Advancements in Nanomaterial Dispersion and Stability and Thermophysical Properties of Nano-Enhanced Phase Change Materials for Biomedical Applications
Abstract
:1. Introduction
2. Dispersion and Stability of Nanomaterials in PCMs
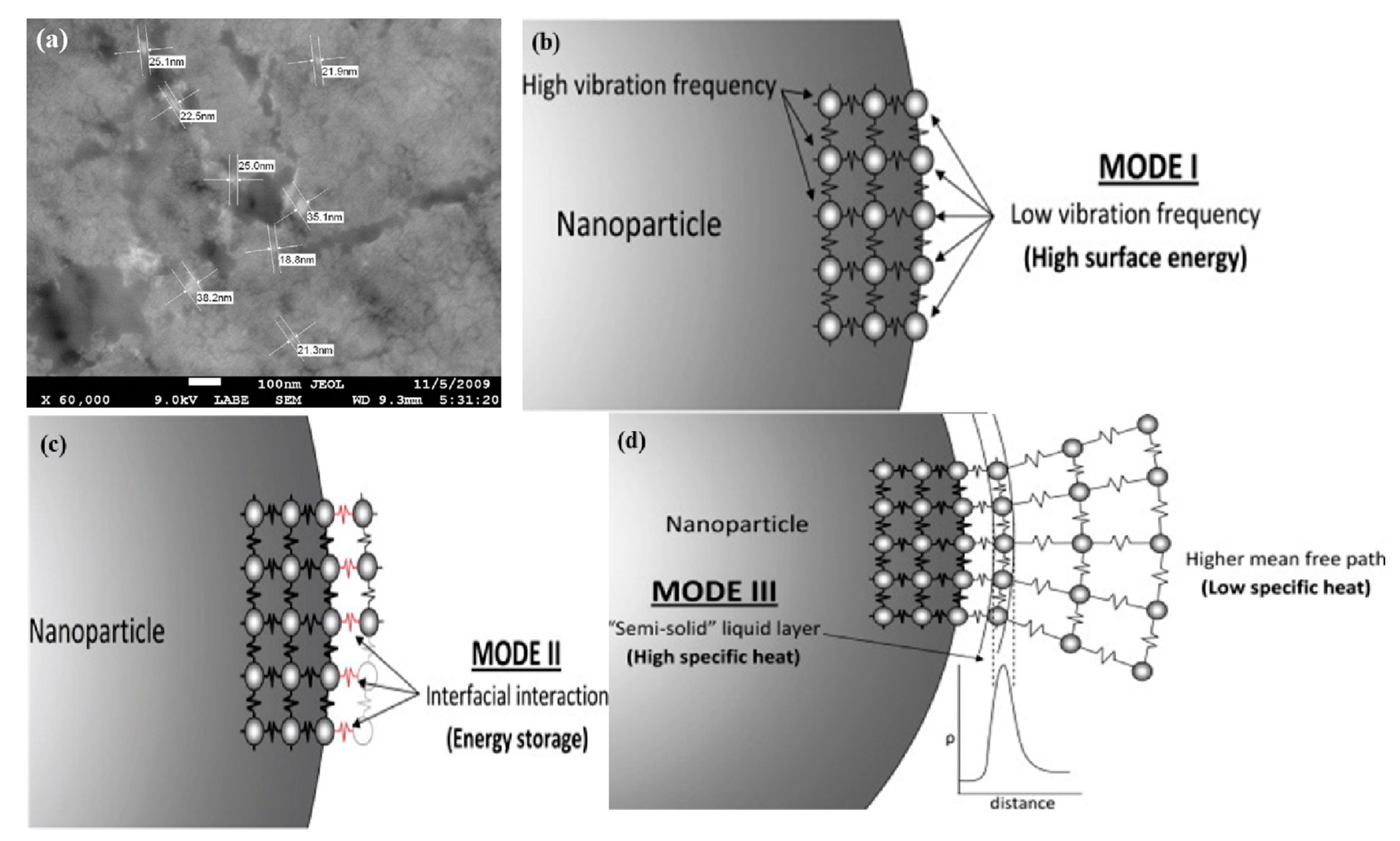
2.1. Agglomeration of Nanomaterials and Their Influence Factors
2.2. Improvement Methods
2.2.1. Physical Methods
- (1)
- Mechanical dispersion method
- (2)
- Ultrasonic dispersion
- (3)
- Dispersants
2.2.2. Chemical Methods
- (1)
- Coupling agent method
- (2)
- Esterification modification
- (3)
- Polymer grafting method
3. The Effect of Nano-Modification on the Thermophysical Properties of PCMs
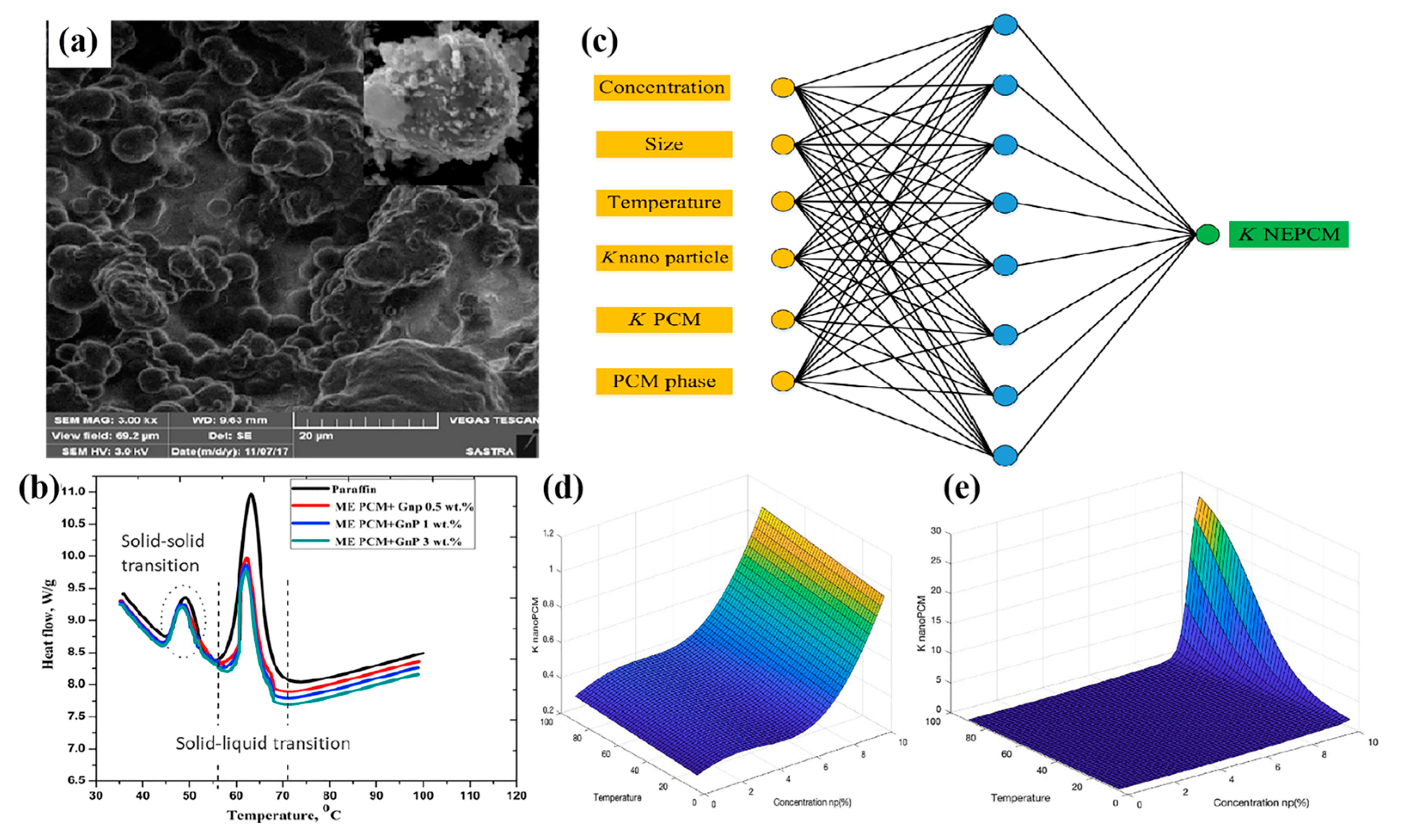
3.1. Thermal Conductivity
3.1.1. Nano Metal
3.1.2. Nano-Metal Oxide
3.1.3. Carbon Nanomaterials
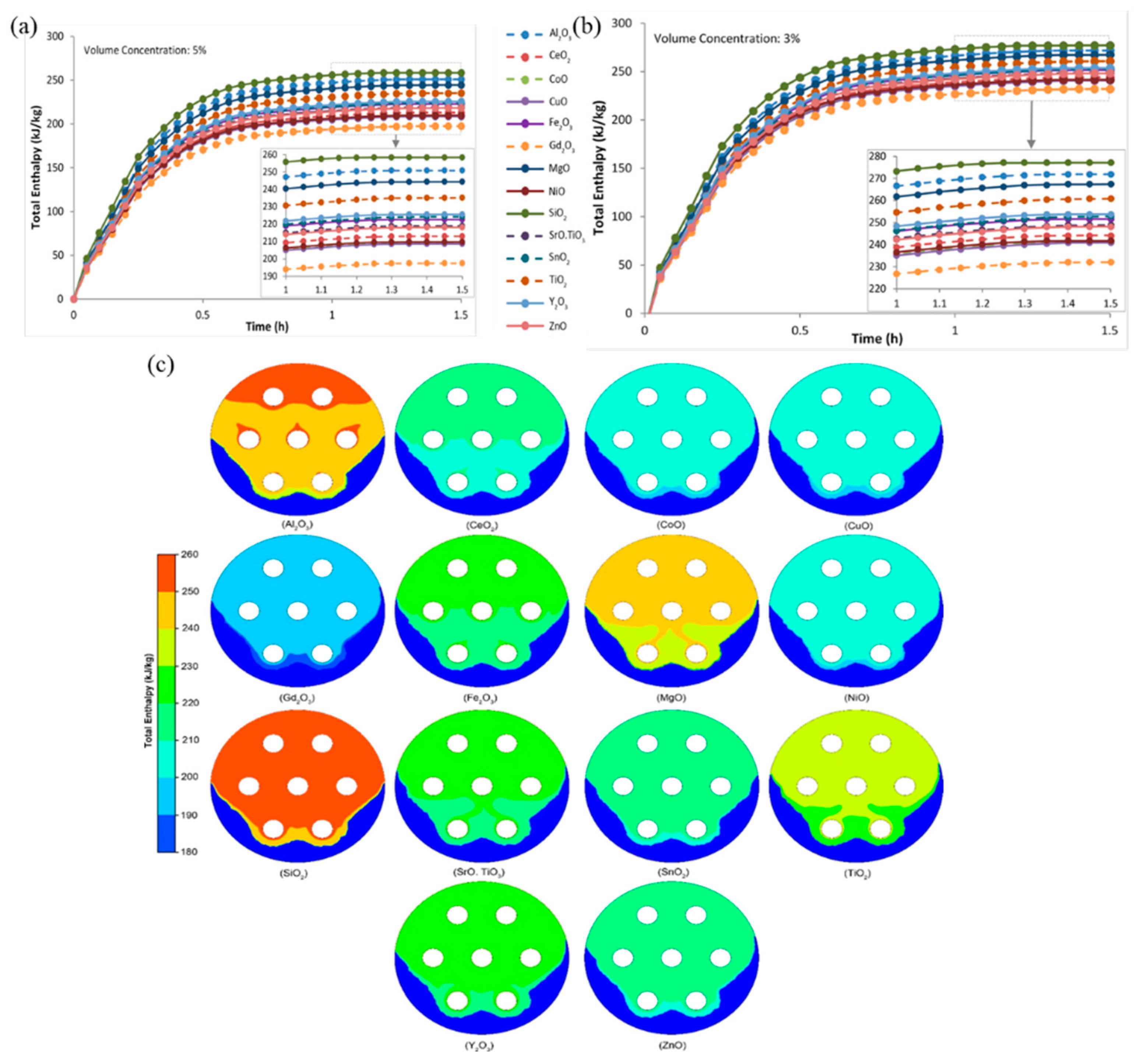
3.2. Latent Heat
3.2.1. Influence Principles
3.2.2. Carbon Nanomaterials
3.2.3. Nano-Metal and Metal Oxide
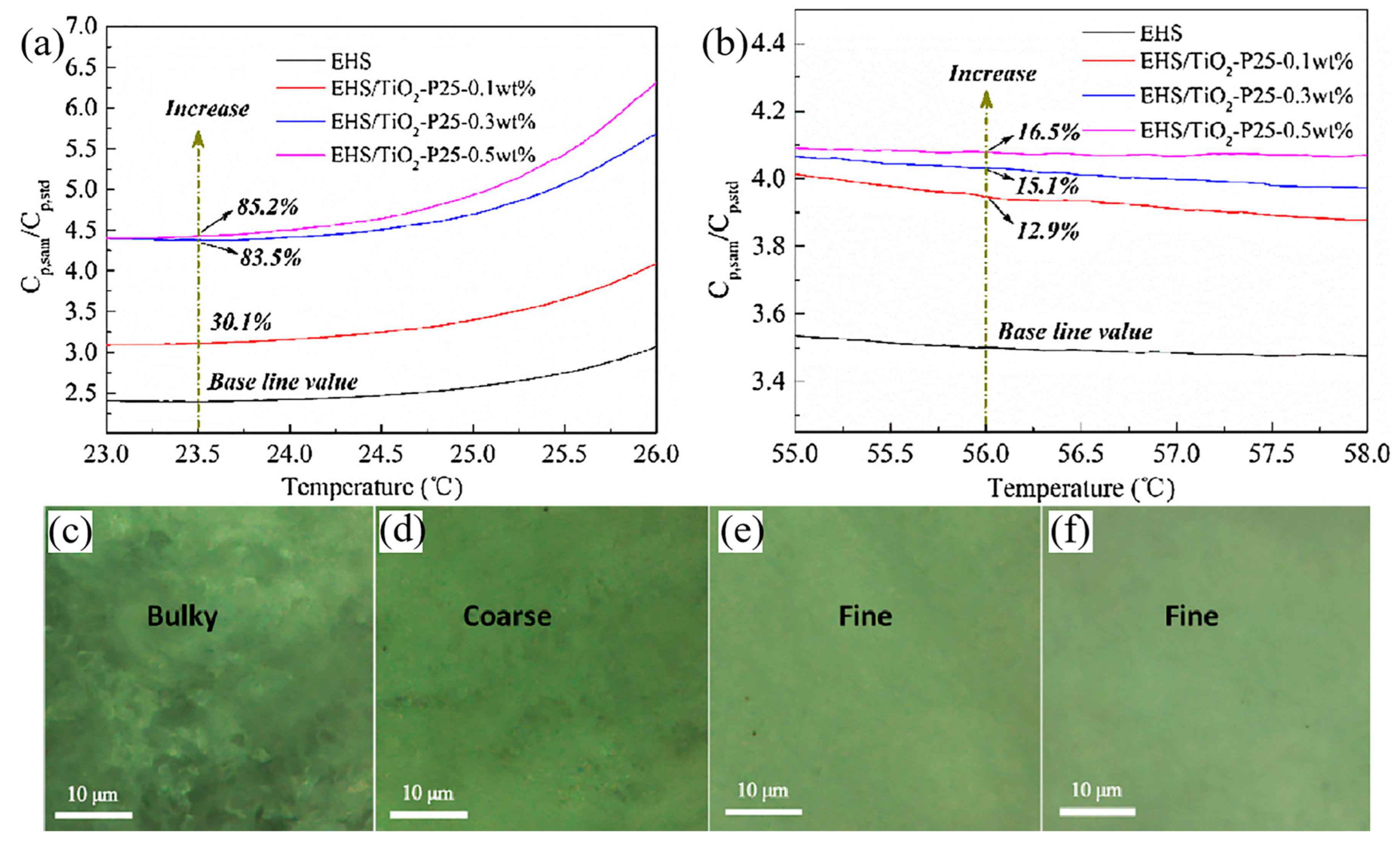
3.3. Specific Heat Capacity
4. Medical Applications of Nano-Modified PCMs
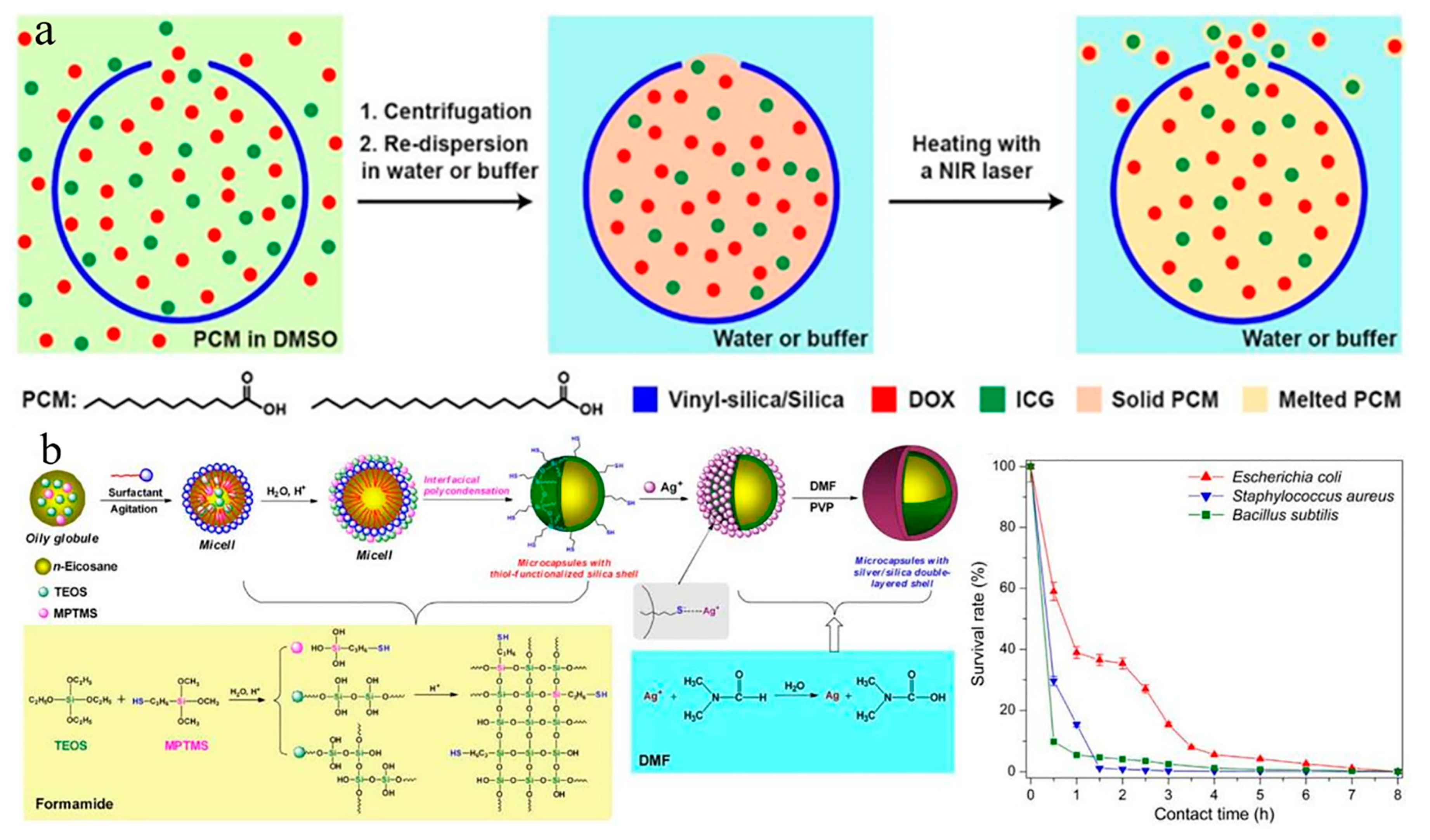
5. Current Challenges and Future Trends
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, Y.; Jiang, Y.; Pan, Y.; Zou, X.; Zhang, Q.; Gao, X.; Zhang, J.; Yu, K.; Yang, Y.; Liu, Y. Recent advances in energy storage and applications of form-stable phase change materials with recyclable skeleton. Carbon Neutraliz. 2024, 3, 313–343. [Google Scholar] [CrossRef]
- Shukla, A.; Sharma, A.; Shukla, M.; Chen, C.R. Development of thermal energy storage materials for biomedical applications. J. Med. Eng. Technol. 2015, 39, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, G.; Xing, X.; Liu, J.; Cheng, Y.; Ye, T.; Wang, Q.; Xiao, X.; Li, Z.; Deng, H. Near-Infrared Light-Triggered Porous AuPd Alloy Nanoparticles to Produce Mild Localized Heat to Accelerate Bone Regeneration. J. Phys. Chem. Lett. 2019, 10, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Lu, S.; Jiang, S.; Li, B.; Liu, B.; Sun, Q.; Li, J.; Wang, F.; Wei, Y. Engineered Protein Photo-Thermal Hydrogels for Outstanding In Situ Tongue Cancer Therapy. Adv. Mater. 2021, 33, 2100619. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, G.; Zhao, P.; Ou, C. Near-infrared light-controllable bufalin delivery from a black phosphorus-hybrid supramolecular hydrogel for synergistic photothermal-chemo tumor therapy. Nano Res. 2021, 14, 3988–3998. [Google Scholar] [CrossRef]
- Yao, Q.; Lan, Q.-H.; Jiang, X.; Du, C.-C.; Zhai, Y.-Y.; Shen, X.; Xu, H.-L.; Xiao, J.; Kou, L.; Zhao, Y.-Z. Bioinspired biliverdin/silk fibroin hydrogel for antiglioma photothermal therapy and wound healing. Theranostics 2020, 10, 11719–11736. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Win, K.Y.; Yao, X.; Yang, W.; Han, M.Y. Plasmonically Modulated Gold Nanostructures for Photothermal Ablation of Bacteria. Adv. Healthc. Mater. 2021, 10, 2001158. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, H.; Wu, D.; Wang, X. Temperature and pH dual-stimuli-responsive phase-change microcapsules for multipurpose applications in smart drug delivery. J. Colloid Interface Sci. 2021, 583, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, H.; Hai, G.; Jia, D.; Xing, L.; Chen, S.; Cheng, P.; Han, M.; Dong, W.; Wang, G. Carbon nanotube bundles assembled flexible hierarchical framework based phase change material composites for thermal energy harvesting and thermotherapy. Energy Storage Mater. 2020, 26, 129–137. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Yun, B.Y.; Yang, S.; Yuk, H.; Wi, S.; Kim, S. Structurally advanced hybrid support composite phase change materials: Architectural synergy. Energy Storage Mater. 2021, 42, 164–184. [Google Scholar] [CrossRef]
- Lin, Y.X.; Alva, G.; Fang, G.Y. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685–708. [Google Scholar] [CrossRef]
