Research Progress in the Degradation of Chemical Warfare Agent Simulants Using Metal–Organic Frameworks
Abstract
:1. Introduction
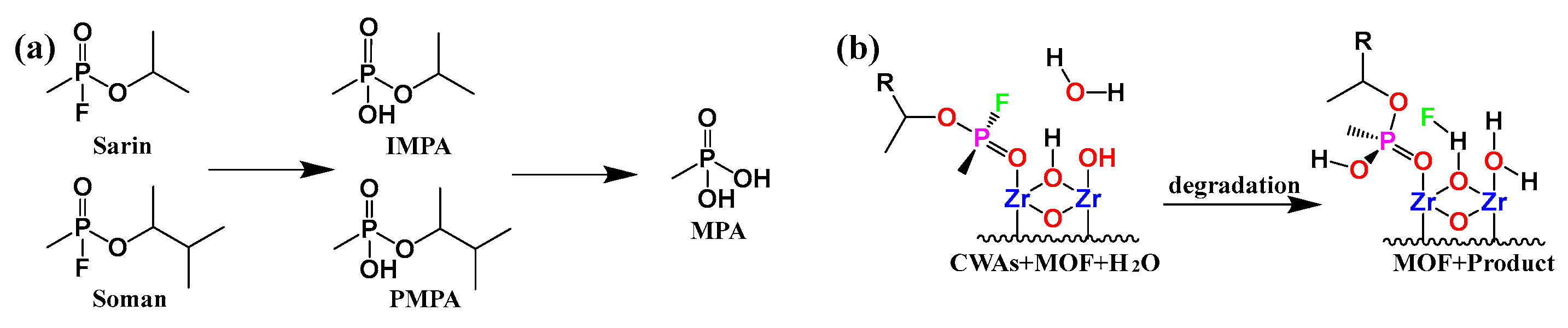
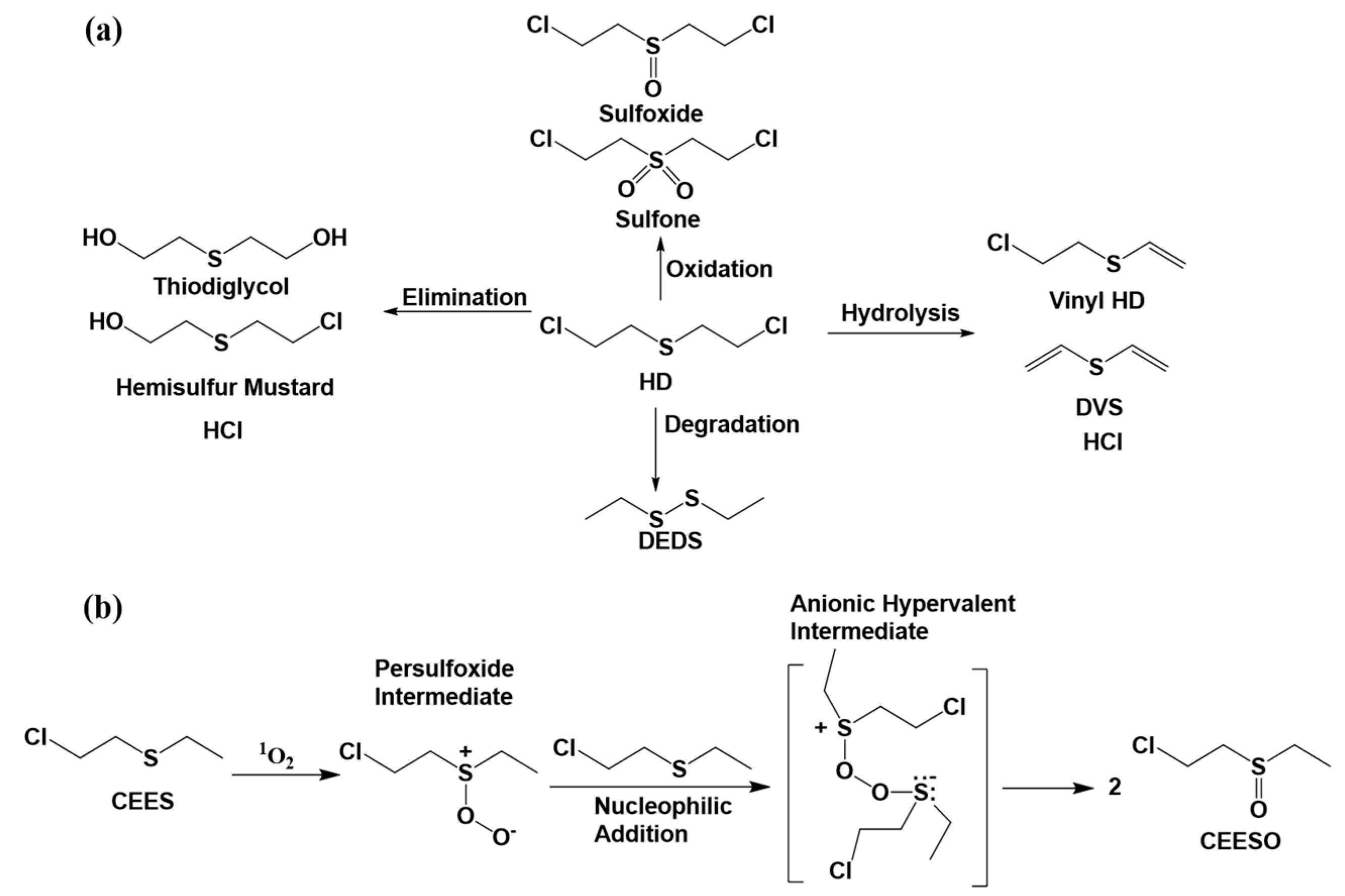
2. Degradation Mechanism of Chemical Warfare Agents
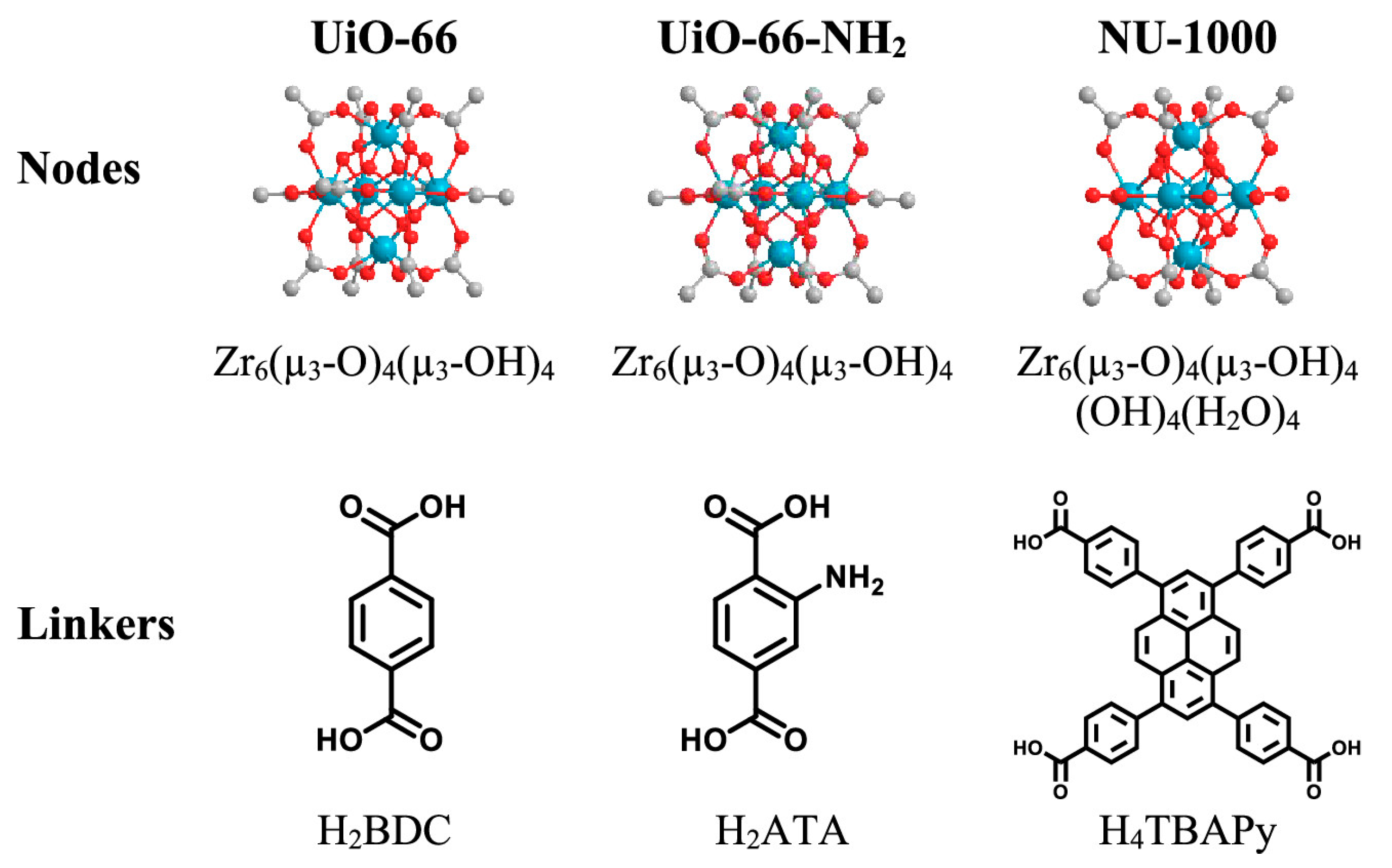
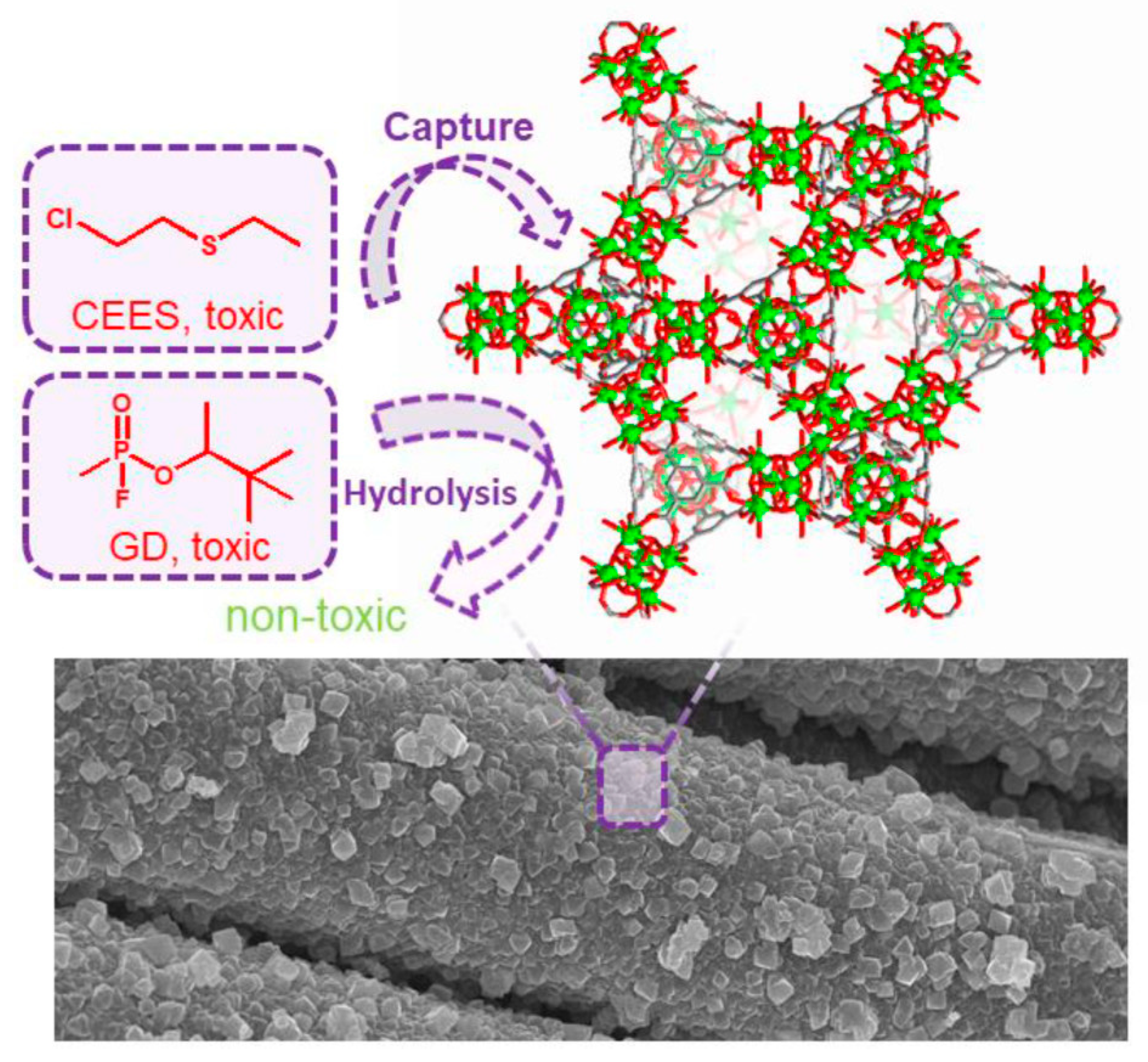
3. MOFs Degradation of Chemical Warfare Agents
3.1. Chemical Nerve Agent Simulants with MOFs
3.2. MOF Degradation of Mustard Gas Simulants
4. MOF Composites Degradation of Chemical Nerve Agents
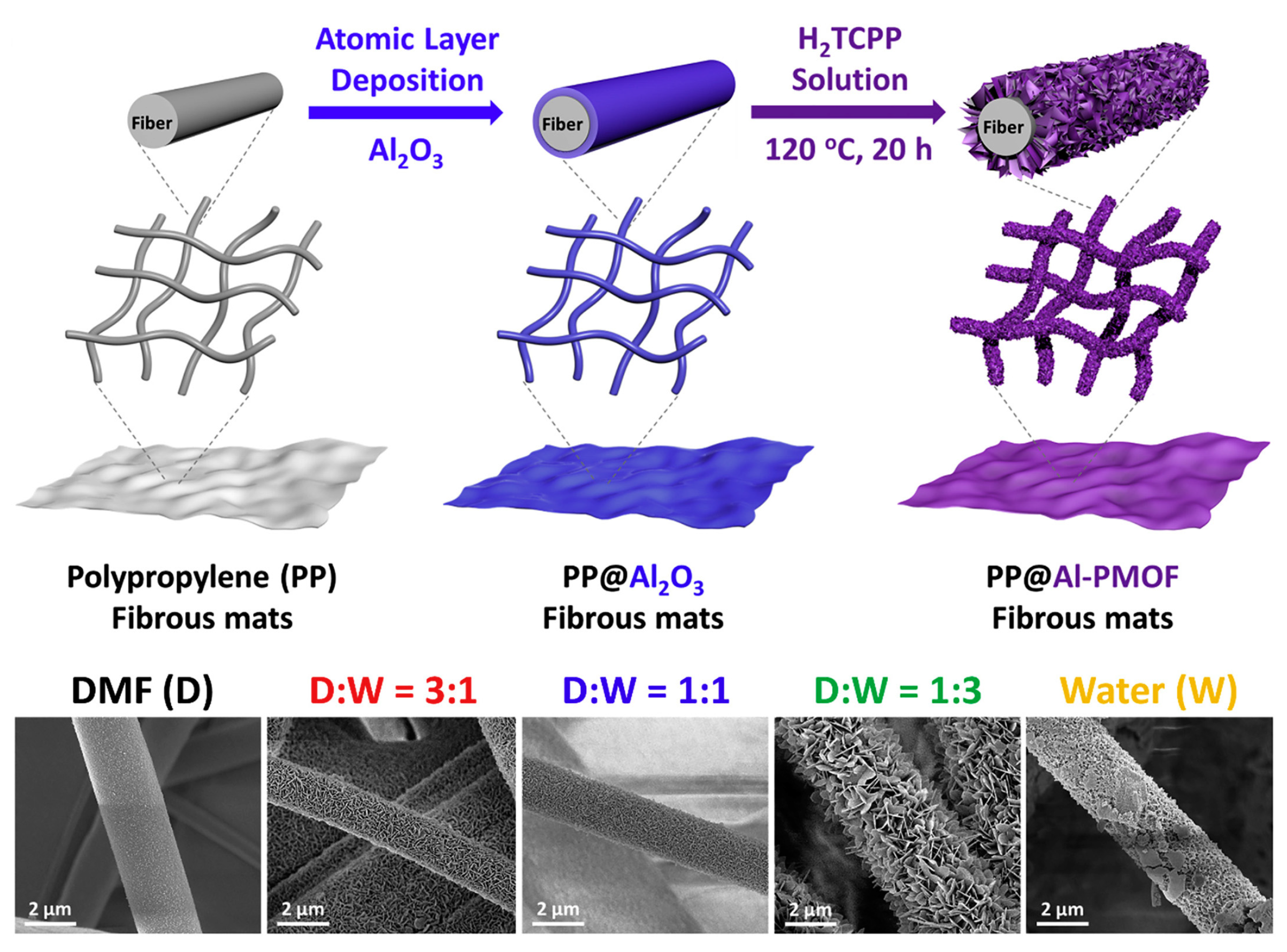
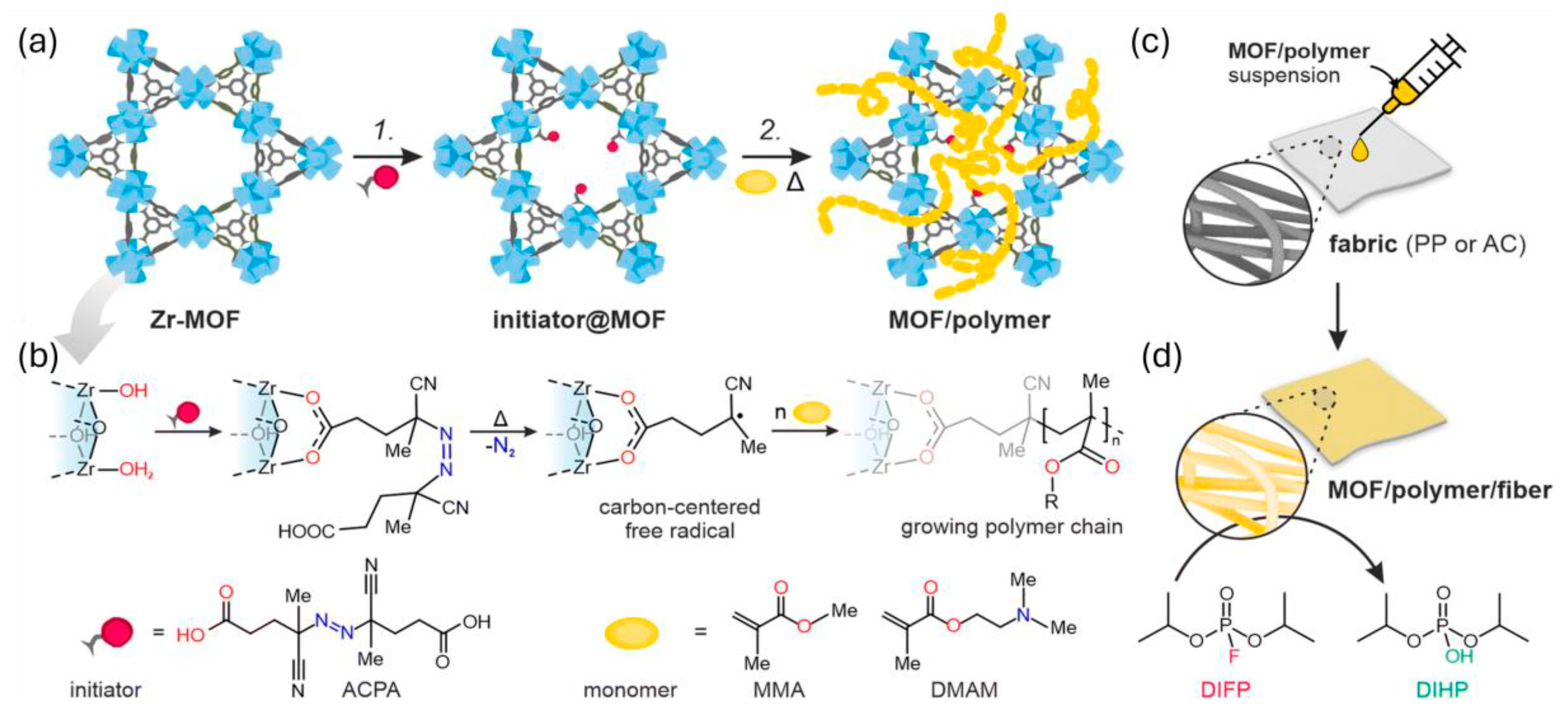
4.1. MOF Composite Degradation of Chemical Nerve Agent Simulants
4.2. MOF Composite Degradation of Mustard Gas Simulants
5. Conclusions
Funding
Conflicts of Interest
References
- Schwenk, M. Chemical warfare agents. classes and targets. Toxicol. Lett. 2018, 293, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Destruction and detection of chemical warfare agents. Chem. Rev. 2011, 111, 5345–5403. [Google Scholar] [CrossRef]
- Jett, D.A.; Spriggs, S.M. Translational research on chemical nerve agents. Neurobiol. Dis. 2020, 133, 104335. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, J.; Prescott, M.; Jamal, R.; Hardawan, S.A.; Abdullah, A.; Galea, S. The long-term psychosocial impact of a surprise chemical weapons attack on civilians in Halabja, Iraqi Kurdistan. J. Nerv. Ment. Dis. 2008, 196, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.; Elbahtimy, H.; Martin, S.B. The future of chemical weapons: Implications from the syrian civil war. Secur. Stud. 2018, 27, 704–733. [Google Scholar] [CrossRef]
- Sakurada, K.; Ohta, H. No promising antidote 25 years after the Tokyo subway sarin attack: A review. Leg. Med. 2020, 47, 101761. [Google Scholar] [CrossRef] [PubMed]
- Vale, J.A.; Marrs Obe, T.C.; Maynard Cbe, R.L. Novichok: A murderous nerve agent attack in the UK. Clin. Toxicol. 2018, 56, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gao, M.; Zhang, M.; Zhang, Y.; Gao, M.; Wang, Z.; Xu, L.; Wang, X.; Shen, B. Advances in the adsorption and degradation of chemical warfare agents and simulants by metal-organic frameworks. Coord. Chem. Rev. 2023, 493, 215289. [Google Scholar] [CrossRef]
- Aroniadou-Anderjaska, V.; Apland, J.P.; Figueiredo, T.H.; De Araujo Furtado, M.; Braga, M.F. Acetylcholinesterase inhibitors (nerve agents) as weapons of mass destruction: History, mechanisms of action, and medical countermeasures. Neuropharmacology 2020, 181, 108298. [Google Scholar] [CrossRef]
- Zhuang, Q.; Franjesevic, A.J.; Corrigan, T.S.; Coldren, W.H.; Dicken, R.; Sillart, S.; DeYong, A.; Yoshino, N.; Smith, J.; Fabry, S.; et al. Demonstration of in vitro resurrection of aged acetylcholinesterase after exposure to organophosphorus chemical nerve agents. J. Med. Chem. 2018, 61, 7034–7042. [Google Scholar] [CrossRef]
