-Porphyrins, and Their Photocatalytic Degradation for Rhodamine B Dye. Molecules 2021, 26, 3598. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Three Isomeric Zn(II)-Sn(IV)-Zn(II) Porphyrin-Triad-Based Supramolecular Nanoarchitectures for the Morphology-Dependent Photocatalytic Degradation of Methyl Orange. ACS Omega 2022, 7, 9775–9784. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, F.; Tian, X.; Chen, T.; Wu, L.; Jin, L.Y. Supramolecular nanostructures constructed by rod–coil molecular isomers: Effect of rod sequences on molecular assembly. Soft Matter 2019, 15, 6718–6724. [Google Scholar] [CrossRef]
- Chen, L.; Feng, J.; Yang, D.; Tian, F.; Ye, X.; Qian, Q.; Wei, S.; Zhou, Y. Sequence isomerism-dependent self-assembly of glycopeptide mimetics with switchable antibiofilm properties. Chem. Sci. 2019, 10, 8171–8178. [Google Scholar] [CrossRef]
- Dibble, J.P.; Troyano-Valls, C.; Tovar, J.D. A Tale of Three Hydrophobicities: Impact of Constitutional Isomerism on Nanostructure Evolution and Electronic Communication in π-Conjugated Peptides. Macromolecules 2020, 53, 7263–7273. [Google Scholar] [CrossRef]
- Chatterjee, S.; Tyagi, A.K.; Ayyub, P. Efficient Photocatalytic Degradation of Rhodamine B Dye by Aligned Arrays of Self Assembled Hydrogen Titanate Nanotubes. J. Nanomater. 2014, 2014, 328618. [Google Scholar] [CrossRef]
- Sundararajan, M.; Sailaja, V.; Kennedy, L.J.; Vijaya, J.J. Photocatalytic degradation of Rhodamine B under visible light using nanostructured zinc doped cobalt ferrite: Kinetics and mechanism. Ceram. Int. 2017, 43, 540–548. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Li, C.; Yu, J.; Jiang, X.; Zheng, Y.; Hu, W.; Jiao, F. Enhanced photocatalytic degradation of rhodamine B, methylene blue and 4-nitrophenol under visible light irradiation using TiO2/MgZnAl layered double hydroxide. J. Mater. Sci. Mater. Electron. 2018, 29, 7002–7014. [Google Scholar] [CrossRef]
- Luna-Flores, A.; Morales, M.A.; Agustín-Serrano, R.; Portillo, R.; Luna-López, J.A.; Pérez-Sánchez, G.F.; Luz, A.D.H.-d.l.; Tepale, N. Improvement of the Photocatalytic Activity of ZnO/Burkeite Heterostructure Prepared by Combustion Method. Catalysts 2019, 9, 817. [Google Scholar] [CrossRef]
- Pontes do Nascimento, N.M.; Machado de Lima, B.R.; Zamian, J.R.; Ferreira da Costa, C.E.; Adriano Santos do Nascimento, L.; Luque, R.; Filho, G.N.d.R. Synthesis of Mesoporous Zn1−xMxAl2O4 Substituted by Co2+ and Ni2+ Ions and Application in the Photodegradation of Rhodamine B. Materials 2020, 13, 2150. [Google Scholar] [CrossRef] [PubMed]
- Suvaitha, P.; Selvam, S.; Ganesan, D.; Rajangam, V.; Raji, A. Green Synthesis of SnO2 Nanoparticles for Catalytic Degradation of Rhodamine B. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 661–676. [Google Scholar]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [Google Scholar] [CrossRef]
- Amakali, T.; Zivkovic, A.; Warwick, M.E.A.; Jones, D.R.; Dunnill, C.W.; Daniel, L.S.; Uahengo, V.; Mitchell, C.E.; Dzade, N.Y.; De Leeuw, N.H. Photocatalytic degradation of rhodamine B dye and hydrogen evolution by hydrothermally synthesized NaBH4-spiked ZnS nanostructures. Front. Chem. 2022, 10, 835832. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Sn(IV)porphyrin-Anchored TiO2 Nanoparticles via Axial-Ligand Coordination for Enhancement of Visible Light-Activated Photocatalytic Degradation. Inorganics 2023, 11, 336. [Google Scholar] [CrossRef]