- Yu, K.; Liu, C.; Li, L.; Tian, W.; Yang, Y.; Liu, Y. Carbon-negative heat-stored limestone calcined clay cement mortar containing form-stable phase change materials. J. Clean. Prod. 2024, 437, 140703. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Yu, K.; Jia, M.; Tian, W.; Yang, Y.; Liu, Y. Enhanced thermo-mechanical properties of cementitious composites via red mud-based microencapsulated phase change material: Towards energy conservation in building. Energy 2024, 290, 130301. [Google Scholar] [CrossRef]
- Yu, K.; Liu, Y.; Yang, Y. Review on form-stable inorganic hydrated salt phase change materials: Preparation, characterization and effect on the thermophysical properties. Appl. Energy 2021, 292, 116845. [Google Scholar] [CrossRef]
- Ho, M.X.; Pan, C. Optimal concentration of alumina nanoparticles in molten Hitec salt to maximize its specific heat capacity. Int. J. Heat Mass Transf. 2014, 70, 174–184. [Google Scholar] [CrossRef]
- Sang, L.; Liu, T. The enhanced specific heat capacity of ternary carbonates nanofluids with different nanoparticles. Sol. Energy Mater. Sol. Cells 2017, 169, 297–303. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Abd Majid, M.Z.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Saleh, T.A. Trends in nanomaterial types, synthesis methods, properties and uses: Toxicity, environmental concerns and economic viability. Nano-Struct. Nano-Objects 2024, 37, 101109. [Google Scholar] [CrossRef]
- Qi, G.Q.; Yang, J.; Bao, R.Y.; Liu, Z.Y.; Yang, W.; Xie, B.H.; Yang, M.B. Enhanced comprehensive performance of polyethylene glycol based phase change material with hybrid graphene nanomaterials for thermal energy storage. Carbon 2015, 88, 196–205. [Google Scholar] [CrossRef]
- Kibria, M.A.; Anisur, M.R.; Mahfuz, M.H.; Saidur, R.; Metselaar, I.H.S.C. A review on thermophysical properties of nanoparticle dispersed phase change materials. Energy Convers. Manag. 2015, 95, 69–89. [Google Scholar] [CrossRef]
- Ma, Z.; Lin, W.; Sohel, M.I. Nano-enhanced phase change materials for improved building performance. Renew. Sustain. Energy Rev. 2016, 58, 1256–1268. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, H.; Ali, M.K.A.; Liu, H.; Su, D.; Tian, Z. Dispersion behavior assessment of the molybdenum disulfide nanomaterials dispersed into poly alpha olefin. J. Mol. Liq. 2020, 311, 113303. [Google Scholar] [CrossRef]
- Siddiqui, F.R.; Tso, C.Y.; Chan, K.C.; Fu, S.C.; Chao, C.Y.H. On trade-off for dispersion stability and thermal transport of Cu-Al2O3 hybrid nanofluid for various mixing ratios. Int. J. Heat Mass Transf. 2019, 132, 1200–1216. [Google Scholar] [CrossRef]
- Jafri, R.I.; Arockiados, T.; Rajalakshmi, N.; Ramaprabhu, S. Nanostructured Pt Dispersed on Graphene-Multiwalled Carbon Nanotube Hybrid Nanomaterials as Electrocatalyst for PEMFC. J. Electrochem. Soc. 2010, 157, B874. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, K.; Pan, Y.; Li, J.; Qu, Q.; Pan, S.; Liu, Y. Encapsulation and functionalization strategies of organic phase change materials in medical applications. J. Therm. Anal. Calorim. 2024, 149, 4333–4366. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc. 2017, 12, 355–371. [Google Scholar] [CrossRef]
- Gholami, R.; Raza, A.; Rabiei, M.; Fakhari, N.; Balasubramaniam, P.; Rasouli, V.; Nagarajan, R. An approach to improve wellbore stability in active shale formations using nanomaterials. Petroleum 2021, 7, 24–32. [Google Scholar] [CrossRef]
- Zhang, Q.; Chi, H.; Tang, M.; Chen, J.; Li, G.; Liu, Y.; Liu, B. Mixed surfactant modified graphene oxide nanocarriers for DOX delivery to cisplatin-resistant human ovarian carcinoma cells. RSC Adv. 2016, 6, 87258. [Google Scholar] [CrossRef]