- Cavalcante, S.F.d.A.; Simas, A.B.C.; Barcellos, M.C.; de Oliveira, V.G.M.; Sousa, R.B.; Cabral, P.A.d.M.; Kuča, K.; França, T.C.C. Acetylcholinesterase: The “hub” for neurodegenerative diseases and chemical weapons convention. Biomolecules 2020, 10, 414. [Google Scholar] [CrossRef]
- Gorecki, L.; Soukup, O.; Korabecny, J. Countermeasures in organophosphorus intoxication: Pitfalls and prospects. Trends Pharmacol. Sci. 2022, 43, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Etemad, L.; Moshiri, M.; Balali-Mood, M. Advances in treatment of acute sulfur mustard poisoning—A critical review. Crit. Rev. Toxicol. 2019, 49, 191–214. [Google Scholar] [CrossRef]
- Sezigen, S.; Kenar, L. Recent sulfur mustard attacks in middle east and experience of health professionals. Toxicol. Lett. 2020, 320, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Shahri, S.H.G.; Balali-Mood, M.; Heidarzadeh, H.R.; Abrishami, M. Ophthalmic complications and managements of sulfur mustard exposure: A narrative review. Arch. Iran. Med. 2022, 25, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ping, E.M.; Sun, J.M.; Zhang, L.J.; Zhou, Y.S.; Zhong, Y.X.; Zhou, Y.Y.; Wang, Y.A. Multifunctional Ag@MOF-5@chitosan non-woven cloth composites for sulfur mustard decontamination and hemostasis. Dalton Trans. 2019, 48, 6951–6959. [Google Scholar] [CrossRef] [PubMed]
- Geravand, E.; Farzaneh, F.; Gil-San-Millan, R.; Carmona, F.J.; Navarro, J.A.R. Mixed-metal cerium/zirconium MOFs with improved nerve agent detoxification properties. Inorg. Chem. 2020, 59, 16160–16167. [Google Scholar] [CrossRef]
- Roy, A.; Srivastava, A.K.; Singh, B.; Shah, D.; Mahato, T.H.; Srivastava, A. Kinetics of degradation of sulfur mustard and sarin simulants on HKUST-1 metal organic framework. Dalton Trans. 2012, 41, 12346–12348. [Google Scholar] [CrossRef]
- Roy, A.; Srivastava, A.K.; Singh, B.; Mahato, T.H.; Shah, D.; Halve, A.K. Degradation of sulfur mustard and 2-chloroethyl ethyl sulfide on Cu-BTC metal organic framework. Microporous Mesoporous Mater. 2012, 162, 207–212. [Google Scholar] [CrossRef]
- Yu, M.Y.; Yang, J.; Guo, T.T.; Ma, J.F. Efficient catalytic oxidative desulfurization toward thioether and sulfur mustard stimulant by polyoxomolybdate-resorcin[4]arene-based metal-organic materials. Inorg. Chem. 2020, 59, 4985–4994. [Google Scholar] [CrossRef]
- Vu, A.T.; Jiang, S.; Ho, K.; Lee, J.B.; Lee, C.H. Mesoporous magnesium oxide and its composites: Preparation, characterization, and removal of 2-chloroethyl ethyl sulfide. Chem. Eng. J. 2015, 269, 82–93. [Google Scholar] [CrossRef]
- Mahayoni, E.; Min, S.; Kim, J.; Jeong, K.; Kim, S.H. Effective degradation of sulfur mustard simulant using novel sulfur-doped mesoporous zinc oxide under ambient conditions. J. Hazard. Mater. 2021, 411, 125144. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Arcibar-Orozco, J.A.; Bandosz, T.J. Reactive removal of 2-chloroethyl ethyl sulfide vapors under visible light irradiation by cerium oxide modified highly porous zirconium (hydr) oxide. Appl. Surf. Sci. 2016, 390, 735–743. [Google Scholar] [CrossRef]