- Nagamine, M.; Osial, M.; Jackowska, K.; Krysinski, P.; Widera-Kalinowska, J. Tetracycline Photocatalytic Degradation under CdS Treatment. J. Mar. Sci. Eng. 2020, 8, 483. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Liu, X.; Li, Q.; Zheng, Y. A Novel PVDF-TiO2@g-C3N4 Composite Electrospun Fiber for Efficient Photocatalytic Degradation of Tetracycline under Visible Light Irradiation. Ecotoxicol. Environ. Saf. 2021, 210, 111866. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, A.O.; Olatunji, O.S. Photocatalytic degradation of tetracycline in aqueous systems under visible light irridiation using needle-like SnO2 nanoparticles anchored on exfoliated g-C3N4. Environ. Sci. Eur. 2022, 34, 5. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Kim, H. Photocatalytic degradation of tetracycline antibiotics using hydrothermally synthesized two-dimensional molybdenum disulfide/titanium dioxide composites. J. Colloid Interface Sci. 2022, 606, 454–463. [Google Scholar] [CrossRef]
- Wei, X.; Feng, H.; Li, L.; Gong, J.; Jiang, K.; Xue, S.; Chu, P.K. Synthesis of tetragonal prismatic γ-In2Se3 nanostructures with predominantly {110} facets and photocatalytic degradation of tetracycline. Appl. Catal. B-Environ. 2020, 260, 118218. [Google Scholar] [CrossRef]
- Li, M.Y.; Tang, Y.-B.; Shi, W.-L.; Chen, F.-Y.; Shi, Y.; Gu, H.C. Design of visible-light-response core–shell Fe2O3/CuBi2O4 heterojunctions with enhanced photocatalytic activity towards the degradation of tetracycline: Z-scheme photocatalytic mechanism insight. Inorg. Chem. Front. 2018, 5, 3148–3154. [Google Scholar] [CrossRef]
- Yu, X.; He, J.; Zhang, Y.; Hu, J.; Chen, F.; Wang, Y.; He, G.; Liu, J.; He, Q. Effective photodegradation of tetracycline by narrow-energy band gap photocatalysts La2−xSrxNiMnO6 (x = 0, 0.05, 0.10, and 0.125). J. Alloys Compd. 2019, 806, 451–463. [Google Scholar] [CrossRef]
- Linh, N.X.D.; Hanh, N.T.; Cuong, L.M.; Huong, N.T.; Ha, N.T.T.; Trinh, T.D.; Van Noi, N.; Cam, N.T.D.; Pham, T.D. Facile Fabrication of α-Fe2O3/g-C3N4 Z-Scheme Heterojunction for Novel Degradation of Residual Tetracycline. Top. Catal. 2023, 66, 139–148. [Google Scholar] [CrossRef]
- Jiang, L.B.; Yuan, X.Z.; Zeng, G.M.; Liang, J.; Chen, X.H.; Yu, H.B.; Wang, H.; Wu, Z.B.; Zhang, J.; Xiong, T. In-situ synthesis of direct solid-state dual Z-scheme WO3/g-C3N4/Bi2O3 photocatalyst for the degradation of refractory pollutant. Appl. Catal. B-Environ. 2018, 227, 376–385. [Google Scholar] [CrossRef]
- Yuan, X.; Jiang, L.; Chen, X.; Leng, L.; Wang, H.; Wu, Z.; Xiong, T.; Liang, J.; Zeng, G. Highly Efficient Visible-Light-Induced Photoactivity of Z-Scheme Ag2CO3/Ag/WO3 Photocatalysts for Organic Pollutant Degradation. Environ. Sci. Nano 2017, 4, 2175–2185. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, L.; Ding, P.; Wang, C.; Wang, Q.; Wang, H.; Li, Y.; Xu, H.; Li, H. One-step oxygen vacancy engineering of WO3-x/2D g-C3N4 heterostructure: Triple effects for sustaining photoactivity. J. Alloys Compd. 2019, 795, 426–435. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Integration of Sn (IV) porphyrin on Mesoporous Alumina Support and Visible Light Catalytic Photodegradation of Methylene Blue. Mater. Today Commun. 2024, 39, 109033. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Q.Z.; Wang, Z.P.; Zhu, Y. An all-organic 0D/2D supramolecular porphyrin/g-C3N4 heterojunction assembled via π-π interaction for efficient visible photocatalytic oxidation. Appl. Catal. B Environ. 2021, 291, 120059. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Surface Modification of ZnO with Sn(IV)-Porphyrin for Enhanced Visible Light Photocatalytic Degradation of Amaranth Dye. Molecules 2023, 28, 6481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jiang, Y.; Wang, T.; Lu, Q.; Zhao, K.; Pan, J. Photothermal-photocatalytic route of MOF-based membrane with nanosheet array structures for solar-driven water purification. Chem. Eng. J. 2023, 475, 146268. [Google Scholar] [CrossRef]
- Zhu, K.; Li, X.; Chen, Y.; Huang, Y.; Yang, Z.; Guan, G.; Yan, K. Recent advances on the spherical metal oxides for sustainable degradation of antibiotics. Coord. Chem. Rev. 2024, 510, 215813. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Jia, X.; Yang, J.; Miao, X.; Shao, D.; Wu, J.; Song, H. 2D/2D ultrathin polypyrrole heterojunct aerogel with synergistic photocatalytic-photothermal evaporation performance for efficient water purification. Desalination 2024, 574, 117295. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Yan, S.; Yang, J.; Jia, X.; Song, H. Bioinspired Graphene Aerogels with Hybrid Wettability for Solar-Driven Purification of Complex Wastewater. ACS Appl. Mater. Interfaces 2024, 16, 1794–1804. [Google Scholar] [CrossRef]