- Estrada-Monje, A.; Zitzumbo-Guzmán, R.; Bañuelos-Díaz, J.A.; Zaragoza-Contreras, E.A. Ultrasonic dispersion and activation of TiO2 nanoparticles and its effect on bacterial inhibition in EVA films. Mater. Chem. Phys. 2019, 235, 121760. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, B.; Kang, H.; Guo, E.; Li, J.; Du, G.; Chen, Z.; Wang, T. Ultrasound-assisted dispersion of TiB2 nanoparticles in 7075 matrix hybrid composites. Mater. Sci. Eng. A 2022, 840, 142958. [Google Scholar] [CrossRef]
- Nishad, J.; Dutta, A.; Saha, S.; Rudra, S.G.; Varghese, E.; Sharma, R.R.; Tomar, M.; Kumar, M.; Kaur, C. Ultrasound-assisted development of stable grapefruit peel polyphenolic nano-emulsion: Optimization and application in improving oxidative stability of mustard oil. Food Chem. 2021, 334, 127561. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Huang, M.; Zhu, Z.; Huang, T.; Huang, M. Effect of ultrasound assisted emulsification in the production of Pickering emulsion formulated with chitosan self-assembled particles: Stability, macro, and micro rheological properties. LWT 2022, 154, 112595. [Google Scholar] [CrossRef]
- Sunu Surendran, K.T.; Gnanavelbabu, A. Efficacy of ultrasonic treatment for dispersing nanoparticles: A correlation with microstructure. Mater. Today Proc. 2021, 45, 7845–7849. [Google Scholar] [CrossRef]
- Xue, T.; Yu, C.; Zhou, Z.; Zhang, F. Imidization of styrene–maleic anhydride copolymer for dispersing nano-SiO2 in water. J. Coat. Technol. Res. 2023, 20, 1867–1880. [Google Scholar] [CrossRef]
- Ettelaie, R.; Zengin, A.; Lishchuk, S.V. Novel food grade dispersants: Review of recent progress. Curr. Opin. Colloid Interface Sci. 2017, 28, 46–55. [Google Scholar] [CrossRef]
- Lee, J.; Jo, S.H.; Lim, J. Effect of surface modification of CaCO3 nanoparticles by a silane coupling agent methyltrimethoxysilane on the stability of foam and emulsion. J. Ind. Eng. Chem. 2019, 74, 63–70. [Google Scholar] [CrossRef]
- Dário, B.S.; Pereira, R.; Petri, D.F.S. Tristyrylphenol based surfactants as efficient dispersants of TiO2 particles in dilute and concentrated dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130170. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Elansezhian, R. Dispersion stability of zinc oxide nanoparticles in an electroless bath with various surfactants. Mater. Today Proc. 2022, 51, 369–373. [Google Scholar] [CrossRef]
- Kubo, T.; Wang, X.; Tong, Q.; Yan, M. Polymer-Based Photocoupling Agent for the Efficient Immobilization of Nanomaterials and Small Molecules. Langmuir 2011, 27, 9372–9378. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Abdolmaleki, A.; Mallakpour, S.; Borandeh, S. Effect of silane-modified ZnO on morphology and properties of bionanocomposites based on poly(ester-amide) containing tyrosine linkages. Polym. Bull. 2012, 69, 15–28. [Google Scholar] [CrossRef]
- Li, M.; Du, Z.; Fang, X.; Ge, X.; Yu, S. Esterified styrene-maleic anhydride ester copolymer–modified MWCNTs dispersion with excellent dispersibility and long storage stability. J. Nanopart. Res. 2023, 25, 216. [Google Scholar] [CrossRef]
- Guglielmi, M.; Kickelbick, G.; Martucci, A. Sol-Gel Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Ma, T.; Li, Z.; Zhao, J. Photovoltaic panel integrated with phase change materials (PV-PCM): Technology overview and materials selection. Renew. Sustain. Energy Rev. 2019, 116, 109406. [Google Scholar] [CrossRef]
- Kenisarin, M.M.; Mahkamov, K.; Costa, S.C.; Makhkamova, I. Melting and solidification of PCMs inside a spherical capsule: A critical review. J. Energy Storage 2020, 27, 101082. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.-n.; Zhou, F. Thermophysical properties and applications of nano-enhanced PCMs: An update review. Energy Convers. Manag. 2020, 214, 112876. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.; Farid, M. Innovative method of metal coating of microcapsules containing phase change materials. Sol. Energy 2016, 129, 54–64. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; Qian, T.; Guan, W.; Li, Y.; Yin, X. Thermal conductivity enhancement of polyethylene glycol/expanded vermiculite shape-stabilized composite phase change materials with silver nanowire for thermal energy storage. Chem. Eng. J. 2016, 295, 427–435. [Google Scholar] [CrossRef]