- Totten, R.K.; Kim, Y.-S.; Weston, M.H.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. Enhanced catalytic activity through the tuning of micropore environment and supercritical co2 processing: Al(Porphyrin)-based porous organic polymers for the degradation of a nerve agent simulant. J. Am. Chem. Soc. 2013, 135, 11720–11723. [Google Scholar] [CrossRef]
- Kim, H.; Shin, J.; Kang, D.W.; Kim, Y.; Kim, J.H.; Kang, M.; Choe, J.H.; Park, S.; Kim, J.S.; Hong, C.S. Photocatalytic detoxification of a sulfur mustard simulant under realistic conditions by imidazoline-based porous organic polymer composites. Cell Rep. Phy. Sci. 2022, 3, 100888. [Google Scholar] [CrossRef]
- Wang, H.; Wagner, G.W.; Lu, A.X.; Nguyen, D.L.; Buchanan, J.H.; McNutt, P.M.; Karwacki, C.J. Photocatalytic oxidation of sulfur mustard and its simulant on BODIPY-incorporated polymer coatings and fabrics. ACS Appl. Mater. Interfaces 2018, 10, 18771–18777. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, J.R.; Bustone, G.P.; Clark, E.R. Decontamination and remediation of the sulfur mustard simulant cees with "off-the-shelf" reagents in solution and gel states: A proof-of-concept study. ChemistryOpen 2017, 6, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, L.; Kuang, Y.; Ouyang, G. Universal strategy for metal-organic framework growth: From cascading-functional films to MOF-on-MOFs. Small 2024, 11, 2307976. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.R.; Wang, X.; Wang, X.; Kirlikovali, K.O.; Xie, H.; Ma, K.; Fahy, K.M.; Chen, H.; Islamoglu, T.; Snurr, R.Q.; et al. Structure-activity relationship insights for organophosphonate hydrolysis at TI(IV) active sites in metal-organic frameworks. J. Am. Chem. Soc. 2023, 145, 7435–7445. [Google Scholar] [CrossRef]
- Ma, K.K.; Cheung, Y.H.; Kirlikovali, K.O.; Xie, H.M.; Idrees, K.B.; Wang, X.L.; Islamoglu, T.; Xin, J.H.; Farha, O.K. Fibrous Zr-mof nanozyme aerogels with macro-nanoporous structure for enhanced catalytic hydrolysis of organophosphate toxins. Adv. Mater. 2023, 36, 2300951. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.B.; Lin, F.R.; Liu, Z.Y.; Kong, Y.R.; Idrees, K.B.; Liu, Y.; Zou, Y.; Farha, O.K.; Ren, X.M. MOf-polymer mixed matrix membranes as chemical protective layers for solid-phase detoxification of toxic organophosphates. ACS Appl. Mater. Interfaces 2023, 15, 2933–2939. [Google Scholar] [CrossRef]
- Acharya, D.; Muthurasu, A.; Ko, T.H.; Bhattarai, R.M.; Kim, T.; Chae, S.-H.; Saidin, S.; Chhetri, K.; Kim, H.Y. Iron mof-derived Fe2O3/npc decorated on MIL-88A converted Fe3C implanted electrospun porous carbon nanofibers for symmetric supercapacitors. ACS Appl. Energy Mater. 2023, 6, 9196–9206. [Google Scholar] [CrossRef]
- Acharya, D.; Pathak, I.; Dahal, B.; Lohani, P.C.; Bhattarai, R.M.; Muthurasu, A.; Kim, T.; Ko, T.H.; Chhetri, K.; Kim, H.Y. Immoderate nanoarchitectures of bimetallic MOF derived Ni–Fe–O/NPC on porous carbon nanofibers as freestanding electrode for asymmetric supercapacitors. Carbon 2023, 201, 12–23. [Google Scholar] [CrossRef]