| Photocatalyst | Rate Constant (min−1) | Reference |
|---|---|---|
| H2Ti3O7 nanotube | 0.002 | [52] |
| TiO2 (P-25) | 0.001 | [52] |
| Co0.6Zn0.4Fe2O4 | 0.015 | [53] |
| TiO2 | 0.0015 | [54] |
| TiO2/MgZnAl | 0.005 | [54] |
| ZnO | 0.009 | [55] |
| ZnO/Burkeite | 0.028 | [55] |
| Ni0.5Zn0.5Al2O4 | 0.005 | [56] |
| SnO2-Acalypha Indica | 0.0062 | [57] |
| Au/ZnO | 0.030 | [58] |
| ZnS-NaBH4 | 0.0123 | [59] |
| SnP | 0.0016 | [60] |
| TiO2 | 0.006 | [60] |
| SnP@TiO2 | 0.0078 | [60] |
| SnP/AA@TiO2 | 0.0366 | [60] |
| T1 | 0.014 | this study |
| T2 | 0.037 | this study |
| Photocatalyst | Irradiation Time (min) | Catalyst Dosage (mg) | TC Concentration (mg/L) | Rate Constant (min−1) | Reference |
|---|---|---|---|---|---|
| CdS | 60 | 10 | 40 | 0.0056 | [61] |
| poly vinylidene fluoride-TiO2@g-C3N4-0.2g | 300 | 100 | 50 | 0.012 | [62] |
| SnO2 | 120 | 50 | 30 | 0.00324 | [63] |
| g-C3N4 | 120 | 50 | 30 | 0.00473 | [63] |
| 3% SnO2/g-C3N4 | 120 | 50 | 30 | 0.0205 | [63] |
| TiO2 | 100 | 25 | 10 | 0.0055 | [64] |
| 20 wt% MoS2/TiO2 | 100 | 25 | 10 | 0.0185 | [64] |
| γ-In2Se3 (0.04 M EDTA) | 120 | 50 | 20 | 0.0175 | [65] |
| Fe2O3 | 120 | 50 | 10 | 0.00485 | [66] |
| 30%- Fe2O3/CuBi2O4 | 120 | 50 | 10 | 0.01246 | [66] |
| La2-xSrxNiMnO6 (x = 0.10) | 240 | 100 | 10 | 0.0101 | [67] |
| α-Fe2O3/g-C3N4 | 120 | 50 | 10 | 0.0222 | [68] |
| WO3/g-C3N4/Bi2O3 | 60 | 100 | 10 | 0.0240 | [69] |
| Ag2CO3/Ag/WO3 | 90 | 100 | 10 | 0.0179 | [70] |
| WO3/g-C3N4 | 100 | 100 | 20 | 0.0210 | [71] |
| T1 | 45 | 50 | 100 | 0.012 | This study |
| T2 | 45 | 50 | 100 | 0.026 | This study |
| Structural Isomers | T1 | T2 |
|---|---|---|
| Starting materials | ZnL + SnP1 (2:1) | ZnL + SnP2 (2:1) |
| Chemical formula | C178H176N14O2SnZn2 | C178H176N14O2SnZn2 |
| Color | reddish-brown | dark green |
| Melting point | 338−340 °C | 346−348 °C |
| Solubility in toluene | highly soluble | sparingly soluble |
| Morphology (FE-SEM) | nanosphere | nanofiber |
| Self-assembly | intramolecular coordination for orthogonal alignment and intermolecular π-π interaction | intramolecular coordination for linear alignment and intermolecular π-π interaction |
| BET surface area (m2/g) | 63 | 97 |
| UV–vis absorption | 428 nm (Soret band); 564, 612 nm (Q-band) | 455 nm (Soret band); 572, 614 nm (Q-band) |
| Emission (excited at 550 nm) | 597 nm and 646 nm | 590 nm and 640 nm |
| Band gap energy (Eg) | 2.72 eV | 2.56 eV |
| First-order degradation rate constant (RhB dye) | 0.014 min−1 | 0.037 min−1 |
| First-order degradation rate constant (TC antibiotic) | 0.012 min−1 | 0.026 min−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shee, N.K.; Kim, H.-J. Supramolecular Self-Assembled Nanostructures Derived from Amplified Structural Isomerism of Zn(II)−Sn(IV)−Zn(II) Porphyrin Triads and Their Visible Light Photocatalytic Degradation of Pollutants. Nanomaterials 2024, 14, 1104. https://doi.org/10.3390/nano14131104
Shee NK, Kim H-J. Supramolecular Self-Assembled Nanostructures Derived from Amplified Structural Isomerism of Zn(II)−Sn(IV)−Zn(II) Porphyrin Triads and Their Visible Light Photocatalytic Degradation of Pollutants. Nanomaterials. 2024; 14(13):1104. https://doi.org/10.3390/nano14131104
Chicago/Turabian StyleShee, Nirmal Kumar, and Hee-Joon Kim. 2024. "Supramolecular Self-Assembled Nanostructures Derived from Amplified Structural Isomerism of Zn(II)−Sn(IV)−Zn(II) Porphyrin Triads and Their Visible Light Photocatalytic Degradation of Pollutants" Nanomaterials 14, no. 13: 1104. https://doi.org/10.3390/nano14131104