- Zeng, J.L.; Cao, Z.; Yang, D.W.; Sun, L.X.; Zhang, L. Thermal conductivity enhancement of Ag nanowires on an organic phase change material. J. Therm. Anal. Calorim. 2010, 101, 385–389. [Google Scholar] [CrossRef]
- Sreethawong, T.; Shah, K.W.; Zhang, S.Y.; Ye, E.; Lim, S.H.; Maheswaran, U.; Mao, W.Y.; Han, M.Y. Optimized production of copper nanostructures with high yields for efficient use as thermal conductivity-enhancing PCM dopant. J. Mater. Chem. A 2014, 2, 3417. [Google Scholar] [CrossRef]
- Molefi, J.A.; Luyt, A.S.; Krupa, I. Investigation of thermally conducting phase-change materials based on polyethylene/wax blends filled with copper particles. J. Appl. Polym. Sci. 2010, 116, 1766–1774. [Google Scholar] [CrossRef]
- Tang, B.; Qiu, M.; Zhang, S. Thermal conductivity enhancement of PEG/SiO2 composite PCM by in situ Cu doping. Sol. Energy Mater. Sol. Cells 2012, 105, 242–248. [Google Scholar] [CrossRef]
- Babapoor, A.; Karimi, G. Thermal properties measurement and heat storage analysis of paraffinnanoparticles composites phase change material: Comparison and optimization. Appl. Therm. Eng. 2015, 90, 945–951. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V.; Metselaar, H.S.C.; Sandaran, S.C. Thermal properties and heat storage analysis of palmitic acid-TiO2 composite as nano-enhanced organic phase change material (NEOPCM). Appl. Therm. Eng. 2016, 99, 1254–1262. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Deng, Y.; Guan, W.; Wang, X.; Qian, T. Preparation of paraffin/porous TiO2 foams with enhanced thermal conductivity as PCM, by covering the TiO2 surface with a carbon layer. Appl. Energy 2016, 171, 37–45. [Google Scholar] [CrossRef]
- Şahan, N.; Fois, M.; Paksoy, H. Improving thermal conductivity phase change materials—A study of paraffin nanomagnetite composites. Sol. Energy Mater. Sol. Cells 2015, 137, 61–67. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, R.; Peng, F.; Fang, Y.; Akiyama, T.; Wang, S. Synthesis, characterization and thermal properties of paraffin microcapsules modified with nano-Al2O3. Appl. Energy 2015, 137, 731–737. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Su, Y. The thermal conductivity improvements of phase change materials using modified carbon nanotubes. Diam. Relat. Mater. 2022, 125, 109023. [Google Scholar] [CrossRef]
- Yu, K.; Liu, Y.; Sun, F.; Jia, M.; Yang, Y. Graphene-Modified Hydrate Salt/UV-Curable Resin Form-Stable Phase Change Materials: Continuously Adjustable Phase Change Temperature and Ultrafast Solar-to-Thermal Conversion. Energy Fuels 2019, 33, 7634–7644. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, K.; Xie, M.; Lu, S.; Yang, Y.; Wang, H.; Jia, H. Hydrate salt/self-curing acrylic resin form-stable phase change materials with enhanced surface stability and thermal properties via the incorporation of graphene oxide. Int. J. Energy Res. 2020, 44, 5791–5805. [Google Scholar] [CrossRef]
- Xu, C.; Wang, W.; Zhang, H.; Fang, G. Thermal properties of lauric acid-palmitic acid eutectics/polyvinyl butyral/carbon nanofibers as shape-stable phase change materials. Thermochim. Acta 2022, 715, 179300. [Google Scholar] [CrossRef]
- Yu, K.; Jin, B.; Liu, Y.; Li, L. Enhanced thermal conductivity of form-stable phase change materials using carbon nanofiber-expanded graphite hybrid structure. Mater. Res. Express 2019, 6, 125503. [Google Scholar] [CrossRef]