- Adhikari, A.; Chhetri, K.; Rai, R.; Acharya, D.; Kunwar, J.; Bhattarai, R.M.; Jha, R.K.; Kandel, D.; Kim, H.Y.; Kandel, M.R. (Fe-Co-Ni-Zn)-based metal–organic framework-derived electrocatalyst for zinc–air batteries. Nanomaterials 2023, 13, 2612. [Google Scholar] [CrossRef]
- Kiaei, K.; Brunson, K.; Gładysiak, A.; Smith, K.; Hunter, K.; Thomas, A.; Radke, D.; Zuehlsdorff, T.; Stylianou, K.C. Instantaneous degradation of nerve agent simulants using zirconium-based metal–organic polyhedra. J. Mater. Chem. A 2023, 11, 14265–14271. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, C.; Wang, Y.; Huang, J.; Gu, S.; Yang, H.; Liu, X.; Xu, W.; Ye, D. Construction of mof-loaded polypropylene nonwoven fabrics for fast catalytic hydrolysis of chemical warfare agent simulants. Mater. Lett. 2022, 326, 132916. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Forgan, R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef]
- Couzon, N.; Ferreira, M.; Duval, S.; El-Achari, A.; Campagne, C.; Loiseau, T.; Volkringer, C. Microwave-assisted synthesis of porous composites mof-textile for the protection against chemical and nuclear hazards. ACS Appl. Mater. Interfaces 2022, 14, 21497–21508. [Google Scholar] [CrossRef]
- Tang, J.; Li, P.; Islamoglu, T.; Li, S.; Zhang, X.; Son, F.A.; Chen, Z.; Mian, M.R.; Lee, S.-J.; Wu, J.; et al. Micropore environment regulation of zirconium MOFs for instantaneous hydrolysis of an organophosphorus chemical. Cell Rep. Phys. Sci. 2021, 2, 100612. [Google Scholar] [CrossRef]
- Chen, X.; Mendes, B.B.; Zhuang, Y.; Conniot, J.; Mercado Argandona, S.; Melle, F.; Sousa, D.P.; Perl, D.; Chivu, A.; Patra, H.K.; et al. A fluorinated bodipy-based zirconium metal-organic framework for in vivo enhanced photodynamic therapy. J. Am. Chem. Soc. 2024, 146, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Luczak, J.; Kroczewska, M.; Baluk, M.; Sowik, J.; Mazierski, P.; Zaleska-Medynska, A. Morphology control through the synthesis of metal-organic frameworks. Adv. Colloid Interface Sci. 2023, 314, 102864. [Google Scholar] [CrossRef] [PubMed]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.-C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.S.; Yu, J.L.; Zhang, L.J.; Zhou, Y.S.; Zhong, Y.X.; Huang, C.C.; Wang, Y.A. Integrating two highly active components into one for decontaminating sulfur mustard and sarin. Ind. Eng. Chem. Res. 2021, 60, 14193–14202. [Google Scholar] [CrossRef]
- Son, Y.R.; Kim, M.K.; Ryu, S.G.; Kim, H.S. Rapid capture and hydrolysis of a sulfur mustard gas in silver-ion-exchanged zeolite Y. ACS Appl. Mater. Interfaces 2018, 10, 40651–40660. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Yekta, S.; Mirzaei, D. A novel CuO NPs/AgZSM-5 zeolite composite adsorbent: Synthesis, identification and its application for the removal of sulfur mustard agent simulant. J. Alloys Compd. 2018, 748, 995–1005. [Google Scholar] [CrossRef]