- Ji, H.; Sellan, D.P.; Pettes, M.T.; Kong, X.; Ji, J.; Li, S.; Ruoff, R.S. Enhanced thermal conductivity of phase change materials with ultrathin-graphite foams for thermal energy storage. Energy Environ. Sci. 2014, 7, 1185–1192. [Google Scholar] [CrossRef]
- Shi, J.-N.; Ger, M.-D.; Liu, Y.-M.; Fan, Y.-C.; Wen, N.-T.; Lin, C.-K.; Pu, N.-W. Improving the thermal conductivity and shape-stabilization of phase change materials using nanographite additives. Carbon 2013, 51, 365–372. [Google Scholar] [CrossRef]
- Praveen, B.; Suresh, S.; Pethurajan, V. Heat transfer performance of graphene nano-platelets laden micro-encapsulated PCM with polymer shell for thermal energy storage based heat sink. Appl. Therm. Eng. 2019, 156, 237–249. [Google Scholar] [CrossRef]
- Oya, T.; Nomura, T.; Tsubota, M.; Okinaka, N.; Akiyama, T. Thermal conductivity enhancement of erythritol as PCM by using graphite and nickel particles. Appl. Therm. Eng. 2013, 61, 825–828. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, C.; Hu, S.; Yu, X. The experimental exploration of carbon nanofiber and carbon nanotube additives on thermal behavior of phase change materials. Sol. Energy Mater. Sol. Cells 2011, 95, 1208–1212. [Google Scholar] [CrossRef]
- Taheri, M.; Pourfayaz, F.; Hemmati, S. A highly accurate model for prediction of thermal conductivity of carbon-based nano-enhanced PCMs using an artificial neural network. Energy Rep. 2023, 10, 1249–1258. [Google Scholar] [CrossRef]
- Colla, L.; Fedele, L.; Mancin, S.; Danza, L.; Manca, O. Nano-PCMs for enhanced energy storage and passive cooling applications. Appl. Therm. Eng. 2017, 110, 584–589. [Google Scholar] [CrossRef]
- Teng, T.P.; Yu, C.C. Characteristics of phase-change materials containing oxide nano-additives for thermal storage. Nanoscale Res. Lett. 2012, 7, 611. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Lugo, L.; Legido, J.L.; Piñeiro, M.M. Rheological non-Newtonian behaviour of ethylene glycol-based Fe2O3 nanofluids. Nanoscale Res. Lett. 2011, 6, 560. [Google Scholar] [CrossRef] [PubMed]
- Younes, H.; Christensen, G.; Luan, X.; Hong, H.; Smith, P. Effects of alignment, pH, surfactant, and solvent on heat transfer nanofluids containing Fe2O3 and CuO nanoparticles. J. Appl. Phys. 2012, 111, 064308. [Google Scholar] [CrossRef]
- Shaikh, S.; Lafdi, K.; Hallinan, K. Carbon nanoadditives to enhance latent energy storage of phase change materials. J. Appl. Phys. 2008, 103, 094302. [Google Scholar] [CrossRef]
- Karaipekli, A.; Biçer, A.; Sarı, A.; Tyagi, V.V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Elbahjaoui, R.; El Qarnia, H. Thermal analysis of nanoparticle-enhanced phase change material solidification in a rectangular latent heat storage unit including natural convection. Energy Build. 2017, 153, 1–17. [Google Scholar] [CrossRef]
- Rufuss, D.D.W.; Suganthi, L.; Iniyan, S.; Davies, P.A. Effects of nanoparticle-enhanced phase change material (NPCM) on solar still productivity. J. Clean. Prod. 2018, 192, 9–29. [Google Scholar] [CrossRef]
- Praveen, B.; Suresh, S. Experimental study on heat transfer performance of neopentyl glycol/CuO composite solid-solid PCM in TES based heat sink. Eng. Sci. Technol. Int. J. 2018, 21, 1086–1094. [Google Scholar] [CrossRef]
- Ho, C.J.; Gao, J.Y. Preparation and thermophysical properties of nanoparticle-in-paraffin emulsion as phase change material. Int. Commun. Heat Mass Transf. 2009, 36, 467–470. [Google Scholar] [CrossRef]
- Zeng, J.-L.; Zhu, F.-R.; Yu, S.-B.; Zhu, L.; Cao, Z.; Sun, L.-X.; Deng, G.-R.; Yan, W.-P.; Zhang, L. Effects of copper nanowires on the properties of an organic phase change material. Sol. Energy Mater. Sol. Cells 2012, 105, 174–178. [Google Scholar] [CrossRef]
- Salyan, S.; Suresh, S. Study of thermo-physical properties and cycling stability of d-Mannitol-copper oxide nanocomposites as phase change materials. J. Energy Storage 2018, 15, 245–255. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.A.; Sewell, P. Heat transfer evaluation of metal oxides based nano-PCMs for latent heat storage system application. Int. J. Heat Mass Transf. 2019, 144, 118619. [Google Scholar] [CrossRef]
- Pomianowski, M.; Heiselberg, P.; Jensen, R.L.; Cheng, R.; Zhang, Y. A new experimental method to determine specific heat capacity of inhomogeneous concrete material with incorporated microencapsulated-PCM. Cem. Concr. Res. 2014, 55, 22–34. [Google Scholar] [CrossRef]
- Zhang, Y. Modified computational methods using effective heat capacity model for the thermal evaluation of PCM outfitted walls. Int. Commun. Heat Mass Transf. 2019, 108, 104278. [Google Scholar] [CrossRef]
- Bao, X.; Tian, Y.; Yuan, L.; Cui, H.; Tang, W.; Fung, W.H.; Qi, H. Development of high performance PCM cement composites for passive solar buildings. Energy Build. 2019, 194, 33–45. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Enhancement of specific heat capacity of high-temperature silica-nanofluids synthesized in alkali chloride salt eutectics for solar thermal-energy storage applications. Int. J. Heat Mass Transf. 2011, 54, 1064–1070. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M. Effect of nanoparticles on heat capacity of nanofluids based on molten salts as PCM for thermal energy storage. Nanoscale Res. Lett. 2013, 8, 448. [Google Scholar] [CrossRef] [PubMed]
- Dudda, B.; Shin, D. Effect of nanoparticle dispersion on specific heat capacity of a binary nitrate salt eutectic for concentrated solar power applications. Int. J. Therm. Sci. 2013, 69, 37–42. [Google Scholar] [CrossRef]
- He, Q.; Wang, S.; Tong, M.; Liu, Y. Experimental study on thermophysical properties of nanofluids as phase-change material (PCM) in low temperature cool storage. Energy Convers. Manag. 2012, 64, 199–205. [Google Scholar] [CrossRef]
- Tao, Y.B.; Liu, Y.K.; He, Y.L. Effect of carbon nanomaterial on latent heat storage performance of carbonate salts in horizontal concentric tube. Energy 2019, 185, 994–1004. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Investigation of specific heat and latent heat enhancement in hydrate salt based TiO2 nanofluid phase change material. Appl. Therm. Eng. 2017, 124, 533–538. [Google Scholar] [CrossRef]
- Cao, L.; Fang, M.; Cong, Y. Effect of nanoparticle clusters on the specific heat capacity of a molten carbonate for thermal energy storage. J. Energy Storage 2023, 68, 107664. [Google Scholar] [CrossRef]
- Ryu, T.K.; Baek, S.W.; Kang, R.H.; Jeong, K.Y.; Jun, D.R.; Choi, S.W. Photodynamic and photothermal tumor therapy using phase-change material nanoparticles containing chlorin e6 and nanodiamonds. J. Control. Release Off. J. Control. Release Soc. 2018, 270, 237–245. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Z.; Bo, R.; Li, C.; Meng, X. Effect of the cooling clothing integrating with phase change material on the thermal comfort of healthcare workers with personal protective equipment during the COVID-19. Case Stud. Therm. Eng. 2023, 42, 102725. [Google Scholar] [CrossRef]
- Lv, J.; Wang, J.; Wang, J.; Zhang, T.; Yang, B.; Zhen, Z.; Zheng, Y.; Wang, Y. Nanofibrous films embedded with phase change material: Flexible and photo-responsive dressings for thermal compress therapy. J. Energy Storage 2023, 60, 106609. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Qu, Q.; Pan, S.; Yu, K.; Liu, Y. Graphene oxide modified sodium alginate/polyethylene glycol phase change material hydrogel scaffold composite with photothermal temperature control for potential bone tissue regeneration. J. Mater. Res. Technol. 2024, 30, 2446–2457. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, X.; Ji, J.; Han, L.; Ding, X.; Xie, W. Application and research progress of phase change materials in biomedical field. Biomater. Sci. 2021, 9, 5762–5780. [Google Scholar] [CrossRef]