- Harvey, J.A.; Pearce, C.J.; Hall, M.G.; Bruni, E.J.; DeCoste, J.B.; Sava Gallis, D.F. Insights into the solvent-assisted degradation of organophosphorus compounds by a Zr-based metal–organic framework. Dalton Trans. 2019, 48, 16153–16157. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Liu, Y.; Howarth, A.J.; Hupp, J.T.; Farha, O.K. Selective photooxidation of a mustard-gas simulant catalyzed by a porphyrinic metal–organic framework. Angew. Chem. Int. Ed. 2015, 127, 9129–9133. [Google Scholar] [CrossRef]
- Ma, F.-J.; Liu, S.-X.; Sun, C.-Y.; Liang, D.-D.; Ren, G.-J.; Wei, F.; Chen, Y.-G.; Su, Z.-M. A sodalite-type porous metal−organic framework with polyoxometalate templates: Adsorption and decomposition of dimethyl methylphosphonate. J. Am. Chem. Soc. 2011, 133, 4178–4181. [Google Scholar] [CrossRef]
- Wang, S.; Bromberg, L.; Schreuder-Gibson, H.; Hatton, T.A. Organophophorous ester degradation by chromium(III) terephthalate metal–organic framework (MIL-101) chelated to n,n-dimethylaminopyridine and related aminopyridines. ACS Appl. Mater. Interfaces 2013, 5, 1269–1278. [Google Scholar] [CrossRef]
- de Koning, M.C.; Peterson, G.W.; van Grol, M.; Iordanov, I.; McEntee, M. Degradation and detection of the nerve agent vx by a chromophore-functionalized zirconium mof. Chem. Mater. 2019, 31, 7417–7424. [Google Scholar] [CrossRef]
- Yao, A.; Jiao, X.; Chen, D.; Li, C. Photothermally enhanced detoxification of chemical warfare agent simulants using bioinspired core–shell dopamine–melanin@metal–organic frameworks and their fabrics. ACS Appl. Mater. Interfaces 2019, 11, 7927–7935. [Google Scholar] [CrossRef]
- Chen, Z.; Li, P.; Wang, X.; Otake, K.-i.; Zhang, X.; Robison, L.; Atilgan, A.; Islamoglu, T.; Hall, M.G.; Peterson, G.W.; et al. Ligand-directed reticular synthesis of catalytically active missing zirconium-based metal–organic frameworks. J. Am. Chem. Soc. 2019, 141, 12229–12235. [Google Scholar] [CrossRef]
- Cho, K.Y.; Seo, J.Y.; Kim, H.-J.; Pai, S.J.; Do, X.H.; Yoon, H.G.; Hwang, S.S.; Han, S.S.; Baek, K.-Y. Facile control of defect site density and particle size of UiO-66 for enhanced hydrolysis rates: Insights into feasibility of Zr(IV)-based metal-organic framework (MOF) catalysts. Appl. Catal. B Environ. 2019, 245, 635–647. [Google Scholar] [CrossRef]
- Wang, H.; Mahle, J.J.; Tovar, T.M.; Peterson, G.W.; Hall, M.G.; DeCoste, J.B.; Buchanan, J.H.; Karwacki, C.J. Solid-phase detoxification of chemical warfare agents using zirconium-based metal organic frameworks and the moisture effects: Analyze via digestion. ACS Appl. Mater. Interfaces 2019, 11, 21109–21116. [Google Scholar] [CrossRef]
- Garibay, S.J.; Farha, O.K.; DeCoste, J.B. Single-component frameworks for heterogeneous catalytic hydrolysis of organophosphorous compounds in pure water. Chem. Commun. 2019, 55, 7005–7008. [Google Scholar] [CrossRef]
- Lee, D.T.; Jamir, J.D.; Peterson, G.W.; Parsons, G.N. Protective Fabrics: Metal-organic framework textiles for rapid photocatalytic sulfur mustard simulant detoxification. Matter 2020, 2, 404–415. [Google Scholar] [CrossRef]