- Shen, S.; Zhu, C.; Huo, D.; Yang, M.; Xue, J.; Xia, Y. A Hybrid Nanomaterial for the Controlled Generation of Free Radicals and Oxidative Destruction of Hypoxic Cancer Cells. Angew. Chem. 2017, 56, 8801–8804. [Google Scholar] [CrossRef]
- Qiu, J.; Xie, M.; Wu, T.; Qin, D.; Xia, Y. Gold nanocages for effective photothermal conversion and related applications. Chem. Sci. 2020, 11, 12955–12973. [Google Scholar] [CrossRef]
- Moon, G.D.; Choi, S.W.; Cai, X.; Li, W.; Cho, E.C.; Jeong, U.; Wang, L.V.; Xia, Y. A new theranostic system based on gold nanocages and phase-change materials with unique features for photoacoustic imaging and controlled release. J. Am. Chem. Soc. 2011, 133, 4762–4765. [Google Scholar] [CrossRef]
- Cheng, H.; Huo, D.; Zhu, C.; Shen, S.; Wang, W.; Li, H.; Zhu, Z.; Xia, Y. Combination cancer treatment through photothermally controlled release of selenous acid from gold nanocages. Biomaterials 2018, 178, 517–526. [Google Scholar] [CrossRef]
- Wang, Y.; Brown, P.; Xia, Y. Swarming towards the target. Nat. Mater. 2011, 10, 482–483. [Google Scholar] [CrossRef]
- Seo, S.H.; Kim, B.M.; Joe, A.; Han, H.W.; Chen, X.; Cheng, Z.; Jang, E.S. NIR-light-induced surface-enhanced Raman scattering for detection and photothermal/photodynamic therapy of cancer cells using methylene blue-embedded gold nanorod@SiO2 nanocomposites. Biomaterials 2014, 35, 3309–3318. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Gai, S.; Lin, J. Review—Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef]
- Qiu, J.; Huo, D.; Xue, J.; Zhu, G.; Xia, Y. Encapsulation of a Phase-Change Material in Nanocapsules with a Well-Defined Hole in the Wall for the Controlled Release of Drugs. Angew. Chem. Int. Ed. 2019, 58, 10606–10611. [Google Scholar] [CrossRef]
- Zhang, E. What controls the antibacterial activity of Ti-Ag alloy, Ag ion or Ti2Ag particles? Mater. Sci. Eng. C 2019, 109, 110548. [Google Scholar]
- Zhang, X.; Wang, X.; Wu, D. Design and synthesis of multifunctional microencapsulated phase change materials with silver/silica double-layered shell for thermal energy storage, electrical conduction and antimicrobial effectiveness. Energy 2016, 111, 498–512. [Google Scholar] [CrossRef]
- Ilić, V.; Šaponjić, Z.; Vodnik, V.; Molina, R.; Dimitrijević, S.; Jovančić, P.; Nedeljković, J.; Radetić, M. Antifungal efficiency of corona pretreated polyester and polyamide fabrics loaded with Ag nanoparticles. J. Mater. Sci. 2009, 44, 3983–3990. [Google Scholar] [CrossRef]
- Zhang, X. 3—Heat-storage and thermo-regulated textiles and clothing. In Smart Fibres, Fabrics and Clothing; Tao, X., Ed.; Woodhead Publishing: Sawston, UK, 2001; pp. 34–57. [Google Scholar]
- Shao, Y.-W.; Hu, W.-W.; Gao, M.-H.; Xiao, Y.-Y.; Huang, T.; Zhang, N.; Yang, J.-H.; Qi, X.-D.; Wang, Y. Flexible MXene-coated melamine foam based phase change material composites for integrated solar-thermal energy conversion/storage, shape memory and thermal therapy functions. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106291. [Google Scholar] [CrossRef]
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, H.; Zheng, Y.; Yao, Y.; Li, X.; Yu, J.; Ding, B. Corrigendum to “Nanofibrous hydrogels embedded with phase-change materials: Temperature-responsive dressings for accelerating skin wound healing” [Compos. Commun. 25 (2021) 100752]. Compos. Commun. 2022, 30, 101091. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Zhang, T.; Yuan, X.; Chen, L.; Lv, J.; Wang, Y. Flexible and photo-responsive composite phase change materials for thermal therapy. J. Energy Storage 2024, 75, 109595. [Google Scholar] [CrossRef]