- Yao, A.; Jiao, X.; Chen, D.; Li, C. Bio-inspired polydopamine-mediated Zr-mof fabrics for solar photothermal-driven instantaneous detoxification of chemical warfare agent simulants. ACS Appl. Mater. Interfaces 2020, 12, 18437–18445. [Google Scholar] [CrossRef]
- Jang, W.J.; Lee, T.Y.; Kim, Y.J.; Lee, S.C.; Shin, M.S.; Lee, S.J. Photothermally active core–shell catalyst based on UiO-66 and polydopamine for highly effective detoxification of nerve agents. ACS Appl. Mater. Interfaces 2023, 15, 31525–31532. [Google Scholar] [CrossRef]
- Atilgan, A.; Cetin, M.M.; Yu, J.; Beldjoudi, Y.; Liu, J.; Stern, C.L.; Cetin, F.M.; Islamoglu, T.; Farha, O.K.; Deria, P.; et al. Post-synthetically elaborated BODIPY-based porous organic polymers (pops) for the photochemical detoxification of a sulfur mustard simulant. J. Am. Chem. Soc. 2020, 142, 18554–18564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cao, M.; Sun, Z.-B.; Han, Z.; Wang, Z.-Y.; Wang, Q.-Y.; Zang, S.-Q. Modulating bodipy-based silver chalcogenide cluster-based metal–organic frameworks for real-time decontamination of a gaseous sulfur mustard simulant. Chem. Mater. 2023, 35, 10238–10246. [Google Scholar] [CrossRef]
- Yang, J.; He, X.; Dai, J.; Tian, R.; Yuan, D. Photo-assisted enhancement performance for rapid detoxification of chemical warfare agent simulants over versatile ZnIn2S4/UiO-66-NH2 nanocomposite catalysts. J. Hazard. Mater. 2021, 417, 126056. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Li, Z.W.; Li, X.S.; Guo, P.W.; Wang, H.B.; Guo, X.; Zhong, J.Y. Plasma jet decontamination of sulfur mustard and its analogues in water by oxidation effect. J. Water Process. Eng. 2023, 53, 103647. [Google Scholar] [CrossRef]
- Oliver, M.C.; Huang, L. Advances in metal–organic frameworks for the removal of chemical warfare agents: Insights into hydrolysis and oxidation reaction mechanisms. Nanomaterials 2023, 13, 2178. [Google Scholar] [CrossRef] [PubMed]
- Giles, S.L.; Sousa-Castillo, A.; Santiago, E.Y.; Purdy, A.P.; Correa-Duarte, M.A.; Govorov, A.O.; Baturina, O.A. Visible light driven oxidation of harmful 2-Chloroethyl ethyl sulfide using SiO2-TiO2 composite particles and air. Colloid Interface Sci. Commun. 2021, 41, 100362. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Gao, Q.; Zhang, L.J.; Zhou, Y.S.; Zhong, Y.X.; Yu, J.L.; Liu, J.; Huang, C.C.; Wang, Y.A. Combining two into one: A dual-function H5PV2Mo10O40@Mof-808 composite as a versatile decontaminant for sulfur mustard and soman. Inorg. Chem. 2020, 59, 11595–11605. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.R.; Ryu, S.G.; Kim, H.S. Rapid adsorption and removal of sulfur mustard with zeolitic imidazolate frameworks ZIF-8 and ZIF-67. Microporous Mesoporous Mater. 2020, 293, 109819. [Google Scholar] [CrossRef]
- Hao, Y.; Papazyan, E.K.; Ba, Y.; Liu, Y. Mechanism-guided design of metal–organic framework composites for selective photooxidation of a mustard gas simulant under solvent-free conditions. ACS Catal. 2022, 12, 363–371. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Bandosz, T.J. Defectous UiO-66 MOF nanocomposites as reactive media of superior protection against toxic vapors. ACS Appl. Mater. Interfaces 2020, 12, 14678–14689. [Google Scholar] [CrossRef]
- Son, F.A.; Wasson, M.C.; Islamoglu, T.; Chen, Z.; Gong, X.; Hanna, S.L.; Lyu, J.; Wang, X.; Idrees, K.B.; Mahle, J.J.; et al. Uncovering the role of metal–organic framework topology on the capture and reactivity of chemical warfare agents. Chem. Mater. 2020, 32, 4609–4617. [Google Scholar] [CrossRef]
- Tian, H.R.; Zhang, Z.; Liu, S.M.; Dang, T.Y.; Li, X.H.; Lu, Y.; Liu, S.X. A novel polyoxovanadate-based Co-MOF: Highly efficient and selective oxidation of a mustard gas simulant by two-site synergetic catalysis. J. Mater. Chem. A 2020, 8, 12398–12405. [Google Scholar] [CrossRef]
- Zou, X.N.; Zhang, D.; Luan, T.X.; Li, Q.; Li, L.; Li, P.Z.; Zhao, Y. Incorporating photochromic triphenylamine into a zirconium-organic framework for highly effective photocatalytic aerobic oxidation of sulfides. ACS Appl. Mater. Interfaces 2021, 13, 20137–20144. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.H.; Luo, D.; Wu, K.; Chen, Z.Y.; Wu, M.M.; Zhou, X.P.; Li, D. Superoxide ion and singlet oxygen photogenerated by metalloporphyrin-based metal-organic frameworks for highly efficient and selective photooxidation of a sulfur mustard simulant. ACS Appl. Mater. Interfaces 2021, 13, 37102–37110. [Google Scholar] [CrossRef]
- Shen, C.; Mao, Z.; Xu, H.; Zhang, L.; Zhong, Y.; Wang, B.; Feng, X.; Tao, C.-a.; Sui, X. Catalytic MOF-loaded cellulose sponge for rapid degradation of chemical warfare agents simulant. Carbohydr. Polym. 2019, 213, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ma, K.; Mahle, J.J.; Wang, H.; Syed, Z.H.; Atilgan, A.; Chen, Y.; Xin, J.H.; Islamoglu, T.; Peterson, G.W.; et al. Integration of metal–organic frameworks on protective layers for destruction of nerve agents under relevant conditions. J. Am. Chem. Soc. 2019, 141, 20016–20021. [Google Scholar] [CrossRef] [PubMed]
- Gil-San-Millan, R.; Delgado, P.; Lopez-Maya, E.; Martin-Romera, J.D.; Barea, E.; Navarro, J.A.R. Layer-by-layer integration of zirconium metal–organic frameworks onto activated carbon spheres and fabrics with model nerve agent detoxification properties. ACS Appl. Mater. Interfaces 2021, 13, 50491–50496. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Islamoglu, T.; Farha, O.K. Toward base heterogenization: A zirconium metal–organic framework/dendrimer or polymer mixture for rapid hydrolysis of a nerve-agent simulant. ACS Appl. Nano Mater. 2019, 2, 1005–1008. [Google Scholar] [CrossRef]
- Kalinovskyy, Y.; Wright, A.J.; Hiscock, J.R.; Watts, T.D.; Williams, R.L.; Cooper, N.J.; Main, M.J.; Holder, S.J.; Blight, B.A. Swell and destroy: A metal–organic framework-containing polymer sponge that immobilizes and catalytically degrades nerve agents. ACS Appl. Mater. Interfaces 2020, 12, 8634–8641. [Google Scholar] [CrossRef] [PubMed]
- Bunge, M.A.; Pasciak, E.; Choi, J.; Haverhals, L.; Reichert, W.M.; Glover, T.G. Ionic liquid welding of the UiO-66-NH2 mof to cotton textiles. Ind. Eng. Chem. Res. 2020, 59, 19285–19298. [Google Scholar] [CrossRef]