- Meng, B.; Zhang, X.; Hua, W.; Liu, L.; Ma, K. Development and application of phase change material in fresh e-commerce cold chain logistics: A review. J. Energy Storage 2022, 55, 105373. [Google Scholar] [CrossRef]
- Ashok, A.; Brison, M.; LeTallec, Y. Improving cold chain systems: Challenges and solutions. Vaccine 2017, 35, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, L.; Lu, W.; Wu, Z. Production of ternary organic phase change material combined with expanded graphite and its application in cold chain transportation. Therm. Sci. Eng. Prog. 2023, 46, 102204. [Google Scholar] [CrossRef]

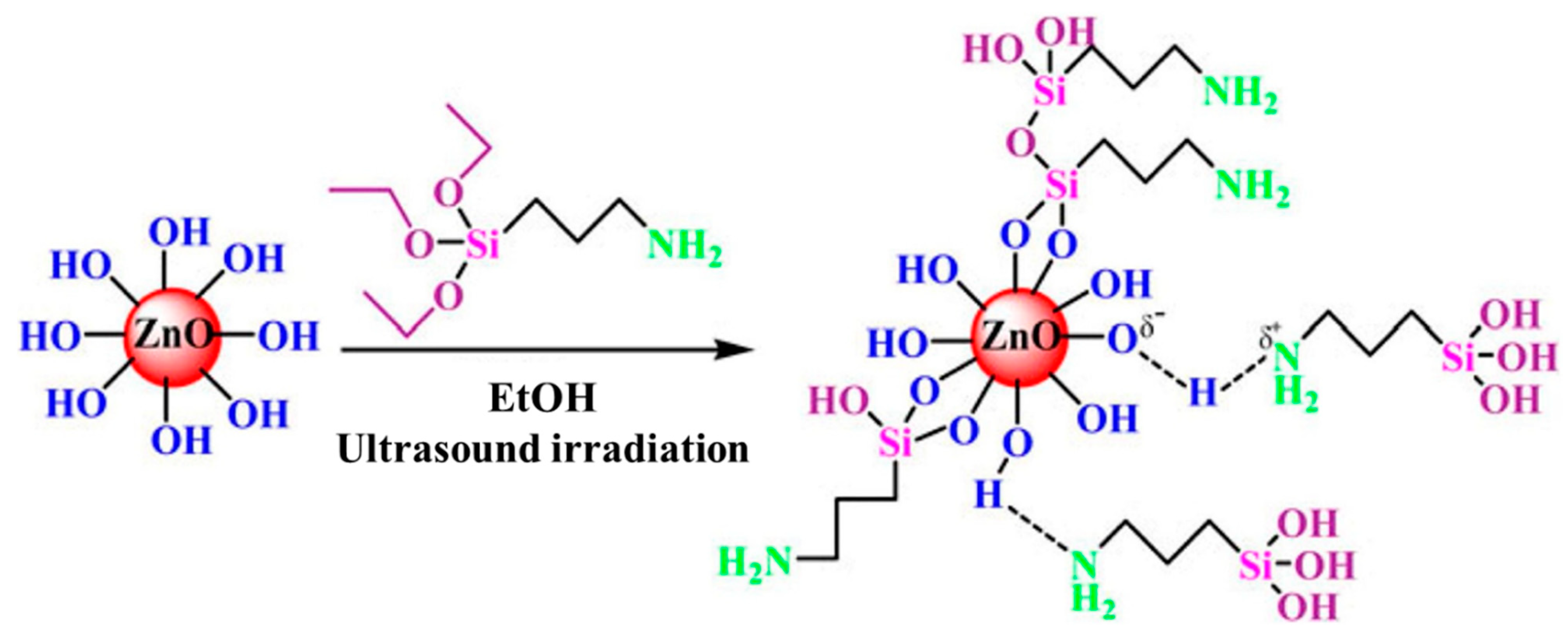
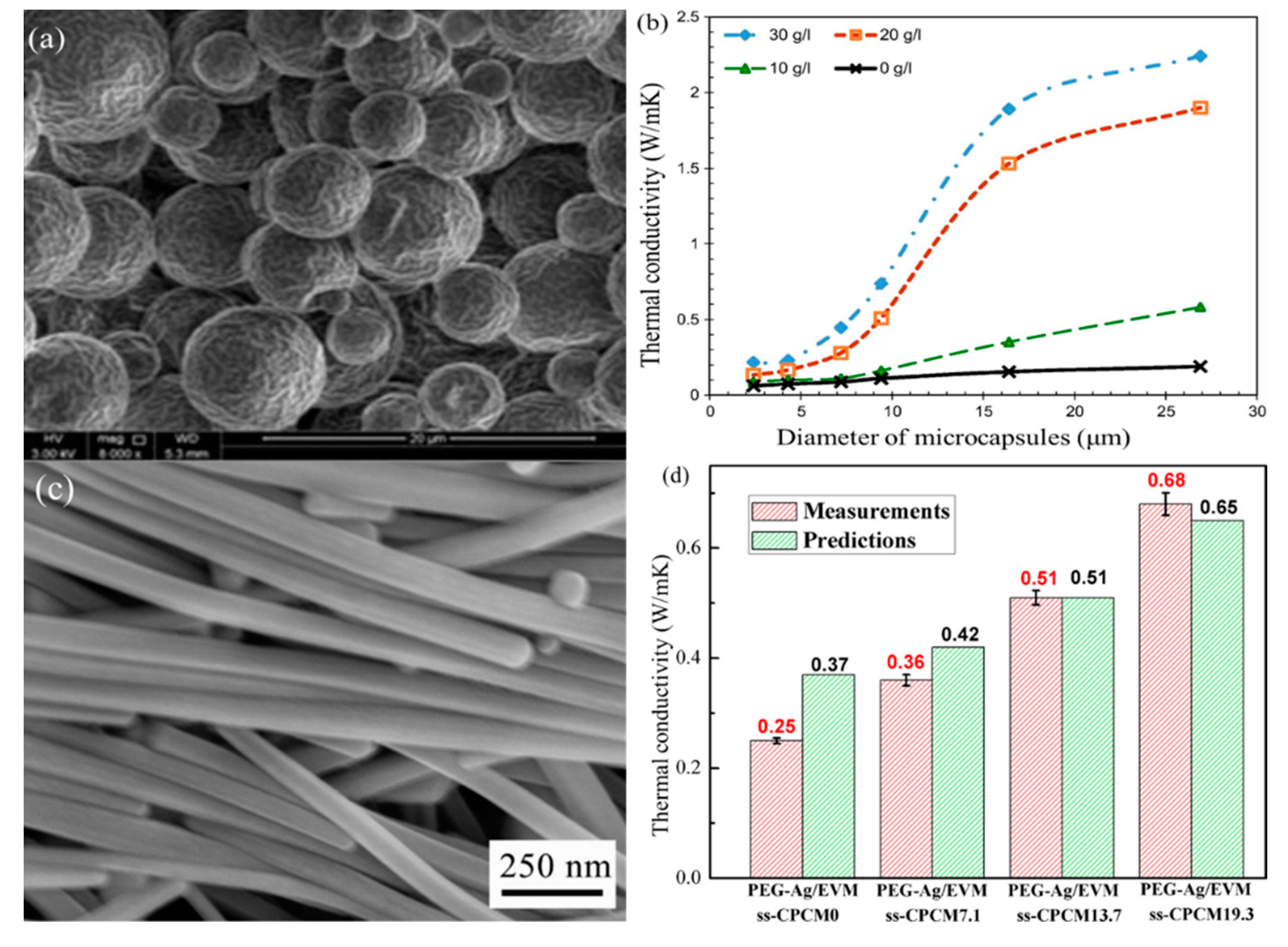
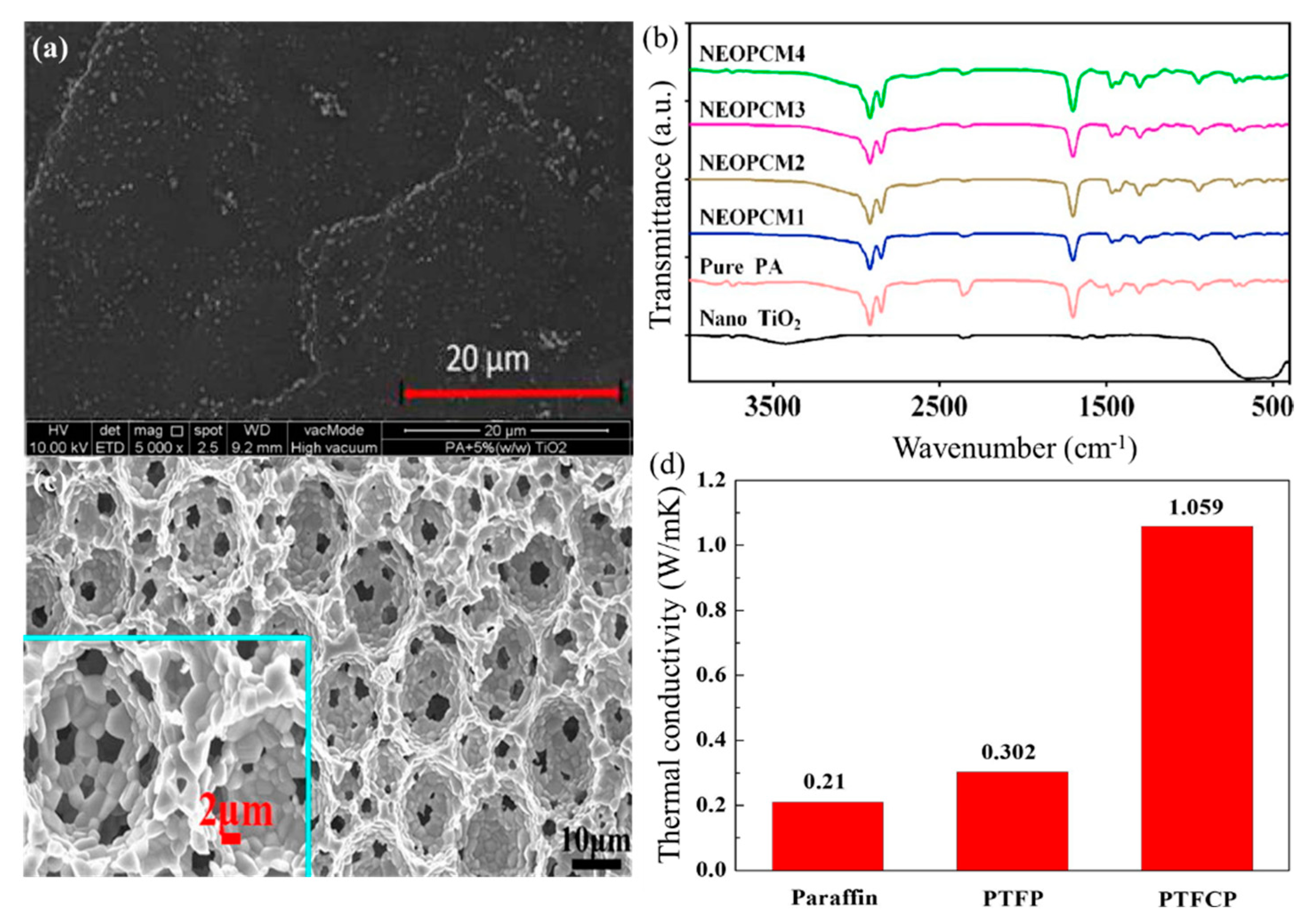
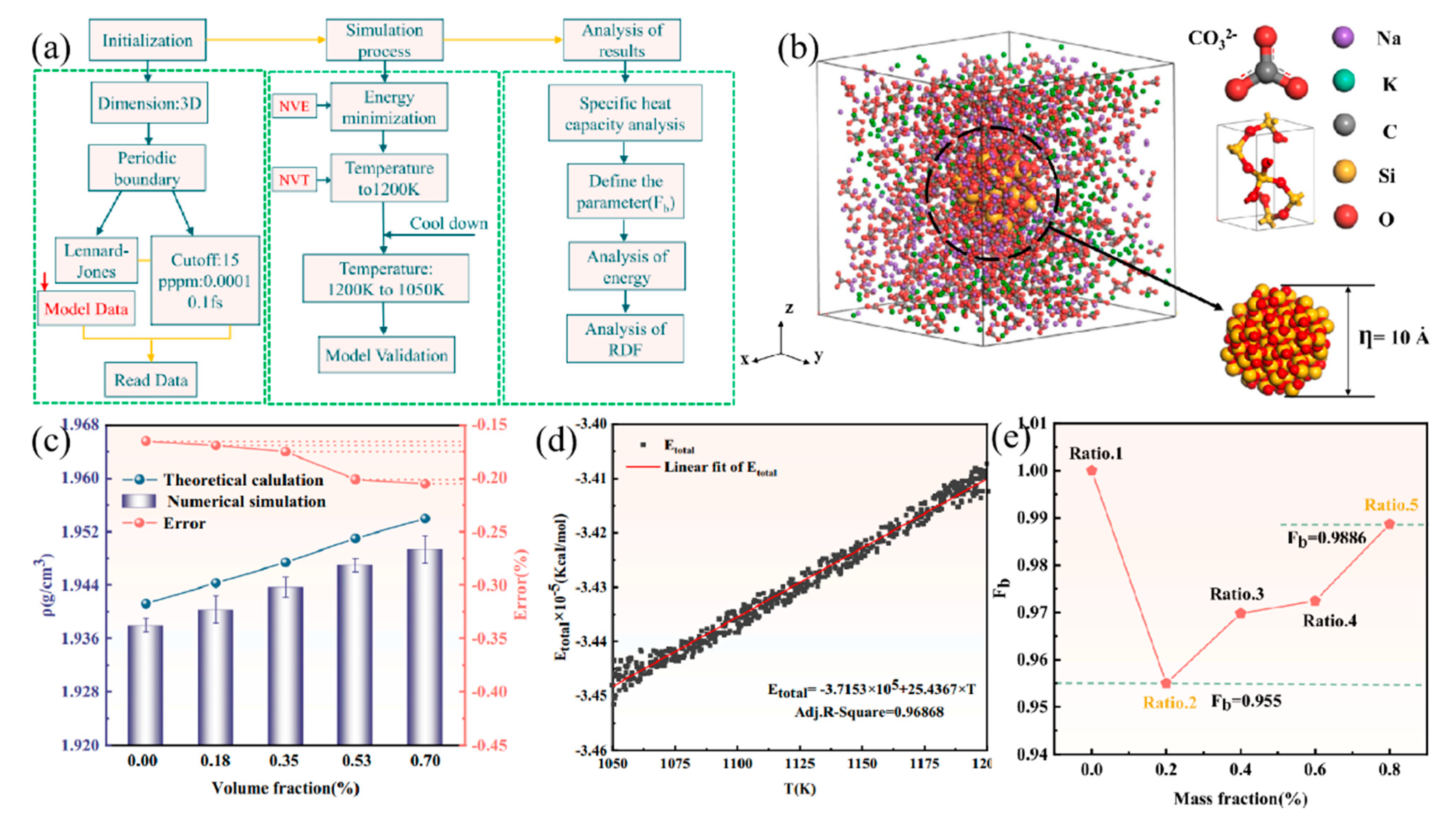
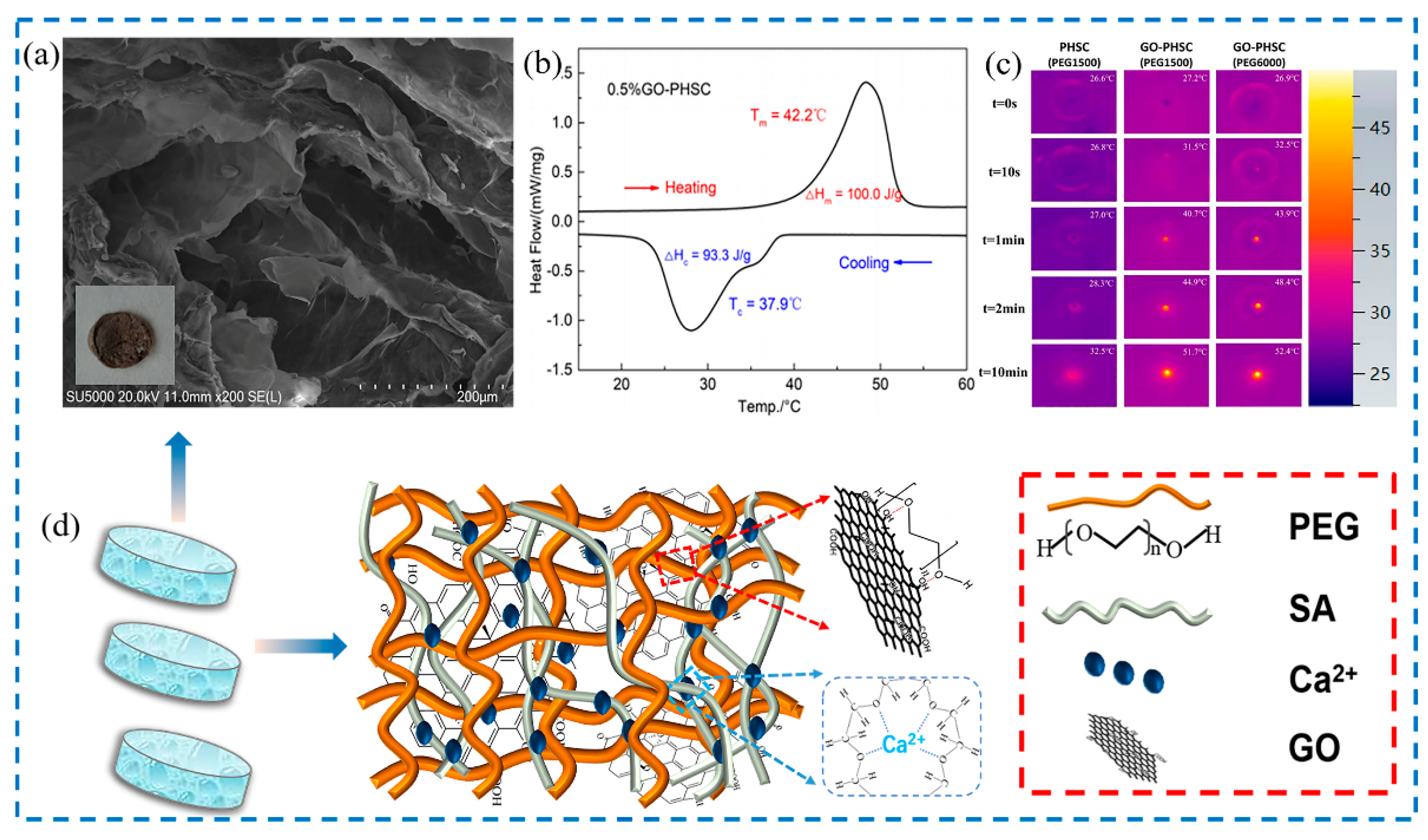
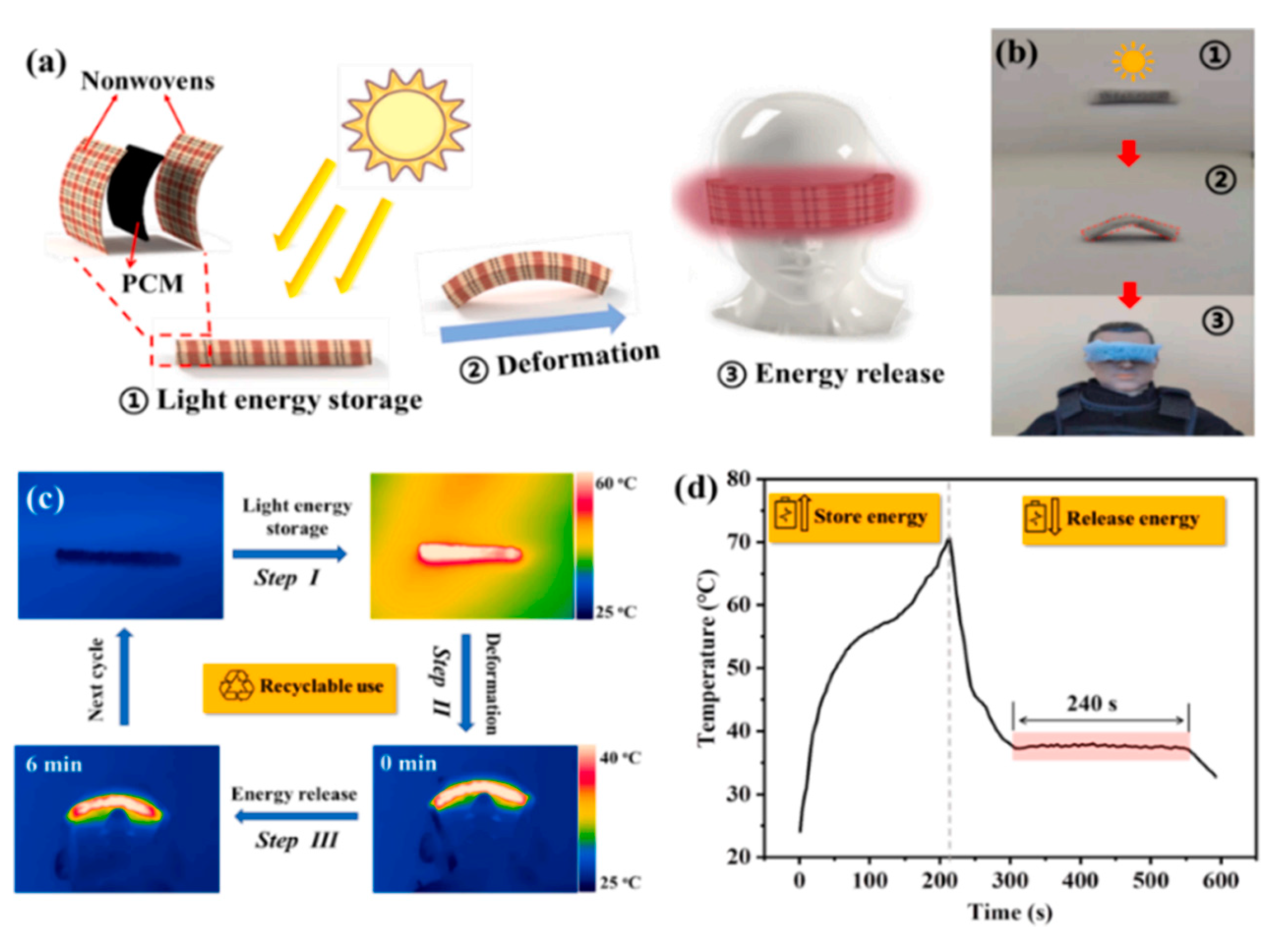
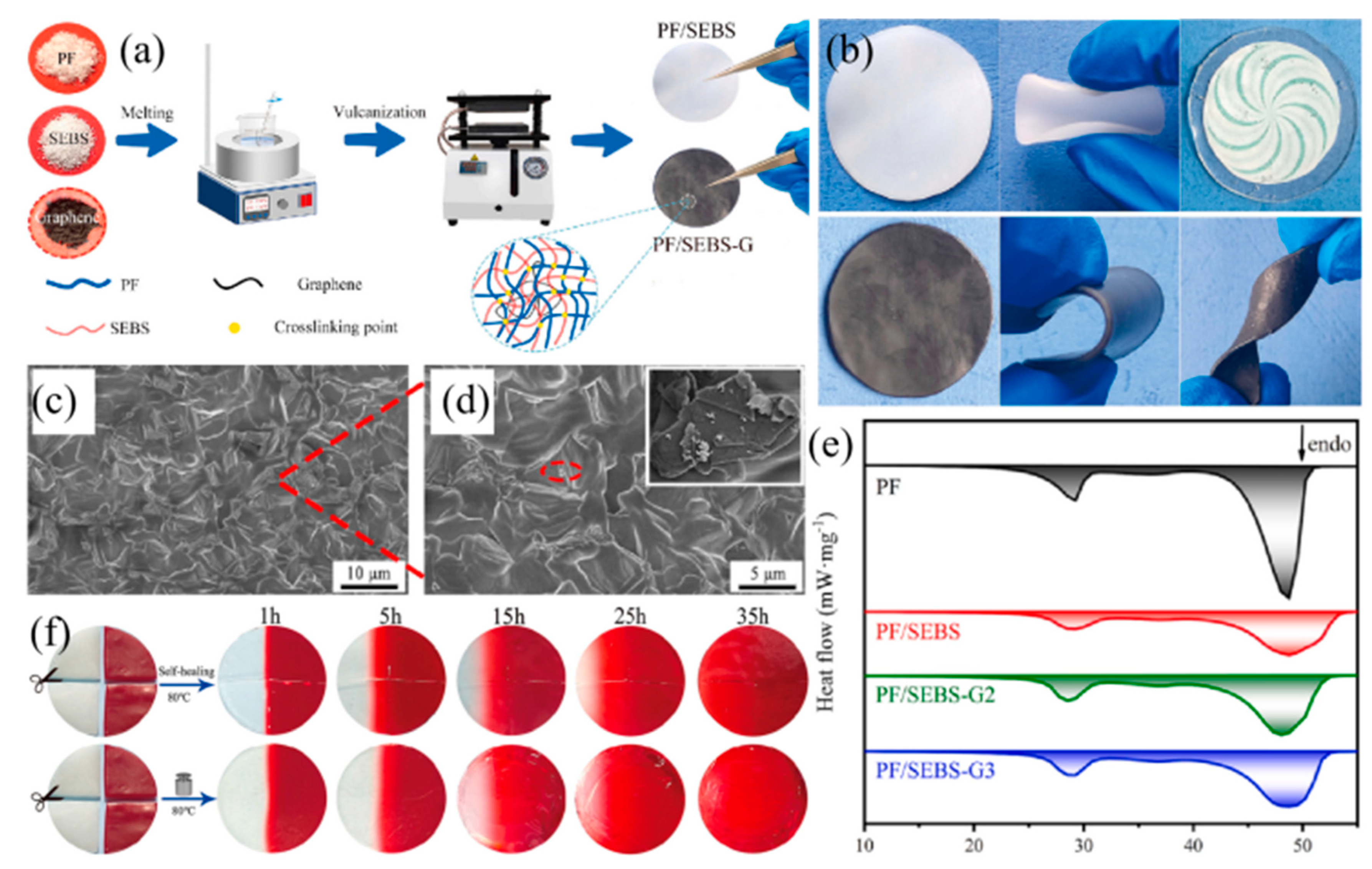

| Materials | Heating | Cooling | ||
|---|---|---|---|---|
| Melting Temperature (°C) | Melting Latent Heat (J/g) | Crystallization Temperature (°C) | Crystallization Latent Heat (J/g) | |
| C20 | 36.18 | 275.91 | 32.92 | 268.13 |
| ExP/C20 (60%) | 36.12 | 161.18 | 34.32 | 155.26 |
| ExP/C20 (60%)/Carbon nanotubes (0.3 wt%) | 36.24 | 160.38 | 34.32 | 155.26 |
| ExP/C20 (60%)/Carbon nanotubes (0.5 wt%) | 36.34 | 159.02 | 35.55 | 145.92 |
| ExP/C20 (60%)/Carbon nanotubes (1 wt%) | 36.47 | 157.43 | 35.53 | 141.15 |
| PCM | Application | Description | References |
|---|---|---|---|
| Polyethylene glycol (PEG) | Tissue Engineering | PCM embedded in hydrogel scaffold for temperature control during photothermal therapy. | [96] |
| Lauric acid (LA) | Drug Delivery | Utilization of PCMs for precise temperature-controlled drug release, enhancing therapeutic efficacy. | [98] |
| N-eicosane PCM | Antibacterial Dressings | Incorporation of silver-modified PCMs in medical bandages for wound care, reducing infection risks. | [107] |
| Polyethylene glycol (PEG) | Hygiene Products | PCM-treated fabrics in hygiene products like diapers for moisture control and antimicrobial benefits. | [109] |
| Polyethylene glycol (PEG) | Thermotherapy Masks | CNT-based aerogel/PEG composites in masks for thermotherapy, offering relief from nasal conditions. | [9] |
| Polyethylene glycol (PEG) | Thermal Eye Patches | Flexible PCM composites for thermal eye patches, ensuring comfort and temperature control. | [110] |
| Fatty acids (FAs) | Wound Healing Dressings | Temperature-responsive PCM hydrogel dressings for promoting wound healing. | [112] |
| Paraffin | Thermal Therapy Films | PF/SEBS-G3 films with graphene for thermal therapy, featuring flexibility and self-healing properties. | [113] |
| Tetradecane–dodecanol–decanoic acid (TDDA) | Cold Chain Logistics | TDDA/EG PCM for maintaining temperature stability in cold storage boxes during the transportation of vaccines and medical products. | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Le, T.C.; Zhao, S.; Shang, C.; Hu, M.; Zhang, S.; Liu, Y.; Pan, S. Advancements in Nanomaterial Dispersion and Stability and Thermophysical Properties of Nano-Enhanced Phase Change Materials for Biomedical Applications. Nanomaterials 2024, 14, 1126. https://doi.org/10.3390/nano14131126
Zhang Q, Le TC, Zhao S, Shang C, Hu M, Zhang S, Liu Y, Pan S. Advancements in Nanomaterial Dispersion and Stability and Thermophysical Properties of Nano-Enhanced Phase Change Materials for Biomedical Applications. Nanomaterials. 2024; 14(13):1126. https://doi.org/10.3390/nano14131126
Chicago/Turabian StyleZhang, Qian, Tkhu Chang Le, Shuang Zhao, Chenxi Shang, Menglin Hu, Su Zhang, Yushi Liu, and Shuang Pan. 2024. "Advancements in Nanomaterial Dispersion and Stability and Thermophysical Properties of Nano-Enhanced Phase Change Materials for Biomedical Applications" Nanomaterials 14, no. 13: 1126. https://doi.org/10.3390/nano14131126