- Pander, M.; Gil-San-Millan, R.; Delgado, P.; Perona-Bermejo, C.; Kostrzewa, U.; Kaczkowski, K.; Kubicki, D.J.; Navarro, J.A.R.; Bury, W. MOF/polymer hybrids through in situ free radical polymerization in metal-organic frameworks. Mater. Horiz. 2023, 10, 1301–1308. [Google Scholar] [CrossRef]
- Ma, K.; Wasson, M.C.; Wang, X.; Zhang, X.; Idrees, K.B.; Chen, Z.; Wu, Y.; Lee, S.-J.; Cao, R.; Chen, Y.; et al. Near-instantaneous catalytic hydrolysis of organophosphorus nerve agents with zirconium-based MOF/hydrogel composites. Chem. Catal. 2021, 1, 721–733. [Google Scholar] [CrossRef]
- Tao, C.-a.; Zhao, H.; Dong, R.; Li, Y.; Wang, F.; Zhao, S.; Wang, J. Zr-MOF nanosheets vertically grown on fabrics for rapid catalytic elimination of nerve agent simulants. ACS Appl. Nano Mater. 2024, 7, 13681–13692. [Google Scholar] [CrossRef]
- Ma, K.; Islamoglu, T.; Chen, Z.; Li, P.; Wasson, M.C.; Chen, Y.; Wang, Y.; Peterson, G.W.; Xin, J.H.; Farha, O.K. Scalable and template-free aqueous synthesis of zirconium-based metal–organic framework coating on textile fiber. J. Am. Chem. Soc. 2019, 141, 15626–15633. [Google Scholar] [CrossRef]
- Zhou, C.; Yuan, B.; Zhang, S.X.; Yang, G.; Lu, L.; Li, H.G.; Tao, C.A. Ultrafast degradation and high adsorption capability of a sulfur mustard simulant under ambient conditions using granular uio-66-nh2 metal-organic gels. ACS Appl. Mater. Interfaces 2022, 14, 23383–23391. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Qiu, F.; Xu, R.; Zhao, Q.; Guo, L.; Chen, D.; Li, C.; Jiao, X. Dual-function detoxifying nanofabrics against nerve agent and blistering agent simulants. ACS Appl. Mater. Interfaces 2023, 15, 1265–1275. [Google Scholar] [CrossRef]
- Zhou, C.; Li, L.; Qin, H.; Wu, Q.; Wang, L.; Lin, C.; Yang, B.; Tao, C.A.; Zhang, S. Humidity enhances the solid-phase catalytic ability of a bulk mof-808 metal-organic gel toward a chemical warfare agent simulant. ACS Appl. Mater. Interfaces 2023, 15, 54582–54589. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Chen, Q.; Jiang, H.; Zhang, K. Research Progress in the Degradation of Chemical Warfare Agent Simulants Using Metal–Organic Frameworks. Nanomaterials 2024, 14, 1108. https://doi.org/10.3390/nano14131108
Huang T, Chen Q, Jiang H, Zhang K. Research Progress in the Degradation of Chemical Warfare Agent Simulants Using Metal–Organic Frameworks. Nanomaterials. 2024; 14(13):1108. https://doi.org/10.3390/nano14131108
Chicago/Turabian StyleHuang, Taotao, Qian Chen, Hui Jiang, and Kui Zhang. 2024. "Research Progress in the Degradation of Chemical Warfare Agent Simulants Using Metal–Organic Frameworks" Nanomaterials 14, no. 13: 1108. https://doi.org/10.3390/nano14131108
APA StyleHuang, T., Chen, Q., Jiang, H., & Zhang, K. (2024). Research Progress in the Degradation of Chemical Warfare Agent Simulants Using Metal–Organic Frameworks. Nanomaterials, 14(13), 1108. https://doi.org/10.3390/nano14131108




